-
PDF
- Split View
-
Views
-
Cite
Cite
Candice D. Fike, Marta Sidoryk-Wegrzynowicz, Michael Aschner, Marshall Summar, Lawrence S. Prince, Gary Cunningham, Mark Kaplowitz, Yongmei Zhang, Judy L. Aschner, Prolonged hypoxia augments l-citrulline transport by System A in the newborn piglet pulmonary circulation, Cardiovascular Research, Volume 95, Issue 3, 1 August 2012, Pages 375–384, https://doi.org/10.1093/cvr/cvs186
Close - Share Icon Share
Abstract
Pulmonary arterial endothelial cells (PAECs) express the enzymes needed for generation of l-arginine from intracellular l-citrulline but do not express the enzymes needed for de novol-citrulline synthesis. Hence, l-citrulline levels in PAECs are dependent on l-citrulline transport. Once generated, l-arginine can be converted to l-citrulline and nitric oxide (NO) by the enzyme NO synthase. We sought to determine whether hypoxia, a condition aetiologically linked to pulmonary hypertension, alters the transport of l-citrulline and the expression of the sodium-coupled neutral amino acid transporters (SNATs) in PAECs from newborn piglets.
PAECs isolated from newborn piglets were cultured under normoxic and hypoxic conditions and used to measure SNAT1, 2, 3, and 5 protein expression and 14C-l-citrulline uptake. SNAT1 protein expression was increased, while SNAT2, SNAT3, and SNAT5 expression was unaltered in hypoxic PAECs. 14C-l-citrulline uptake was increased in hypoxic PAECs. Studies with inhibitors of System A (SNAT1/2) and System N (SNAT3/5) revealed that the increased 14C-l-citrulline uptake was largely due to System A-mediated transport. Additional studies were performed to evaluate SNAT protein expression and l-citrulline levels in lungs of piglets with chronic hypoxia-induced pulmonary hypertension and comparable age controls. Lungs from piglets raised in chronic hypoxia exhibited greater SNAT1 expression and higher l-citrulline levels than lungs from controls.
Increased SNAT1 expression and the concomitant enhanced ability to transport l-citrulline in PAECs could represent an important regulatory mechanism to counteract NO signalling impairments known to occur during the development of chronic hypoxia-induced pulmonary hypertension in newborns.
1. Introduction
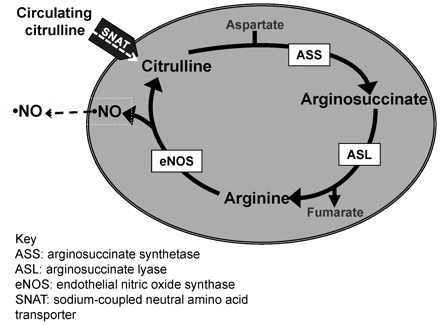
l-Citrulline provides an intracellular source for l-arginine via a biosynthetic pathway involving the enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL).1l-Arginine is the substrate for nitric oxide synthase (NOS), which generates nitric oxide (NO) and citrulline; together these two pathways form a mechanism for citrulline recycling1 (Figure 1). The regulation of NO biosynthesis via intracellular metabolism of l-citrulline is poorly understood and may represent a novel target for therapeutic intervention for conditions associated with NO dysfunction, such as some forms of neonatal pulmonary hypertension.2–4 While pulmonary arterial endothelial cells (PAECs) express ASS and ASL, they do not express the enzymes needed for de novo synthesis of l-citrulline.5 Thus, intracellular citrulline concentrations depend, at least in part, on the uptake of circulating blood-borne l-citrulline produced primarily in the intestines.6,7 Remarkably, little is known about the neutral amino acid transporters responsible for l-citrulline transport into PAECs.8
Schematic of the citrulline recycling pathway in endothelial cells. Note that there are currently five known SNAT proteins (SNATs 1–5).
The Na+-dependent neutral amino acid transporters (SNATs) are one of the major systems responsible for neutral amino acid transport in mammalian cells.9–11 SNATs are expressed in most cell types and are categorized as System A or System N, dependent in part on their functional properties. Recently, the SLC38 gene family of transporters were identified, renamed SNATs 1–5, and subdivided based on the similarity of their transport properties to System A or System N.12 Many factors, including hormones, growth factors, and hyperosmotic stress, have been associated with changes in the activities and expression of SNAT proteins.9–15 However, to date, little is known about the impact on SNAT expression or l-citrulline transport in PAECs from conditions such as hypoxia that are associated with the development of pulmonary hypertension.16
Newborn piglets exposed to prolonged hypoxia develop pulmonary hypertension.17 The primary goal of this study was to determine whether prolonged hypoxia alters expression of SNAT proteins and l-citrulline uptake by PAECs isolated from newborn piglets. We also performed studies to determine whether SNAT expression and citrulline levels are altered in lungs of piglets exposed to 3 or 10 days of hypoxia.
2. Methods
2.1 Animals: in vivo hypoxia model
York-Landrace mixed breed piglets were obtained from the vendor on day of life 2 (n = 14) and raised in a normobaric hypoxic environment until either day of life 5 (3 days of hypoxia; n = 7) or day of life 12 (10 days of hypoxia; n = 7) following our previously described methods.2,17 O2 content was regulated at 10–12% O2. CO2 was absorbed with soda lime, and PCO2 was maintained at 3–6 Torr. The chamber was opened twice each day to clean the chamber and to weigh the animals. The piglets were fed artificial sow milk ad libitum. Normoxic, age-matched control animals were either 5 days old (n = 7) or 12 days old (n = 7) when obtained from the vendor and studied on the day of arrival, i.e. at the same post-natal ages as the hypoxic piglets.
To obtain the tissue used in these experiments, 5-day-old or 12-day-old piglets were pre-anaesthetized with ketamine (30 mg/kg im) and acepromazine (2 mg/kg im) and then anaesthetized with pentobarbital sodium (10–20 mg/kg iv). All animals were given heparin (1000 IU/kg iv) and then exsanguinated. The depth of anaesthesia prior to exsanguination was monitored by assessing consciousness and response to painful stimuli. The thorax was opened and the lungs were removed. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23) and was approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Use.
2.2 Whole lung tissue and pulmonary artery isolation
Distal pieces of whole lung and pulmonary arteries >1 mm were dissected from both age-matched control and hypoxic piglet groups, frozen in liquid nitrogen, and stored at −80°C until use.
2.3 PAEC isolation
The main pulmonary artery was isolated from the lungs of 5-day-old piglets (n = 5) and used to obtain PAECs following modified methods.18 Each pulmonary artery was flushed with PBS, then filled with 0.25% trypsin-EDTA and incubated for 5 min. The pulmonary artery was then gently flushed with endothelial growth medium (EGM-2, Lonza) and supplemental foetal bovine serum (FBS, 10%) to remove the endothelial cells. The harvested endothelial cells were cultured in EBM-2 in 100 mm plates in a humidified, normoxic incubator (21% O2, 5% CO2) at 37°C. PAECs were identified by their cobblestone morphology and positive staining for endothelial nitric oxide synthase (eNOS). Cells were subcultured at near confluence and used at passages 4–10.
2.4 Pulmonary artery smooth muscle cell isolation
Pulmonary arteries <1 mm were dissected from the lungs of 5-day-old piglets (n = 5) and the surrounding adventitia and connective tissue removed. Pulmonary artery smooth muscle cell (PASMCs) were obtained from the cleaned arteries by enzymatic digestion with collagenase (5%) using modified methods.19,20 The PASMCs were cultured in Dulbecco's modified Eagle Medium (DMEM) and supplemental FBS (10%) in 100 mm plates in a humidified, normoxic incubator (21% O2, 5% CO2) at 37°C. PASMCs were identified by their typical elongated morphology19 and positive staining for smooth muscle cell myosin heavy chain and α-actin. Cells were subcultured at near confluence and used at passages 3–5.
2.5 PAEC and PASMC protocols
To assess the effect of hypoxia on SNAT1, 2, 3, or 5 protein expression and citrulline uptake, confluent monolayers of PAECs or PASMCs from piglet primary cell lines were passaged from 100 mm plates to 6-well plates and cultured overnight in EGM-2 or DMEM under normoxic conditions. The next morning, the media was changed and the PAECs or PASMCs were placed into either a normoxic (21% O2, 5% CO2, 37°C) or hypoxic (4% O2, 5% CO2, 37°C) humidified environment. The hypoxic environment was carefully maintained at the desired levels of oxygen and CO2 using PROOX model 110 oxygen and CO2 control systems (Biospherix, Ltd., Redfield, NY, USA).
2.6 Citrulline uptake
After 24, 48, or 72 h in normoxic or hypoxic culture conditions, PAECs or PASMCs were washed three times with 2 mL of fresh sodium-HEPES buffer composed of the following: 122 mM NaCl, 3.3 mM KCl, 0.4 mM MgSO4, 1.2 mM CaCl2, 1.2 mM KH2PO4, 10 mM glucose and 25 mM HEPES adjusted to pH of 7.4 with 10 mM NaOH. Cells were incubated under normoxic conditions (21% O2, 5% CO2, 37°C) with 0.25 μCi/mL 14C-l-citrulline (specific activity: 56.3 mCi/mmol; Perkin Elmer, Norwalk, CT, USA) in the presence of a saturating concentration (200 μM total concentration) of unlabelled citrulline using our previously described methods.21 Some PAECs or PASMCs were also incubated in the presence of system-selective competing amino acids. Competitors used to select for system A were glutamine, histidine, threonine, and leucine, each 10 mM and chosen for use because these amino acids are preferentially transported by Systems N, ASC, or L, respectively. Competitors to select for system N were 2-methylaminoisobutyric acid, leucine, and threonine, each 10 mM and chosen for use because these amino acids are transported by Systems A, L, or ASC, respectively. Incubations were stopped after 1, 5, and 10 min with ice-cold sodium-HEPES buffer using cells in different wells at each of the three time points. All cells were lysed with 1 M NaOH for 30 min at 37°C. An aliquot of the lysate was transferred to a scintillation vial, LSC cocktail (Fisher Scientific, Pittsburgh, PA, USA) was added and radioactivity was measured in a scintillation counter (Beckman LS 6500; Brea, CA, USA). The remainder of the lysate was used for protein determination by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Uptake of l-citrulline was expressed as CPM per mg protein.
2.7 Protein analysis by immunoblot technique
After 24, 48, or 72 h in normoxic or hypoxic environments, PAECs or PASMCs were washed two times with PBS, then collected and stored at −80°C. Frozen PAECs, PASMCs, pulmonary arteries, or lung samples were crushed under liquid N2 in a pre-chilled mortar and pestle into a fine powder, transferred to a tube containing homogenization buffer with protease inhibitors, and then sonicated. Protein concentrations for all homogenates were determined by the protein assay (Bradford). Using our previously described methods,22 supernatants were applied to tris–glycine pre-cast 4–20% polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) so that equal amounts of protein were loaded. Electrophoresis was carried out and the proteins were transferred from the gel to a nitrocellulose membrane. The membrane was incubated at room temperature in PBS containing 7.5% non-fat dried milk and 0.1% Tween-20 to block non-specific protein binding. To detect the protein of interest, the nitrocellulose membrane was incubated overnight with the primary antibody from ABCAM (Cambridge, MA, USA) (SNAT1, 1:800 for PAECs or 1:1000 for PASMCs, the lung, and pulmonary artery homogenates; SNAT2, 1:500 for PAECs or 1: 1000 for the lung and pulmonary artery homogenates; SNAT3, 1:1000 for PAECs or 1:500 for the lung and pulmonary artery homogenates; SNAT5, 1:900 for PAECs or 1:1000 for the lung and pulmonary artery homogenates) diluted in PBS containing 0.1% Tween-20 and 1% non-fat dried milk (carrier buffer); followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Zymed) diluted in the carrier buffer (1:5000). The membranes were developed using enhanced chemiluminescence reagents (ECL, Amersham CO.) and the chemiluminescent signal was captured on X-ray film (ECL Hyperfilm, Kodak). Similar procedures were followed to reprobe the membranes for either β-actin (Sigma, 1:50,000 on membranes used for PAECs) or GAPDH (ABCAM, 1:1000 on membranes used for the lung, pulmonary artery, and PASMC homogenates). The bands for each protein were quantified using densitometry.
2.8 Immunolocalization of SNAT1 in lungs
Paraffin-embedded lung sections from 5-day-old control piglets were subjected to antigen retrieval using high pH Target Retrieval Solution (Dako, Carpinteria, CA, USA). Sections were labelled using primary antibodies against SNAT1 (1:100, ABCAM) or eNOS (1:100, BD, San Jose, CA, USA). Alexa-fluorophore containing secondary antibodies (Invitrogen) and TO-PRO-3 (Invitrogen) nuclear stain were used for visualization. Images were acquired using a TCS SP2 laser scanning confocal system (Leica Microsystems, Buffalo Grove, IL, USA).
2.9 Lung amino acid determination
Frozen lung samples were used to measure levels of the following amino acids: citrulline, glutamine (known to be transported by System N and System A), alanine (known to be transported by System A but not by System N), and histidine (known to be preferentially transported by System N). For amino acid analyses, a 20 mg piece of frozen lung tissue was homogenized in 100 μL 1ysis buffer, allowed to sit on ice for 30 min, and then centrifuged at 400× g to remove the largest pieces of lung tissue. Next, 100 μL of the supernatant was diluted with 200 mL of 0.15 M sulfosalicylic acid, allowed to sit on ice for 30 min, and then centrifuged to precipitate the larger protein molecules. Amino acid levels were measured in the supernatant using a Hitachi L8800 amino acid analyzer (Hitachi USA, San Jose, CA, USA).2
2.10 Data analyses
Data are presented as mean ± SEM. An unpaired t-test was used to compare data between normoxic and hypoxic tissues. P-values <0.05 were considered statistically significant.
3. Results
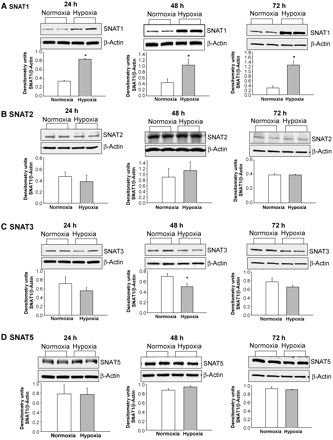
SNAT1 protein expression was greater in PAECs cultured under hypoxic conditions compared with PAECs cultured under normoxic conditions for all time periods studied: 24, 48, and 72 h (Figure 2A). SNAT2 (Figure 2B) and SNAT5 (Figure 2D) protein expression did not differ between PAECs cultured under normoxic vs. hypoxic conditions at any time point. SNAT3 protein (Figure 2C) was reduced in PAECs cultured in hypoxia for 48 h but not for 24 or 72 h compared with PAECs cultured in normoxia.
Immunoblot results and corresponding densitometry for SNAT1, 2, 3, and 5 protein expression normalized to β-actin in PAECs cultured under normoxic and hypoxic conditions for 24, 48, or 72 h. (A–D) Representative immunoblots and corresponding densitometry of studies that were performed in duplicate or triplicate using PAECs from four to five different piglets. *Different from normoxia; P < 0.05.
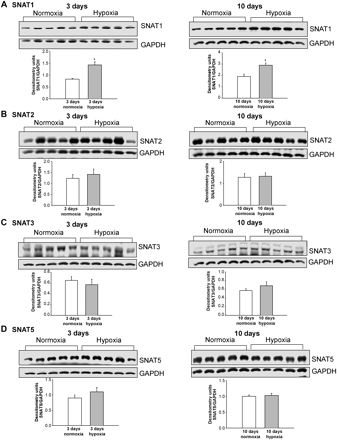
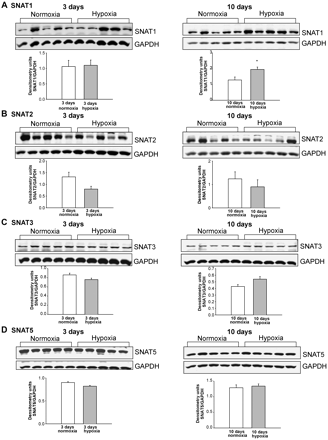
We also determined whether in vitro findings in PAECs cultured under prolonged hypoxic conditions correlate with in vivo changes in SNAT expression in the lungs and pulmonary arteries of piglets with pulmonary hypertension induced by prolonged exposure to hypoxia. Consistent with the in vitro data (Figure 2A), SNAT1 protein expression was increased in lungs from both age groups of hypoxic piglets (Figure 3A). Although not discernibly altered in pulmonary arteries from piglets exposed to in vivo hypoxia for 3 days (Figure 4A), SNAT1 protein expression was increased in pulmonary arteries from piglets exposed to in vivo hypoxia for 10 days (Figure 4A). We found no difference in SNAT2, SNAT3, or SNAT5 protein expression in lungs (Figure 3) or pulmonary arteries (Figure 4) of hypoxic piglets vs. lungs or pulmonary arteries of their comparable age normoxic control piglets.
(A–D) Immunoblot results and corresponding densitometry for SNAT protein expression normalized to GAPDH in lungs from piglets raised under normoxic and hypoxic conditions for either 3 days (n = 5 normoxic and n = 5 hypoxic piglets) or 10 days (n = 5 normoxic piglets and n = 5 hypoxic piglets); *different from normoxic; P < 0.05.
(A–D) Immunoblot results and corresponding densitometry for SNAT protein expression normalized to GAPDH in pulmonary arteries from piglets raised under normoxic and hypoxic conditions for either 3 days (n = 5 normoxic piglets and n = 5 hypoxic piglets) or 10 days (n = 5 normoxic piglets and n = 5 hypoxic piglets); *different from normoxic; P < 0.05.
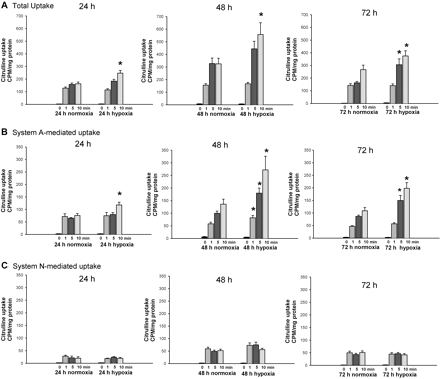
Next, we performed a series of studies to determine whether exposure to prolonged hypoxia alters l-citrulline uptake in PAECs from newborn piglets. 14C-l-citrulline uptake was increased in PAECs cultured under hypoxic conditions for all three time periods, 24, 48, and 72 h (Figure 5A). By selectively inhibiting System A (SNAT1/2) or System N (SNAT3/5), we found that for all three time points the enhanced 14C-l-citrulline uptake was largely due to System A transport (Figure 5B).
Citrulline uptake in PAECs cultured under normoxic and hypoxic conditions for 24, 48, or 72 h: (A) total uptake; (B) System A-mediated uptake (determined by the use of amino acid competitors to select for System A: glutamine, histidine, threonine, and leucine, amino acids preferentially transported by Systems N, ASC, and L, respectively); (C) System N-mediated uptake (determined by the use of amino acid competitors to select for System N: 2-methylaminoisobutyric acid, leucine, and threonine, amino acids preferentially transported by Systems A, L, or ASC, respectively). All studies were performed in triplicate using PAECs from three to four different piglets. *Different from normoxic; P < 0.05.
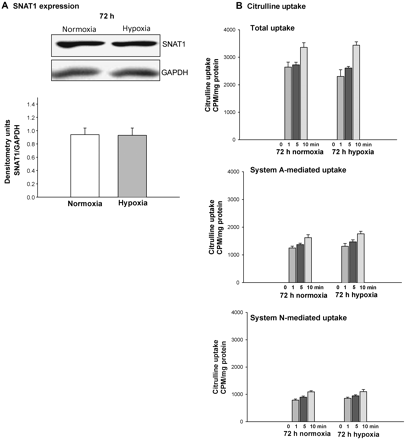
We also determined whether exposure to hypoxia for 72 h alters SNAT1 expression or l-citrulline uptake in PASMCs from newborn piglets. Unlike PAECs (Figure 2 and Figure 5), neither SNAT1 expression (Figure 6A) nor 14C-l-citrulline uptake (Figure 6B) were altered in PASMCs exposed to prolonged in vitro hypoxia.
SNAT1 protein expression and citrulline uptake in PASMC cultured under normoxic and hypoxic conditions for 72 h. (A) Representative immunoblot for SNAT1 and corresponding densitometry for SNAT1 normalized to GAPDH in studies performed using PASMC from five different piglets. (B) Total, System A, and System N-mediated citrulline uptake in studies performed in triplicate with PASMC from two different piglets.
Immunohistochemistry was performed to determine whether cell types in addition to PAECs and PASMC express SNAT1 in lungs of piglets. As shown in Supplementary material online, Figure S1, SNAT1 is widely expressed in multiple cell types, including in airway epithelial cells.
Amino acid analysis revealed that citrulline levels were greater in the lungs of both groups of hypoxic piglets than in lungs of their comparable age control piglets (Table 1). Alanine levels were increased in lungs of piglets exposed to 10 days of hypoxia, whereas histidine levels were decreased in lungs of both groups of hypoxic piglets.
Amino acid levels in piglet lungs
| . | Citrulline, μM . | Alanine, μM . | Glutamine, μM . | Histidine, μM . |
|---|---|---|---|---|
| Three-day exposure group | ||||
| Normoxia (n = 4) | 3 ± 1 | 331 ± 41 | 1389 ± 50 | 21 ± 3 |
| Hypoxic (n = 7) | 10a ± 2 | 370 ± 34 | 1230 ± 73 | 8a ± 2 |
| Ten-day exposure group | ||||
| Normoxia (n = 7) | 4 ± 1 | 272 ± 19 | 1380 ± 57 | 20 ± 1 |
| Hypoxia (n = 7) | 16a ± 3 | 419a ± 50 | 1334 ± 89 | 10a ± 1 |
| . | Citrulline, μM . | Alanine, μM . | Glutamine, μM . | Histidine, μM . |
|---|---|---|---|---|
| Three-day exposure group | ||||
| Normoxia (n = 4) | 3 ± 1 | 331 ± 41 | 1389 ± 50 | 21 ± 3 |
| Hypoxic (n = 7) | 10a ± 2 | 370 ± 34 | 1230 ± 73 | 8a ± 2 |
| Ten-day exposure group | ||||
| Normoxia (n = 7) | 4 ± 1 | 272 ± 19 | 1380 ± 57 | 20 ± 1 |
| Hypoxia (n = 7) | 16a ± 3 | 419a ± 50 | 1334 ± 89 | 10a ± 1 |
aDifferent from preceding control; P < 0.05.
Amino acid levels in piglet lungs
| . | Citrulline, μM . | Alanine, μM . | Glutamine, μM . | Histidine, μM . |
|---|---|---|---|---|
| Three-day exposure group | ||||
| Normoxia (n = 4) | 3 ± 1 | 331 ± 41 | 1389 ± 50 | 21 ± 3 |
| Hypoxic (n = 7) | 10a ± 2 | 370 ± 34 | 1230 ± 73 | 8a ± 2 |
| Ten-day exposure group | ||||
| Normoxia (n = 7) | 4 ± 1 | 272 ± 19 | 1380 ± 57 | 20 ± 1 |
| Hypoxia (n = 7) | 16a ± 3 | 419a ± 50 | 1334 ± 89 | 10a ± 1 |
| . | Citrulline, μM . | Alanine, μM . | Glutamine, μM . | Histidine, μM . |
|---|---|---|---|---|
| Three-day exposure group | ||||
| Normoxia (n = 4) | 3 ± 1 | 331 ± 41 | 1389 ± 50 | 21 ± 3 |
| Hypoxic (n = 7) | 10a ± 2 | 370 ± 34 | 1230 ± 73 | 8a ± 2 |
| Ten-day exposure group | ||||
| Normoxia (n = 7) | 4 ± 1 | 272 ± 19 | 1380 ± 57 | 20 ± 1 |
| Hypoxia (n = 7) | 16a ± 3 | 419a ± 50 | 1334 ± 89 | 10a ± 1 |
aDifferent from preceding control; P < 0.05.
4. Discussion
In this investigation, we report a number of novel findings regarding prolonged hypoxia, SNATs protein expression, and l-citrulline transport in lungs of newborn piglets. In particular, we show for the first time that prolonged hypoxia augments both SNAT1 protein expression and l-citrulline uptake in PAECs from newborn piglets.
SNATs are classically categorized as System A or System N, dependent in part on their functional properties and patterns of regulation. System A subtypes, which include SNAT1, SNAT2, and SNAT4 are characterized by a broader amino acid substrate profile than System N subtypes, which include SNAT3 and SNAT5.12,23 More specifically, SNAT1 and SNAT2 (also referred to as ATA1 and ATA2 respectively) show a high affinity to alanine, serine, proline, and glycine but also recognize N-methylated amino acids, like 2-methylaminisobutyric acid.12,24 SNAT3 (also referred to as SN1 or NAT) recognizes the classic System N substrates,23 glutamine, asparagine, and histidine, whereas SNAT5 (also known as SN2) favours serine.25 SNATs are found in most mammalian cells.11,12 Our finding that SNAT1 is expressed in multiple lung cell types, including airway epithelial cells, was therefore not surprising. Moreover, it is well known that a number of different transport systems may occur in the same cell membrane. In this regard and consistent with our findings, SNAT1, SNAT2, SNAT3, and SNAT5 have previously been reported to be expressed in lung tissue.12,25,26 SNATs expression may differ between tissues. For example, SNAT4 is considered to be liver specific12 and was not evaluated by us.
The transport of neutral amino acids into endothelial cells has been studied for decades.9 However, likely due to the unknown molecular identity of these transporters until very recently,12 information about the expression of SNAT1, 2, 3, 4, and 5 in endothelial cells from any vascular bed is scarce. Specifically, cells from the human umbilical venous endothelial cell (HUVEC)-derived permanent line EA.hy926 and primary HUVECs were shown by quantitative real-time RT-PCR to express SNAT3.27 To our knowledge, there are no reports regarding the expression of the SNAT family in pulmonary vascular endothelial cells. We add to the literature and show that SNAT1, 2, 3, and 5 are expressed in PAECs from newborn piglets.
There is also very limited information available about the impact of hypoxia on SNAT expression and/or activity in any tissue or cell type. In cultured cells from the human placenta, 24 h of hypoxia reduced the expression and function of System A transporters.28 Six hours of hypoxia reduced the substrate affinity of System A transporters in cultured human lung fibroblasts.29 SNAT1 expression decreased in rat pup brains 24 h after hypoxia-ischaemia but increased 7 days later.30 We now report that 24, 48, and 72 h of in vitro hypoxia increases SNAT1 expression in PAECs, that 10 days in vivo hypoxia increases SNAT1 expression in pulmonary arteries, and that 3 and 10 days in vivo exposure to hypoxia increases lung SNAT1 expression.
In addition to altering transporter expression, we found that prolonged in vitro hypoxia for durations of 24, 48, and 72 h increases the functional ability of PAECs to transport citrulline. Previous investigators found no change in citrulline transport in PAECs from adult pigs cultured for up to 24 h in hypoxia.16 The discrepancy in findings could be due to the age of the animals, the degree of hypoxia, or to different experimental conditions. Of great interest, our findings with competitive transport inhibitors show that the hypoxia-induced increase in citrulline transport is due to System A, which includes SNAT1. Our findings are in line with the many studies that have shown that changes in function and expression of System A transporters occur in the placenta and mammary gland during pregnancy.13,31–33 Moreover, changes in SNAT1 have recently been described in cardiomyocytes and astrocytes during oxidative stress.14,15 It is also important to note that our findings point to a role for System A in citrulline transport in normoxic PAECs.
There is some, albeit limited, information available about citrulline transport in organs other than lungs and in cells other than endothelial cells. For example, studies with rat kidney slices found that basolateral l-citrulline uptake was largely due to the Na+-independent organic anion transporter, OAT1.34 Yet, in studies with rat renal proximal tubular epithelial cells and the human kidney proximal tubular epithelial cell line, HK-2, apical uptake of l-citrulline was shown to be mediated by at least two distinct Na+-dependent transport systems and one Na+-independent transport system.35 Likewise, studies with Caco-2 cells, a model of human intestinal epithelial cells, found that apical l-citrulline uptake was mediated both by Na+-dependent (via System B(0,+)) and Na+-independent (via Systems L and b(0,+)) systems.36 In contrast, a study with everted rat small intestinal sacs indicated that l-citrulline uptake is predominantly Na+ dependent.37 Na+-independent System L was shown to mediate l-citrulline uptake in a variety of neural cell cultures;38 whereas l-citrulline transport in rat aortic smooth muscle cells was shown to be primarily mediated by System L but was also partially dependent on System N.39l-Citrulline transport in macrophages also appears to be partially Na+ dependent.40 Thus, the transporters involved in l-citrulline uptake may not be the same in all organs and cells.
More relevant to our novel findings, we know of only a few other studies that have evaluated citrulline transport in endothelial cells of any vascular bed. Citrulline uptake by bovine aortic endothelial cells was shown to be modulated by P1,P4-diadenosine 5′-tetraphosphate (Ap4A)2, an α,ω-dinucleotide found in platelets.41 Glutamine, an amino acid that is transported by both System A and N, has been shown to compete with and thereby inhibit citrulline transport in endothelial cells of the systemic circulation, including HUVECs,27 bovine aortic endothelial cells, bovine coronary endothelial cells, and human mesenteric microvascular endothelial cells.42 Notably, whether System A or N transporters were involved with citrulline transport was not evaluated in any of these studies.
Of interest, a few studies have evaluated transport of neutral amino acids other than l-citrulline in PAECs. In particular, the l-glutamine influx in porcine PAECs43 and rat lung microvascular endothelial cells44 was shown to be predominantly mediated by the Na+-dependent system, ASC. l-Histidine uptake in rat lung microvascular endothelial cells was shown to involve both the Na+-dependent System N and the Na+-independent System L.8l-Glutamic acid uptake in bovine PAECs was previously shown to be mostly Na+ dependent.45 Taken together, these studies show that Na+-dependent systems are involved in neutral amino acid transport in PAECs. However, the role of these systems in citrulline transport in PAECs has not been previously evaluated.
Our results indicate that Systems A and N account for ∼60% of total citrulline uptake in PAECs (Figure 5). Delineation of the other transport systems, including Na+-independent systems, involved in citrulline uptake in PAECs will require future studies. Regardless, our findings are provocative as they identify the system of transporters, System A, responsible for an increase in citrulline transport under hypoxic conditions in PAECs.
It is important to note that our findings with PASMCs contrast with our findings with PAECs. Indeed, the lack of change in SNAT1 and citrulline transport in hypoxic PASMCs indicates that exposure to prolonged hypoxia does not influence citrulline transport similarly in all lung cell types. Moreover, findings with PASMCs indicate that hypoxia-induced alterations in lung levels of citrulline are unlikely to be due to any contribution from PASMCs.
Our findings of increased citrulline levels in lungs of hypoxic piglets merit some comment. First, the concomitant elevation in alanine and reduction in histidine support the idea that System A transporters are responsible for the enhanced amino acid levels. This is because alanine is transported by System A and not by System N and histidine is preferentially transported by System N.12 Secondly, we know of no other studies that have evaluated lung citrulline levels in an animal model of chronic hypoxia-induced pulmonary hypertension. However, lungs of lambs with pulmonary hypertension induced by an elevated blood flow were found to have diminished citrulline levels.46 The physiological impact of altered lung citrulline levels in the context of pulmonary hypertension is not yet clear.
In summary, the novel findings in this study provide important insights into a little explored area of NO biology in the pulmonary circulation. In particular, we show that prolonged hypoxia increases SNAT1 expression with accompanying increases in System A-mediated citrulline transport in PAECs of newborn piglets. These in vitro findings appear to have in vivo relevance as both citrulline levels and SNAT1 protein are increased in lungs of piglets with chronic hypoxia-induced pulmonary hypertension. In light of recent publications demonstrating the ameliorative effects of supplemental citrulline in two different models of neonatal pulmonary hypertension,2,4 an increase in SNAT1 expression and concomitant enhanced ability to transport citrulline could be an important regulatory mechanism capable of modulating NO production, particularly under conditions of chronic hypoxia. Manipulation of SNAT1 expression or citrulline transport (Figure 1) merits further investigation as a novel therapeutic approach for neonatal patients with pulmonary hypertension.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: C.F., J.A., and M.S. are listed as inventors on a patent application to treat lung diseases with intravenous citrulline.
Funding
This work was supported by the National Institutes of Health (HL97566 to C.F., HL68572 to C.F., HL075511 to J.A., ES07331 to M.A., ES10563 to M.A. and HD061221 to M.S.).