-
PDF
- Split View
-
Views
-
Cite
Cite
J Tamargo, G Rosano, T Walther, J Duarte, A Niessner, JC Kaski, C Ceconi, H Drexel, K Kjeldsen, G Savarese, C Torp-Pedersen, D Atar, BS Lewis, S Agewall, Gender differences in the effects of cardiovascular drugs, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 3, Issue 3, July 2017, Pages 163–182, https://doi.org/10.1093/ehjcvp/pvw042
Close - Share Icon Share
Abstract
Although sex-specific differences in cardiovascular medicine are well known, the exact influences of sex on the effect of cardiovascular drugs remain unclear. Women and men differ in body composition and physiology (hormonal influences during the menstrual cycle, menopause, and pregnancy) and they present differences in drug pharmacokinetics (absorption, distribution, metabolism, and excretion) and pharmacodynamics, so that is not rare that they may respond differently to cardiovascular drugs. Furthermore, women are also less often treated with evidence-based drugs thereby preventing optimization of therapeutics for women of all ages, experience more relevant adverse drug reactions than men, and remain underrepresented in most clinical trials. Thus, current guidelines for prevention, diagnosis, and medical treatment for cardiovascular diseases are based on trials conducted predominantly in middle-aged men. A better understanding of these sex-related differences is fundamental to improve the safety and efficacy of cardiovascular drugs and for developing proper individualized cardiovascular therapeutic strategies both in men and women. This review briefly summarizes gender differences in the pharmacokinetics and pharmacodynamics of cardiovascular drugs and provides recommendations to close the gaps in our understanding of sex-specific differences in drug efficacy and safety.
Introduction
Cardiovascular diseases (CVD) are the leading cause of morbidity and mortality in both sexes.1–6 In the past, the risk of CVD was underestimated in women due to a misperception that females were protected against CVD.1–6 Furthermore, women develop coronary artery disease (CAD) around 10 years later than men and at that time present a higher prevalence of cardiovascular risk factors, so they were more likely to be excluded from clinical trials.5–9 Even nowadays CVD are commonly perceived to be a health problem only for men, leaving women with an inadequate prevention vulnerable to CVD. However, even when women during the fertile period have a lower risk of cardiovascular events, this protection decreases after menopause, so that CVD is the major cause of death in women older than 65 years of age.1–10 In Europe, CVD cause a greater proportion of deaths among women (51%) than men (42%) overall, i.e. they kill twice as many women as all forms of cancer combined.1,2
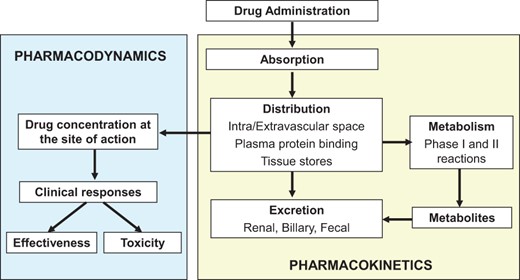
Men and women differ in the anatomy and physiology of the cardiovascular system (body composition, role of hormonal changes during menstrual cycle/pregnancy/menopause) and in risk factors, prevalence, symptoms, management, and outcomes of CVD.11–22 There are also gender-related differences in the pharmacokinetics (PK) (i.e. the way drugs are absorbed, distributed, biotransformed, and excreted) and pharmacodynamics (PD) (the relationship between drug effect and drug concentration at the site of action) of some widely used cardiovascular drugs12,13 (Figure 1). Thus, it would not be a surprise that efficacy and safety of these drugs can differ between men and woman.13–21 However, the reported clinical relevance of these differences in PK/PD is moderate or remains uncertain, mainly because women are underrepresented in clinical trials.14 Thus, current guidelines for CVD are based on studies conducted predominantly on middle-aged men. As expected, the lack of evidence on the gender difference in the efficacy and safety of cardiovascular therapeutic interventions leads to poor appropriateness. For these reasons, there has been growing attention of the European Society of Cardiology on the gender-related differences in the effects of cardiovascular drugs.1,2,4,13,20 Taking into account these issues, the aims of this review are to summarize the effects of gender on PK/PD of cardiovascular drugs; to identify the scientific gaps that exist regarding to cardiovascular therapy in women; and to improve the treatment of CVD from a gender perspective. Throughout the text the terms ‘sex’, which is genetically determined, and ‘gender’, which refers to the socially constructed characteristics of women and men (such as norms, roles and relationships of and between groups of women and men), will be used as synonyms.
Schematic representation of the interrelationship of the absorption, distribution, metabolism, and excretion of a drug (pharmacokinetics) and its concentration at the site of action (pharmacodynamics).
Gender differences in pharmacokinetics
Sex-based differences in PK may arise from differences in body composition, drug absorption, plasma and tissue distribution, metabolizing enzymes and transporters, and drug excretion12–19,23–29 (Table 1). Oral drug absorption is influenced by gastric pH, gastrointestinal transit times, blood flow and presystemic gut, and hepatic metabolism. Gastric acid secretion is lower and gastrointestinal transit times are slower in women, whereas gut metabolism does not consistently vary by sex.15–19,23–30 A prolonged gastrointestinal transit can decrease the absorption of metoprolol or verapamil and drugs requiring an acidic environment for absorption may have lower oral bioavailability in women and they should wait longer after eating before taking drugs that should be administered on an empty stomach.27 Formulations designed to be absorbed in the duodenum (i.e. enteric-coated aspirin) may exhibit reduced/delayed absorption in women, particularly after a meal.31 However, transdermal absorption is similar in both sexes.12,15,29
Gender differences in absorption, distribution, metabolism, and excretion
| Parameter . | Sex differences . |
|---|---|
| Drug bioavailability | |
| Absorption | M > W |
| Gastric acid secretion | M > W > P. Decreases absorption of weak acids but increases absorption of weak bases in M |
| Gastric emptying | M > W > P. E inhibit gastric empting |
| Gastrointestinal transit times | |
| Gut metabolism | M = W |
| Body composition | |
| Body surface area | M > P > W. Absorption increases when body surface is larger |
| Organ (heart) size | M > W |
| Organ blood flow | Greater blood flow to skeletal muscle and liver in M; greater to adipose tissue in W. Blood flow increases during P |
| Total body water | M > P > W |
| Plasma volume | P > M > W. Varies during the menstrual cycle and P |
| Body fat content | W > M |
| Cardiac output | M > P > W. Increase rate of distribution in M |
| Pulmonary function | M > P > W. Increase pulmonary elimination in M |
| Drug distribution | |
| Volume of distribution | W > M. Higher Vd for lipophilic drugs in W |
| M > W. Higher Vd for hydrophilic drugs in M | |
| Plasma protein binding to | |
| Albumin | M = W. P and OCP reduce plasma albumin and increases free drug plasma levels |
| α1-acid glycoprotein | M > W. E, OC and P decrease its plasma levels |
| Globulins | E increase sex-hormone binding, corticosteroid-binding and thyroxine-binding globulins |
| Drug transporters | |
| Hepatic P-glycoprotein | M > W |
| OCT2 | M > W. E downregulates OCT2 |
| OATP1B1-3 | M > W |
| Drug metabolizing enzymes and transporters | |
| Phase I metabolic reactions (hydrolysis, oxidation, reduction) mediated via cytochrome P450 (CYP) isoforms | CYP1A2: M > W. Decreased in pregnancy and by OCP |
| CYP2B6: W > M | |
| CYP2C9: M = W | |
| CYP2C19: M = W | |
| Decreases in pregnancy and by OCP | |
| CYP3A4: W > M. Increases by OCP | |
| CYP2D6: M > W. E induces and OCP decreases CYP2D6 activity | |
| CYP2E1: M > W. Increases by OCP | |
| Phase II metabolism | |
| Uridine diphosphate glucuronosyltransferases (UGTs 1/2) | M > W. Increase by OCP and E and during pregnancy |
| N-Acetyltransferases | M = W |
| Catechol-O-methyltransferase | M > W |
| Acetyl-/Butyryl-cholinesterase | M > W |
| Xantine-oxidase | W > M |
| Gastric alcohol dehydrogenase | M > W. Higher alcohol plasma levels in W |
| Drug excretion | |
| M > W. Renal Cl increases during P |
| Drugs actively secreted by the kidney may show sex differences in renal excretion | |
| Parameter . | Sex differences . |
|---|---|
| Drug bioavailability | |
| Absorption | M > W |
| Gastric acid secretion | M > W > P. Decreases absorption of weak acids but increases absorption of weak bases in M |
| Gastric emptying | M > W > P. E inhibit gastric empting |
| Gastrointestinal transit times | |
| Gut metabolism | M = W |
| Body composition | |
| Body surface area | M > P > W. Absorption increases when body surface is larger |
| Organ (heart) size | M > W |
| Organ blood flow | Greater blood flow to skeletal muscle and liver in M; greater to adipose tissue in W. Blood flow increases during P |
| Total body water | M > P > W |
| Plasma volume | P > M > W. Varies during the menstrual cycle and P |
| Body fat content | W > M |
| Cardiac output | M > P > W. Increase rate of distribution in M |
| Pulmonary function | M > P > W. Increase pulmonary elimination in M |
| Drug distribution | |
| Volume of distribution | W > M. Higher Vd for lipophilic drugs in W |
| M > W. Higher Vd for hydrophilic drugs in M | |
| Plasma protein binding to | |
| Albumin | M = W. P and OCP reduce plasma albumin and increases free drug plasma levels |
| α1-acid glycoprotein | M > W. E, OC and P decrease its plasma levels |
| Globulins | E increase sex-hormone binding, corticosteroid-binding and thyroxine-binding globulins |
| Drug transporters | |
| Hepatic P-glycoprotein | M > W |
| OCT2 | M > W. E downregulates OCT2 |
| OATP1B1-3 | M > W |
| Drug metabolizing enzymes and transporters | |
| Phase I metabolic reactions (hydrolysis, oxidation, reduction) mediated via cytochrome P450 (CYP) isoforms | CYP1A2: M > W. Decreased in pregnancy and by OCP |
| CYP2B6: W > M | |
| CYP2C9: M = W | |
| CYP2C19: M = W | |
| Decreases in pregnancy and by OCP | |
| CYP3A4: W > M. Increases by OCP | |
| CYP2D6: M > W. E induces and OCP decreases CYP2D6 activity | |
| CYP2E1: M > W. Increases by OCP | |
| Phase II metabolism | |
| Uridine diphosphate glucuronosyltransferases (UGTs 1/2) | M > W. Increase by OCP and E and during pregnancy |
| N-Acetyltransferases | M = W |
| Catechol-O-methyltransferase | M > W |
| Acetyl-/Butyryl-cholinesterase | M > W |
| Xantine-oxidase | W > M |
| Gastric alcohol dehydrogenase | M > W. Higher alcohol plasma levels in W |
| Drug excretion | |
| M > W. Renal Cl increases during P |
| Drugs actively secreted by the kidney may show sex differences in renal excretion | |
References are presented in Supplementary material online, Table S1. Cl, clearance; E, oestrogens; GFR, glomerular filtration rate; GI, gastrointestinal; M, men; OCP, oral contraceptives; OATP, organic anion-transporter polypeptide; OCT, organic cationic transporter; P, pregnancy; P-gp, P-glycoprotein; Vd, volume of distribution; W, women.
Gender differences in absorption, distribution, metabolism, and excretion
| Parameter . | Sex differences . |
|---|---|
| Drug bioavailability | |
| Absorption | M > W |
| Gastric acid secretion | M > W > P. Decreases absorption of weak acids but increases absorption of weak bases in M |
| Gastric emptying | M > W > P. E inhibit gastric empting |
| Gastrointestinal transit times | |
| Gut metabolism | M = W |
| Body composition | |
| Body surface area | M > P > W. Absorption increases when body surface is larger |
| Organ (heart) size | M > W |
| Organ blood flow | Greater blood flow to skeletal muscle and liver in M; greater to adipose tissue in W. Blood flow increases during P |
| Total body water | M > P > W |
| Plasma volume | P > M > W. Varies during the menstrual cycle and P |
| Body fat content | W > M |
| Cardiac output | M > P > W. Increase rate of distribution in M |
| Pulmonary function | M > P > W. Increase pulmonary elimination in M |
| Drug distribution | |
| Volume of distribution | W > M. Higher Vd for lipophilic drugs in W |
| M > W. Higher Vd for hydrophilic drugs in M | |
| Plasma protein binding to | |
| Albumin | M = W. P and OCP reduce plasma albumin and increases free drug plasma levels |
| α1-acid glycoprotein | M > W. E, OC and P decrease its plasma levels |
| Globulins | E increase sex-hormone binding, corticosteroid-binding and thyroxine-binding globulins |
| Drug transporters | |
| Hepatic P-glycoprotein | M > W |
| OCT2 | M > W. E downregulates OCT2 |
| OATP1B1-3 | M > W |
| Drug metabolizing enzymes and transporters | |
| Phase I metabolic reactions (hydrolysis, oxidation, reduction) mediated via cytochrome P450 (CYP) isoforms | CYP1A2: M > W. Decreased in pregnancy and by OCP |
| CYP2B6: W > M | |
| CYP2C9: M = W | |
| CYP2C19: M = W | |
| Decreases in pregnancy and by OCP | |
| CYP3A4: W > M. Increases by OCP | |
| CYP2D6: M > W. E induces and OCP decreases CYP2D6 activity | |
| CYP2E1: M > W. Increases by OCP | |
| Phase II metabolism | |
| Uridine diphosphate glucuronosyltransferases (UGTs 1/2) | M > W. Increase by OCP and E and during pregnancy |
| N-Acetyltransferases | M = W |
| Catechol-O-methyltransferase | M > W |
| Acetyl-/Butyryl-cholinesterase | M > W |
| Xantine-oxidase | W > M |
| Gastric alcohol dehydrogenase | M > W. Higher alcohol plasma levels in W |
| Drug excretion | |
| M > W. Renal Cl increases during P |
| Drugs actively secreted by the kidney may show sex differences in renal excretion | |
| Parameter . | Sex differences . |
|---|---|
| Drug bioavailability | |
| Absorption | M > W |
| Gastric acid secretion | M > W > P. Decreases absorption of weak acids but increases absorption of weak bases in M |
| Gastric emptying | M > W > P. E inhibit gastric empting |
| Gastrointestinal transit times | |
| Gut metabolism | M = W |
| Body composition | |
| Body surface area | M > P > W. Absorption increases when body surface is larger |
| Organ (heart) size | M > W |
| Organ blood flow | Greater blood flow to skeletal muscle and liver in M; greater to adipose tissue in W. Blood flow increases during P |
| Total body water | M > P > W |
| Plasma volume | P > M > W. Varies during the menstrual cycle and P |
| Body fat content | W > M |
| Cardiac output | M > P > W. Increase rate of distribution in M |
| Pulmonary function | M > P > W. Increase pulmonary elimination in M |
| Drug distribution | |
| Volume of distribution | W > M. Higher Vd for lipophilic drugs in W |
| M > W. Higher Vd for hydrophilic drugs in M | |
| Plasma protein binding to | |
| Albumin | M = W. P and OCP reduce plasma albumin and increases free drug plasma levels |
| α1-acid glycoprotein | M > W. E, OC and P decrease its plasma levels |
| Globulins | E increase sex-hormone binding, corticosteroid-binding and thyroxine-binding globulins |
| Drug transporters | |
| Hepatic P-glycoprotein | M > W |
| OCT2 | M > W. E downregulates OCT2 |
| OATP1B1-3 | M > W |
| Drug metabolizing enzymes and transporters | |
| Phase I metabolic reactions (hydrolysis, oxidation, reduction) mediated via cytochrome P450 (CYP) isoforms | CYP1A2: M > W. Decreased in pregnancy and by OCP |
| CYP2B6: W > M | |
| CYP2C9: M = W | |
| CYP2C19: M = W | |
| Decreases in pregnancy and by OCP | |
| CYP3A4: W > M. Increases by OCP | |
| CYP2D6: M > W. E induces and OCP decreases CYP2D6 activity | |
| CYP2E1: M > W. Increases by OCP | |
| Phase II metabolism | |
| Uridine diphosphate glucuronosyltransferases (UGTs 1/2) | M > W. Increase by OCP and E and during pregnancy |
| N-Acetyltransferases | M = W |
| Catechol-O-methyltransferase | M > W |
| Acetyl-/Butyryl-cholinesterase | M > W |
| Xantine-oxidase | W > M |
| Gastric alcohol dehydrogenase | M > W. Higher alcohol plasma levels in W |
| Drug excretion | |
| M > W. Renal Cl increases during P |
| Drugs actively secreted by the kidney may show sex differences in renal excretion | |
References are presented in Supplementary material online, Table S1. Cl, clearance; E, oestrogens; GFR, glomerular filtration rate; GI, gastrointestinal; M, men; OCP, oral contraceptives; OATP, organic anion-transporter polypeptide; OCT, organic cationic transporter; P, pregnancy; P-gp, P-glycoprotein; Vd, volume of distribution; W, women.
Drug distribution depends on body composition, plasma volume, organ blood flow, and tissue and plasma protein binding.15,18,24,25 Sex hormones modulate drug plasma protein binding but limited data support that these gender differences significantly affect pharmacological effects. Women have higher percent of body fat and lower body weight, plasma volume and organ size, and blood flow. This explains the faster onset, higher volume of distribution (Vd), and longer effects of lipophilic drugs (anaesthetics, benzodiazepines, neuromuscular blockers) (Table 2), while the Vd of hydrophilic drugs is smaller, reaching higher peak plasma levels (Cmax) and greater effects as compared with men.15–18,24,25 Therefore, drugs requiring loading-dosages [i.e. some antiarrhythmics (amiodarone, lidocaine, procainamide), digoxin, heparin, thrombolytics] can reach higher Cmax and produce a higher risk of adverse drug reactions (ADRs) in women.27,29 In patients with obesity or marked increases in extracellular volume (e.g. heart failure), differences in body composition may alter drug distribution.29,32
Sex-related differences in drug pharmacokinetic parameters
| Drug class . | Outcomes in females . |
|---|---|
| Anaesthetics: propofol | Plasma propofol levels decline more rapidly in W at the end of infusion |
| Alcohol | Lower gastric alcohol dehydrogenase activity in W. Higher plasma concentrations in W as compared with M following an equivalent drink |
| Antidepressants | Higher AUC and Cmax in W |
| H1-antihistamines | Slower metabolism and elimination in W |
| Antipsychotic drugsa | Higher plasma levels and Vd and lower Cl in W. Reduce the dosage in W or increase dosage in M. Olanzapine is more rapidly eliminated in M than in W |
| Aspirin | Bioavailability and plasma levels of aspirin and salicylate are higher in W possibly due to lower activity of aspirin esterase, larger Vd and lower Cl in W than in M. Differences disappear with OCP |
| Benzodiazepines | Lower initial plasma levels due to larger Vd, and possibly higher Cl, in W. OC reduce their Cl. Higher plasma levels of free diazepam in W |
| Beta-receptor agonists | W are less sensitive |
| Beta blockers: metoprolol, propranolol | W have higher plasma levels due to a smaller Vd and slower Cl. Drug exposure to metoprolol increases by OC |
| Renal Cl of atenolol and metoprolol increases during P due to enhanced hepatic metabolism | |
| Calcium channel blockers | Faster Cl of verapamil, and nifedipine in W. Increased bioavailability and decreased clearance of oral verapamil in W compared with M |
| Digoxin | W have higher serum digoxin concentrations due to reduced Vd and lower Cl. Drug Cl increases during P |
| Glucocorticoids | Oral Cl and Vd of prednisolone are higher in M. Prednisolone clearance was reduced by OC |
| Heparin | W had higher plasma levels and APTT values than M due to a lower Cl |
| Iron | Oral absorption of iron is greater in W than in M |
| Isosorbide mononitrate | W had significantly higher serum plasma concentrations compared with men, probably due to the lower body weights in females |
| Labetalol | Labetalol concentrations are 80% higher in W |
| Lidocaine | W has a larger Vd and may require a higher i.v. bolus dose than M. Higher free plasma levels in W receiving OCP, as alpha 1-acid glycoprotein levels are reduced by oestrogens |
| μ-opioid (OP3) receptor agonistsb | Slower onset and offset of action in W |
| Neuromuscular blocking drugsc | Lower Vd, higher plasma levels, faster onset and prolonged duration in W due to the higher body fat and lower Vd |
| Paracetamol | Lower plasma levels and higher Cl in M due to increased activity of the glucuronidation pathway. OCP increase drug clearance |
| Procainamide | Plasma levels are higher (30%) in W due to a lower BMI and Vd |
| Quinidine | Plasma protein binding decreases during P |
| Selective serotonin reuptake inhibitorsd | W present higher plasma levels, probably related to sex-related activity of various CYP enzymes |
| Statins | Higher plasma levels of lovastatin and simvastatin in W |
| Theophylline | Metabolism is faster and half-life is shorter in W than in M. Plasma protein binding decreases and the Vd increases during P |
| Torasemide | Higher Cmax and lower Cl in W than in M |
| Tricyclic antidepressants | Free plasma concentrations of imipramine, clomipramine, and nortriptyline are higher during pregnancy |
| Verapamil | W display faster Cl of verapamil after i.v. administration probably due to the higher activity of CYP3A4 or lower activity of P-gp; lower Cl in W after oral administration |
| Vorapaxar | Cmax and AUC are 30% higher in women but no dose adjustment is required |
| Warfarin | Higher free plasma levels in W |
| Zolpidem | Plasma levels and AUC are higher, and Cl is lower in W |
| Drug class . | Outcomes in females . |
|---|---|
| Anaesthetics: propofol | Plasma propofol levels decline more rapidly in W at the end of infusion |
| Alcohol | Lower gastric alcohol dehydrogenase activity in W. Higher plasma concentrations in W as compared with M following an equivalent drink |
| Antidepressants | Higher AUC and Cmax in W |
| H1-antihistamines | Slower metabolism and elimination in W |
| Antipsychotic drugsa | Higher plasma levels and Vd and lower Cl in W. Reduce the dosage in W or increase dosage in M. Olanzapine is more rapidly eliminated in M than in W |
| Aspirin | Bioavailability and plasma levels of aspirin and salicylate are higher in W possibly due to lower activity of aspirin esterase, larger Vd and lower Cl in W than in M. Differences disappear with OCP |
| Benzodiazepines | Lower initial plasma levels due to larger Vd, and possibly higher Cl, in W. OC reduce their Cl. Higher plasma levels of free diazepam in W |
| Beta-receptor agonists | W are less sensitive |
| Beta blockers: metoprolol, propranolol | W have higher plasma levels due to a smaller Vd and slower Cl. Drug exposure to metoprolol increases by OC |
| Renal Cl of atenolol and metoprolol increases during P due to enhanced hepatic metabolism | |
| Calcium channel blockers | Faster Cl of verapamil, and nifedipine in W. Increased bioavailability and decreased clearance of oral verapamil in W compared with M |
| Digoxin | W have higher serum digoxin concentrations due to reduced Vd and lower Cl. Drug Cl increases during P |
| Glucocorticoids | Oral Cl and Vd of prednisolone are higher in M. Prednisolone clearance was reduced by OC |
| Heparin | W had higher plasma levels and APTT values than M due to a lower Cl |
| Iron | Oral absorption of iron is greater in W than in M |
| Isosorbide mononitrate | W had significantly higher serum plasma concentrations compared with men, probably due to the lower body weights in females |
| Labetalol | Labetalol concentrations are 80% higher in W |
| Lidocaine | W has a larger Vd and may require a higher i.v. bolus dose than M. Higher free plasma levels in W receiving OCP, as alpha 1-acid glycoprotein levels are reduced by oestrogens |
| μ-opioid (OP3) receptor agonistsb | Slower onset and offset of action in W |
| Neuromuscular blocking drugsc | Lower Vd, higher plasma levels, faster onset and prolonged duration in W due to the higher body fat and lower Vd |
| Paracetamol | Lower plasma levels and higher Cl in M due to increased activity of the glucuronidation pathway. OCP increase drug clearance |
| Procainamide | Plasma levels are higher (30%) in W due to a lower BMI and Vd |
| Quinidine | Plasma protein binding decreases during P |
| Selective serotonin reuptake inhibitorsd | W present higher plasma levels, probably related to sex-related activity of various CYP enzymes |
| Statins | Higher plasma levels of lovastatin and simvastatin in W |
| Theophylline | Metabolism is faster and half-life is shorter in W than in M. Plasma protein binding decreases and the Vd increases during P |
| Torasemide | Higher Cmax and lower Cl in W than in M |
| Tricyclic antidepressants | Free plasma concentrations of imipramine, clomipramine, and nortriptyline are higher during pregnancy |
| Verapamil | W display faster Cl of verapamil after i.v. administration probably due to the higher activity of CYP3A4 or lower activity of P-gp; lower Cl in W after oral administration |
| Vorapaxar | Cmax and AUC are 30% higher in women but no dose adjustment is required |
| Warfarin | Higher free plasma levels in W |
| Zolpidem | Plasma levels and AUC are higher, and Cl is lower in W |
References are presented in Supplementary material online, Table S2.
AUC, area under the curve; BMI, body mass index; Cl, clearance; Cmax, peak plasma drug concentrations; CYP, cytochrome P450 isoforms; i.v., intravenous; M, men; OC, oral contraceptives; P, pregnancy; P-gp, P-glycoprotein; Vd, volume of distribution; W, women.
Olanzapine, clozapine, pimozide, haloperidol.
Fentanyl, morphine, pentazocine, ramifentanil.
Atracurium, pancuronium, rocuronium vecuronium.
Citalopram, dapoxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline.
Sex-related differences in drug pharmacokinetic parameters
| Drug class . | Outcomes in females . |
|---|---|
| Anaesthetics: propofol | Plasma propofol levels decline more rapidly in W at the end of infusion |
| Alcohol | Lower gastric alcohol dehydrogenase activity in W. Higher plasma concentrations in W as compared with M following an equivalent drink |
| Antidepressants | Higher AUC and Cmax in W |
| H1-antihistamines | Slower metabolism and elimination in W |
| Antipsychotic drugsa | Higher plasma levels and Vd and lower Cl in W. Reduce the dosage in W or increase dosage in M. Olanzapine is more rapidly eliminated in M than in W |
| Aspirin | Bioavailability and plasma levels of aspirin and salicylate are higher in W possibly due to lower activity of aspirin esterase, larger Vd and lower Cl in W than in M. Differences disappear with OCP |
| Benzodiazepines | Lower initial plasma levels due to larger Vd, and possibly higher Cl, in W. OC reduce their Cl. Higher plasma levels of free diazepam in W |
| Beta-receptor agonists | W are less sensitive |
| Beta blockers: metoprolol, propranolol | W have higher plasma levels due to a smaller Vd and slower Cl. Drug exposure to metoprolol increases by OC |
| Renal Cl of atenolol and metoprolol increases during P due to enhanced hepatic metabolism | |
| Calcium channel blockers | Faster Cl of verapamil, and nifedipine in W. Increased bioavailability and decreased clearance of oral verapamil in W compared with M |
| Digoxin | W have higher serum digoxin concentrations due to reduced Vd and lower Cl. Drug Cl increases during P |
| Glucocorticoids | Oral Cl and Vd of prednisolone are higher in M. Prednisolone clearance was reduced by OC |
| Heparin | W had higher plasma levels and APTT values than M due to a lower Cl |
| Iron | Oral absorption of iron is greater in W than in M |
| Isosorbide mononitrate | W had significantly higher serum plasma concentrations compared with men, probably due to the lower body weights in females |
| Labetalol | Labetalol concentrations are 80% higher in W |
| Lidocaine | W has a larger Vd and may require a higher i.v. bolus dose than M. Higher free plasma levels in W receiving OCP, as alpha 1-acid glycoprotein levels are reduced by oestrogens |
| μ-opioid (OP3) receptor agonistsb | Slower onset and offset of action in W |
| Neuromuscular blocking drugsc | Lower Vd, higher plasma levels, faster onset and prolonged duration in W due to the higher body fat and lower Vd |
| Paracetamol | Lower plasma levels and higher Cl in M due to increased activity of the glucuronidation pathway. OCP increase drug clearance |
| Procainamide | Plasma levels are higher (30%) in W due to a lower BMI and Vd |
| Quinidine | Plasma protein binding decreases during P |
| Selective serotonin reuptake inhibitorsd | W present higher plasma levels, probably related to sex-related activity of various CYP enzymes |
| Statins | Higher plasma levels of lovastatin and simvastatin in W |
| Theophylline | Metabolism is faster and half-life is shorter in W than in M. Plasma protein binding decreases and the Vd increases during P |
| Torasemide | Higher Cmax and lower Cl in W than in M |
| Tricyclic antidepressants | Free plasma concentrations of imipramine, clomipramine, and nortriptyline are higher during pregnancy |
| Verapamil | W display faster Cl of verapamil after i.v. administration probably due to the higher activity of CYP3A4 or lower activity of P-gp; lower Cl in W after oral administration |
| Vorapaxar | Cmax and AUC are 30% higher in women but no dose adjustment is required |
| Warfarin | Higher free plasma levels in W |
| Zolpidem | Plasma levels and AUC are higher, and Cl is lower in W |
| Drug class . | Outcomes in females . |
|---|---|
| Anaesthetics: propofol | Plasma propofol levels decline more rapidly in W at the end of infusion |
| Alcohol | Lower gastric alcohol dehydrogenase activity in W. Higher plasma concentrations in W as compared with M following an equivalent drink |
| Antidepressants | Higher AUC and Cmax in W |
| H1-antihistamines | Slower metabolism and elimination in W |
| Antipsychotic drugsa | Higher plasma levels and Vd and lower Cl in W. Reduce the dosage in W or increase dosage in M. Olanzapine is more rapidly eliminated in M than in W |
| Aspirin | Bioavailability and plasma levels of aspirin and salicylate are higher in W possibly due to lower activity of aspirin esterase, larger Vd and lower Cl in W than in M. Differences disappear with OCP |
| Benzodiazepines | Lower initial plasma levels due to larger Vd, and possibly higher Cl, in W. OC reduce their Cl. Higher plasma levels of free diazepam in W |
| Beta-receptor agonists | W are less sensitive |
| Beta blockers: metoprolol, propranolol | W have higher plasma levels due to a smaller Vd and slower Cl. Drug exposure to metoprolol increases by OC |
| Renal Cl of atenolol and metoprolol increases during P due to enhanced hepatic metabolism | |
| Calcium channel blockers | Faster Cl of verapamil, and nifedipine in W. Increased bioavailability and decreased clearance of oral verapamil in W compared with M |
| Digoxin | W have higher serum digoxin concentrations due to reduced Vd and lower Cl. Drug Cl increases during P |
| Glucocorticoids | Oral Cl and Vd of prednisolone are higher in M. Prednisolone clearance was reduced by OC |
| Heparin | W had higher plasma levels and APTT values than M due to a lower Cl |
| Iron | Oral absorption of iron is greater in W than in M |
| Isosorbide mononitrate | W had significantly higher serum plasma concentrations compared with men, probably due to the lower body weights in females |
| Labetalol | Labetalol concentrations are 80% higher in W |
| Lidocaine | W has a larger Vd and may require a higher i.v. bolus dose than M. Higher free plasma levels in W receiving OCP, as alpha 1-acid glycoprotein levels are reduced by oestrogens |
| μ-opioid (OP3) receptor agonistsb | Slower onset and offset of action in W |
| Neuromuscular blocking drugsc | Lower Vd, higher plasma levels, faster onset and prolonged duration in W due to the higher body fat and lower Vd |
| Paracetamol | Lower plasma levels and higher Cl in M due to increased activity of the glucuronidation pathway. OCP increase drug clearance |
| Procainamide | Plasma levels are higher (30%) in W due to a lower BMI and Vd |
| Quinidine | Plasma protein binding decreases during P |
| Selective serotonin reuptake inhibitorsd | W present higher plasma levels, probably related to sex-related activity of various CYP enzymes |
| Statins | Higher plasma levels of lovastatin and simvastatin in W |
| Theophylline | Metabolism is faster and half-life is shorter in W than in M. Plasma protein binding decreases and the Vd increases during P |
| Torasemide | Higher Cmax and lower Cl in W than in M |
| Tricyclic antidepressants | Free plasma concentrations of imipramine, clomipramine, and nortriptyline are higher during pregnancy |
| Verapamil | W display faster Cl of verapamil after i.v. administration probably due to the higher activity of CYP3A4 or lower activity of P-gp; lower Cl in W after oral administration |
| Vorapaxar | Cmax and AUC are 30% higher in women but no dose adjustment is required |
| Warfarin | Higher free plasma levels in W |
| Zolpidem | Plasma levels and AUC are higher, and Cl is lower in W |
References are presented in Supplementary material online, Table S2.
AUC, area under the curve; BMI, body mass index; Cl, clearance; Cmax, peak plasma drug concentrations; CYP, cytochrome P450 isoforms; i.v., intravenous; M, men; OC, oral contraceptives; P, pregnancy; P-gp, P-glycoprotein; Vd, volume of distribution; W, women.
Olanzapine, clozapine, pimozide, haloperidol.
Fentanyl, morphine, pentazocine, ramifentanil.
Atracurium, pancuronium, rocuronium vecuronium.
Citalopram, dapoxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline.
Drug elimination from the body occurs by two processes: biotransformation and excretion. Hepatic clearance is a function of cardiac output and liver blood flow, which are lower in women, and sex-based differences in drug-metabolizing enzymes and transporters (Table 1), which play a greater role in PK variability than any of the other parameter.15–19,23–25,33–39 CYP3A and the transporter P-glycoprotein (P-gp) present appreciable substrate overlap so that the increased clearance of CYP3A4 substrates in women might be the result of their lower hepatic P-gp activity.12,15,17,35–39 Renal clearance depends on glomerular filtration rate (GFR) and tubular secretion and reabsorption. GFR is 10–25% lower in women, mostly older women, and drugs primarily excreted unchanged in the urine are cleared more slowly in women, but sex-related differences in renal excretion disappear after normalization for body weight or GFR.12,17,18,26,40
Differences in body composition and PK parameters may affect drug disposition leading to differences in drug efficacy and safety. However, only a few sex-based differences in PKs may lead to clinically relevant changes in drug efficacy or safety as most of the differences disappear after adjusting drug dosages for total body weight/size or GFR.29 Sex-based differences in PK and weight-dosing recommendations may be warranted for drugs with a narrow therapeutic margin (e.g. antiarrhythmics, digoxin, anticoagulants, antithrombotics, and thrombolytics) to avoid an increase in the incidence of ADRs.12,15–21,23–26
Gender differences in pharmacodynamics
Prospective and mainly retrospective analysis of clinical trials revealed sex-related differences in the efficacy and safety of several widely used cardiovascular drugs (Tables 3and4).1,12,15–20,23–29,41 PD differences have not been studied as extensively as the PK differences and can be difficult to quantify as women are often underrepresented in trials and differences can be partly modulated by sex hormones [e.g. oral contraceptives (OCs) and hormone replacement therapy (HRT)].41 This explains why differences in clinical outcomes are still uncertain for some cardiovascular drugs routinely used in clinical practice. Next, we shall review several sex-related PD differences.
Sex differences in drug pharmacodynamics
| Drug class . | Outcomes . |
|---|---|
| Alcohol | Higher vulnerability of W to acute and chronic complications of alcoholism |
| Anaesthetics: propofol | W are less sensitive to propofol. W wake up faster and require higher doses than M for the same effect |
| ACEIs | No mortality benefit in W with asymptomatic LV systolic dysfunction |
| Antidepressants | W respond better to selective serotonin/noradrenaline uptake inhibitors. M respond better to TCA and MAO inhibitors than W |
| Antipsychotic drugs | More effective in W. They require lower doses to control symptoms |
| Aspirin | Higher protective effect against stroke in W and against MI in M. Aspirin is more active in male platelets. Aspirin resistance is more frequent in W |
| Benzodiazepines | Diazepam impairs psychomotor skills to a greater extent in W. They should be initiated at lower dosages in W |
| Beta blockers | Greater reduction in blood pressure and heart rate in W treated with metoprolol and propranolol |
| Digoxin | W with HF have an increased risk of mortality on digoxin therapy. W require lower doses and lower plasma levels (< 0.8 ng/mL) |
| Glucocortioids | Females are more sensitive to the effects of methylprednisolone |
| Heparin | W had increased partial thromboplastin time, even after weight-adjusted dosing, suggesting an increased sensitivity |
| Ibuprofen | Less effective in W |
| Lidocaine | W may require a higher i.v. bolus doses to achieve the same plasma levels |
| μ-opioid (OP3) and κ* (OP2) receptor agonistsa | W experience more pain and are more sensitive to opioid receptor agonists. M require 30–60% greater dose of morphine and κ receptor agonists for the same pain relief |
| Neuromuscular blocking drugsb | W are more sensitive and require lower (20–30%) doses than M due to a smaller Vd. If a rapid onset of action is required the dose should be increased in M |
| Paracetamol | W displayed lower Cl and Vd compared with M. OCP increase drug Cl |
| rt-PA | W with acute ischaemic stroke obtain more benefit from rt-PA than M |
| SSRIsc | W respond better than M, being the preferred therapy |
| Verapamil | Greater reduction in blood pressure and heart rate in W |
| Warfarin | W need less warfarin per week than M. Doses should be modified to reduce the risk of excessive anticoagulation in W |
| Zolpidem | The recommended initial dose is lower in W |
| Drug class . | Outcomes . |
|---|---|
| Alcohol | Higher vulnerability of W to acute and chronic complications of alcoholism |
| Anaesthetics: propofol | W are less sensitive to propofol. W wake up faster and require higher doses than M for the same effect |
| ACEIs | No mortality benefit in W with asymptomatic LV systolic dysfunction |
| Antidepressants | W respond better to selective serotonin/noradrenaline uptake inhibitors. M respond better to TCA and MAO inhibitors than W |
| Antipsychotic drugs | More effective in W. They require lower doses to control symptoms |
| Aspirin | Higher protective effect against stroke in W and against MI in M. Aspirin is more active in male platelets. Aspirin resistance is more frequent in W |
| Benzodiazepines | Diazepam impairs psychomotor skills to a greater extent in W. They should be initiated at lower dosages in W |
| Beta blockers | Greater reduction in blood pressure and heart rate in W treated with metoprolol and propranolol |
| Digoxin | W with HF have an increased risk of mortality on digoxin therapy. W require lower doses and lower plasma levels (< 0.8 ng/mL) |
| Glucocortioids | Females are more sensitive to the effects of methylprednisolone |
| Heparin | W had increased partial thromboplastin time, even after weight-adjusted dosing, suggesting an increased sensitivity |
| Ibuprofen | Less effective in W |
| Lidocaine | W may require a higher i.v. bolus doses to achieve the same plasma levels |
| μ-opioid (OP3) and κ* (OP2) receptor agonistsa | W experience more pain and are more sensitive to opioid receptor agonists. M require 30–60% greater dose of morphine and κ receptor agonists for the same pain relief |
| Neuromuscular blocking drugsb | W are more sensitive and require lower (20–30%) doses than M due to a smaller Vd. If a rapid onset of action is required the dose should be increased in M |
| Paracetamol | W displayed lower Cl and Vd compared with M. OCP increase drug Cl |
| rt-PA | W with acute ischaemic stroke obtain more benefit from rt-PA than M |
| SSRIsc | W respond better than M, being the preferred therapy |
| Verapamil | Greater reduction in blood pressure and heart rate in W |
| Warfarin | W need less warfarin per week than M. Doses should be modified to reduce the risk of excessive anticoagulation in W |
| Zolpidem | The recommended initial dose is lower in W |
References are presented in Supplementary material online, Table S3.
ACEIs, angiotensin-converting enzyme inhibitors; Cl, clearance; E, oestrogens; HF, heart failure; i.v., intravenous; LV, left ventricular; M, men; MAO, monoamine oxidase; MI, myocardial infarction; OCP, oral contraceptives; rt-PA, recombinant tissue plasminogen activator; SSRIs, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants; Vd, volume of distribution; W, women.
aAlfentanyl, butorphanol*, fentanyl, morphine, nalbuphine* pentazocine*, remifentanyl.
bAtracurium, pancuronium, rocuronium and vecuronium.
cCitalopram, dapoxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline. *refers to κ (OP2) receptor agonists.
Sex differences in drug pharmacodynamics
| Drug class . | Outcomes . |
|---|---|
| Alcohol | Higher vulnerability of W to acute and chronic complications of alcoholism |
| Anaesthetics: propofol | W are less sensitive to propofol. W wake up faster and require higher doses than M for the same effect |
| ACEIs | No mortality benefit in W with asymptomatic LV systolic dysfunction |
| Antidepressants | W respond better to selective serotonin/noradrenaline uptake inhibitors. M respond better to TCA and MAO inhibitors than W |
| Antipsychotic drugs | More effective in W. They require lower doses to control symptoms |
| Aspirin | Higher protective effect against stroke in W and against MI in M. Aspirin is more active in male platelets. Aspirin resistance is more frequent in W |
| Benzodiazepines | Diazepam impairs psychomotor skills to a greater extent in W. They should be initiated at lower dosages in W |
| Beta blockers | Greater reduction in blood pressure and heart rate in W treated with metoprolol and propranolol |
| Digoxin | W with HF have an increased risk of mortality on digoxin therapy. W require lower doses and lower plasma levels (< 0.8 ng/mL) |
| Glucocortioids | Females are more sensitive to the effects of methylprednisolone |
| Heparin | W had increased partial thromboplastin time, even after weight-adjusted dosing, suggesting an increased sensitivity |
| Ibuprofen | Less effective in W |
| Lidocaine | W may require a higher i.v. bolus doses to achieve the same plasma levels |
| μ-opioid (OP3) and κ* (OP2) receptor agonistsa | W experience more pain and are more sensitive to opioid receptor agonists. M require 30–60% greater dose of morphine and κ receptor agonists for the same pain relief |
| Neuromuscular blocking drugsb | W are more sensitive and require lower (20–30%) doses than M due to a smaller Vd. If a rapid onset of action is required the dose should be increased in M |
| Paracetamol | W displayed lower Cl and Vd compared with M. OCP increase drug Cl |
| rt-PA | W with acute ischaemic stroke obtain more benefit from rt-PA than M |
| SSRIsc | W respond better than M, being the preferred therapy |
| Verapamil | Greater reduction in blood pressure and heart rate in W |
| Warfarin | W need less warfarin per week than M. Doses should be modified to reduce the risk of excessive anticoagulation in W |
| Zolpidem | The recommended initial dose is lower in W |
| Drug class . | Outcomes . |
|---|---|
| Alcohol | Higher vulnerability of W to acute and chronic complications of alcoholism |
| Anaesthetics: propofol | W are less sensitive to propofol. W wake up faster and require higher doses than M for the same effect |
| ACEIs | No mortality benefit in W with asymptomatic LV systolic dysfunction |
| Antidepressants | W respond better to selective serotonin/noradrenaline uptake inhibitors. M respond better to TCA and MAO inhibitors than W |
| Antipsychotic drugs | More effective in W. They require lower doses to control symptoms |
| Aspirin | Higher protective effect against stroke in W and against MI in M. Aspirin is more active in male platelets. Aspirin resistance is more frequent in W |
| Benzodiazepines | Diazepam impairs psychomotor skills to a greater extent in W. They should be initiated at lower dosages in W |
| Beta blockers | Greater reduction in blood pressure and heart rate in W treated with metoprolol and propranolol |
| Digoxin | W with HF have an increased risk of mortality on digoxin therapy. W require lower doses and lower plasma levels (< 0.8 ng/mL) |
| Glucocortioids | Females are more sensitive to the effects of methylprednisolone |
| Heparin | W had increased partial thromboplastin time, even after weight-adjusted dosing, suggesting an increased sensitivity |
| Ibuprofen | Less effective in W |
| Lidocaine | W may require a higher i.v. bolus doses to achieve the same plasma levels |
| μ-opioid (OP3) and κ* (OP2) receptor agonistsa | W experience more pain and are more sensitive to opioid receptor agonists. M require 30–60% greater dose of morphine and κ receptor agonists for the same pain relief |
| Neuromuscular blocking drugsb | W are more sensitive and require lower (20–30%) doses than M due to a smaller Vd. If a rapid onset of action is required the dose should be increased in M |
| Paracetamol | W displayed lower Cl and Vd compared with M. OCP increase drug Cl |
| rt-PA | W with acute ischaemic stroke obtain more benefit from rt-PA than M |
| SSRIsc | W respond better than M, being the preferred therapy |
| Verapamil | Greater reduction in blood pressure and heart rate in W |
| Warfarin | W need less warfarin per week than M. Doses should be modified to reduce the risk of excessive anticoagulation in W |
| Zolpidem | The recommended initial dose is lower in W |
References are presented in Supplementary material online, Table S3.
ACEIs, angiotensin-converting enzyme inhibitors; Cl, clearance; E, oestrogens; HF, heart failure; i.v., intravenous; LV, left ventricular; M, men; MAO, monoamine oxidase; MI, myocardial infarction; OCP, oral contraceptives; rt-PA, recombinant tissue plasminogen activator; SSRIs, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants; Vd, volume of distribution; W, women.
aAlfentanyl, butorphanol*, fentanyl, morphine, nalbuphine* pentazocine*, remifentanyl.
bAtracurium, pancuronium, rocuronium and vecuronium.
cCitalopram, dapoxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline. *refers to κ (OP2) receptor agonists.
Examples of sex differences in adverse drug reactions
| Drug class . | Outcomes in females . |
|---|---|
| Analgesic drugs | W report more adverse effects to perioperative analgesic drugs |
| Anaphylactic shock | Anaphylactic shock induced by neuromuscular blocking agents, hypnotics, opioids and benzodiazepines is more frequent in W |
| Anaesthetic drugs | W are more prone to ADR postoperatively |
| Angiotensin converting enzyme inhibitors | Dry cough is 2 to 3 times more frequent in W. No gender preference for angioedema/urticaria |
| Anorectics | Cardiac valvulopathy is more frequent in W exposed to phentermine, dexfenfluramine, or fenfluramine |
| Antiarrhythmic drugs | Higher risk of QT prolongation and TdP in W |
| Anticoagulants | More frequent and severe bleedings in W |
| H1-Antihistamines | W are more vulnerable to sedation and drowsiness |
| Antiplatelets | More frequent and severe bleedings in W |
| Antipsychotics | W present more extrapyramidal and anticholinergic effects and QTc prolongation. M reported more sexual problems |
| Aspirin | Increased risk of bleeding in W. More ulcer complications in M |
| Beta blockers | Enhanced BP lowering and heart rate reduction with metoprolol in W |
| Benzodiazepines | Diazepam impaired the psychomotor skills more in W than in M. Dependency is more frequent in W |
| Calcium channel blockers | Higher risk of oedema in W. Women taking OCP and diazepam during menstruation become relatively intoxicated |
| Digoxin | Higher mortality in W with HF. Digoxin plasma levels < 0.8 ng/mL are recommended in W |
| Diuretics | Higher rates of hospitalizations due to hypo-osmolarity, hypokalaemia and hyponatraemia and higher risk of arrhythmias in W |
| Drug-induced TdP | W have a longer QTc intervals and development of TdP more frequently than M |
| GPIIb/IIIa inhibitors | W experience more bleeding than M |
| Heparin | W present higher bleeding risk |
| Opioid receptor agonists | W experience more ADRs (nausea and vomiting, respiratory depression) despite smaller dose requirements for pain control |
| NSAIDs | M display a higher prevalence of ADRs than W |
| Paracetamol | Acute liver failure due to paracetamol overdose is more common in W |
| Procainamide | Systemic lupus erythematosus more common in W |
| Skin diseases | W > M (systemic lupus erythematosus and photosensitivity) |
| Statins | Myopathy is more frequent in older W with low body weight |
| Thiazides | More hyponatraemia and hypokalaemia in W |
| Thiazolidinediones | Double the risk of fractures among diabetic W, but not among M |
| Thrombolytics | Higher risk of bleeding and intracranial haemorrhagic in W |
| Unfractioned heparin | W develop higher plasma levels and higher bleeding risk |
| Zolpidem | To reduce the risk of morning-after activity impairment decrease the dose of zolpidem by 50% in W |
| Drug class . | Outcomes in females . |
|---|---|
| Analgesic drugs | W report more adverse effects to perioperative analgesic drugs |
| Anaphylactic shock | Anaphylactic shock induced by neuromuscular blocking agents, hypnotics, opioids and benzodiazepines is more frequent in W |
| Anaesthetic drugs | W are more prone to ADR postoperatively |
| Angiotensin converting enzyme inhibitors | Dry cough is 2 to 3 times more frequent in W. No gender preference for angioedema/urticaria |
| Anorectics | Cardiac valvulopathy is more frequent in W exposed to phentermine, dexfenfluramine, or fenfluramine |
| Antiarrhythmic drugs | Higher risk of QT prolongation and TdP in W |
| Anticoagulants | More frequent and severe bleedings in W |
| H1-Antihistamines | W are more vulnerable to sedation and drowsiness |
| Antiplatelets | More frequent and severe bleedings in W |
| Antipsychotics | W present more extrapyramidal and anticholinergic effects and QTc prolongation. M reported more sexual problems |
| Aspirin | Increased risk of bleeding in W. More ulcer complications in M |
| Beta blockers | Enhanced BP lowering and heart rate reduction with metoprolol in W |
| Benzodiazepines | Diazepam impaired the psychomotor skills more in W than in M. Dependency is more frequent in W |
| Calcium channel blockers | Higher risk of oedema in W. Women taking OCP and diazepam during menstruation become relatively intoxicated |
| Digoxin | Higher mortality in W with HF. Digoxin plasma levels < 0.8 ng/mL are recommended in W |
| Diuretics | Higher rates of hospitalizations due to hypo-osmolarity, hypokalaemia and hyponatraemia and higher risk of arrhythmias in W |
| Drug-induced TdP | W have a longer QTc intervals and development of TdP more frequently than M |
| GPIIb/IIIa inhibitors | W experience more bleeding than M |
| Heparin | W present higher bleeding risk |
| Opioid receptor agonists | W experience more ADRs (nausea and vomiting, respiratory depression) despite smaller dose requirements for pain control |
| NSAIDs | M display a higher prevalence of ADRs than W |
| Paracetamol | Acute liver failure due to paracetamol overdose is more common in W |
| Procainamide | Systemic lupus erythematosus more common in W |
| Skin diseases | W > M (systemic lupus erythematosus and photosensitivity) |
| Statins | Myopathy is more frequent in older W with low body weight |
| Thiazides | More hyponatraemia and hypokalaemia in W |
| Thiazolidinediones | Double the risk of fractures among diabetic W, but not among M |
| Thrombolytics | Higher risk of bleeding and intracranial haemorrhagic in W |
| Unfractioned heparin | W develop higher plasma levels and higher bleeding risk |
| Zolpidem | To reduce the risk of morning-after activity impairment decrease the dose of zolpidem by 50% in W |
References are presented in Supplementary material online, Table S4.
ACEIs, angiotensin-converting enzyme inhibitors; ADR, adverse drug reactions; BP, blood pressure; CV, cardiovascular; E: oestrogens; GP, glycoprotein; HF, heart failure; M, men; NSAIDs, non-steroidal anti-inflammatory drugs; OCP, oral contraceptives; QTc, corrected QT interval; TdP, torsades de pointes; W, women.
Examples of sex differences in adverse drug reactions
| Drug class . | Outcomes in females . |
|---|---|
| Analgesic drugs | W report more adverse effects to perioperative analgesic drugs |
| Anaphylactic shock | Anaphylactic shock induced by neuromuscular blocking agents, hypnotics, opioids and benzodiazepines is more frequent in W |
| Anaesthetic drugs | W are more prone to ADR postoperatively |
| Angiotensin converting enzyme inhibitors | Dry cough is 2 to 3 times more frequent in W. No gender preference for angioedema/urticaria |
| Anorectics | Cardiac valvulopathy is more frequent in W exposed to phentermine, dexfenfluramine, or fenfluramine |
| Antiarrhythmic drugs | Higher risk of QT prolongation and TdP in W |
| Anticoagulants | More frequent and severe bleedings in W |
| H1-Antihistamines | W are more vulnerable to sedation and drowsiness |
| Antiplatelets | More frequent and severe bleedings in W |
| Antipsychotics | W present more extrapyramidal and anticholinergic effects and QTc prolongation. M reported more sexual problems |
| Aspirin | Increased risk of bleeding in W. More ulcer complications in M |
| Beta blockers | Enhanced BP lowering and heart rate reduction with metoprolol in W |
| Benzodiazepines | Diazepam impaired the psychomotor skills more in W than in M. Dependency is more frequent in W |
| Calcium channel blockers | Higher risk of oedema in W. Women taking OCP and diazepam during menstruation become relatively intoxicated |
| Digoxin | Higher mortality in W with HF. Digoxin plasma levels < 0.8 ng/mL are recommended in W |
| Diuretics | Higher rates of hospitalizations due to hypo-osmolarity, hypokalaemia and hyponatraemia and higher risk of arrhythmias in W |
| Drug-induced TdP | W have a longer QTc intervals and development of TdP more frequently than M |
| GPIIb/IIIa inhibitors | W experience more bleeding than M |
| Heparin | W present higher bleeding risk |
| Opioid receptor agonists | W experience more ADRs (nausea and vomiting, respiratory depression) despite smaller dose requirements for pain control |
| NSAIDs | M display a higher prevalence of ADRs than W |
| Paracetamol | Acute liver failure due to paracetamol overdose is more common in W |
| Procainamide | Systemic lupus erythematosus more common in W |
| Skin diseases | W > M (systemic lupus erythematosus and photosensitivity) |
| Statins | Myopathy is more frequent in older W with low body weight |
| Thiazides | More hyponatraemia and hypokalaemia in W |
| Thiazolidinediones | Double the risk of fractures among diabetic W, but not among M |
| Thrombolytics | Higher risk of bleeding and intracranial haemorrhagic in W |
| Unfractioned heparin | W develop higher plasma levels and higher bleeding risk |
| Zolpidem | To reduce the risk of morning-after activity impairment decrease the dose of zolpidem by 50% in W |
| Drug class . | Outcomes in females . |
|---|---|
| Analgesic drugs | W report more adverse effects to perioperative analgesic drugs |
| Anaphylactic shock | Anaphylactic shock induced by neuromuscular blocking agents, hypnotics, opioids and benzodiazepines is more frequent in W |
| Anaesthetic drugs | W are more prone to ADR postoperatively |
| Angiotensin converting enzyme inhibitors | Dry cough is 2 to 3 times more frequent in W. No gender preference for angioedema/urticaria |
| Anorectics | Cardiac valvulopathy is more frequent in W exposed to phentermine, dexfenfluramine, or fenfluramine |
| Antiarrhythmic drugs | Higher risk of QT prolongation and TdP in W |
| Anticoagulants | More frequent and severe bleedings in W |
| H1-Antihistamines | W are more vulnerable to sedation and drowsiness |
| Antiplatelets | More frequent and severe bleedings in W |
| Antipsychotics | W present more extrapyramidal and anticholinergic effects and QTc prolongation. M reported more sexual problems |
| Aspirin | Increased risk of bleeding in W. More ulcer complications in M |
| Beta blockers | Enhanced BP lowering and heart rate reduction with metoprolol in W |
| Benzodiazepines | Diazepam impaired the psychomotor skills more in W than in M. Dependency is more frequent in W |
| Calcium channel blockers | Higher risk of oedema in W. Women taking OCP and diazepam during menstruation become relatively intoxicated |
| Digoxin | Higher mortality in W with HF. Digoxin plasma levels < 0.8 ng/mL are recommended in W |
| Diuretics | Higher rates of hospitalizations due to hypo-osmolarity, hypokalaemia and hyponatraemia and higher risk of arrhythmias in W |
| Drug-induced TdP | W have a longer QTc intervals and development of TdP more frequently than M |
| GPIIb/IIIa inhibitors | W experience more bleeding than M |
| Heparin | W present higher bleeding risk |
| Opioid receptor agonists | W experience more ADRs (nausea and vomiting, respiratory depression) despite smaller dose requirements for pain control |
| NSAIDs | M display a higher prevalence of ADRs than W |
| Paracetamol | Acute liver failure due to paracetamol overdose is more common in W |
| Procainamide | Systemic lupus erythematosus more common in W |
| Skin diseases | W > M (systemic lupus erythematosus and photosensitivity) |
| Statins | Myopathy is more frequent in older W with low body weight |
| Thiazides | More hyponatraemia and hypokalaemia in W |
| Thiazolidinediones | Double the risk of fractures among diabetic W, but not among M |
| Thrombolytics | Higher risk of bleeding and intracranial haemorrhagic in W |
| Unfractioned heparin | W develop higher plasma levels and higher bleeding risk |
| Zolpidem | To reduce the risk of morning-after activity impairment decrease the dose of zolpidem by 50% in W |
References are presented in Supplementary material online, Table S4.
ACEIs, angiotensin-converting enzyme inhibitors; ADR, adverse drug reactions; BP, blood pressure; CV, cardiovascular; E: oestrogens; GP, glycoprotein; HF, heart failure; M, men; NSAIDs, non-steroidal anti-inflammatory drugs; OCP, oral contraceptives; QTc, corrected QT interval; TdP, torsades de pointes; W, women.
Antithrombotic drugs
Antithrombotic therapy, including anticoagulants and antiplatelet drugs, is the cornerstone for prevention and treatment of arterial thrombosis (e.g. myocardial infarction and stroke), venous thromboembolic disorders, and the complications of atrial fibrillation (AF).42 Women with acute coronary syndromes (ACS) have a higher risk of major bleedings than men, probably due to their smaller body, older age, reduced creatinine clearance, higher prevalence of comorbidities (hypertension, diabetes, renal dysfunction), higher risk of antithrombotics overdosing, and, perhaps, differences in response to antithrombotics between women and men.42–45
Anticoagulants
Indirect thrombin inhibitors
In men, unfractioned heparin (UFH) distributes into plasma volume, which is proportional to body weight, and is eliminated more rapidly; so, higher doses are required in heavy patients46,47 Women treated with UFH for acute myocardial infarction (AMI) achieve higher activated partial thromboplastin time than men, a finding associated with an increasing bleeding risk, even after weight-adjusted dosing.48 The main suggested risk factors for bleeding included a smaller body size, older age, reduced creatinine clearance, higher prevalence of comorbidities, and an increased sensitivity to heparin.46,48,49
A posthoc analysis of the TIMI 11A study showed similar PK/PD profiles of enoxaparin in men and women with non-ST-segment elevation ACS (NSTEMI-ACS).50,51 The meta-analysis of two large trials (ESSENCE and TIMI 11B) reported that enoxaparin was more effective than intravenous (i.v.) dose-adjusted UFH in reducing the risk of death, MI, or recurrent angina prompting urgent revascularization, but the benefit was greater in women.52 In the FRISC study, dalteparin reduced the risk of death and MI in patients with ACS, but women showed larger absolute and relative reduction of the primary endpoint compared with men.53 However, minor bleeding was more frequent and anti-Xa activity during the acute phase treatment was higher in women.54 The ExTRACT-TIMI 25 study randomized ST-segment elevation MI (STEMI) patients with planned fibrinolysis to enoxaparin or UFH. Women had a similar relative benefit and greater absolute benefit than men when treated with enoxaparin, despite they presented higher baseline risk and increased short term mortality.55 In the SYNERGY study, enoxaparin was not superior but also non-inferior to UFH across multiple subgroups, including those stratified by sex, with a modest increase in the risk of major bleeding.56
Direct thrombin inhibitors
Clearance of argatroban is faster in women, but no sex-related differences in anticoagulant response were reported.57,58 In the pooled analysis of REPLACE-2, ACUITY, and HORIZONS-AMI trials men and women undergoing percutaneous coronary interventions (PCI) experience similar safety benefits of bivalirudin in reducing bleeding complications, but women experienced a more pronounced benefit of bivalirudin in reducing 12-month mortality than men.59,60 In the ACUITY trial, no differences were observed in rates of 1-year composite ischaemia or mortality in women who received bivalirudin vs. heparin plus GPI.61 Bleeding complications were higher in women, likely because of comorbidities, as they were older and had more diabetes, hypertension, and renal impairment.59,60,62–64 In the REPLACE-2 trial, female gender was associated with higher rates of death and bleeding complications in univariate analyses, but multivariate analyses eliminated nearly all outcome differences between sexes.60,65,66 Similar results were observed in another study.67
Parenteral anti-factor Xa inhibitors
In the OASIS-5 trial, fondaparinux and enoxaparin showed similar efficacy in reducing the composite endpoint (death, MI, or refractory ischaemia at 9 days) or major bleeding in men and women with ACS.68 In the OASIS-6 trial, fondaparinux reduced the primary composite endpoint (death or reinfarction at 30 days) with a non-significant trend towards fewer severe haemorrhages in men and women with STEMI treated with primary PCI, thrombolysis, or no reperfusion therapy.69,70
Oral anticoagulants
Warfarin is equally effective in reducing the risk of thromboembolism in men and women and did not pose a greater risk of major haemorrhagic complications in women.29,71–73 In five randomized trials, warfarin consistently decreased (68%) the risk of stroke in patients with AF with virtually no increase in the frequency of major bleeding.74 However, women had more minor bleeding complications than men75,76 and they require less mg per week than men to maintain a therapeutic International Normalized Ratio (INR), older women requiring the lowest doses.73 Thus, starting and maintenance doses should be modified to reduce the risk of inadequate therapy in young females, and excessive anticoagulation in elderly patients.77 Surprisingly, there is little and contradictory information regarding the possible interactions of OCs and HRT and oral anticoagulants. Thus, it is recommended frequent monitoring of INR when this combination is used.78
Novel anti-factor II and anti-factor X antagonists
Gender had no significant influence on the PK of rivaroxaban,79–81 apixaban,82 and edoxaban83. Dabigatran exposure is ∼30% higher in females, but no sex-related interactions were observed.84–86 Major phase three trials in patients with non-valvular AF (NVAF) recruited approximately 30-40% of women.84,87–90 Dose adjustments were made according to weight and renal function in some trials, which implies some correction for smaller female patients. There were small trends towards reduction of stroke and systemic embolism for dabigatran 150 mg84 and reduction of major bleedings for edoxaban 60 mg90 and apixaban88 in women compared with men. An analysis of RELY, ARISTOTLE, and ROCKET AF trials (17 336 women) showed that compared with warfarin, novel anti-factor II and anti-factor X antagonists (NOACs) reduced the event rate in both sexes, but women suffered significant lower bleeding rates with NOACs compared with warfarin, while men had similar bleeding rates with both drugs. Thus, women appear to derive more benefits in terms of increased efficacy and improved safety from NOACs compared with men.91 In a secondary analysis of the ARISTOTLE trial, women (35.3%) had a similar rate of stroke or systemic embolism, but among patients with previous history of stroke or transient ischaemic attack, women had a lower risk of recurrent stroke compared with men. Women also had a lower risk of all-cause death and cardiovascular death and a trend towards less major bleeding and major or non-major clinically relevant bleeding than men.92
In a meta-analysis of 13 studies (> 100 000 patients) NOACs appeared to have a similar efficacy and safety compared with vitamin K antagonists in females and males treated for NVAF and acute venous thromboembolism (VTE).93,94 However, in another two meta-analysis women with acute VTE presented more bleeding complications than men when treated with NOACs, although all-cause mortality was not reported by sex in these patients.94–96 Finally, in a meta-analysis of six trials women with NVAF treated with warfarin have a greater residual risk of cerebrovascular accidents/systemic embolisms (CVA/SE) and an equivalent major bleeding risk, whereas those treated with NOACs deemed superior to warfarin are at equivalent residual risk of CVA/SE and less major bleeding risk compared with men.97 These results suggested an increased net benefit of NOACs compared with warfarin in treating women with AF.
Antiplatelet drugs
Women have longer bleeding times, higher baseline platelet reactivity, and stronger spontaneous and adenosine diphosphate- or collagen-induced aggregation and their glycoprotein (GP) IIb/IIIa receptors are more prone to be activated by multiple stimuli as compared with men.42,98–104 Differences in platelet reactivity may result from direct platelet effects of sex hormones or indirect effect on the vasculature. Oestrogens via oestrogen receptor α decrease platelet aggregation and stimulate prostacyclin and NO synthesis and release from vascular endothelial cells105–107 and decrease the levels of fibrinogen, antithrombin III, protein S, and plasminogen activator inhibitor 1.42,108 Conversely, testosterone increases the production of thromboxane A2 and the expression of TXA2 receptors.42,109,110 These changes may explain why platelets from premenopausal women are less prothrombotic than platelets from age-matched men, although post-menopausal HRT does not exert cardioprotective effects111,112 and OCs increase the risk of thrombotic events.113
Acetylsalicylic acid
Low-dose aspirin has been the cornerstone of treatment for patients with various atherosclerotic disease manifestations.114,115 Its antiplatelet effect is similar in both sexes when COX-1 direct pathways are considered, but pathways indirectly related to COX-1, i.e. those stimulated by collagen, adenosine diphosphate (ADP), and epinephrine are less inhibited in female subjects.116,In vitro, aspirin produces a greater inhibition of platelet aggregation in men, while women retained a higher prevalence of ‘aspirin resistance’ because of increased baseline platelet reactivity.98,116–118 In ex vivo platelet aggregation studies, aspirin was less effective at inhibiting platelet aggregation in women with a history of ischaemic stroke or transient ischaemic attack.119 Thus, inhibition of platelet aggregation in women treated with aspirin may be insufficient, and females might benefit from higher maintenance dosages or the use of alternative antiplatelet drugs. There are some potential explanations for these gender-specific differences, including (i) PK differences. Oral bioavailability, area under the plasma concentration–time curve (AUC), and elimination half-life of aspirin are significantly greater in women, probably because men conjugate more aspirin with glycine and glucuronic acid, while salicylic acid clearance is higher in males due to enhanced activity of the glycine conjugation pathway.116,120–122 These differences in biotransformation disappear in women taking OCs.122 (ii) The role of sex hormones. The inhibitory effect of aspirin is not affected by oestrogens,42,123 but it is reduced in orchiectomized males and restored by testosterone, which confirms its role in aspirin-mediated antiaggregant effects.123,124 (iii) Sex-related differences in platelet and vascular functions and disease pathogenesis. Men with stable ischaemic heart disease are more likely to respond to mental stress increasing blood pressure (BP), while women exhibit higher platelet aggregation.125
In a primary prevention trial in 39 876 women, subgroup analyses showed that aspirin significantly reduced the risk of major cardiovascular events, ischaemic stroke, and MI only among women 65 years of age or older.126 In a sex-specific meta-analysis of six primary prevention trials (51 342 women), aspirin reduced the risk of cardiovascular events in both sexes.127 Women derived benefit from a reduction in the risk of ischaemic stroke, without an increase in haemorrhagic stroke or a significant effect on MI, cardiovascular, and all-cause mortality. In men, benefit derived from a reduction in MI, but there was no significant effect on stroke (haemorrhagic strokes increased), cardiovascular, and all-cause mortality. However, aspirin also increased the risk of major bleeding (≈70%) in both sexes; thus, the overall benefit and risk requires careful consideration by the physician and patient before initiating aspirin for primary prevention of CVD. In 14 trials enrolling 107 686 participants without pre-existing CVD low-dose aspirin reduced major cardiovascular events, MI, ischaemic stroke, and all-cause mortality, but increases haemorrhagic stroke and major bleedings in both sexes.128 In subgroup analysis, aspirin use reduced MI among men and ischaemic stroke among women. Aspirin had no significant effect on CVD in the diabetic population, but reduced the risk of MI among diabetic men.128
The benefits of aspirin in secondary prevention trials are well documented in both sexes. The meta-analyses of 287 trials, comprising predominantly studies with aspirin, showed that aspirin reduces serious cardiovascular events (non-fatal MI, non-fatal stroke, or vascular deaths) by ≈25% in high-risk patients although the absolute risk reduction mainly depends on the individual’s absolute risk without treatment.129 In 23 trials (n = 113 494 participants) aspirin reduced (27%) the risk of non-fatal, but not of fatal MI. Trials that recruited predominantly men demonstrated the largest risk reduction (38%), while trials that recruited predominately women failed to demonstrate any benefit.130 Another meta-analyses compared long-term aspirin treatment on serious vascular events (MI, stroke, or vascular death) and major bleeds in 6 primary prevention trials (95 000 individuals at low-average risk) and 16 secondary prevention trials (17 000 individuals at high-average risk).115 In primary prevention trials, aspirin produced a 12% reduction in serious vascular events, due to mainly a reduction of about a fifth in non-fatal MI; the net effect on stroke and vascular mortality was not significant. In secondary prevention trials, aspirin yielded a greater absolute reduction in serious vascular events with a non-significant increase in haemorrhagic stroke but reductions of about a fifth in total stroke and in coronary events. In both primary and secondary prevention trials, the proportional reductions in the aggregate of all serious vascular events seemed similar for men and women. However, aspirin also increased (≈70%) the risk of major bleeding in both sexes to a similar degree. Thus, for secondary prevention, the net benefits aspirin substantially exceed the bleeding hazards, irrespective of age or sex, while the balance of beneficial effects and bleeding hazards in primary prevention was less clear.
Glycoprotein IIb/IIIa inhibitors
In a meta-analysis of 6 randomized trials in >31 000 patients with NST-ACS undergoing PCI, i.v. glycoprotein IIb/IIIa inhibitors (GPIs) reduced 30-day rate of death or MI at 30 days in males, but not in females,131 apparently because a higher percentage of men with positive baseline troponins. Once patients were stratified according to troponin levels, there was no evidence of a sex difference in treatment response.131 A pooled analysis from EPIC, EPILOG, and EPISTENT trials (6595 patients) found that women and men obtain equivalent short- and long-term benefit in clinical outcomes from abciximab during PCI.132 In the ESPRIT trial, eptifibatide reduced to a similar extent the rates of death, MI, or urgent target vessel revascularization in both sexes.133
Women had higher rates of both major and minor bleeding after PCIs than men,131–134 but after adjustment for weight, age, and comobidities, differences in bleeding between men and women were non-significant. In the CRUSADE study, women with NSTE-ACS experienced more bleeding than men whether or not they were treated with GPIs. However, because of frequent excessive dosing in women, ∼25% of this excess bleeding risk is avoidable by appropriate dose adjustment.44 In STEMI patients, early administration of abciximab use improved patency of the infarct-related artery before primary PCI and improved epicardial flow and reduced mortality after primary PCI in women.135 The frequency of bleeding events was similar in both women and men.
Adenosine diphosphate P2Y12receptor antagonists
Although ex-vivo studies found that women are more often hyporesponsive to clopidogrel, there are no differences in the plasma levels of its active metabolite between sexes.12,136–138 In a sex-specific meta-analysis of 5 randomized trials (79 613 patients, 30% women), clopidogrel reduced the risk of major cardiovascular events in both women and men.139 In women, the overall effect of clopidogrel was driven by a reduction of MI; in men, by a significant reduction in MI, stroke, and all-cause mortality. Additionally, clopidogrel increased the risk of major bleeding in both men and women.
Another meta-analysis of 20 trials (233 285 participants) confirmed that cardiovascular risk (defined as MI, stroke, or cardiovascular death) reduction with clopidogrel did not significantly differ by gender. Results for other inhibitors were comparable, although available data were sparse.140
Systemic exposure of prasugrel and its active metabolite are not appreciably affected by gender.141,142 In the TRITON-TIMI 38 study which compared prasugrel with clopidogrel in patients with ACS and scheduled PCI unadjusted data showed a higher incidence of primary efficacy endpoints (cardiovascular death, nonfatal MI, or nonfatal stroke, individually and in combination) in women, but this difference disappeared after adjustment for baseline characteristics.143,144 Similarly, in the PROMETHEUS study comparing outcomes in patients with ACS treated with clopidogrel and prasugrel, 1-year major adverse cardiac events (MACE) was significantly higher in women, but differences were no longer significant after adjustment for baseline risk.145 In both trials, female gender was the strongest independent predictor of non-CABG-related serious bleeding, possibly due to some extent to lower body weight.144,145
Ticagrelor exposure was higher and its elimination half-life slightly longer in women, but dose adjustment is not required.146 In a pre-specified analysis of the PLATO trial, female sex was not an independent risk factor for adverse clinical outcomes in moderate-to-high risk ACS patients and ticagrelor showed similar safety profile in men and women.147,148 In a pre-specified subgroup analysis of the CHAMPION PHOENIX trial, cangrelor reduced the odds of major adverse cardiovascular events and stent thrombosis in women and men and appeared to offer greater net clinical benefit than clopidogrel.149
Beta blockers
Oestrogens and progesterone inhibit the cardiac expression of β1-adrenoceptors and reduce β-adrenergic-mediated stimulation exerting cardioprotective effects.150,151 Thus, gender-specific differences in the PDs of β-blockers might be expected.
Women present higher Cmax and AUC to metoprolol and propranolol than men due to an enhanced absorption, lower Vd, and slower clearance via CYP2D6, leading to a greater reduction in heart rate and systolic BP during exercise.12,152–155 Drug exposure to metoprolol is further increased by OCs,153,156 while increased expression of CYP2D6 by testosterone can lead to faster drug clearance in men.12,153 Surprisingly, metoprolol might exert a greater effect on stress-induced angina pectoris in men than in women in spite of higher plasma levels in females.157
Some trials found that β-blockers improved survival in males, but not in females, with hypertension152 or CAD158 or heart failure with reduced ejection fraction (HFrEF).159,160 However, the posthoc analysis of several trials confirmed a similar and significant survival benefits of β-blockers (bisoprolol, carvedilol, metoprolol) on all-cause mortality/all-cause hospitalizations in women and men with HFrEF.161–164 Similarly, pooling total mortality data by sex from MERIT-HF, CIBIS-II, and COPERNICUS showed similar and significant survival benefits in women and men with HFrEF.165 In the BEST trial, the survival advantage was confined to women with non-ischaemic aetiology, while in the ischaemic group, there was a trend for a better survival in men,166 while the meta-analysis of 5 studies (CIBIS-II, COPERNICUS, MERIT-HF, BEST, and U.S. Carvedilol) recruiting 2134 women with HFrEF confirmed a similar reduction in mortality in both sexes.167 These contrasting results were attributed to the fact that β-blockers were underused in females with MI, the underrepresentation of women in these trials (<25%), and women were older and sicker than the male cohort. In a recent a meta-analysis of 11 trials enrolling 13 833 patients (24% women) with HFrEF in sinus rhythm β-blockers reduced all-cause mortality and HF admissions for HF, irrespective of age or sex.168 Thus, β blockers should not be withheld from women with HFrEF.
Calcium channel blockers
Gender-specific PK differences have been described for verapamil169,170 and nifedipine,171 but not for amlodipine.172 Women display faster clearance and lower plasma levels for nifedipine171 and faster clearance of verapamil after i.v. administration; however, after oral administration women showed slower clearance than men,169 which may be attributed to the lower body weight, higher activity of CYP3A4 and/or lower activity of P-gp compared with men.170,173 Verapamil clearance decreases with age in women, which explains why older women show a greater antihypertensive response.174 In an 18-week open study, amlodipine produced a greater BP reduction and incidence of oedema in women than in men.175 However, major hypertension trials with calcium channel blockers (ALLHAT, INSIGHT, STOP-Hypertension-2, NORDIL) found no evidence for gender-specific differences in outcomes.11 In a subanalysis of the HOT trial, the incidence of acute MI was significantly less in women with a lower diastolic BP target (<85 mmHg); a non-significant trend was found in men.176
Digoxin
A posthoc analysis of the DIG study found that digoxin increased all-cause mortality among women, but not men, with HFrEF.177 However, another retrospective analysis of the DIG trial reported a beneficial effect of digoxin on morbidity and no excess mortality in women at serum concentrations between 0.5 and 0.9 ng/mL, while at concentrations ≥1.2 ng/mL was harmful.178 Thus, recommended digoxin plasma concentrations should be 0.5–0.9 ng/mL in women.179,180 Similarly, the SOLVD trial enrolling patients with HFrEF did not find differences in mortality between men and women treated with digoxin.181 However, in this study digoxin was not randomly assigned and women represented only 20% of the population.
The increased mortality reported in the DIG trial was related to: (i) supratherapeutic plasma levels (>2.0 ng/mL) due to the reduced Vd and slower renal clearance in women.25,179 However, no sex-based differences in digoxin PK were found when actual or ideal body weight was used.182 (ii) Women present fewer Na+ pumps in erythrocytes and skeletal muscle than men, which may predispose to fatal arrhythmias.183 (iii) Hormone replacement therapy, because a subgroup analysis of the HERS trial found a higher incidence of coronary events only in women on HRT treated with digoxin.184 Thus, it was speculated that progestin inhibits P-gp increasing serum digoxin concentrations. However, in this study digoxin was not randomized and women on digoxin were sicker.
Diuretics
Women experience more frequent electrolyte disturbances (e.g. hyponatraemia and hypokalaemia).185 The Cmax and AUC of torasemide are 30–40% higher due to a reduced elimination in women than in men, which may explain why in the German Pharmacovigilance Project the majority of hospitalizations occurred in women.186 However, no dose adjustments are recommended for torasemide.
Ivabradine
This is a selective and specific inhibitor of the hyperpolarization-activated mixed Na+/K+ inward If current, the primary modulator of the spontaneous diastolic depolarization in the sino-atrial node.187 No differences in the efficacy or safety of ivabradine were observed in patients with stable angina pectoris,188–190 with stable CAD and left-ventricular systolic dysfunction191 or with symptomatic chronic HF, LV systolic dysfunction (LVEF ≤ 35%), and heart rate ≥ 70 bpm.192
LCZ699 (entresto)
This angiotensin II receptor/neprilysin inhibitor results in systemic exposure to sacubitril (inactive prodrug of LBQ657), LBQ657 (neprilysin inhibitor), and valsartan (angiotensin II receptor blocker).193 Pharmacokinetic parameters of LCZ696 analytes (LBQ657 and valsartan) are similar in men and women.194 In patients with chronic HF (New York Heart Association class II-IV) and LVEF ≤ 40% (amended later to ≤35%), the PARADIGM-HF trial found that the risk reduction of death or HF hospitalizations remain consistent in both men and women.195,196
Nitrates
The Cmax and AUC of isosorbide-5-mononitrate are higher in women, probably due to their lower body weight.197 Thus, dosing should be based on dose/kg or titrated to the required clinical effect.
PCSK9 inhibitors
Alirocumab and evolocumab bind selectively to proprotein convertase subtilisin/kexin type 9 (PCSK9) and prevent circulating PCSK9 from binding to the low-density lipoprotein receptor (LDLR) on the hepatocyte surface. Thus, PCSK9 inhibitors prevent PCSK9-mediated LDLR degradation leading to a reduction in serum LDL-cholesterol (LDL-C). Gender has no impact on the PK of alirocumab198 and evolocumab,199 and a similar reduction in LDL-C levels is observed in both men and women with primary hypercholesterolaemia and mixed dyslipidaemia.200–202
Renin-angiotensin-aldosterone system inhibitors
Oestrogens increase angiotensinogen synthesis and angiotensin II plasma levels, but down-regulate renin, angiotensin-converting enzyme (ACE) activity and angiotensin II type-1 receptors expression, while androgens up-regulate the renin-angiotensin-aldosterone system (RAAS).203–205 Thus, the premenopausal cardioprotective effects of oestrogens may result in part from RAAS inhibition12,203 No sex-differences have been described in the PK or the antihypertensive effects of ACE-inhibitors (ACEI), angiotensin receptor blockers (ARB), and aliskiren.12,19,206,207 A posthoc analysis of an Australian trial showed a significant reduction of cardiovascular events with ACEIs in men, but not in women, despite similar reductions in BP in both sexes,208 but this result has not been confirmed. Hypertensive men are treated more frequently with renin-angiotensin-aldosterone system inhibitors (RAASIs), while diuretics are more frequently prescribed in women.209 The lower use of RAASIs in young women may be related to their potential teratogenic effects.210–212 However, this lower use persists in women in all age groups, possibly due to the higher incidence of ADRs, including renal dysfunction and ACEI-induced cough.213–215 This gender bias may contribute to persistent HF symptoms in women.180
Early trials (CONSENSUS-1, SAVE, SOLVD)216–218 suggested that the reduction in mortality and HF hospitalization with enalapril and captopril were observed in men, but not in women, which can be explained by the small percentage of women enrolled.7 However, the AIRE and HOPE trials found a significant benefit for women, especially in the secondary prevention of cardiovascular events in high-risk patients.219,220 A meta-analysis of 30 studies (5399 men; 1991 women) confirmed comparable benefits of ACEI on total mortality and the combined endpoint of mortality or HF hospitalization for HF in males and females with HFrEF.221 Another meta-analysis found a similar reduction in death, MI, and HF admissions in patients with LV dysfunction after MI in both genders.222 However, women with asymptomatic LV dysfunction may not achieve a mortality benefit with ACEIs.167 The large studies (CHARM, LIFE, ELITE, VALHEFT, VALUE, VALIANT, OPTIMAAL) showed that ARBs produced a similar reduction in mortality or HF hospitalization in women and men with HFrEF.7,19,223–230 In patients with acute MI and LV dysfunction, the EPHESUS trial showed a trend towards greater benefit for 30-days all-cause mortality in women treated with eplerenone,231 but no differences were observed in the RALES trial with spironolactone.232
Statins
Dyslipidaemia has the highest population-adjusted risk among women compared with all other known risk factors for atherosclerotic CVD.233 This greater atherosclerotic risk is typically not observed before menopause when the prevalence of hypercholesterolaemia is lower in women compared with men, even if cholesterol levels are elevated.5 However, after menopause total cholesterol, LDL-C and triglyceride levels increase, while HDL-C levels decrease, so that women are at higher cardiovascular risk.234 Despite this evidence, women are less likely than men to have LDL-C levels < 100 mg/dL or to receive evidence-based high-intensity statin therapy as recommended in the guidelines, although the use of statins remains low in both sexes.5,235 This could reflect the perception of a lower risk of recurrent cardiovascular events in females with CVD despite they have a higher calculated cardiovascular risk than men men,1–6 although the risk of higher incidence of real (i.e. new-onset diabetes) or perceived ADRs could drive these differences.236–239 Plasma concentrations of statins are 15–40% higher in women, but dose adjustment is unnecessary.12,240
In secondary prevention trials, statins are equally effective in women and men for reducing coronary events, strokes, and all-cause mortality with no increase in non-coronary mortality.241–244 Interestingly, recent evidence confirmed their beneficial effects in primary prevention trials in women.242,243,245–249 In a meta-analysis of 27 trials (174 000 participants, 47 000 women), the relative risk reductions in major coronary events, coronary revascularizations, stroke, and all-cause mortality did not differ significantly between men and women, showing that statin therapy is of similar effectiveness in both sexes.243 The NICE guidelines recommended statin therapy for primary prevention in people with a predicted 10-year risk of a cardiovascular event of at least 10%250 and the 2013 ACC/AHA guidelines recommended statin use in asymptomatic adults aged 40–75 years without a history of CVD who have (i) LDL-C levels > 189 mg/dL, (ii) LDL-C levels of 70–189 mg/dL, if they also have DM (moderate-to-high dose statin use is recommended, depending on 10-year CVD event risk), or (iii) an estimated 10-year CVD event risk of ≥ 7.5%, as calculated on the pooled cohort equation risk calculator.251
Female sex and advanced age are recognized risk factors for statin-associated ADRs, i.e. muscle symptoms and new-onset diabetes.7,236,237,239,252–255 The lower metabolism, body mass index, and plasma volume and the reduced muscle mass of women compared with men, predispose to statin-induced myalgias.7,236–239,252 However, the risk of diabetes is low both in absolute terms and when compared with the reduction in coronary events.237,256
Thrombolytic agents
There are no gender differences in drug PK and women with STEMI obtain a similar reduction in morbidity and mortality with fibrinolytic therapy as men. However, women have an increased risk of major bleeding and haemorrhagic stroke42,257–266 probably because women enrolled in clinical trials were generally older and more often had co-morbidities, i.e. they were at greater risk, which may partly explain the observed higher rates of mortality compared with men.259,260,266–273 The finding that the increased risk of bleeding can only be partly reduced by adjusting the dose for body weight and renal function suggests an involvement of PD mechanisms.264,274 In a pooled analysis of randomized clinical trials, women with acute ischaemic stroke appear benefit more from recombinant tissue plasminogen activator (rtPA) than men and the usual gender difference in outcome favouring men was not observed in the thrombolytic therapy group.275
Gender differences in adverse drug reactions
Women present a greater (1.5–1.7-fold) incidence of ADRs and they tend to be more severe than in men requiring more often hospital admissions.7,23,26,36,276–282 Specifically, women have a higher risk of drug-induced torsades de pointes (TdP), hepatotoxicity and skin diseases, bleeding complications with anticoagulants, platelet antiaggregants and thrombolytics, electrolyte abnormalities with diuretics, myopathy with statins and cough, and rise in creatinine with ACEIs12,17,26,36,42,44,61,185,186,237,263,276–282 (Table 4). This is in line with the evidence that 8 of 10 drugs dropped out from US market between 1997 and 2000 posed greater health risks for women than for men.283
The reasons for the higher incidence of ADRs are unclear, but may result from (i) increased polypharmacy, as women consume more drugs than men, including over-the-counter medications and herbal remedies, which increases the risk of ADRs from drug–drug interactions11,36; (ii) differences in prescribing guideline-based drug therapy2; (iii) sex-related differences in PD (alterations in drug-target expression and/or in signal transduction pathways), immunological and hormonal factors.26 However, sex-related differences can be explained simply because women present higher drug plasma levels than men due to lower clearance and/or smaller Vd and if doses are not corrected for body weight, women are more frequently overdosed than men.18,19 Thus, when interpreting clinical trials, it is important to analyse whether the dose was given on a mg/kg basis or the same total dose was given to all subjects irrespective of body weight.
Drug-induced torsade de pointes
A prolonged heart-rate corrected QT interval (QTc) is a marker for an increased risk of polymorphic ventricular tachyarrhythmias, specifically TdP. Even after careful dosing based on body weight and creatinine clearance, equivalent drug plasma concentrations and men-predominance in the use of class I and III antiarrhythmics two-thirds of the TdP induced by cardiovascular or non-cardiovascular QT-prolonging drugs occurred in women.284–291
Women have longer QTc intervals and female gender is an independent risk factor for TdP, particularly when taking QT-prolonging drugs, as compared with men.286 The greatest QTc prolongation is observed during menstruation and ovulatory phase of the menstrual cycle, while shorter QT intervals are observed during the luteal phase which was correlated with the increase in serum progesterone.292–295 This observation and the finding that the QT shortens after puberty in men, but not in women,295 suggest that sex hormones can modulate cardiac Ca2+ and K+ channels involved in ventricular repolarization.288,294–300 Female hearts show reduced expression of several cardiac K+ channel subunits (Kv1.4, HERG, minK, KChiP2, SUR2, Kir2.3, Kir6.2), L-type Ca2+ channels, connexin-43 and phospholamban.294,296–298 Testosterone increases the rapid (IKr) and slow (IKs) components of the delayed rectifier and the inward rectifier K+ currents (IK1) that may account for the shorter QTc interval in men.288,294,297,299,300 Progesterone decreases the L-type Ca2+ current (ICaL) and IKr.294,297,300 However, in post-menopausal women HRT did not modify the QTc interval, suggesting that oestrogens and/or progesterone did not explain the gender differences in myocardial repolarization299 and that other unrecognized mechanisms, may be important in determining sex-related differences in the risk of developing drug-induced QT prolongation.301
Gender differences treatment
There are important differences in the prescription, adherence, and response to cardiovascular drugs between men and women, but translation of this information into clinical practice is slow.302,303 A recent study in ∼30 million American adults found that women were prescribed more medications than men but were less adherent (possibly related to the higher incidence of ADRs).1–4,10,11,21 Particularly, women with CVD are less likely to receive preventive treatments or guidance and treated less aggressively with guideline-recommended medication than men at similar cardiovascular risk and are less likely to undergo cardiac procedures.1–5,10,11,304–306 Women receive diuretics more often, but less nitrates, antiplatelets, lipid-modifying agents, ACEI, ARB, or beta blockers than men even after adjusting for all known variables.381,307–309 Sex differences in the treatment of CVD may be related to the gender of the physician (male physicians used significantly less medications and lower doses in female patients), differences in physicians’ interpretation of women’s symptoms and time of treatment with respect to the progression of CVD.11,304,310 Sometimes the reason is that women are older, forgetting that they live longer than men. These differences in cardiovascular treatment and care further suggest the need for interventions tailored to address gender disparities. An evidence-based pharmacotherapy in women is therefore auspicabile for women’s health.
Different gender representation in cardiovascular clinical trials
Women have been underrepresented in clinical trials, particularly in early phases, possibly due to hormonal changes during menstrual cycle and menopause, the influence of OCs and HRT on drug PK/PD, the fear related to drug administration during childbearing age or lactation, the underestimation of cardiovascular risk, the misconception of symptoms of CAD, and the lower occurrence of outcomes.1–5,8–14,26,305,311 This underrepresentation has important implications. First, it is a key factor contributing to limited recognition of sex-based differences in prescription, adherence, and responses to cardiovascular drugs, thereby preventing optimization of therapy for women of all ages. Second, quite often clinical trials are not powered to draw sex-specific conclusions and posthoc analyses are often used or simply data obtained in men are often extrapolated to women. Because translation of evidence into clinical practice only occurs in populations adequately represented in clinical trials, current guidelines for prevention and treatment for CVD are based on trials conducted predominantly in middle-aged men.6 Third, inadequate inclusion of female cells/animals in preclinical research and inadequate analysis of clinical data by sex might contribute to the lack of reproducibility of biomedical research.312 Thus, gender-based analyses are essential to elucidate possible differences in cardiovascular drug efficacy and safety.
On the regulatory end, there are continued efforts by regulatory agencies to increase the enrolment of both sexes in all phases of drug development, from preclinical studies to large-scale phase III trials. The National Institutes of Health (NIH) Revitalization Act of 1993 required the inclusion of women in NIH-funded clinical reseach.313 The guidelines for implementation, amended in 2001, required researchers to address inclusion of women in funding proposals and stated that phase III drug trials must be designed and carried out to allow for the valid analysis of differences between women and men when prior research has indicated that it may be important.314 The Office of Research on Womeńs Health plays a critical role in funding basic and clinical research to study the role of sex and gender in health and disease and sets NIH research priorities in diseases, disorders, and conditions that primarily affect women. The Women at Heart Initiative launched by the ESC highlights the growing burden and under-appreciation of women’s heart disease and promotes improved handling of women at risk of CVD in clinical practice.
Unfortunately, inclusion of women and sex-specific analysis and reporting remain low. In a Cochrane Review of 258 clinical trials, women comprised only 27% of the population. In 196 trials that included both men and women, only 33% examined outcomes by gender and in trials that performed a gender-based analysis, 20% reported significant differences in cardiovascular-related outcomes by gender.315 When analysed by year of publication, before or after 1993, there was no difference in the frequency of gender-based analyses. In another analysis of mixed-gender NHLBI-sponsored randomized controlled trials with primary outcomes of stroke, MI, or death published between 1997 and 2006 the median enrolment of women was 27% and only 13 out of 19 studies reported gender-based outcomes.311 Another study analysed the current level of compliance with the NIH guidelines in 56 federally funded randomized controlled trials. The median enrolment of women in trials including both sexes was 37% and 75% of the studies did not report any outcomes by sex.316 Even in recent large-scale trials with NOACs, women accounted for only 25–40% of the recruited patients.81,84,87–90
Conclusions
The response to cardiovascular drugs may differ among women and men because of differences in body composition, PK/PD properties of some drugs and fluctuations in endogenous sex hormone levels (menstrual cycle, pregnancy), or the administration of OCs or HRT. Additionally, women present a higher incidence of ADRs and ADRs tend to be more severe in women, probably as a result of administration of fixed doses, not adapted to body weight, leading to higher plasma levels and potential over dosage as compared with men. The identification of sex differences in dosing, efficacy, and safety of cardiovascular drugs is an essential first step in personalizing treatment. Gender-specific PD/PK differences have not been investigated for many drugs and the clinical relevance of many sex-related differences remains unproven. This should stimulate basic and clinical research to better understand sex-related differences in the efficacy and safety of cardiovascular drugs and the role of sex on the PD/PK variations induced by pathological conditions. Table 5 lists some recommendations for the design and dissemination of future CVD trials in women. Future trials should enrol an adequate number of females depending on the question being addressed and the design should include the analysis of sex-specific cardiovascular endpoints important for women. Cost-effectiveness analysis should be conducted and quality-of-life measures should be part of outcomes evaluated by gender. All this information would allow a better understanding of sex-related differences in the efficacy and safety of cardiovascular drugs and a more personalized drug selection for CVD prevention and treatment, particularly for those syndromes (i.e. diastolic dysfunction) that are more prevalent in women. Finally, sex-related differences in cardiovascular drug efficacy and safety should be part of medical education and presented as an intrinsic characteristic of the drugs on their labels. Nowadays, even among drugs with a greater than 40% difference in PK between men and women, sex-related recommendations for drug dosages are not included on their labels.25 Nevertheless, the most effective strategy to minimize the higher incidence of ADR in women is the development and implementation of sex-specific pharmacological guidelines.
Suggestions to improve our understanding of gender differences in the effects of cardiovascular drugs
|
|
ADR, adverse drug responses; CV, cardiovascular; CVD, cardiovascular diseases; PD, pharmacodynamics; PK, pharmacokinetics.
Suggestions to improve our understanding of gender differences in the effects of cardiovascular drugs
|
|
ADR, adverse drug responses; CV, cardiovascular; CVD, cardiovascular diseases; PD, pharmacodynamics; PK, pharmacokinetics.
Supplementary material
Supplementary material is available at European Heart Journal–Cardiovascular Pharmacotherapy online.
Conflicts of interest: none declared.
References
[No authors listed].
Praluent® Summary of Product Characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003882/WC500194521.pdf (
Repatha® Summary of Product Characteristics. 1http://ec.europa.eu/health/documents/community-register/2015/20150717132330/anx_132330_es.pdf (
NICE Clinical guideline [CG181], Cardiovascular disease: risk assessment and reduction, including lipid modification. www.nice.org.uk/guidance/cg181/evidence/lipid-modification-update-full-guideline-243786637 (