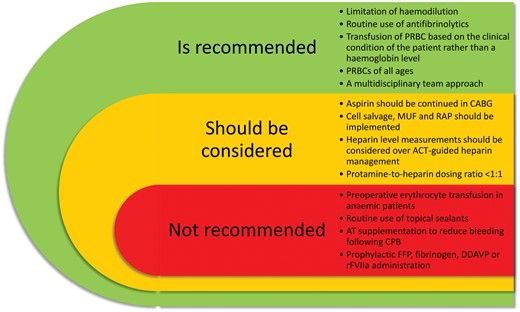
-
PDF
- Split View
-
Views
-
Cite
Cite
Domenico Pagano, Milan Milojevic, Michael I Meesters, Umberto Benedetto, Daniel Bolliger, Christian von Heymann, Anders Jeppsson, Andreas Koster, Ruben L Osnabrugge, Marco Ranucci, Hanne Berg Ravn, Alexander B A Vonk, Alexander Wahba, Christa Boer, 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 1, January 2018, Pages 79–111, https://doi.org/10.1093/ejcts/ezx325
Close - Share Icon Share
The Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA)
TABLE OF CONTENTS
Abbreviations and acronyms 80
1. Preamble 81
2. Introduction 81
3. Methods 81
4. Preoperative management 82
4.1 Laboratory and point-of-care tests to predict perioperative bleeding 82
4.2 Management of preoperative anticoagulant and antiplatelet drugs 83
4.2.1 Acetylsalicylic acid 83
4.2.2 Dual antiplatelet therapy 84
4.2.3 Glycoprotein IIb/IIIa inhibitors 85
4.2.4 Low-molecular-weight heparin 85
4.2.5 Vitamin K antagonists 86
4.2.6 Direct oral anticoagulant 86
4.3 Preoperative anaemia 87
4.3.1 Implications for preoperative anaemia 87
4.3.2 Iron supplementation 87
4.3.3 Erythropoietin 88
4.3.4 Blood transfusion to treat preoperative anaemia 88
5. Intraoperative management 88
5.1 Surgical techniques 88
5.1.1 Off-pump surgery 89
5.1.2 Minimally invasive extracorporeal circulation 89
5.1.3 Minimally invasive surgery 89
5.1.4 Topical haemostatics 90
5.2 Cardiopulmonary bypass 90
5.2.1 Closed versus open cardiopulmonary bypass 91
5.2.2 Biocompatible coating 91
5.2.3 Cell salvage 91
5.2.4 Ultrafiltration 92
5.2.5 Retrograde and antegrade autologous priming 92
5.2.6 Coagulation-friendly environment 92
5.3 Intraoperative anticoagulation 92
5.3.1 Heparin and anticoagulation monitoring 93
5.3.2 Protamine 93
5.3.3 Antithrombin 93
5.3.4 Heparin-induced thrombocytopenia anticoagulation management 94
5.4 Intravascular volume 94
5.4.1 Goal-directed haemodynamic therapy 94
5.4.2 Use of crystalloids and colloids 95
5.4.3 Haemodilution and cardioplegia 95
5.4.4 Predonation of blood 95
5.4.5 Acute normovolaemic haemodilution 96
6. Coagulation and transfusion 96
6.1 Procoagulant interventions 96
6.1.1 Antifibrinolytics 97
6.1.2 Fresh-frozen plasma 97
61.3 Factor XIII 97
6.1.4 Fibrinogen concentrates 98
6.1.5 Prothrombin complex concentrate 98
6.1.6 Desmopressin 98
6.1.7 Recombinant factor VIIa 99
6.2 Transfusion strategies 99
6.2.1 Quality of blood products 99
6.2.2 Algorithm-guided therapy for perioperative bleeding 100
6.2.3 Transfusion triggers for packed red blood cells and platelet concentrate 100
7. Anticoagulation management during extracorporeal life support 102
8. Final remarks 102
Conflict of interest 103
References 103
ABBREVIATIONS AND ACRONYMS
Abbreviations and acronyms
- ACS
Acute coronary syndrome
- ACT
Activated clotting time
- AKI
Acute kidney injury
- ANH
Acute normovolaemic haemodilution
- aPTT
Activated partial thromboplastin time
- ASA
Acetylsalicylic acid
- AT
Antithrombin
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CKD
Chronic kidney disease
- CPB
Cardiopulmonary bypass
- DAPT
Dual antiplatelet therapy
- DDAVP
Desmopressin
- DOAC
Direct oral anticoagulant
- EACA
ε-Aminocaproic acid
- EACTA
European Association of Cardiothoracic Anaesthesiology
- EACTS
European Association for Cardio-Thoracic Surgery
- ECC
Extracorporeal circulation
- ECLS
Extracorporeal life support
- ECMO
Extracorporeal membrane oxygenation
- EPO
Erythropoietin
- FFP
Fresh-frozen plasma
- FXIII
Factor XIII
- GPIIb/IIIa
Glycoprotein IIb/IIIa
- Hb
Haemoglobin
- HES
Hydroxyethyl starches
- HIT
Heparin-induced thrombocytopenia
- HR
Hazard ratio
- INR
International normalized ratio
- LMWH
Low-molecular-weight heparin
- MI
Myocardial infarction
- MiECC
Minimally invasive extracorporeal circulation circuit
- OR
Odds ratio
- PBM
Patient blood management
- PCC
Prothrombin complex concentrate
- PLTC
Platelet concentrate
- PMEA
Poly2-methoxyethylacrylate
- POC
Point-of-care
- PRBCs
Packed red blood cells
- RAP
Retrograde autologous priming
- RCT
Randomized controlled trial
- rFVIIa
Recombinant activated factor seven
- RR
Risk ratio
- SD
Solvent–detergent
- TEG
Thromboelastography
- TEM
Thromboelastometry
- TRALI
Transfusion-related acute lung injury
- TRIM
Transfusion-related immune modulation
- TXA
Tranexamic acid
- UFH
Unfractionated heparin
- VKA
Vitamin K antagonist
1. PREAMBLE
Cardiac surgery is associated with perioperative blood loss and a high risk of allogeneic blood transfusion. Patient blood management (PBM) in cardiac surgery contributes to the maintenance of perioperative haemostasis and the minimization of bleeding, which reduce blood transfusion requirements. PBM in cardiac surgery comprises an interaction between the cardiothoracic surgeon, the anaesthesiologist and the clinical perfusionist. The impact of cardiopulmonary bypass distinguishes this discipline from other surgical specialities.
In a joint effort, the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA) provide evidence-based recommendations for PBM in adult-acquired cardiac surgery. Literature searches were based on the Population, Intervention, Comparison, Outcome and Time (PICOT) method using standardized Medical Subject Headings (MeSH) terms from the National Library of Medicine, PubMed and Embase database lists of search terms. The PICOT study end points included bleeding, transfusions and reoperations for bleeding. The guideline was reviewed by an external review method and endorsed by the EACTS and the EACTA in collaboration with the editors of the European Journal of Cardio-Thoracic Surgery and the Journal of Cardiothoracic and Vascular Anesthesia.
This guideline provides practical recommendations for all clinicians working in the field of PBM in cardiac surgery, with emphasis on preoperative patient optimization and risk reduction, intraoperative maintenance of haemostasis and postoperative treatment for bleeding complications.
2. INTRODUCTION
Cardiac surgery is known to be associated with a high risk of perioperative blood loss and allogeneic blood transfusions due to the invasiveness of the procedures, the need for high-dose anticoagulation and the exposure to cardiopulmonary bypass (CPB). Both high blood product transfusion requirements and reoperation for bleeding have been associated with adverse clinical outcomes [1, 2]. The implementation of a multidisciplinary patient blood management (PBM) programme, the goal of which is meticulous surgical and perioperative haemostasis and minimization of blood loss, may, therefore, contribute to a reduction in transfusion requirements, a decrease in health care costs and an improvement in patient outcomes.
Even the transfusion of 1 or 2 units of packed red blood cells (PRBCs) has been associated with a dramatic increase in morbidity, mortality and costs in patients undergoing coronary artery bypass grafting (CABG) [3]. However, it remains unclear whether these complications are independent predictors of outcome or more surrogate markers of surgical complexity and complications. In contrast, re-exploration for bleeding and tamponade appears to be a strong risk factor for an increase in immediate postoperative mortality and morbidity. There is, however, no general consensus on when exploration for postoperative bleeding is indicated, and surgical practice varies considerably in this regard [4]. The first step towards creating algorithms to decrease blood loss and transfusions is to identify patients at high risk of bleeding, transfusion requirements and reoperation for bleeding. Also, there is little consensus on what constitutes a massive blood transfusion and what thresholds might be associated with adverse outcomes [5, 6].
Several factors have been found to be associated with an increased risk of bleeding, transfusion and reoperation [7–9], including advanced age, preoperative dual antiplatelet therapy (DAPT) use, poor platelet function, preoperative anaemia, small body surface area, the female gender, non-elective surgery, non-isolated surgery, non-CABG surgery and redo surgery. In an attempt to facilitate the preoperative prediction of the need for transfusion, several scoring methods have been developed [7, 10–15]. However, most of these risk models are of uncertain relevance, because they have not been externally validated. Risk prediction algorithms are, therefore, mostly used to stratify outcomes and allow risk-adjusted benchmarking in the context of quality and outcome monitoring.
PBM in cardiac surgery comprises an interaction between the surgeon, the anaesthesiologist and the clinical perfusionist, and the use of CPB distinguishes this discipline from other surgical specialities. Despite the availability of recent guidelines on PBM [9, 16, 17], there are currently no PBM guidelines available that specifically address the unique context of cardiac surgery and that are endorsed by the European societies. In a joint effort, the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA) collaborated on a common guideline with evidence-based recommendations for PBM in adult-acquired cardiac surgery conditions.
3. METHODS
As an expression of their declared and consolidated interest in medical care and scientific research in the field of PBM in cardiac surgery, the EACTS Council and the Board of Directors of EACTA selected a task force of experts, including a clinical methodologist. After the scope of the clinical guideline had been agreed upon by the task force members, a preliminary review of previous guidelines was performed. The scope and table of contents were established by co-chairs, and topics were allocated to the writing group. A systematic review of the published evidence in the field of blood conservation during adult-acquired cardiac surgery was performed based on the Population, Intervention, Comparison, Outcome and Time (PICOT) questions. Task force members undertook an evidence review; they were assisted by 2 research fellows with an MD/PhD in anaesthesiology and an MSc in clinical epidemiology, respectively. After study selection and quality assessment, tables of recommendations were drafted based on the synthesis of the best medical evidence. The experts conducted a review of the available evidence and followed the Methodology Manual for EACTS clinical guidelines [18]. All chapters were written through a collaboration between an EACTS member and an EACTA member. Agreement was reached through conference calls and face-to-face meetings, without excluding members with a conflict of interest. When no agreement could be obtained, a consensus was reached following a modified Delphi process [19]. Task force members who were unable to attend a face-to-face meeting voted by email. Three Delphi rounds were required to reach a consensus, and a final decision was made through a series of teleconference calls. Chapters were written by task force members who did not have a disclosure for the specific topic.
The literature was restricted to the years 2001–2017 to focus on contemporary evidence. The guideline only focused on adult-acquired cardiac surgery and did not include studies in the areas of transplantation, trauma, circulatory arrest or long-term circulatory support. Outcomes were defined as bleeding volume or chest drain output in the first 24 h following surgery, transfusion requirements and reoperation for bleeding at any time point during hospitalization. The definitions of bleeding, time frames of measurement and thresholds for transfusions varied significantly between studies. Wherever a distinction in time frame beyond 24 h was made, it is emphasized in the text.
The medical evidence was critically appraised for quality, including internal validity, external validity for the population of interest and publication bias. Randomized controlled trials (RCTs) and meta-analyses of RCTs were considered as the highest level of evidence, followed by other study designs. When a meta-analysis was the basis for a recommendation, the quality was assessed by a group of specialists from the EACTS/EACTA. In the absence of published evidence, expert consensus statements were made to cover specific issues that are essential to daily practice. The level of evidence and the strength of the recommendations were weighed and graded according to predefined scales, as outlined in Table 1 and 2.
Classes of recommendations
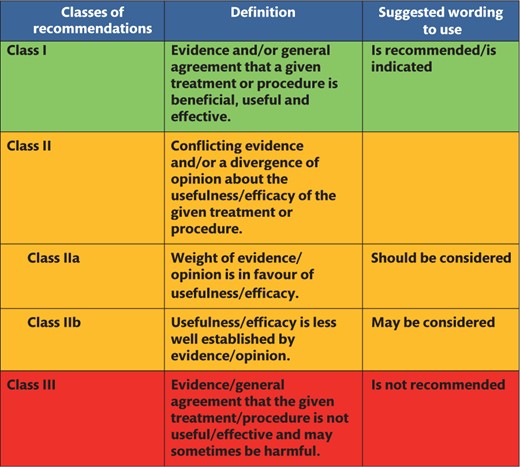 |
 |
Classes of recommendations based on the Methodology Manual for European Association for Cardio-Thoracic Surgery clinical guidelines [18].
Classes of recommendations
 |
 |
Classes of recommendations based on the Methodology Manual for European Association for Cardio-Thoracic Surgery clinical guidelines [18].
Levels of evidence
 |
 |
Levels of evidence based on the Methodology Manual for European Association for Cardio-Thoracic Surgery clinical guidelines [18].
Levels of evidence
 |
 |
Levels of evidence based on the Methodology Manual for European Association for Cardio-Thoracic Surgery clinical guidelines [18].
4. PREOPERATIVE MANAGEMENT
Inadequate patient optimization before surgery increases the risk of intraoperative anaemia and bleeding. Preoperative measures include management of antithrombotic medication, optimization of haemoglobin (Hb) levels in the context of patient comorbidities and body surface dimensions and the assessment and weighing of patient haemostatic risk factors, including the presence of congenital disorders.
4.1 Laboratory and point-of-care tests to predict perioperative bleeding
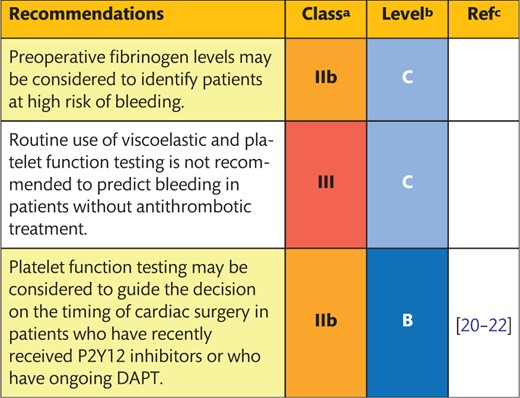 |
 |
Class of recommendation.
Level of evidence.
References.
DAPT: dual antiplatelet therapy.
 |
 |
Class of recommendation.
Level of evidence.
References.
DAPT: dual antiplatelet therapy.
Background
Preoperative assessment of haemostatic parameters by standard laboratory testing, viscoelastic testing and/or platelet function tests has been proposed to predict perioperative bleeding in cardiac surgery.
Description of the evidence
Standard laboratory testing
The use of routine preoperative screening has been much debated regarding its ability to identify high-risk patients for postoperative bleeding and transfusion requirements. There is no association between preoperative prothrombin time or the activated partial thromboplastin time (aPTT) and perioperative blood loss or transfusion requirements [23–25]. So far, the most commonly identified risk factor for postoperative bleeding is a low-fibrinogen level [23, 26–28]. However, despite its association with bleeding, the positive predictive value of a low-fibrinogen level remains poor (positive predictive value <20%) [26]. A low-platelet count <100 × 10−9/l has been associated with increased risk of transfusion [12], and patients with the highest postoperative blood loss volumes show the lowest platelet counts [24]. Finally, it has been shown that patients with the highest postoperative blood loss volumes show the lowest thrombin generation rates [24, 29], but this test is mainly used for research purposes and is not routinely used in everyday practice.
Preoperative fibrinogen levels and thrombin generation assays may be considered to identify patients at high risk of bleeding, e.g. during aortic or emergency surgery, although thrombin generation assays are not yet validated for routine clinical application.
Viscoelastic testing and platelet function tests
Preoperative assessment of haemostatic parameters using viscoelastic tests, such as rotational thromboelastometry (TEM) or thromboelastography (TEG), has been found to have a limited association with the risk of postoperative bleeding [29–31]. Preoperative platelet function testing has not been demonstrated to be associated with an increased risk of bleeding complications in patients without ongoing or recently stopped DAPT. In contrast, several studies have shown a significant association between impaired platelet function and bleeding complications in patients on DAPT. This finding indicates that platelet function testing may be used to guide the timing of surgery in this group of patients [21, 22, 32, 33].
4.2 Management of preoperative anticoagulant and antiplatelet drugs
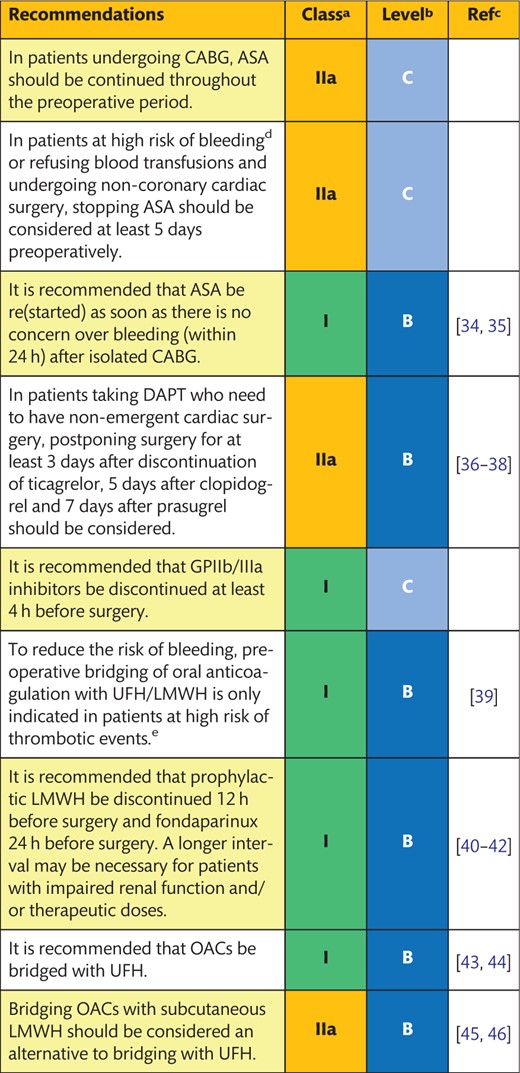 |
 |
 |
 |
Class of recommendation.
Level of evidence.
References.
Complex and redo operation, severe renal insufficiency, haematological diseases and hereditary deficiencies in platelet function.
Mechanical prosthetic heart valve, atrial fibrillation with rheumatic valvular disease, an acute thrombotic event within the previous 4 weeks and atrial fibrillation with a CHA2DS2-VASc score >4.
ASA: acetylsalicylic acid; CABG: coronary artery bypass grafting; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 (2 points), diabetes, prior stroke (2 points) – vascular disease, age 65–74, sex category (female); DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulant; GP: glycoprotein; INR: international normalized ratio; LMWH: low-molecular-weight heparin; OAC: oral anticoagulants; UFH: unfractionated heparin; VKA: vitamin K antagonist.
 |
 |
 |
 |
Class of recommendation.
Level of evidence.
References.
Complex and redo operation, severe renal insufficiency, haematological diseases and hereditary deficiencies in platelet function.
Mechanical prosthetic heart valve, atrial fibrillation with rheumatic valvular disease, an acute thrombotic event within the previous 4 weeks and atrial fibrillation with a CHA2DS2-VASc score >4.
ASA: acetylsalicylic acid; CABG: coronary artery bypass grafting; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 (2 points), diabetes, prior stroke (2 points) – vascular disease, age 65–74, sex category (female); DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulant; GP: glycoprotein; INR: international normalized ratio; LMWH: low-molecular-weight heparin; OAC: oral anticoagulants; UFH: unfractionated heparin; VKA: vitamin K antagonist.
4.2.1 Acetylsalicylic acid
Background
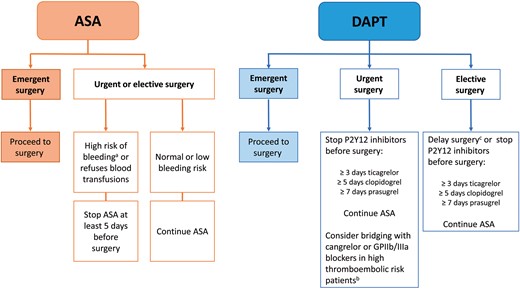
Acetylsalicylic acid (ASA) is one of the cornerstones for the treatment of acute and chronic cardiovascular disease. Primary and secondary prevention with ASA has been shown to reduce mortality, myocardial infarction (MI) and stroke [47] but to increase the risk of bleeding complications. Virtually, all patients requiring CABG—independent of whether they require emergent, urgent or elective surgery—are treated with ASA. Figure 1 provides an overview of the management of antiplatelet therapy in patients undergoing CABG surgery.
Management of antiplatelet therapy in patients having coronary artery bypass grafting surgery. aComplex and redo operations, severe renal insufficiency, haematological diseases and hereditary deficiencies in platelet function. bRecent stent implantation, recent thromboembolic event and alarming angiographic results. cUntil the recommended DAPT period is completed. ASA: acetylsalicylic acid; DAPT: dual antiplatelet therapy; GPIIb/IIIa: glycoprotein IIb/IIIa.
Description of the evidence
Discontinuation before surgery A recent meta-analysis comparing preoperative ASA administration with distinct dosing regimens versus no treatment or placebo in patients having CABG included 13 RCTs (n = 2399 patients) [48]. The meta-analysis showed that ASA reduced the risk of perioperative MI [odds ratio (OR) 0.56, 95% confidence interval (CI) 0.33–0.96] but not the risk of death (OR 1.16, 95% CI 0.42–3.22). Twelve-hour blood loss, PRBC transfusions and surgical re-exploration increased with ASA. The meta-analysis was limited by substantial heterogeneity but confirmed findings from a previous meta-analysis [49]. A large RCT compared the administration of ASA (100 mg) on the day of surgery versus placebo in patients having CABG [50]. The study showed no effect of treatment with ASA on 24-h bleeding (mean blood loss: 780 vs 740 ml; P = 0.30) or on the incidence of death or thrombotic complications (19.3% vs 20.4%; P = 0.55). Because patients were only eligible for inclusion if they were not using ASA or stopped ASA >4 days before surgery, these findings are difficult to generalize to other settings [50]. Another RCT demonstrated that preoperative administration of ASA (300 mg) was associated with significantly more patients having post-surgical drainage losses >1000 ml (OR 1.60, 95% CI 1.17–2.18) but with a lower rate of major cardiovascular events at the 53-month follow-up compared with placebo [hazard ratio (HR) 0.65, 95% CI 0.41–1.03] [51]. In a small RCT in patients with a platelet glycoprotein (GP) IIIa polymorphism, treatment with ASA (300 mg) increased the risk of postoperative bleeding (+25%) [52].
If one considers all of the results from the different studies, continuation of ASA is associated with more blood loss but also reduces ischaemic events in patients having CABG. In patients who refuse blood transfusions, who are undergoing non-CABG surgery or who are at a high risk of re-exploration for bleeding (e.g. in cases of complex and redo operations, severe renal insufficiency, haematological disease and hereditary platelet function deficiencies), ASA should be stopped at least 5 days before surgery. In other patients, the prevention of thrombotic events outweighs the risk of postoperative bleeding events. Moreover, recent data suggest that the inhibiting effect of ASA on platelet aggregation is reversible by platelet transfusion [53, 54], which also argues for the continuation of ASA.
Because current evidence supports the idea that ASA should be continued before surgery, the question of restarting applies only to a minority of patients. In a large prospective observational trial [34], patients who restarted ASA within 48 h of CABG had a mortality rate of 1.3% compared to 4.0% in the group of patients who did not receive ASA during this period (P < 0.001).
In conclusion, there is sufficient evidence to recommend continuation of ASA before cardiac surgery, and, if discontinued, it should be given to all patients having CABG as soon as there is no concern over bleeding to prevent thromboembolic complications.
4.2.2 Dual antiplatelet therapy
Background
DAPT with ASA and a P2Y12-receptor antagonist (clopidogrel, ticagrelor and prasugrel) reduces the risk of thrombotic complications in patients with acute coronary syndrome (ACS) compared with ASA treatment only [55–57]. The risk of thrombotic complications is further reduced if a second-generation P2Y12 antagonist (ticagrelor/prasugrel) is used instead of clopidogrel [56, 57], but the risk of both spontaneous and surgical bleeding complications increases with the newer antagonists [56–58]. The duration of DAPT treatment varies depending on the indication (recent ACS and/or stent implantation, coronary endarterectomy or off-pump surgery), bleeding risks and concomitant medications [59]. Recently, cangrelor, a new reversible intravenous P2Y12 inhibitor with an ultrashort half-life to offset the effect after discontinuation, was introduced [60].
Description of the evidence
Discontinuation of P2Y12 inhibitors before surgery
Continuation of DAPT until surgery increases the risk of postoperative bleeding, transfusions and re-exploration for bleeding, as shown in RCTs [36, 61, 62], observational studies [37, 63] and meta-analyses [64, 65]. It is therefore recommended that P2Y12 inhibitors be discontinued before elective surgery whenever feasible [66, 67]. Of note is the fact that, if P2Y12-receptor inhibitors are discontinued, ASA therapy should be continued until surgery. Alternatively, elective non-cardiac and cardiac procedures may be postponed until the DAPT treatment period is completed. In urgent cases, the risk of thromboembolic episodes must be weighed against the risk of perioperative bleeding complications. In extremely high-risk patients (e.g. patients with recent stent implantation), bridging therapy with cangrelor or a GPIIb/IIIa blocker may be considered [66, 67].
The safe discontinuation interval differs among distinct P2Y12 inhibitors due to variations in the platelet inhibitory effect and pharmacodynamic and pharmacokinetic properties [67]. In the CABG substudy of the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial, >5 days’ discontinuation of clopidogrel before surgery did not increase the risk of bleeding [risk ratio (RR) 0.83, 95% CI 0.46–1.48] [61]. For prasugrel, a time interval of 7 days is recommended due to the longer offset time compared with clopidogrel [68] and the high incidence of bleeding complications reported in the CABG substudy of the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38 trial [36]. Although it was previously recommended that ticagrelor be discontinued at least 5 days before surgery [69], a large observational study in CABG patients showed that discontinuation of ticagrelor for 3 or 4 days before surgery did not relate to a higher incidence of bleeding complications (OR 0.93, 95% CI 0.53–1.64) [37]; this observation is also supported by others [38, 63]. Current guidelines recommend restarting DAPT in all patients with ACS, independent of the revascularization strategy [66, 67].
Because of the individual variation in the magnitude and duration of the antiplatelet effect [21, 69, 70], the use of platelet function tests may help to optimize the timing of surgery or establish the grade of platelet inhibition in patients in whom the time since discontinuation is unclear. The guidance is that the interruption of therapy may be preferred over an arbitrary, specified period [66, 67]. The platelet inhibitory response assessed by platelet function testing to clopidogrel [22, 32, 33, 71], prasugrel [22, 32, 33, 71] and ticagrelor [21] is associated with CABG-related bleeding. A strategy based on preoperative platelet function testing to determine the timing of CABG in clopidogrel-treated patients led to 50% shorter waiting times for surgical treatment compared to those for a discontinuation time-based strategy [20]. It should, however, be pointed out that no RCT or observational study has investigated the incidence of perioperative bleeding complications in relation to the time since discontinuation-based or platelet function test-based surgery and that defined cut-off levels for acceptable platelet function are unavailable.
Taken together, in patients taking DAPT who need to undergo non-emergent cardiac surgery, postponement of surgery for at least 3 days after discontinuation of ticagrelor, 5 days after clopidogrel and 7 days after prasugrel should be considered to reduce the risk of postoperative bleeding (Fig. 1). During discontinuation of P2Y12 inhibitors, ASA therapy should be continued until surgery. Platelet function testing may be used to guide the timing of surgery in patients taking DAPT.
4.2.3 Glycoprotein IIb/IIIa inhibitors
Background
Glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors (eptifibatide, tirofiban and abciximab) are today almost exclusively used in conjunction with percutaneous coronary interventions but may also be used for bridging risk patients on oral P2Y12 inhibitors to surgery [66, 67, 72].
Description of the evidence
The optimal time for discontinuation of GPIIb/IIIa inhibitors before surgery is mainly based on pharmacokinetic calculations. Platelet function recovery is obtained 24–48 h after discontinuation of abciximab and 4–8 h after discontinuation of eptifibatide and tirofiban [73]. Cessation at 4 h before surgery is sufficient for patients treated with GPIIb/IIIa inhibitors. In a small prospective study, discontinuation of eptifibatide 4 h before CABG resulted in lower bleeding rates than discontinuation 2 h before the procedure [74]. For abciximab, no difference in bleeding was noted in a pooled analysis of 82 patients in the Evaluation in Percutaneous Transluminal Coronary Angioplasty to Improve Long-Term Outcome with Abciximab GP IIb/IIIa Blockade (EPILOG) and Evaluation of Platelet IIb/IIIa Inhibitor for Stenting (EPISTENT) trials who underwent acute CABG when abciximab was stopped <6 h before CABG in 61% of the patients [75]. In a small retrospective study, tirofiban-treated patients having CABG showed more bleeding than patients without tirofiban, but no difference was found between different discontinuation times [76].
In conclusion, GPIIb/IIIa inhibitor discontinuation at least 4 h before surgery should be considered to minimize the risk of postoperative bleeding.
4.2.4 Low-molecular-weight heparin
Background
Low-molecular-weight heparin (LMWH; enoxaparin and fondaparinux) mainly inhibits activated FX (FXa), with plasma concentration peaks 3–4 h after administration. It is the preferred strategy for prophylactic and therapeutic anticoagulation in patients after certain surgeries (e.g. orthopaedic surgery) and in patients with malignancies. The half-life is around 5 h in patients with normal renal function. Monitoring of the anticoagulant effects of LMWH can be achieved by plasma anti-FXa activity. LMWH-induced bleeding may be treated with protamine, but this therapy does not completely reverse the anticoagulant effect of LMWH.
Description of the evidence
Cardiac surgery patients receiving enoxaparin versus unfractionated heparin (UFH) more frequently underwent re-exploration for bleeding (HR 2.6, 95% CI 1.1–5.9) [44]. Patients receiving LMWH within 12 h before surgery showed more blood loss and transfusion requirements than patients receiving UFH or a dose of LMWH >12 h before surgery [77]. However, other studies could not confirm this finding [41]. Fondaparinux versus enoxaparin before CABG surgery resulted in the same rate of bleeding events, but when it was not discontinued 36 h before surgery, fondaparinux was associated with higher 12-h postoperative bleeding rates [42]. The half-life of fondaparinux is increased in patients with chronic kidney disease.
Overall, preoperative bridging of oral anticoagulation with UFH/LMWH is only indicated in patients at high risk of thrombotic events. It is further recommended that prophylactic LMWH be discontinued 12 h and fondaparinux 24 h before surgery; a longer interval may be necessary for patients with impaired renal function.
4.2.5 Vitamin K antagonists
Background
Vitamin K antagonists (VKAs) are commonly used to prevent and treat thromboembolism in patients with atrial fibrillation, venous thromboembolic disease and mechanical heart valves (particularly when they are in the mitral or tricuspid position). The anticoagulation effect can be monitored by the prothrombin time and is most often expressed as an international normalized ratio (INR).
Description of the evidence
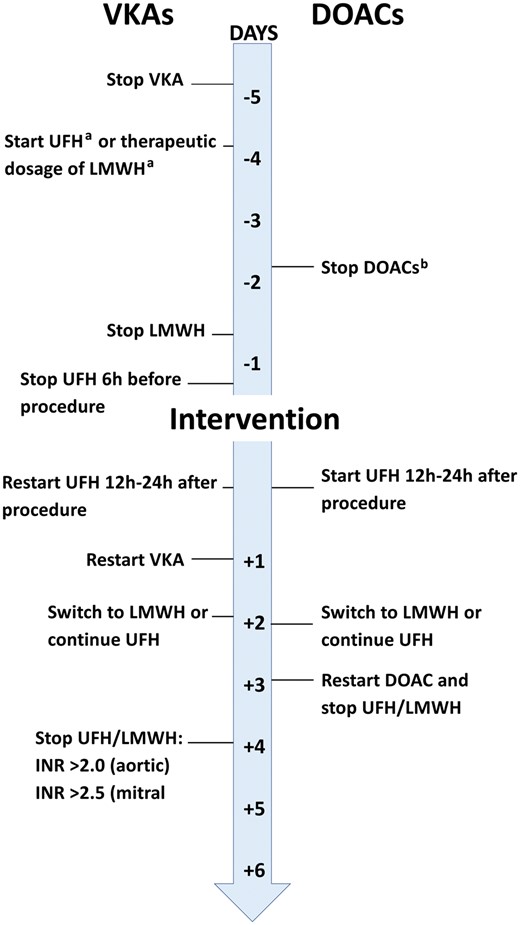
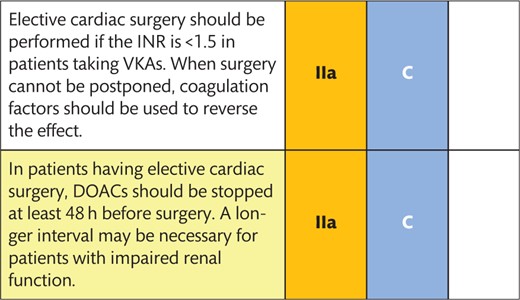
VKAs are regularly stopped 3–5 days before surgery to obtain an INR <1.5. In patients having urgent or emergency surgery, the effect of VKA can be completely reversed by administering prothrombin complex concentrate (PCC). Bridging non-cardiac surgery patients who are taking VKA with a full therapeutic dose of LMWH after surgery is associated with increased bleeding but not with a meaningful reduction in thrombotic events [39]. Thus, bridging to cardiac surgery is only recommended in patients at a very high risk of thrombotic events, e.g. those with a recent (<4 weeks) pulmonary embolism or a prosthetic mechanical valve, atrial fibrillation with the rheumatic valvular disease or atrial fibrillation with a CHA2DS2-VASc [congestive heart failure, hypertension, age ≥75 (2 points), diabetes, prior stroke (2 points) – vascular disease, age 65–74, sex category (female)] score >4. In these patients, VKA should be discontinued 5 days before surgery and bridged by LMWH (Fig. 2). Bridging with UFH after surgery until the INR reaches the therapeutic range in patients on VKAs (bridging with subcutaneous LMWH may be considered an alternative to bridging with UFH) is recommended. Elective cardiac surgery should not be performed if the INR is >1.5 in patients taking VKAs. When surgery cannot be postponed, coagulation factors should be used to reverse the effect.
Management of oral anticoagulation in patients with an indication for pre- and/or postoperative bridging (reproduced with permission from Sousa-Uva. Stuart J. Head, Milan Milojevic, Jean-Philippe Collet, Giovanni Landoni, Manuel Castella et al. 2017 EACTS Guidelines on Perioperative Medication in Adult Cardiac Surgery. Eur J Cardiothorac Surg 2017; doi:10.1093/ejcts/ezx314). aBridging with UFH/LMWH should start when INR values are below specific therapeutic ranges. bDiscontinuation should be prolonged to >72 h if creatinine clearance is 50–79 ml/min/1.73 m2 or ≥96 h if creatinine clearance is <50 ml/min/1.73 m2. DOACs: direct oral anticoagulants; INR: international normalized ratio; LMWH: low-molecular-weight heparin; UFH: unfractionated heparin; VKAs: vitamin K antagonists.
4.2.6 Direct oral anticoagulant
Background
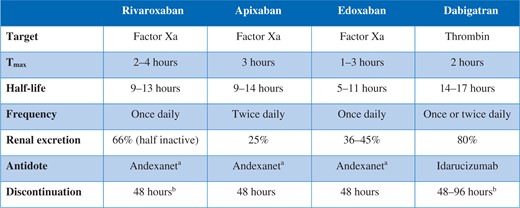
Direct oral anticoagulants (DOACs) include direct thrombin inhibitors (dabigatran) and FXa inhibitors (rivaroxaban, apixaban and edoxaban), and novel formulations are under development. DOACs are increasingly used as an alternative anticoagulation strategy for VKAs.
Description of the evidence
Because emergency surgery in patients under dabigatran treatment has been associated with severe or even fatal bleeding, it is recommended that DOACs be discontinued at least 48 h before cardiac surgery [78–80]. DOACs may be bridged by heparins in patients with acute thrombotic events <4 weeks. The half-life of DOACs may be prolonged in case of impaired renal function (Table 3).
Different types of DOACs
 |
 |
Not yet approved by EMA.
Discontinue >48 h if creatinine clearance is >80 ml/min/1.73 m2; discontinue >72 h if creatinine clearance is 50–79 ml/min/1.73 m2; and discontinue >96 h if creatinine clearance is <50 ml/min/1.73 m2.
EMA: European Medicines Agency; DOACs: direct oral anticoagulants.
Different types of DOACs
 |
 |
Not yet approved by EMA.
Discontinue >48 h if creatinine clearance is >80 ml/min/1.73 m2; discontinue >72 h if creatinine clearance is 50–79 ml/min/1.73 m2; and discontinue >96 h if creatinine clearance is <50 ml/min/1.73 m2.
EMA: European Medicines Agency; DOACs: direct oral anticoagulants.
Whenever feasible in patients with impaired renal function, the concentration of DOAC should be measured in terms of the diluted thrombin time (dabigatran) or calibrated with anti-FXa activities (rivaroxaban, apixaban or edoxaban). The use of viscoelastic tests is still debated in the diagnosis of DOAC-induced prolonged clotting time. Ecarin clotting times may be used when available for patients taking dabigatran. For emergency reversal of dabigatran, the newly released antidote (idarucizumab) can be used in the pre- and postoperative settings. Treatment of postoperative FXa-related bleeding includes PCC, activated PCC (FEIBA®, Shire US Inc., Lexington, MA, USA) and recombinant activated factor VII (rFVIIa), because no specific antidote is approved at the moment. These agents have been found to be effective in some studies (PCC in volunteer studies, aPCC and rFVIIa mainly in vitro studies) on FXa inhibitors. Due to the relatively low level of protein binding, haemodialysis is effective for increasing the clearance of dabigatran but not for that of FXa-inhibitors (protein binding >85%).
Based on the available evidence, it is recommended that DOACs be stopped at least 48 h prior to surgery in patients having elective cardiac surgery; a longer interval may be necessary for patients with impaired renal function (Fig. 2).
4.3 Preoperative anaemia
 |
 |
Class of recommendation.
Level of evidence.
References.
AKI: acute kidney injury; EPO: erythropoietin; Hb: haemoglobin.
 |
 |
Class of recommendation.
Level of evidence.
References.
AKI: acute kidney injury; EPO: erythropoietin; Hb: haemoglobin.
4.3.1 Implications for preoperative anaemia
Background
Anaemia is a frequent comorbid condition in the elderly patients, and up to 40% of patients present with mild anaemia (women, Hb 100–120 g/l; men, Hb 100–130 g/l) or severe anaemia (both genders, Hb ≤100 g/l) of any cause prior to cardiac surgery [84]. Although anaemia is recognized to be a predictor of postoperative adverse outcomes, this evidence is mostly based on observational studies.
Description of the evidence
Preoperative anaemia is associated with a worsening of the clinical outcome after cardiac surgery in terms of blood transfusion requirements, acute kidney injury (AKI) and death [85–87]. Several retrospective studies showed a significant interaction between preoperative anaemia and blood transfusions on outcome and a pronounced detrimental effect of blood transfusions for anaemic patients [88, 89]. The risk for intraoperative transfusion is significantly higher in mildly anaemic (HR 1.44, 95% CI 1.20–1.73) or severely anaemic patients (HR 1.81, 95% CI 1.34–2.44) [88, 89].
Derived from retrospective studies, preoperative anaemia is, therefore, considered a risk factor for adverse clinical outcomes and transfusion requirements after cardiac surgery.
4.3.2 Iron supplementation
Background
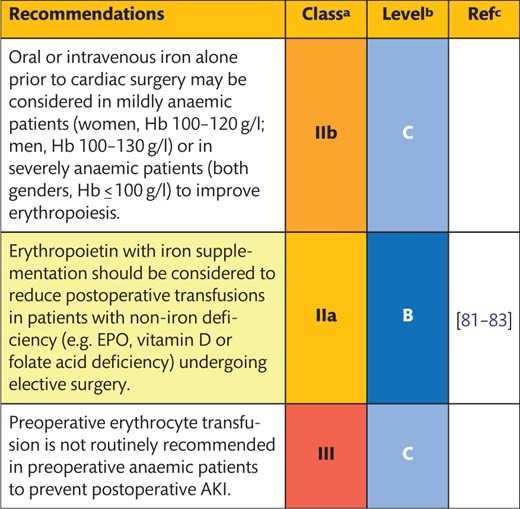
Supplementation of oral or intravenous iron is the treatment of choice for iron deficiency anaemia. Preoperative iron supplementation may reduce transfusion requirements in patients undergoing cardiac surgery with iron deficiency anaemia.
Description of the evidence
A recent RCT in non-anaemic patients, based on a 3-arm parallel group design, compared the effect of 3 preoperative doses of intravenous iron sucrose (100 mg) and daily oral iron (125 mg) with placebo until 1 month following discharge. No difference was found between the transfusion requirements of the various groups [90]. The PROphylaxis for ThromboEmbolism in Critical Care Trial (PROTECT) investigated the perioperative administration of iron isomaltoside (1000 mg) on postoperative Hb levels and transfusion requirements in non-anaemic patients having elective surgery [91]. Although there was a lower number of non-anaemic patients in the iron isomaltoside-treated group compared with the group receiving a placebo 1 month after surgery (8.0% vs 38.5%, P = 0.019), transfusion requirements up to 4 weeks after surgery were not different between groups (13.3% vs 20.0%, P = 0.52). Apart from the significant effect of iron supplementation on stimulation of erythropoiesis, there are limited data supporting the association between the administration of iron and reduced postoperative transfusion requirements in patients with anaemia undergoing cardiac surgery. The optimal time to start iron therapy before surgery is unclear from the current evidence.
4.3.3 Erythropoietin
Background
Preoperative anaemia is associated with adverse outcomes after cardiac surgery in several retrospective studies [92, 93]. Erythropoietin (EPO) with or without iron supplementation may be used to treat preoperative anaemia and to reduce the risk of postoperative transfusion requirements.
Description of the evidence
Erythropoietin, with or without iron, has been used to treat non-iron deficiency anaemia and to increase red cell mass in patients who refuse blood transfusions or who are at high risk of postoperative anaemia, such as patients with renal failure [9, 94, 95]. Administration of EPO (80 000 IU) and oral iron (40 mg) or iron alone in 600 mildly or non-anaemic patients reduced the perioperative PRBC transfusion requirements in patients treated with EPO more than in controls (17% vs 39%, P <0.0005), but this difference was diminished after controlling for baseline Hb levels [81]. In 320 patients undergoing off-pump CABG surgery, preoperative high-dose EPO treatment (cumulative dose of 52 000 IU) starting 2 days prior to and lasting until 2 days after surgery was compared to the effects of placebo [82]. Postoperative transfusion requirements were lower in the EPO group compared with the placebo group (0.33 vs 0.76 units per patient, P = 0.008). A single dose of EPO (500 IU/kg) with iron sucrose (200 mg) 1 day prior to surgery in primary heart valve procedures reduced transfusion requirements during surgery until 4 days postoperatively compared with the control group (59.5% vs 86.5%, P = 0.009) [83]. In all studies, the investigators were blinded to the study treatment (single blinded).
In summary, EPO with or without iron supplementation should be considered in patients with non-iron deficiency undergoing elective surgery to reduce postoperative transfusions. Even an EPO infusion in the days before surgery may have benefits in terms of transfusion requirements. It should be noted that, in part, data showing a treatment benefit for EPO were obtained from non-anaemic treated patients undergoing cardiac surgery.
4.3.4 Blood transfusion to treat preoperative anaemia
Background
Preoperative blood transfusions to treat anaemia are frequently used to symptomatically treat low Hb values. Transfusion prior to cardiac surgery or with initiation of CPB has been suggested for patients with pre-existing anaemia.
Description of the evidence
One small RCT investigated the effect of preoperative PRBC transfusion in anaemic patients on intraoperative transfusion requirements and AKI as outcomes [96]. Prophylactic transfusion of 2 PRBC units was associated with a reduced rate of intraoperative transfusion [0 (0–2) vs 2 (1–4) units, P <0.001] compared with standard care but not with a reduced rate of AKI [96]. Given the lack of additional published evidence, an expert consensus was reached by the guideline committee against the routine use of transfusions for patients with preoperative anaemia. However, in the case of emergency surgery and life-threatening anaemia, it is legitimate to use preoperative blood transfusions to increase Hb levels.
5. INTRAOPERATIVE MANAGEMENT
Intraoperative preservation of patient haemostasis is a multidisciplinary and multifactorial challenge. Even though the surgeon applies meticulous haemostasis and patience with respect to clot formation, these measures are only effective when paralleled by interventions that minimize haemodilution, normothermia, appropriate anticoagulation and haemostatic monitoring during the procedure.
5.1 Surgical techniques
Special surgical or perfusion techniques, such as off-pump CABG, minimally invasive extracorporeal circulation circuits (MiECC) and minimally invasive cardiac surgery are not universally implemented. These procedures might only be safe and efficiently performed when integrated into the daily clinical routine. Therefore, any recommendations regarding the use of these techniques are addressed to centres with adequate experience in these procedures. However, especially for severely anaemic patients, it is highly recommended that the members of the multidisciplinary team (cardiologists, surgeons, anaesthesiologists and perfusionists) discuss optimal treatment strategies, including surgical techniques, the limitations of haemodilution and improved CPB systems to avoid massive transfusions and bleeding.
 |
 |
Class of recommendation.
Level of evidence.
References.
Surgeons, cardiologists, anaesthesiologists and perfusionists.
Dialysis-dependent patients, patients operated on under DAPT and anaemic patients with low body surface area.
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; DAPT: dual antiplatelet therapy; MiECC: minimally invasive extracorporeal circulation circuits.
 |
 |
Class of recommendation.
Level of evidence.
References.
Surgeons, cardiologists, anaesthesiologists and perfusionists.
Dialysis-dependent patients, patients operated on under DAPT and anaemic patients with low body surface area.
CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; DAPT: dual antiplatelet therapy; MiECC: minimally invasive extracorporeal circulation circuits.
5.1.1 Off-pump surgery
Background
Several hypothetical mechanisms by which off-pump CABG surgery might reduce bleeding and/or the need for blood product transfusions, including lower dosing of systemic heparin, the standard use of cell saving and the fact that haemodilution and blood trauma caused by CPB, are obviated with this technique.
Description of the evidence
There are no RCTs comparing off-pump and on-pump CABG where bleeding, transfusion or the need for reoperation was the primary end point; the data are therefore extrapolated. A meta-analysis comprising 102 RCTs compared off-pump with on-pump CABG in 19 101 patients [98]. The secondary end points of PRBC transfusion (OR 0.49, 95% CI 0.33–0.72) and blood product transfusion (OR 0.66, 95% CI 0.55–0.81) were significantly lower in patients having off-pump than in those having on-pump CABG surgery, without differences in re-exploration for bleeding [98]. However, a large variability across studies was observed, suggesting that such a benefit could not be reproduced in several of the RCTs included. A recent meta-analysis of RCTs showed similar results [97] but was also confounded by a high grade of heterogeneity for transfusion requirements and chest tube drainage among RCTs. In the 2 largest RCTs (the Coronary Artery Bypass Surgery [CABG] Off or On Pump Revascularization Study [CORONARY] [105] and the German Off-Pump Coronary Artery Bypass Grafting in Elderly Patients [GOPCABE] trial [106]), off-pump surgery was associated with lower transfusion rates (50.7% vs 63.3% and 56.3% vs 62.7%, respectively; both P <0.001). However, in these RCTs, physicians were not blinded to treatment allocation, and the transfusion protocol was not prespecified. Therefore, the treatment received might have influenced the decision to transfuse.
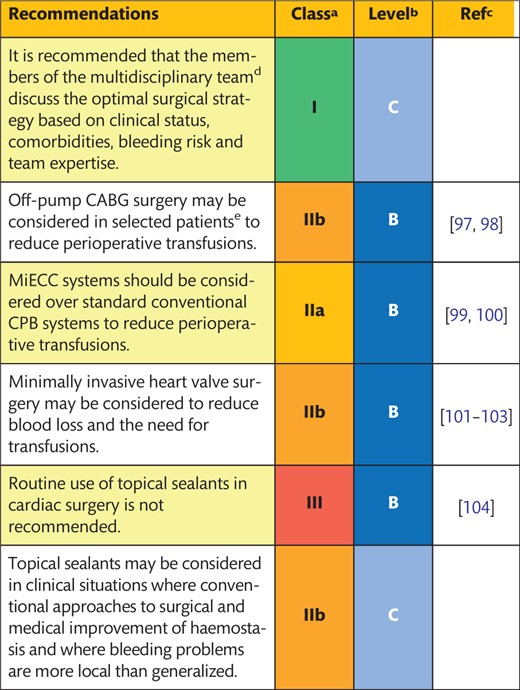
In summary, off-pump surgery may be considered a surgical technique that is associated with fewer transfusion requirements than on-pump surgery.
5.1.2 Minimally invasive extracorporeal circulation
Background
The common features of MiECC are a small priming volume (∼600–750 ml), a reduced artificial surface due to the absence of the venous reservoir, a biocompatible coated system and a centrifugal pump. Systems are completely closed to avoid blood–air contact, and they include the separation of shed blood [107].
Description of the evidence
RCTs comparing MiECC versus conventional CPB were too underpowered to detect a significant difference in transfusion rates. The available meta-analyses [99, 100] show a reduced rate of transfusion with MiECC but include open studies and studies without a predefined transfusion protocol. The type of circuit used may also have influenced the decision to transfuse. These aspects limit the generalizability of current comparisons. Two recent meta-analyses compared MiECC with conventional CPB procedures [99, 100]. An analysis of the results of 24 studies (n = 2770) showed that the use of MiECC was associated with a reduced risk of PRBC transfusion (OR 0.24, 95% CI 0.16–0.37; P <0.001) but without a reduction in reoperations for bleeding [99]. In a meta-analysis that assessed 29 studies with 2335 patients, the use of MiECC resulted in a significant reduction in the risk of transfusions (OR 0.35, 95% CI 0.23–0.53), whereas reoperation rates for persistent bleeding revealed no difference [100].
In summary, the use of MiECC systems, when compared with conventional CPB systems, is associated with a reduction in PRBC transfusions. Therefore, in an attempt to reduce allogeneic blood transfusions, MiECC systems can be considered. However, the use of MiECC systems does not contribute towards reducing reoperation rates for bleeding.
5.1.3 Minimally invasive surgery
Background
Minimally invasive heart valve surgery may play a role in reducing the need for transfusion and reoperation for bleeding, because it requires a smaller incision and less tissue dissection and retraction to open up the surgical field.
Description of the evidence
The available evidence consists almost entirely of observational studies and should not be considered definitive. The 2010 International Society for Minimally Invasive Cardiothoracic Surgery consensus statement concluded that the risk of transfusion for PRBCs was reduced after minimal mitral valve surgery compared with the conventional approach (difference of −1.9 units; 95% CI −2.5 to −1.2 units), but reoperation for bleeding was not different between the groups [102]. A systematic review of mitral valve surgery through a right lateral minithoracotomy versus sternotomy identified a total of 45 studies, including 3 RCTs [101]. The minimally invasive approach was associated with a small reduction in bleeding (mean difference −142.1 ml, 95% CI −199.2 to −85.1 ml) and risk for transfusion (RR 0.67, 95% CI 0.51–0.88). However, pooled data from the small RCTs showed that minimally invasive surgery did not reduce total blood loss (mean difference −149 ml; 95% CI −491 to 193 ml) or the incidence of transfusion (RR 0.94, 95% CI 0.61–1.45). In a systematic review on aortic valve replacement through minimally invasive access versus full sternotomy [103], pooled data from 3 RCTs showed no difference in the rate of blood product transfusions (RR 0.77, 95% CI 0.58–1.03), whereas pooled data from observational studies suggested otherwise. Minimally invasive heart valve surgery is practised in high volumes in only a limited number of centres.
Until minimally invasive valve surgery becomes more widely adopted and large RCTs are performed, there is not enough evidence to suggest broad adaption for the purpose of blood conservation.
5.1.4 Topical haemostatics
Background
In addition to systemic haemostatic action, topical haemostatic agents are locally applied and include haemostatic sealants and topical antifibrinolytic agents [aprotinin, tranexamic acid (TXA) and ε-aminocaproic acid (EACA)] [108, 109]. Passive haemostatic agents that activate platelets, including collagen, gelatines and regenerated oxidized cellulose, do not contain biologically active clotting factors. The last category of topical agents includes synthetic (cyanoacrylates) and semi-synthetic (glutaraldehyde–albumin) sealants, which have sealing properties through a rapid polymerization process without having intrinsic haemostatic activity.
Description of the evidence
A Cochrane systematic review [108] on 18 RCTs comparing fibrin sealant versus no fibrin sealant concluded that fibrin sealants are efficacious in reducing both postoperative blood loss (mean difference −161 ml, 95% CI −98.25 to −224.53) and perioperative exposure to allogeneic PRBC transfusions (RR 0.63, 95% CI 0.45–0.88). However, this review was limited by the inclusion of only 1 cardiac surgery study and RCTs with small sample sizes. A meta-analysis [109] including 8 RCTs (n = 622 patients) showed that topical antifibrinolytic agents reduced the amount of 24-h chest tube blood loss by 220 ml (95% CI −318 to −126) and resulted in a saving of 1 PRBC unit per patient (95% CI −1.54 to −0.53). However, high heterogeneity among RCTs was found. No adverse effects were reported following topical use of the medications. A more recent Cochrane systematic review [110] including 7 RCTs (n = 511) supported a protective role of topical antifibrinolytic agents in cardiac surgery (RR 0.63, 95% CI 0.61–0.66), but the overall quality of the studies included was low. A recent RCT including off-pump CABG patients did not find any benefit from topical EACA regarding postoperative bleeding and transfusion [111].
Based on the available evidence, the routine use of topical sealants in cardiac surgery is not recommended and may only be considered in cases of persistent bleeding where the bleeding is localized.
5.2 Cardiopulmonary bypass
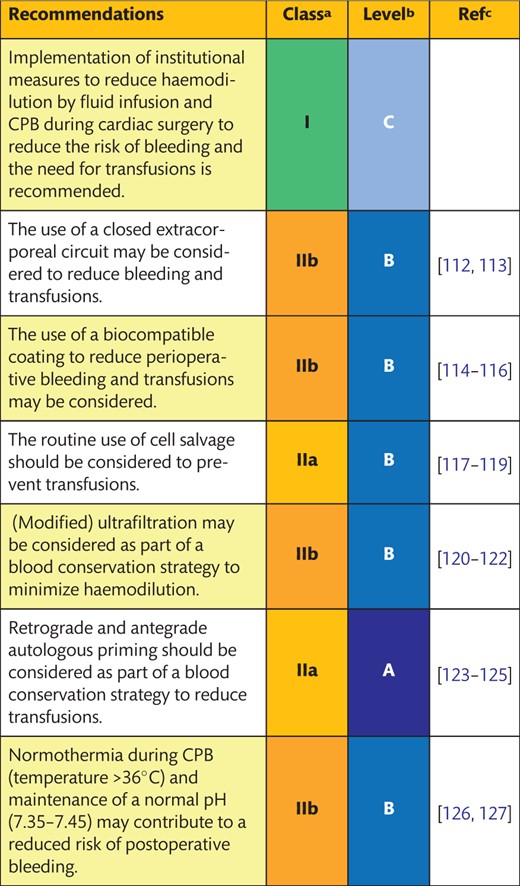 |
 |
Class of recommendation.
Level of evidence.
References.
CPB: cardiopulmonary bypass.
 |
 |
Class of recommendation.
Level of evidence.
References.
CPB: cardiopulmonary bypass.
5.2.1 Closed versus open cardiopulmonary bypass
Background
The contact of blood with ambient air in the reservoir and in other parts of the circuit in extracorporeal circulation (ECC) is regarded as one of the several causes of coagulation factor activation during CPB [128]. Closed systems minimize the blood/air interface and platelet activation and are an integral part of MiECC systems but result in more complicated perfusion that requires active trapping of air bubbles [129]. The use of cardiotomy suction requires a separate reservoir, and vacuum-assisted venous drainage cannot be applied to the collapsible reservoir bag.
Description of the evidence
A large number of studies combined several components of blood conservation, including closed circuits. Some studies combined the elimination of cardiotomy suction with the use of a closed system or investigated the combined effect of surface coating, a closed system and different pumps [113, 130]. In an RCT, closed systems had no advantage over open systems for patient haemostasis [113]. In a small, single-centre RCT, a closed system reduced transfusion requirements compared with an open system (378 ± 364 ml vs 717 ± 486 ml; P = 0.003) [112]. Taken together, the use of a closed ECC may be considered to reduce bleeding and transfusions, but adequately sized RCTs on closed versus open systems are lacking.
5.2.2 Biocompatible coating
Background
Biocompatible coating of tubing and oxygenator mimics the natural endothelial lining of blood vessels and improves the haemocompatibility and hydrophilicity of the system, thereby reducing the risk of clot activation. Among others, biocompatible coatings include ionic or covalent heparin bonding, poly(2-methoxyethylacrylate) (PMEA) and phosphorylcholine. The effect of the biocompatible coating of the ECC on bleeding and transfusion requirements is still under investigation.
Description of the evidence
A systematic review and meta-analysis published in 2009 of 36 RCTs issued between 1992 and 2006 showed that the use of any biocompatible coating reduced the risk of PRBC transfusion (OR 0.8, 95% CI 0.69–0.93) compared with the use of a non-coated circuit [114]. A more recent systematic review of 14 RCTs concluded that second- and third-generation heparin-coated circuits only showed superiority on perioperative blood loss to non-coated circuits in about 50% of the studies included [116]. A large RCT showed that the combination of heparin-coated circuits with low or conventional dose heparinization was associated with a reduced 12-h blood loss compared with non-coated circuits (mean volume 382 vs 431 vs 457 ml; P <0.01, respectively) [131].
Other studies showed that the use of phosphorylcholine-coated circuits might contribute to less perioperative blood loss and fewer transfusion requirements [132–134]. In 2 small studies comparing the effect of PMEA, phosphorylcholine and heparin-coated circuits [135, 136], only 1 study showed that PMEA coating was associated with fewer platelet transfusions compared with heparin coating [135].
Based on the available data, the use of a biocompatible coating may be considered as part of a PBM programme to reduce perioperative bleeding and transfusions.
5.2.3 Cell salvage
Background
Cell salvage of operative blood loss and residual blood from the circuit after CPB may contribute to a reduction in the use of allogeneic blood products, especially PRBCs. A specific rationale for cell salvage is its use during CPB instead of cardiotomy suction, since the retransfusion of whole blood using cardiotomy suction through the ECC may contribute to systemic inflammation and bleeding.
Description of the evidence
A Cochrane review showed that the use of cell salvage reduced the risk of PRBC transfusions (RR 0.78, 95% CI 0.68–0.91) during cardiac surgical procedures [117, 137]. In a meta-analysis comparing cell salvage with cardiotomy suction, it was found that cell salvage reduced the risk of any blood transfusion (OR 0.63, 95% CI 0.43–0.94) without increasing adverse effects or bleeding [138]. Recent studies confirmed that the use of cell salvage is efficient in reducing allogeneic blood exposure [118, 119, 139].
Cardiotomy suction might be associated with thrombin generation and neutrophil and platelet activation and could contribute to higher transfusion requirements [9]. The amelioration of inflammation by the removal of activated plasma through the washing of shed blood has repeatedly been demonstrated [140–142]. Cell saver processing of cardiotomy suction blood only has no beneficial effect on blood conservation and increases the use of fresh-frozen plasma (FFP) transfusions [138, 143, 144]. Extending the use of cell salvage to the postoperative phase may show additional benefits [143], although others did not confirm this [118]. Moreover, large volumes of cell-salvaged blood may lead to a relative loss of plasma and may result in impaired coagulation [145]. Therefore, it may be concluded that the balance between a positive effect of cell salvage on transfusion requirements and a reduction of negative systemic effects (versus the inherent loss of plasma, platelets and leucocytes) depends on the quantity of the salvaged blood.
In the postoperative period, tube drainage and the washing of blood may be implemented to reduce postoperative blood transfusions. The British National Institute for Health and Clinical Excellence conducted an extensive review of the literature and several meta-analyses for postoperative cell salvage [146]. In cardiac surgery, the absolute risk of being exposed to allogeneic blood was approximately 15.6% (95% CI 7.4–21.4%) lower in patients receiving postoperative cell salvage and 1 unit less of allogeneic blood. Postoperative cell salvage resulted in lower rates of infection, lower mortality rates and shorter hospital stays. Unwashed salvaged blood from postoperative surgical drains contains higher concentrations of inflammatory mediators, fibrin split products, interleukins, fat emboli and complement factors and may increase the risk of inflammatory complications [147].
In summary, the routine use of cell salvage should be considered to prevent transfusions, but the retransfusion of large volumes of cell salvaged blood (>1000 ml) may impair coagulation. Overall, postoperative cell salvage and reinfusion of washed erythrocytes may be considered to reduce transfusions in patients with bleeding.
5.2.4 Ultrafiltration
Background
The total volume of blood during CPB can be controlled using ultrafiltration. In modified ultrafiltration, excess fluid is discarded from the remaining blood after the termination of ECC.
Description of the evidence
Ultrafiltration and modified ultrafiltration are accompanied by removal of mediators of inflammation, which may also lead to improved haemostasis. In a meta-analysis, it was shown that ultrafiltration, and particularly modified ultrafiltration, was associated with a reduction in postoperative blood transfusions (−0.73 units; 95% CI −1.16 to −0.31) [121]. In a large RCT, patients subjected to ultrafiltration showed lower transfusion requirements compared to patients in whom ultrafiltration was not implemented [122].
Although most studies hinted at a beneficial effect of ultrafiltration on postoperative transfusion requirements, especially in the presence of preoperative anaemia, the evidence is based on small underpowered studies, with transfusion requirements as a secondary end point.
5.2.5 Retrograde and antegrade autologous priming
Background
The priming of the ECC with an asanguineous fluid results in haemodilution and increases the risk of blood transfusion, particularly in patients with a small body surface area [148]. Antegrade autologous priming and retrograde autologous priming (RAP) are simple, inexpensive, efficient ways to address the issue of haemodilution.
Description of the evidence
Most of the available literature on this topic refers to studies in which several features of minimized bypass systems were combined. However, some smaller RCTs focusing on RAP have been performed. The largest meta-analysis concluded that RAP significantly reduced transfusion requirements (RR 0.53, 95% CI 0.43–0.66; P <0.001) [123]. Similar findings were demonstrated in the meta-analysis of 6 RCTs in which patients treated with RAP received significantly fewer PRBC transfusions during hospitalization (weighted mean difference −0.60 units, 95% CI −0.90 to −0.31; P = 0.001) [124]. These findings were confirmed in an RCT with 120 patients and a body surface area <1.5 m2, showing that RAP resulted in a marked reduction of haemodilution and significantly reduced transfusion requirements [148]. On the basis of the evidence, RAP and antegrade autologous priming should be considered as part of a blood conservation strategy to reduce transfusions.
5.2.6 Coagulation-friendly environment
Background
The enzymatic process of coagulation requires an optimal temperature and acidity level. Both hypothermia and acidosis may reduce the thrombin-generation capacity, thereby impairing patient haemostasis. The number of studies focusing on the association of hypothermia or acidosis with postoperative bleeding is limited.
Description of the evidence
Hypothermia in cardiac surgery is used to enhance myocardial and end-organ preservation, particularly during CPB. However, hypothermia is also associated with coagulopathy. In an RCT of patients who had CABG surgery, it was shown that hypothermia (34°C) was not associated with greater 12-h blood loss compared with normothermic patients (37°C) [149]. A retrospective analysis of patients having off-pump CABG surgery showed that transfusion rates were higher in patients exposed to mild hypothermia (<36°C) [127]. One retrospective study investigated the association between acidosis, post-surgery acidosis and postoperative bleeding [126], showing that even a moderate degree of acidosis (pH <7.35) and hyperlactataemia (lactate >4.0 mmol/l) was associated with a significantly higher volume of postoperative chest drainage compared with no acidosis/hyperlactataemia 12 h after surgery (mean blood loss 576 vs 406 ml; P = 0.001).
Despite the lack of large, prospective studies on temperature management and the maintenance of pH during cardiac surgery, there is general agreement that the coagulation system requires normal physiological circumstances, including normothermia and a pH nearly 7.4.
5.3 Intraoperative anticoagulation
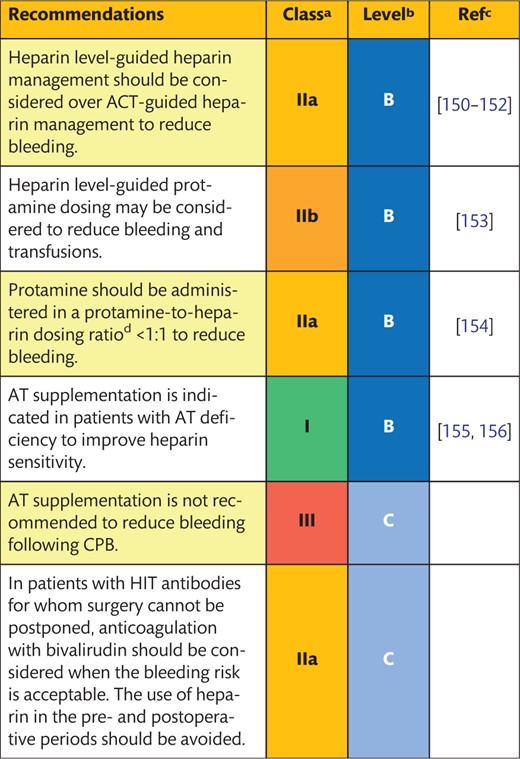 |
 |
Class of recommendation.
Level of evidence.
References.
Protamine-to-heparin dosing ratio based on the initial heparin dose.
ACT: activated clotting time; AT: antithrombin; CPB: cardiopulmonary bypass; HIT: heparin-induced thrombocytopenia.
 |
 |
Class of recommendation.
Level of evidence.
References.
Protamine-to-heparin dosing ratio based on the initial heparin dose.
ACT: activated clotting time; AT: antithrombin; CPB: cardiopulmonary bypass; HIT: heparin-induced thrombocytopenia.
5.3.1 Heparin and anticoagulation monitoring
Background
UFH binds to antithrombin (AT), which then potentiates the inactivation of thrombin and FXa by AT up to a 1000-fold. The sensitivity to heparin is determined by patient-specific characteristics [157] and is assessed by the whole-blood activated clotting time (ACT) test. Target ACT values range from 300 to 600 s depending on the methods used to measure the ACT or due to different heparin dosing strategies. Moreover, the variation in the efficacy of different heparins may need individual anticoagulation strategies.
UFH is usually dosed based on patient body weight (300–600 U/kg), followed by additional doses in the case of a periprocedural decrease in the ACT. Heparin resistance and postoperative heparin rebound are major limitations of this strategy. Heparin resistance may lead to insufficient anticoagulation during the procedure, whereas heparin rebound may contribute to post-surgical bleeding.
Description of the evidence
Individualized heparin management
Individualized heparin management or titration based on a dose–response test is based on the use of the HMS/HepCon (Medtronic, Minneapolis, MN, USA), Hemochron RxDx (Accriva Diagnostics, San Diego, CA, USA) or anti-Xa measurements in addition to the ACT. The number of RCTs that focus on the advantages of heparin dose–response implementation is, however, limited. In most studies, the use of a heparin titration device resulted in an increased heparin dose and a decrease in the protamine dose. In most studies, this situation is associated with decreased blood loss and transfusion requirements as well as with higher platelet counts at the end of the operation [150, 151, 153, 158–160]. In contrast, others did not show favourable results for blood loss and transfusion requirements when heparin titration was compared with an ACT-based protocol [152, 161–163]. Due to the lack of perioperative bleeding and transfusion rates as primary end points in these studies, larger multicentre RCTs are required to determine the added value of individual heparin management. Alternative methods of heparin management and anticoagulation monitoring during ECC consist of the determination of anti-factor Xa activity. The determination of thrombin levels could be promising, but it is not useful with high concentrations of heparin and is currently not available as a bedside clinical test.
Heparinization algorithms
There is increasing interest in the application of heparinization algorithms to tailored heparin and protamine management in cardiac surgery. Application of these algorithms might contribute to improved anticoagulation strategies and PBM, especially in heparin-resistant patients, but RCTs evaluating the effectiveness of these models are currently lacking.
Measures to prevent heparin rebound
Heparin rebound is the result of residual heparin blood concentrations in the postoperative phase. Although most of the literature on this topic originates from the 1980s to the 1990s, the recent literature can be divided into studies focusing on the presence of residual heparin in the blood following weaning from CPB or on the occurrence of postoperative bleeding associated with residual heparin. There is only 1 comparative study that showed that protamine infusion (25 mg/h for 6 h) to neutralize residual heparin resulted in reduced 12-h blood loss compared with the control subjects (525 ± 322 vs 608 ± 385 ml, respectively; P <0.05) but without affecting transfusion rates [164]. The study was, however, limited by the possibility of administering additional protamine to normalize ACT values to preheparin values, which occurred more frequently in the control group and might have enhanced postoperative bleeding [164].
In summary, a statement regarding optimal heparin anticoagulation management is limited by the lack of prospective and comparative studies. Heparin level-guided heparin management should, however, be considered over ACT-guided heparin management to reduce bleeding, especially in patients who are resistant to heparin.
5.3.2 Protamine
Background
The heparin–protamine complex leads to the dissociation of heparin from AT and restoration of the procoagulant properties of blood. Inadequate protamine dosing may, however, influence patient haemostasis and the risk of postoperative bleeding. The dose of protamine is usually based on the initial or total administered dose of heparin throughout the procedure. Protamine administration may be associated with immunological and inflammatory alterations and may induce hypotension, bradycardia, pulmonary vasoconstriction and allergies, which can, in most cases, be prevented by slow infusion rates, prophylactic use of ASA or an antihistaminic drug.
Description of the evidence
In a small RCT, protamine dosing based on the initial heparin dose resulted in prolonged clotting times and microvascular bleeding compared with protamine dosing based on the measured heparin concentration following CPB [165]. In a second RCT, a protamine-to-heparin dosing ratio of 1.3 over the total heparin dose was associated with significantly more postoperative bleeding (615 ml, 95% CI 500–830 vs 470 ml, 95% CI 420–530; P = 0.02) compared with a dosing ratio of 0.8, whereas post-protamine ACT levels were comparable among the groups [154]. None of the patients showed signs of heparin rebound. A recent RCT in patients having CABG showed that a protamine-to-heparin dosing ratio <0.6 was associated with significantly more blood loss within 12 h after surgery [420 ml (interquartile range 337–605 ml) vs 345 ml (interquartile range 230–482 ml); P = 0.0041], but no difference in PRBC transfusion was noted (2.3 units vs 2.7 units; P = 0.83) compared to patients subjected to a ratio >0.8 [152]. Ideally, protamine should be administered in a dose that matches actual heparin levels after the termination of ECC. It is advised not to exceed a protamine dose in a 1:1 ratio to the initial heparin bolus, because protamine overdosing might be associated with perioperative bleeding and enhanced transfusion requirements.
5.3.3 Antithrombin
Background
Patients resistant to heparin may have insufficiently low AT levels or dysfunctional AT and may require additional heparin dosing to reach the target ACT with a concomitant risk of residual heparin in the postoperative phase. Alternatively, AT levels can be restored by recombinant AT supplementation before CPB.
Description of the evidence
In a small RCT (n = 20), preoperative AT supplementation (50 U/kg) was not associated with reduced postoperative bleeding compared with the controls. At 6 and 12 h following surgery, median blood loss was similar between the groups (P = 0.33 and P = 0.24, respectively), with a higher variation in the AT group [166]. In heparin-resistant patients (defined as those demonstrating an inability to reach a target ACT of 480 s upon the administration of 300 + 100 IU/kg heparin), a single bolus of AT (75 U/kg) was associated with a reduced use of FFP. Administration of AT compared with placebo tended to increase 12-h postoperative bleeding (mean blood loss 1290 vs 756 ml; P = 0.05), without differences in transfusion rates between the groups [155]. In a second RCT, AT supplementation also resulted in higher bleeding rates 12 h after surgery compared with the controls (mean blood loss 450 vs 350 ml, respectively; P = 0.011) [167].
In summary, AT supplementation is indicated in patients with AT deficiency to improve heparin sensitivity but should not be used prophylactically to reduce bleeding following CPB. FFP may be considered an alternative to AT supplementation in patients with AT deficiency to improve heparin sensitivity.
5.3.4 Heparin-induced thrombocytopenia anticoagulation management
Background
In patients with Type 2 heparin-induced thrombocytopenia (HIT), antibodies are generated against the antigen complex of heparin and platelet factor 4. This antigen–antibody complex may cause haemostatic and platelet activation and severe thromboembolism, which is diagnosed by highly specific platelet function tests or heparin–platelet factor 4 antibody enzyme-linked immunosorbent assays and platelet serotonin release assays [168]. Usually, HIT antibodies develop after 7–14 days of exposure to heparin with a half-life of 40–100 days. Newer generation ELISA tests are immunoglobulin G-specific, whereas older assays also measured immunoglobulin M and immunoglobulin A antibodies, resulting in a significant overdiagnosis of HIT [168–170]. When HIT is diagnosed, heparin should be replaced by an alternative anticoagulant. The risks of alternative anticoagulation procedures, including increased bleeding, have to be balanced against potential HIT-associated complications.
Description of the evidence
Direct thrombin inhibitors like bivalirudin or argatroban can be used as an alternative anticoagulant during CPB. Bivalirudin is the only agent that has been studied in prospective multicentre trials in patients with and without HIT undergoing on-pump and off-pump cardiac surgery [171–174]. In a cardiac surgery trial in patients without HIT, safety data for bivalirudin were almost comparable to those of the competitor heparin–protamine [173]. Bivalirudin has a short elimination half-life (∼25 min) and no specific antidote. Due to its unique pharmacological characteristics, the surgical and perfusion strategies must be adjusted for the target ACT, use of prime volume and cardioplegia to avoid the stagnation of blood in the CPB circuits [175].
Another strategy is to combine heparin with a potent short-acting antiplatelet agent, such as iloprost or tirofiban, to inhibit or attenuate the HIT reaction [176, 177]. However, when the effects of iloprost or tirofiban are eliminated, patients may again be at risk of developing HIT-associated complications. There are no sufficient data to support reduction of HIT antibodies via perioperative plasmapheresis [178, 179].
In summary, in patients with HIT antibodies, in whom surgery cannot be postponed, bivalirudin anticoagulation may be considered when the bleeding risk is acceptable. The use of heparin in the pre- and postoperative periods should be avoided. If bivalirudin is deemed to be associated with a high risk of bleeding, heparin in combination with an antiplatelet agent may be considered.
5.4 Intravascular volume
5.4.1 Goal-directed haemodynamic therapy
Background
Goal-directed therapy is defined as the use of fluids, inotropes, vasoconstrictors and/or blood transfusions to target systemic haemodynamic goals, such as blood pressure or cardiac output, which improve the perfusion and oxygenation of tissues.
Description of the evidence
An RCT combined with a systematic review [187] was designed to investigate the effect of goal-directed therapy on outcomes in high-risk cardiac surgical patients (EuroSCORE >6 and/or with recent MI <14 days). Although patients in the goal-directed therapy arm received more intravenous fluids compared with the controls, there were no differences in intraoperative PRBC transfusion volumes between the groups [0 (0) units vs 1 (1.6) units, respectively; P = 0.32) [187]. There are currently no other studies available in this area that focus on bleeding and blood transfusions.
Low blood pressure is, among other interventions, treated by vasopressors, but the rise in blood pressure might contribute to enhanced blood loss. On the other hand, maintaining the mean arterial pressure using a vasoconstrictor mitigates the haemodilution associated with anaesthesia and CPB [188]. One RCT showed that goal-directed therapy using fluids, inotropes and vasopressor therapy did not affect the secondary end point transfusion requirements, despite there being more vasopressor therapy than in the control group [189]. Given the availability of limited data, it is not possible to reach evidence-based conclusions on the benefits of goal-directed fluid therapy in this setting.
5.4.2 Use of crystalloids and colloids
Background
There is no consensus regarding optimal fluid strategies in cardiac surgery, but it has been suggested that the use of hydroxyethyl starches (HES) may be associated with increased perioperative blood loss. Most of the available RCTs are limited by sample size and focus on the effect of different priming solutions on patient haemostasis, with bleeding or transfusion requirements as a secondary end point.
Description of the evidence
Priming solutions
In studies addressing the impact of the type of priming solution on postoperative bleeding and blood product transfusion as secondary end points, a comparison of 6% HES 130/0.4 (mean molecular weight 130 kDa; degree of substitution) with 6% HES 400/0.7 prime with gelatine prime [190], albumin prime [191, 192] or Ringer’s acetate [193] was made. There was no effect of 6% HES 130/0.4 on blood loss or transfusion requirements. Two studies showed a reduction in postoperative blood loss when albumin was added to an HES 130/0.4 prime [194] or compared to 6% HES 200/0.5 with 3.5% gelatine [195]. However, a review of prospective studies that compared different priming solutions with blood loss and/or transfusion requirements as primary end points reported no difference in postoperative blood loss when different HES solutions were evaluated [196–199].
Volume therapy
Two studies investigated the impact of different intraoperative volume therapy strategies on postoperative bleeding and/or transfusion as the primary end point. A comparison of 1 litre of 6% HES with 5% albumin during off-pump CABG procedures was associated with significantly more 12-h chest drainage volume (mean blood loss 732 vs 563 ml; P <0.001) [200]. In contrast, 6% HES 130/0.4 vs 3.5% gelatine showed no differences in total blood loss (mean blood loss 19.4 vs 19.2 ml/kg) [201]. In a 3-armed study, there was no difference in 24-h blood loss when 5% albumin or Ringer’s lactate was compared with 6% HES 130/0.4 (mean blood loss 835 vs 670 vs 700 ml, respectively; P = 0.085). However, the transfusion rates of PRBC were higher in the albumin and HES groups compared with that of Ringer’s lactate (58% vs 61% vs 24%, respectively; P = 0.0013), with the highest creatinine levels reported for the HES group [202].
In summary, in studies designed to show a difference in blood loss or transfusion requirements, distinct priming solutions or perioperative volume strategies revealed comparable results. However, due to the suggestion that the use of HES may be associated with kidney failure and increased mortality rates, starches are decreasingly used as volume therapy during cardiac surgery. It is therefore not recommended to use modern low-molecular-weight starches in priming and non-priming solutions to reduce bleeding and transfusions.
5.4.3 Haemodilution and cardioplegia
Background
Haemodilution is advantageous to a certain degree, because it lowers blood viscosity and may improve microcirculatory perfusion. However, haemodilution also leads to a reduced concentration of coagulation factors and blood cells during and following ECC, which may have implications for postoperative haemostasis and organ function.
Description of the evidence
Publications from large database studies have shown that low haematocrit values are associated with increased morbidity [182] and in-hospital deaths [203]. One meta-analysis showed that the use of minimized circuits reduces haemodilution, postoperative bleeding and transfusion requirements [100]. Two studies focused on haemodilution independent of the use of MiECC systems by implementing a multimodality blood conservation scheme or restricting intravenous fluids during surgery [180, 181]. In both studies, a marked reduction in the number of transfusions with increased haematocrit values was achieved [180, 181].
In conclusion, limitation of haemodilution is recommended as part of a blood conservation strategy to reduce bleeding and transfusions.
5.4.4 Predonation of blood
Background
Preoperative autologous blood donation in the hours, days or weeks before surgery may reduce the number of allogeneic blood transfusions. Preoperative blood donation is limited to patients with relatively high Hb and haematocrit levels and with a relatively large body surface area without coagulation abnormalities.
Description of the evidence
A large retrospective study showed that autologous blood donation in elective CABG or valve surgery was associated with a lower occurrence of allogeneic blood transfusion and cost-effectiveness [184]. However, the study lacked statistical adjustment for confounding factors [184]. In a matched-pair analysis with 432 patients, it was shown that preoperative autologous blood donation was associated with a reduction in the transfusion rate of PRBC from 55% to 32% and a 50% reduction in the administration of FFP and platelet concentrate (PLTC) [183]. A nested case-control study (corrected for confounding factors) further revealed that preoperative autologous blood donation reduced PRBC transfusions by 18.3% in valve surgery [204]. This study was, however, limited by the lack of preoperative Hb and haematocrit data [204].
Based on the current evidence, we suggest that preoperative autologous blood donation in patients without severe aortic stenosis, Canadian Cardiovascular Society (CCS) Grade 3–4 angina or ACS <4 weeks and with high Hb levels (>110 g/l) who are having elective surgery may be considered to reduce the number of postoperative transfusions.
5.4.5 Acute normovolaemic haemodilution
Background
During acute normovolaemic haemodilution (ANH), the patient donates blood just before the onset of surgery that is replaced by an equal amount of volume to maintain normovolaemia. The donated blood is retransfused after CPB, but this practice comes at the cost of lower haematocrit values during surgery.
Description of the evidence
A recent systematic review and meta-analysis including 29 RCTs investigated the effect of ANH using a variation of colloid, albumin and crystalloid volume replacement on allogeneic PRBC transfusion requirements in adult and paediatric cardiac surgical procedures [186]. The majority of the studies included had no predefined transfusion protocols. In the 21 studies with allogeneic blood transfusion as a predefined end point, patients subjected to ANH received fewer PRBCs (−0.79 units; 95% CI −1.25 to −0.34; P = 0.001), albeit with a considerable variation in the effect size between studies [186]. Moreover, most of the studies with a large benefit of ANH on PRBC transfusion were published before 2001. In the studies reported after 2001, only half of the studies showed a benefit of ANH on PRBC transfusion. ANH was associated with a significant, but clinically irrelevant, reduction in postoperative bleeding (−0.64 ml; 95% CI −0.97 to −0.31; P <0.0001).
In summary, ANH may have a beneficial effect on postoperative transfusion rates, but this conclusion should be viewed in light of the older studies included in this meta-analysis.
6. COAGULATION AND TRANSFUSION
Postoperative diagnosis and guidance of patient haemostasis and treatment of haemostatic abnormalities by specific procoagulant interventions or blood transfusion products should be considered as closing the circle of PBM in cardiac surgery.
6.1 Procoagulant interventions
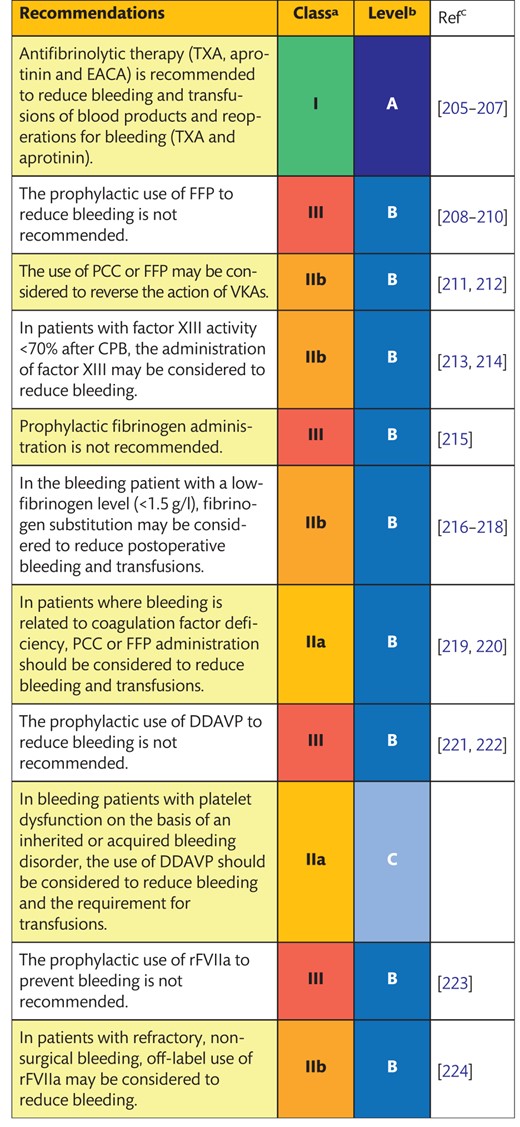 |
 |
Bleeding is defined as persistent, non-surgical microvascular blood loss.
Class of recommendation.
Level of evidence.
References.
CPB: cardiopulmonary bypass; DDAVP: desmopressin; EACA: ε-aminocaproic acid; FFP: fresh-frozen plasma; PCC: prothrombin complex concentrate; rFVIIa: recombinant activated factor VII; TXA: tranexamic acid; VKAs: vitamin K antagonists.
 |
 |
Bleeding is defined as persistent, non-surgical microvascular blood loss.
Class of recommendation.
Level of evidence.
References.
CPB: cardiopulmonary bypass; DDAVP: desmopressin; EACA: ε-aminocaproic acid; FFP: fresh-frozen plasma; PCC: prothrombin complex concentrate; rFVIIa: recombinant activated factor VII; TXA: tranexamic acid; VKAs: vitamin K antagonists.
6.1.1 Antifibrinolytics
Background
Antifibrinolytic therapy is widely used to reduce bleeding, transfusions of blood products and reoperations for bleeding. Three antifibrinolytics are used to reduce reoperation for bleeding and blood product transfusion in cardiac surgery: TXA, aprotinin and EACA. Although aprotinin was withdrawn from the market in 2007, in some countries, the drug remains available through a limited access plan.
Tranexamic acid
The Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) RCT compared TXA with placebo in patients having CABG surgery and demonstrated a reduction in the risk for reoperation due to major haemorrhage (RR 0.36, 95% CI 0.21–0.62; P <0.001) and in the need for the transfusion of any blood products (37.9% vs 54.7%; P <0.001) [205]. This result confirms the findings of previous meta-analyses [206, 225, 226]. In a systematic review and cumulative meta-analysis, it was shown that TXA reduced the pooled risk ratio for blood transfusion in patients having cardiac surgery compared with the controls (RR 0.65, 95% CI 0.60–0.70; P <0.001) [226]. The most significant reported side effect of TXA was convulsive seizure [227, 228]. The findings from the ATACAS trial showed that low-dose (50 mg/kg) versus high-dose (100 mg/kg) TXA was not safer in terms of seizures (0.7 vs 0.6%, respectively), but the higher dose significantly reduced blood loss (P = 0.026) and PRBC transfusion (P = 0.017) [205].
Aprotinin
The use of aprotinin in patients having cardiac surgery has been studied extensively. Several RCTs and meta-analyses demonstrated its efficacy in reducing the risk of reoperation for bleeding (RR 0.46, 95% CI 0.34–0.63; P <0.001) and the need for blood product transfusion (RR 0.68, 95% CI 0.63–0.73; P <0.001) [206]. It was suggested that aprotinin was a more effective agent than lysine analogues in reducing allogeneic blood exposure, but the differences between the antifibrinolytics were small (RR 0.86, 95% CI 0.76–0.96; P = 0.0083) [206]. Moreover, most of the studies were restricted to patients having CABG [207].
Data from observational studies [229, 230] indicated an increased risk of death associated with aprotinin. In these studies, aprotinin was preferentially administered to patients with more risk factors for increased mortality rates at the baseline compared with the control groups. Several observational studies did not report an association between aprotinin and safety outcomes [231–233]. The Blood Conservation Using Antifibrinolytics in a Randomized Trial (BART) [234], which was not designed to look at safety, reported higher mortality rates for patients treated with aprotinin compared to those treated with TXA or EACA, which led to the regulatory marketing suspension of aprotinin. Subsequent recognition of the significant limitations of the BART trial, including crossover of patients in the trial arms [235], and a network meta-analysis showing no adverse effect of aprotinin compared with placebo and other agents [232], led to the lifting of the marketing restriction in Canada and by the European Medicine Agency in 2012 in Europe [236, 237].
ε-Aminocaproic acid
In comparison with TXA, limited data are available to support the use of EACA in cardiac surgery [206]. A meta-analysis of observational studies showed that the use of EACA reduced the risk for blood product transfusions (RR 0.70, 95% CI 0.52–0.93; P = 0.015) but did not decrease the risk of re-exploration for bleeding (RR 0.35, 95% CI 0.11–1.17; P = 0.087).
In summary, safety studies should be designed to define the dose–response relationship of the adverse effects observed following the administration of aprotinin and TXA, given the positive impact these agents have on bleeding.
6.1.2 Fresh-frozen plasma
Background
Fresh frozen plasma is obtained by separating the plasma, including coagulation factors and other proteins and soluble constituents of the blood, from particular elements in donated blood. An increasing number of countries use pooled plasma. Pooled plasma contains plasma from multiple donors and is inactivated for viruses with a lower risk of transfusion-induced lung injury.
Description of the evidence
The largest body of evidence is gathered on the prophylactic administration of FFP to patients without a diagnosed coagulopathy and is summarized in a Cochrane review [208] and 3 other systematic reviews [209, 210, 238]. The RCTs included were limited by small sample sizes and divergent doses of FFP. There was no difference in blood loss or allogeneic blood transfusion in patients undergoing cardiac surgery who received FFP compared with the controls [209, 210, 238]. It was also shown that the therapeutic use of FFP in patients with established coagulopathy or for reversal of oral anticoagulation has no effect on 24-h blood loss [209, 238].
In summary, FFP might be used to reverse the action of oral anticoagulation or in the case of persistent perioperative bleeding, but there is no evidence that prophylactic or therapeutic FFP transfusions reduce blood loss after cardiac surgery.
6.1.3 Factor XIII
Background
Factor XIII (FXIII) is the terminal enzyme in the coagulation cascade and is necessary for cross-linking fibrin monomers to form a stable, strong fibrin clot. Low FXIII levels have been reported after cardiac surgery and are associated with increased bleeding [213, 239, 240]. Specific immunological assays are needed to determine functional FXIII activity. Detection of FXIII deficiency by TEM has been described [241], but there is currently no clinically available point-of-care (POC) test.
Description of the evidence
Three RCTs (n = 527) evaluated the effect of the administration of FXIII after the administration of protamine [213, 242, 243]. The results showed no differences in postoperative bleeding volumes and transfusion rates with any FXIII dosage regimens. There was no difference in adverse events, including thromboembolic events and death, between the intervention and the control groups. However, in patients with a postoperative FXIII level <70%, the bleeding volume was higher compared to patients with FXIII levels >70% [213]; therefore, the latter patients might be the target population.
In summary, there is no evidence that administration of FXIII might be beneficial regarding the reduction of postoperative bleeding volumes and transfusion rates in patients with normal concentrations of FXIII (>70%).
6.1.4 Fibrinogen concentrates
Background
Fibrinogen is the first factor to be depleted during significant bleeding and haemodilution [220, 244]. Preoperative fibrinogen levels <1.5 g/l [25] and hypofibrinogenaemia after cardiac surgery [242, 245] have been associated with increased postoperative bleeding. Fibrinogen supplementation has, therefore, been advocated as one of the primary haemostatic aims in patients who bleed after cardiac surgery [246].
Description of the evidence
Pre- and postoperative fibrinogen substitution
In an RCT including 48 patients, preoperative fibrinogen substitution slightly increased intra- and postoperative fibrinogen concentrations but did not reduce postoperative bleeding in patients who had CABG (mean blood loss 650 vs 730 ml, P = 0.29) [247]. Postoperative fibrinogen administration was tested in 4 studies, with contrasting results. Two studies [248, 249] that included 167 patients undergoing complex cardiac surgery showed reduced requirements for allogeneic blood products in the intervention group. In both studies, fibrinogen concentrate was administered, the goal being a maximal clot firmness of 22 mm in FIBTEM (ROTEM, Basel, Switzerland). These findings are in agreement with a Cochrane systematic review published in 2013, which showed a reduced relative risk of blood transfusion after fibrinogen supplementation (RR 0.47, 95% CI 0.31–0.72) [216]. However, 2 more recent RCTs in bleeding patients after complex cardiac surgery could not confirm the previous findings [215, 250]. Indeed, the largest RCT of 152 bleeding patients without hypofibrinogenaemia undergoing elective aortic surgery, randomly assigned to fibrinogen concentrate or placebo, found that patients treated with a fibrinogen concentrate had a significantly higher rate of any allogeneic blood product use compared with placebo (median units 5.0 vs 3.0, P = 0.026) [250]. A posthoc analysis from an RCT showed that substitution with fibrinogen concentrate to more than 2.87 g/l (or maximal clot firmness of 14 mm in FIBTEM) could not further reduce bleeding volume [251]. Data from a Cochrane review including 124 patients from 3 RCTs [216] and a propensity score analysis in 380 patients undergoing cardiac surgery [252] suggested no increased risk of thromboembolic events or death after fibrinogen substitution. However, additional safety data from larger RCTs, which also addressed different fibrinogen target concentrations, are necessary to confirm this assumption.
In summary, prophylactic fibrinogen administration is not recommended for reducing postoperative bleeding and transfusion risks. However, in patients with a low-fibrinogen level and signs of persistent microvascular bleeding, fibrinogen substitution may be considered to reduce the requirement for transfusions.
6.1.5 Prothrombin complex concentrate
Background
PCCs are lyophilized, human plasma-derived vitamin K-dependent coagulation factors. In Europe, only 4 factor concentrates are available, containing coagulation factors II, VII, IX and X. Although the concentration of factor IX is standardized, the other factors might vary among the different available products. Also, PCC might contain various amounts of AT; proteins C, S and Z and heparin [246, 253].
Description of the evidence
In patients with extremely high INR values (>4–5) taking VKAs for the long term who need urgent or emergent surgery, the use of PCCs might be advantageous compared with FFP due to the more rapid reversal of the anticoagulant effect. A Cochrane review, however, showed no evidence of a difference between PCC and FFP in terms of postoperative bleeding and transfusion risks [212]. In 40 patients with an INR >2.1, the perioperative administration of PCC reversed anticoagulation safely; reversal was faster and postoperative bleeding volume was lower compared with the administration of FFP [211].
Three retrospective cohort studies in 402 patients showed that PCC was associated with fewer re-explorations for bleeding, less blood loss and fewer transfusions of PRBCs compared with FFP [219, 254, 255]. The use of PCCs in patients with bleeding after CPB might be associated with an increased risk of thromboembolic events and acute kidney injury [255]. Recommendations for dosing are not established, and administration might be tailored to the individual patient based on laboratory and clinical variables, including POC coagulation testing [246, 253].
To summarize, in patients in whom bleeding is related to coagulation factor deficiency, PCC or FFP administration should be used to reduce bleeding and transfusions. PCC may be preferred over FFP when rapid normalization of coagulation factors is needed.
6.1.6 Desmopressin
Background
Desmopressin (DDAVP) is a haemostatic agent that was developed to prevent and treat bleeding in patients with mild haemophilia (5–50% of the normal concentration of clotting factors VIII and IX) and patients with von Willebrand’s deficiency, e.g. due to aortic stenosis. DDAVP stimulates the release of von Willebrand factor from endothelial cells. DDAVP is sometimes administered to treat postoperative bleeding in patients undergoing cardiac surgery with impaired platelet function.
Description of the evidence
The effects of DDAVP on bleeding in patients having cardiac surgery are summarized in a Cochrane systematic review of 29 RCTs [256] and 2 meta-analyses [222, 257]. Summing up the evidence, the prophylactic use of DDAVP did not reduce blood loss (weighted mean difference 241 ml, 95% CI −387.55 to −96.01 ml) or the risk of blood transfusions (OR 0.96, 95% CI 0.87–1.06). Recently published meta-analyses concluded that DDAVP has a small effect on the volume of blood loss and allogeneic blood product transfusions [222, 257]. Furthermore, the effect of DDAVP may be more profound in subgroups, e.g. for patients on platelet inhibitors, patients with reduced platelet function and patients exposed to prolonged CPB times [222]. There was no increased risk for MI (RR 1.38, 95% CI 0.77–2.50), stroke (RR 2.40, 95% CI 0.68–8.43) or any thromboembolic complications (RR 1.46, 95% CI 0.64–3.35) after the administration of DDAVP [256, 257]. However, the risk of hypotension during administration of DDAVP that required fluids and/or vasoactive drugs was significantly increased (RR 2.81, 95% CI 1.50–5.27) [256].
In summary, prophylactic use of DDAVP is not recommended, but its administration might be beneficial in patients who are bleeding who have inherited or acquired bleeding disorders or platelet dysfunction [258].
6.1.7 Recombinant factor VIIa
Background
Recombinant factor VIIa (rFVIIa) is used in the treatment and prevention of bleeding in patients with inherited bleeding disorders. The observed haemostatic properties of this agent led to its off-label use in life-threatening bleeding, but nowadays its use is considered to be a last resort in the event of uncontrollable blood loss.
Description of the evidence
A 2012 Cochrane review analysed 29 RCTs with respect to the prophylactic (1361 patients) and therapeutic use (2929 patients) of rFVIIa in patients with or without haemophilia [224]. Prophylactic rFVIIa use just failed to reach significance to reduce transfusion rates (RR 0.85, 95% CI 0.72–1.01) compared with placebo, but this result was associated with an increased risk of thromboembolic adverse events (RR 1.35, 95% CI 0.82–2.25). There was no benefit of the therapeutic use of rFVIIa compared with the use of placebo with respect to transfusion rates, but the administration of rFVIIa was associated with reduced mortality rates (RR 0.91, 95% CI 0.78–1.06) at the cost of more major thromboembolic events (RR 1.14, 95% CI 0.89–1.47).
In summary, prophylactic use of rFVIIa cannot be recommended in cardiac surgery; its therapeutic use should only be considered in patients with uncontrollable bleeding that cannot be managed by other procoagulant interventions.
6.2 Transfusion strategies
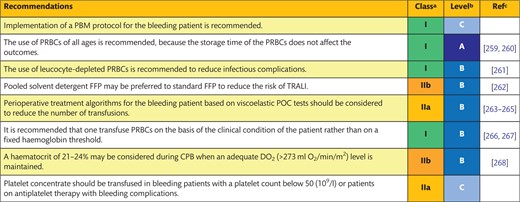 |
 |
Class of recommendation.
Level of evidence.
References.
CPB: cardiopulmonary bypass; FFP: fresh-frozen plasma; PBM: patient blood management; POC: point of care; PRBCs: packed red blood cells; TRALI: transfusion-related acute lung injury.
 |
 |
Class of recommendation.
Level of evidence.
References.
CPB: cardiopulmonary bypass; FFP: fresh-frozen plasma; PBM: patient blood management; POC: point of care; PRBCs: packed red blood cells; TRALI: transfusion-related acute lung injury.
6.2.1 Quality of blood products
Background
Stored PRBCs or FFP reveal comparable efficacy compared to fresh products but differ in terms of the risk of the transmission of bacterial or viral infections [269, 270], transfusion-related acute lung injury (TRALI) [271, 272] and transfusion-related immune modulation (TRIM) [273–275]. To avoid TRALI, FFP and PLTC must be collected from men, women who have not been pregnant and women who have tested negative for human leucocyte antigen antibodies to reduce the risk of TRALI [271]. The effects of TRIM are assumed to be caused by allogeneic mononuclear cells, white blood cell-derived soluble mediators and human leucocyte antigen peptides, transfused mainly via PRBCs [273]. TRIM has been associated with increased rates of mortality and morbidity in cardiac surgery and an increased risk of developing infections [274, 275].
Description of the evidence
Leucocyte reduction of PRBC is the standard practise in Europe and preserves the quality of PRBC during storage and influences favourably on morbidity [261]. In an RCT, patients having cardiac surgery either received buffy coat-depleted or leucocyte-depleted erythrocytes [276]. Although no differences in mortality rates could be shown, patients receiving leucocyte-depleted PRBCs showed a reduced infection rate compared with the controls (21.6% vs 31.6%; OR 1.64, 95% CI 1.08–2.49) [276]. The implementation of new additive solutions further improved PRBC quality during storage.
In the Red-Cell Storage Duration Study (RECESS), 1098 cardiac surgical patients were randomized to receive exclusively ‘new’ (≤10 days) or ‘old’ (≥21 days) leucocyte-depleted PRBCs [259]. There were no differences in multiple-organ dysfunction and mortality rates between either arm. These results are in line with the results of a large Swedish registry that compared the outcomes of 47 071 cardiac surgery patients from 1997 to 2012 who were exclusively transfused with PRBC stored for <14 days (36.6%), 14–27 days (26.8%), 28–42 days (8.9%) or of mixed age (27.8%) [277]. No differences were noted in terms of 30-day-, 2-year- and 10-year mortality rates or in relation to 30-day organ dysfunction or serious infection [277]. A current meta-analysis that included the Red-Cell Storage Duration Study [291] and the large Age of BLood Evaluation (ABLE) trial [260] showed that red blood cell storage time does not affect mortality rates, adverse events or nosocomial infections [278].
PLTCs can be produced as single-donor apheresis PLTC or as pooled (4–5 donors) whole-blood-derived PLTC. Although the risk for TRALI appears to be comparable, an increased risk of transmitting infections is associated with whole-blood-derived PLTC transfusions [279]. One retrospective study in 3272 patients who received single-donor PLTC aged 2–5 days showed no association between PLTC storage age and short-term outcome, survival or postoperative infections [280]. Solvent–detergent treatment and pooling of FFP (SD-FFP) are performed to inactivate capsulated lipid-enveloped viruses and dilute human leucocyte antigen antibodies to reduce TRALI. In a haemovigilance report from Norway (1998–2003), where SD-FFP is exclusively used, no case of TRALI has been associated with SD-FFP transfusion, whereas 3 other European countries have reported incidences of 1.6–8.8 when transfusing FFP [262].
In conclusion, we recommend the use of PRBCs of all ages, because the storage time of the PRBCs does not affect outcomes. In an attempt to reduce infectious complications, the use of leucocyte-depleted PRBCs is recommended; in contrast, the pooled SD-FFP may be preferred over standard FFP to reduce the risk for TRALI.
6.2.2 Algorithm-guided therapy of perioperative bleeding
Background
The introduction of POC tests—in particular, TEG and TEM—has led to the development of specific algorithms to diagnose and, consequently, treat theragnostic perioperative bleeding in cardiac surgery. The first algorithms were based on TEG, and their effectiveness was tested in a small series using historical controlled series, fibrinogen concentration measurements using the Clauss method or a viscoelastic fibrinogen test [251, 281]. Figure 3 provides an overview of possible monitoring options throughout the perioperative period.
Haemostatic monitoring throughout the perioperative period and possible treatment modalities. ACT: activated clotting time; CPB: cardiopulmonary bypass; DAPT: dual antiplatelet therapy; DDAVP: desmopressin; EACA: ε-aminocaproic acid; EPO: erythropoietin; FFP: fresh-frozen plasma; i.v.: intravenous; MiECC: minimally invasive extracorporeal circulation circuit; PCC: prothrombin complex concentrate; PRBC: packed red blood cells.
Description of the evidence
A meta-analysis with 5223 patients shows that exposure to any allogeneic blood product was lower in the POC group (49.6%) than in the conventional group (60.2%; OR 0.63, 95% CI 0.56–0.71; P <0.0001). This finding was confirmed when we considered RCTs only (OR 0.37, 95% CI 0.21–0.68; P = 0.0018). The most relevant decrease in transfusion rate was related to FFP (OR 0.31, 95% CI 0.13–0.74), followed by platelet transfusion (OR 0.62, 95% CI 0.42–0.92) and PRBCs (OR 0.63, CI 0.50–0.78) [263]. Data on chest tube drainage in a subpopulation of 400 patients revealed no differences. Data on the surgical re-exploration rate favoured the POC group versus the conventional group (OR 0.56, 95% CI 0.45–0.71; P <0.00001) [263]. However, a recently published meta-analysis could not show any benefit of algorithm-based therapy on surgical reoperation rates [264]. There was a reduction of thromboembolic events in the POC-guided group versus the conventional group (1.3% vs 2.9%; OR 0.44, 95% CI 0.28–0.70; P = 0.0005). No differences were found with regard to mortality rates (OR 0.92, 95% CI 0.74–1.16; P = 0.45) [263]. The majority of the studies considered algorithms based on viscoelastic tests, whereas a minority reported data inclusive of platelet function tests [263].
In 2016, an updated comprehensive Cochrane report that primarily included cardiac surgery reports assessed the benefits and harms of TEG- and TEM-guided transfusions in adults and children with bleeding [282]. The use of TEG- and TEM-guided management was associated with a reduction in the proportion of patients receiving PRBC transfusions (RR 0.86, 95% CI 0.79–0.94), FFP (RR 0.57, 95% CI 0.33–0.96) and platelets (RR 0.73, 95% CI 0.60–0.88), whereas there was no significant difference in the rate of surgical reoperation or bleeding events and massive transfusions [282]. In 2015, a Dutch/British health technology assessment report confirmed the results of the Cochrane analysis and showed that viscoelastic testing was cost-effective and more efficient than standard laboratory testing [283]. An additional meta-analysis of TEG-/TEM-based coagulation management included 9 RCTs and 8 observational studies comprising 8332 patients [263]. The authors found that TEG-/TEM-based management reduced the odds for transfusion requirements (OR 0.63, 95% CI 0.56–0.71) and re-explorations for bleeding (OR 0.56, 95% CI 0.45–0.71) but made no difference in terms of mortality rate, stroke or chest drain output volume [263]. In a subsequent RCT, 7402 patients were randomized to POC haemostatic testing with an integrated transfusion algorithm or standard of care [265]. POC haemostatic testing reduced the rates of PRBC transfusions (adjusted RR 0.91, 95% CI 0.85–0.98), platelet transfusions (RR 0.77, 95% CI 0.68–0.87) and major bleeding (RR 0.83, 95% CI 0.72–0.94) without affecting complication rates [265].
In summary, the evidence supports the use of perioperative POC testing in patients having cardiac surgery to reduce transfusion requirements. Furthermore, it should be noted that most of the published studies included only viscoelastic coagulation tests, although a specific platelet function test was included in the algorithm in 2 of the more recent studies [265, 284]. Cut-off levels for non-acceptable intraoperative and postoperative platelet function with the different devices and the subsequent interventions are yet to be determined.
6.2.3 Transfusion triggers for packed red blood cells and platelet concentrate
Background
The transfusion threshold is usually defined as the critical haematocrit/Hb value, red cell volume or platelet count/function when patients are transfused with PRBCs or PLTC. However, dynamic strategies also take tissue perfusion/oxygenation into account [285, 286]. Data of RCTs in cardiac surgery are hard to compare, because transfusion thresholds of PRBC transfusion and the quality of blood products vary among studies.
Description of the evidence
A recent meta-analysis compared restrictive (Hb 70–80 g/l) and liberal (Hb 90–100 g/l) transfusion strategies using data from 6 RCTs with 3352 patients, showing a 30% reduction in the number of deaths in the restrictive regimen group (OR 0.70, 95% CI 0.49–1.02; P = 0.060) [287]. The Brazilian Transfusion Requirements After Cardiac Surgery (TRACS) trial compared a liberal transfusion threshold (haematocrit ≥30%) with a restrictive threshold (haematocrit ≥24%) in 502 patients having CABG and/or valve surgery [267]. The per protocol analysis showed that more patients in the liberal group received red blood cell transfusions (78% vs 48%; P <0.001) without a difference in the composite end point of 30-day mortality or severe morbidity [267]. A subanalysis of a small sample of patients older than 60 years (n = 260) showed no difference in the composite end point between the groups, albeit with more cardiogenic shock in the restrictive transfusion threshold group (12.8% vs 5.2%, P = 0.031) [288].
The UK Transfusion Indication Threshold Reduction (TITRe2) trial compared a liberal (Hb <90 g/l) with a restrictive threshold (Hb <75 g/l) for leucocyte-depleted red blood cells in 2000 patients undergoing CABG and/or valve or aortic surgery [266]. Fewer patients in the restrictive group received red blood cells (63.7% vs 94.9%; RR 0.58, 95% CI 0.54–0.62; P <0.001), whereas the 90-day mortality rate was doubled in the restrictive group (4.2% vs 2.6%; OR 1.64, 95% CI 1.00–2.67; P = 0.045). The TITRe2 study was criticized regarding transfusion thresholds and confounders, which might be overcome in the ongoing Transfusion Requirements in Cardiac Surgery (TRICS III) study.
A small RCT conducted with patients undergoing CABG with normothermic CPB and not requiring transfusions compared the effect of a haematocrit of 20% (n = 28) with that of a haematocrit of 25% (n = 26). No differences in oxygen delivery or consumption, blood lactate levels or clinical outcome were found [268]. In a single-centre investigation in which surgery was restricted to CABG (n = 3003), a nadir haematocrit <28% during CPB was associated with an increase in severe morbidity, whereas lowering the nadir haematocrit to 24% was possible when a goal-directed perfusion technique was established, which included maintenance of sufficient oxygen delivery [182]. These findings should highlight the complex interaction of haematocrit, temperature, oxygen supply and tissue perfusion for tissue oxygenation.
There are no studies available in which a certain platelet count or value in a platelet function assay served as a threshold for PLTC transfusion and could be identified as the key effector to stop/reduce microvascular bleeding caused by inadequate platelet count/function [289]. However, in recent American Association of Blood Bank guidelines, a platelet count of 50 (109/l) was recommended as a therapeutic target for the bleeding patient undergoing major surgery [290].
In summary, recent studies seem to be in favour of liberal PRBC transfusions. However, from an expert opinion, we would like to underline that the clinical condition of the patient and the optimization of the balance between oxygen delivery and extraction in the tissues are the most important aspects to be considered rather than a fixed threshold of the level of Hb. The acceptable haematocrit levels during CPB depend on the risk profile of the patient and his or her ability to maintain adequate tissue perfusion and oxygenation. In bleeding patients with a platelet count below 50 (109/l) or patients on antiplatelet therapy, PLTC may be transfused.
7. ANTICOAGULATION MANAGEMENT DURING EXTRACORPOREAL LIFE SUPPORT
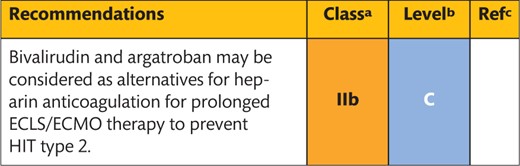 |
 |
Class of recommendation.
Level of evidence.
References.
ECLS: extracorporeal life support; ECMO: extracorporeal membrane oxygenation; HIT: heparin-induced thrombocytopenia.
 |
 |
Class of recommendation.
Level of evidence.
References.
ECLS: extracorporeal life support; ECMO: extracorporeal membrane oxygenation; HIT: heparin-induced thrombocytopenia.
The emerging use of extensive extracorporeal life support (ECLS), e.g. bridge to transplantation, requires novel approaches towards patient anticoagulation. This chapter provides an overview of the current evidence on this topic.
Background
ECLS (venoarterial) and extracorporeal membrane oxygenation (ECMO; venovenous) systems are designed for the temporary support of the failing heart and/or lungs. ECLS systems are small, closed priming volume systems, including a centrifugal pump and coated tubing without any option for removal of air. A cell-saver device replaces cardiotomy suction. ECLS and ECMO require long-term anticoagulation, which is associated with an increased risk of thrombosis or bleeding [291]. The majority of patients exposed to long-term ECC develop acquired von Willebrand syndrome.
UFH anticoagulation is the standard in ECLS/ECMO, with similar dosing challenges to those experienced during cardiac surgery [292, 293]. Reagents, clot detection systems and reference values of different aPTT and ACT assays used during ECLS/ECMO vary greatly [291–293]. Clotting tests do not take into account disturbances of the coagulation system, which are especially frequent in patients with multiple-organ failure requiring ECLS/ECMO support. Strict heparin dosing based on anti-Xa levels may increase the risk of excessive anticoagulation and bleeding [294, 295]. During the use of AT-independent anticoagulants, such as argatroban and bivalirudin, excessive anticoagulation and bleeding are avoided. However, in the absence of antidotes, bivalirudin requires haemofiltration to be eliminated from the circulation [296]. Moreover, there is no strategy for enhanced extracorporeal elimination of argatroban, which is predominantly eliminated via the hepatic system [297].
Description of the evidence
There are significant institutional variations in the anticoagulation management and monitoring of ECLS/ECMO [298]. The dose of heparin anticoagulation depends on the assay, with broad target ranges for the aPTT of 45–80 s and ACT of 140–220 s. Bivalirudin (0.02–0.05 mg/kg/h) and argatroban (0.1–0.2 µg/kg/h) are usually dosed on the basis of the aPTT with target ranges of 45–80 s and 50–60 s, respectively.
Particularly in adult patients, data are limited to small, mostly retrospective, single-centre studies. Clinical outcome studies are rare and mostly too underpowered to allow one to draw valid conclusions. In 1 study with 22 patients on ECMO, low anti-Xa levels were associated with an increase in deep venous thrombosis [299]. This study also showed that the correlation of ACT or aPTT values with anti-Xa levels was poor to moderate, respectively [299]. In 71 patients, a reduction of the ACT target value from 180–220 to 140–160 s reduced bleeding complications and transfusions without increasing thromboembolic events [300]. The role of AT replacement in ECLS/ECMO remains to be clarified, because the evidence is limited to paediatric and small studies [298, 301]. Implementation of viscoelastic tests for anticoagulation monitoring during ECLS/ECMO appears to be promising but needs further validation [298, 302]. Successful use of bivalirudin [303, 304] and argatroban [305] during ECLS/ECMO therapy based on aPTT dosing has been reported in small series in patients with and without HIT. Bivalirudin anticoagulation, when compared to heparin, resulted in more stable conditions of the haemostatic system and reduced bleeding and transfusion rates of bivalirudin [303, 304].
In summary, the evidence for an optimal anticoagulation strategy during ECLS/ECMO is weak, and larger prospective studies are required.
8. FINAL REMARKS
PBM in cardiac surgery comprises a multidisciplinary and multifactorial approach and consists of a sequence of separate strategies and interventions. A key factor in a successful PBM programme is the close collaboration between surgeons, anaesthetists, clinical perfusionists and intensivists. The joint effort of EACTS and EACTA to write this guideline underlines the importance of team efforts, and future guidelines should be extended to enable collaborations with other societies.
Our goal was to provide evidence-based recommendations with respect to blood-conservation strategies, preoperative patient optimization, intraoperative interventions and postoperative monitoring and treatment of bleeding disorders. All recommendations were based on bleeding-related end points, which narrowed our search strategy and reference list (Fig. 4). The lack of consensus on the definition of bleeding outcomes and their relevance for patient recovery warrants a consensus definition for bleeding complications in cardiac surgery for future studies. We also aimed to adhere to recommendations provided by other guidelines in this area.
Key messages of the Joint Effort Patient Blood Management Guidelines for Adult Cardiac Surgery. ACT: activated clotting time; AT: antithrombin; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; DDAVP: desmopressin; FFP: fresh-frozen plasma; MUF: modified ultrafiltration; PRBC: packed red blood cells; RAP: retrograde autologous priming; rFVIIa: recombinant factor VIIa.
A closer evaluation of the recommendations included in this guideline suggests that several fundamental aspects of PBM programmes are supported by the moderate quality of the evidence, and large RCTs are warranted to improve the evidence base of this guideline.
Additionally, it has to be highlighted that a large number of studies included in this guideline, in particular haemovigilance data, are derived from non-European countries. National regulations for the processing and leucodepletion of PRBCs differ among countries, and it is therefore unclear whether non-European data can be translated to the setting of European patients. We hope that the current guidelines represent a further step towards the establishment of a European standard for the quality of blood products, a European haemovigilance database and European standards for PBM.
Conflict of interest: 2014–2017: Umberto Benedetto, Daniel Bolliger, Andreas Koster, Michael Meesters, Domenico Pagano, Hanne Berg Ravn, Alexander Wahba, Milan Milojevic and Ruben Osnabrugge have nothing to disclose. Christa Boer attended consultancy meetings (Nordic Pharma) and received speaker's honoraria from LivaNova Group and Medtronic, Inc. Christian von Heymann received honoraria for lectures or consultancy work and travel reimbursements unrelated to his topic in this guideline from Bayer AG, Boehringer lngelheim, Pfizer GmbH, Bristol Myers Squibb, Daiichi Sankyo, CSL Behring, Shire, Novo Nordisk, Ferring, Nordic Pharma, Mylan Healthcare GmbH, Sanofi-Aventis and HICC GbR during the last 3 years. Anders Jeppsson was a consultant for AstraZeneca, Boeringer-Ingelheim, XVIVO Perfusion, and LFB Corporation, and received speaker honoraria from AstraZeneca, CSL Behring, Roche Diagnostics, Triolab AB and Octapharma. Marco Ranucci was a consultant for LivaNova Group and received speaker's honoraria from Medtronic, Roche Diagnostics, TEM International, Vifor Pharma, Edwards Life Sciences, CSL Behring and Grifols. Alexander B.A. Vonk attended consultancy meetings (Nordic Pharma).
REFERENCES
Author notes
Disclaimer 2017: The EACTS/EACTA Guidelines represent the views of the EACTS and of the EACTA and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The EACTS and the EACTA are not responsible in the event of any contradiction, discrepancy and/or ambiguity between the EACTS/EACTA Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the EACTS/EACTA Guidelines fully into account when exercising their clinical judgement as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the EACTS/EACTA Guidelines do not, in any way whatsoever, override the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and, where appropriate and/or necessary, in consultation with that patient and the patient’s care provider. Nor do the EACTS/EACTA Guidelines exempt health professionals from giving full and careful consideration to the relevant official, updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.