-
PDF
- Split View
-
Views
-
Cite
Cite
Emmanouil S. Brilakis, Joseph P. McConnell, Ryan J. Lennon, Ahmad A. Elesber, Jeffrey G. Meyer, Peter B. Berger, Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up, European Heart Journal, Volume 26, Issue 2, January 2005, Pages 137–144, https://doi.org/10.1093/eurheartj/ehi010
Close - Share Icon Share
Aims We aimed to evaluate the association of lipoprotein-associated phospholipase A2 (Lp-PLA2) with coronary artery disease (CAD) risk factors, with the severity of angiographic CAD, and with the incidence of major adverse events.
Methods and results We measured Lp-PLA2 levels in 504 consecutive patients undergoing clinically indicated coronary angiography. Mean age was 60±11 years and 38% were women. The mean (±SD) Lp-PLA2 level (ng/mL) was 245±91. Lp-PLA2 levels correlated with male gender, LDL, HDL, and total cholesterol, fibrinogen, and creatinine. Lp-PLA2 levels correlated with the extent of angiographic CAD on univariate but not on multivariable analysis. During a median follow-up of 4.0 years, 72 major adverse events occurred in 61 of 466 (13%) contacted patients (20 deaths, 14 myocardial infarctions, 28 coronary revascularizations, and 10 strokes). Higher Lp-PLA2 levels were associated with a greater risk of events: the hazard ratio per SD was 1.28 (95% CI 1.06–1.54, P=0.009), and remained significant after adjusting for clinical and lipid variables and C-reactive protein.
Conclusion Higher Lp-PLA2 levels were associated with a higher incidence of major adverse events at follow-up, independently of traditional CAD risk factors and C-reactive protein.
See page 107 for the editorial comment on this article (doi:10.1093/eurheartj/ehi042)
Introduction
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a 50-kDalton enzyme that belongs to the A2 phospholipase superfamily.1 Lp-PLA2 is produced by macrophages and lymphocytes and 80% of it circulates bound, mainly to LDL.2 Whether Lp-PLA2 is predominantly pro-atherogenic or anti-atherogenic is controversial. Most evidence from animal3 and human2–5 studies suggests it is pro-atherogenic.
The first aim of our study was to examine the association between Lp-PLA2 and: (i) traditional and emerging coronary artery disease (CAD) risk factors: age, gender, smoking, hypertension, obesity, total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol, triglycerides, and homocysteine; (ii) the presence of an acute coronary syndrome; (iii) the presence and extent of angiographic CAD; and (iv) the incidence of major adverse events at follow-up. The second aim was to examine the impact on those associations when other risk factors, such as clinical and lipid parameters and C-reactive protein (CRP) were included in multivariable analyses.
Methods
Patient population
The population included 504 patients (97% Caucasian), aged 26–76 years, undergoing clinically-indicated coronary angiography at our institution between June 1998 and January 1999. Patients were excluded if they had diabetes mellitus, smoking history >50 pack-years, history of organ transplantation, pregnancy, prior coronary revascularization, bleeding disorders, blood transfusion within 30 days, HIV infection, renal failure, or prior chest radiation therapy. The most frequent indications for angiography were acute coronary syndrome (34%), an abnormal nuclear imaging study (25%), and dyspnoea upon exertion (27%). The 504 patients represent >90% of patients eligible for this study during the enrolment period (the remaining patients refused to participate). The study was approved by our Institutional Review Board, and all subjects provided written informed consent. Four hundred and sixty-six patients (92.5%) were contacted by a follow-up questionnaire or by telephone in September 2002. The remaining 38 patients either refused to participate in the follow-up (18, 47%) or could not be contacted (20, 53%). The medical records of the patients who had an event were obtained and reviewed in order to ascertain the type of the event or the cause of death.
Acute coronary syndromes and stroke
Patients were classified as having unstable angina (UA) if they had new-onset chest pain or if they had a significant unexplained change in the pattern of stable angina (such as increased frequency, intensity, or duration, or decreased response to nitrates) in the previous 2 months. Patients were defined as having an acute myocardial infarction (AMI) if they had cardiac marker elevation [total creatinine kinase (CK) more than 3× the upper limit of normal, or cardiac troponin (T) more than the upper limit of normal] in association with chest pain or ischaemic electrocardiographic changes. Stroke was defined as a new neurological defect persisting for >24 h (CT or MRI documentation was available in 9 of 10 patients).
Angiographic analysis
Coronary angiograms were analysed according to the segmental classification proposed by the Coronary Artery Surgery Study (CASS) investigators.6 The maximum-diameter stenosis in each of 27 coronary artery segments was assessed with hand-held callipers or visual analysis. Angiograms were analysed blinded to risk factors and biochemical analyses.
The extent of CAD was quantified as follows: normal coronaries (smooth arteries with no stenosis or stenosis <10%), mild disease (reduction in luminal diameter between 10% and 50%), single-vessel disease (>50% luminal diameter stenosis in one coronary artery or its major branches), two-vessel coronary artery disease (>50% stenoses in two coronary arteries), and three-vessel disease (>50% stenoses in three coronary arteries).
Parameter definitions
The body mass index (BMI) was calculated by dividing the patient's weight in kilograms by the square of the patient's height in metres. Patients were classified as normal weight (BMI 18.5–24.9), overweight (BMI 25–29.9), and obese (BMI ≥30). Patients were considered to be hypertensive if their blood pressure was >140/90 mmHg, or if they were being treated with antihypertensive medications. Major adverse events consisted of any of the following: death, AMI, coronary revascularization, or stroke.
Blood collection and biochemical analyses
Blood was collected in EDTA-treated tubes and divided into aliquots for measurement of plasma risk factors. Fibrinogen was measured using immunoturbidimetric methods on a Roche COBAS MIRA system. Lipids (total cholesterol, triglycerides, and HDL cholesterol) were measured using standard automated enzymatic methods on a Roche COBAS MIRA system. LDL cholesterol was calculated as total cholesterol minus HDL cholesterol and 20% of the triglyceride level (all expressed in mg/dL).
Total plasma homocysteine was measured by high performance liquid chromatography following reduction of the disulfide bonds with Tris (2-carboxyethyl) phosphine hydrochloride and derivatization with SBD-F (7-fluoro-2-oxa-1, 3-diazole-4-sulfonate). Cysteamine was used as an internal standard.
CRP was measured using a sensitive latex particle-enhanced immunoturbidimetric assay on a Hitachi 912 automated analyser, using reagents from Kamiya Biomedical Company. The CRP assay was sensitive to 0.15 mg/L and was standardized against the IFCC/BCR/CAP CRM 470 CRP references.
Lp-PLA2 measurement
Lp-PLA2 mass was measured in plasma aliquots that were collected at the time of enrolment and stored at −70°C using an enzyme-linked immunoassay (PLACTM test, diaDexus, Inc., CA, USA).2,4 Samples were incubated in microtitre plate wells with immobilized monoclonal antibody (2C10) against Lp-PLA2. The enzyme was identified by a second monoclonal anti-Lp-PLA2 antibody (4B4) labelled with horseradish peroxidase. The standard was recombinant Lp-PLA2. The range of detection was 50–1000 ng/mL and the interassay coefficients of variation were 7.8% at 276 ng/mL, 6.1% at 257 ng/mL, and 13.5% at 105 ng/mL. There was no cross-reactivity with other A2 phospholipases.2 All analyses were performed blinded to risk factors, biochemical, and clinical characteristics.
The mean Lp-PLA2 value in our study was 245±91 ng/mL, which was lower than the values reported from previous studies [mean Lp-PLA2 level was 2370±520 ng/mL in the West of Scotland Coronary Prevention Study (WOSCOPS) patients4]. Although the antibodies used in our method were the same antibodies as used in previous studies, the recombinant Lp-PLA2 that we used as calibrator in the Lp-PLA2 assay had greater than five-fold more immunological action than the standard used previously. This accounts for the differences in recovered values in the patient samples (Robert Wolfert, diaDexus Inc., personal communication).
Statistical analysis
Most continuous variables are summarized as mean±standard deviation. Variables with heavily skewed distributions (CRP, homocysteine, triglycerides, and creatinine) are reported as medians, with first and third quartiles in parentheses. Discrete variables are presented as frequencies and group percentages. The association of continuous variables with the extent of CAD (none, mild, one-, two-, or three-vessel disease) was tested using a linear contrast in association with one-way analysis-of-variance. The Armitage trend test was used to assess the association between categorical variables and the extent of CAD. Differences in distribution between other groups were tested using one-way analysis of variance or the Kruskal–Wallis test. Spearman's correlation coefficient was used to assess linear relationships between continuous variables.
Multiple regression models were used to estimate conditional relationships. The covariates used in the logistic models for CAD and Cox proportional hazards models for incidence of major adverse events were age, gender, smoking history, hypertension, total and HDL cholesterol, triglycerides, and CRP. Heavily skewed variables were logarithmically transformed for use in these models. The proportional hazards assumption was satisfied for both Lp-PLA2 and log(CRP). Linearity was assessed for the continuous variables in logistic and Cox regression models by the use of generalized additive models. Splines were fitted and the fitted results, plus point-wise 95% confidence intervals, were plotted. In this way the linearity was assessed visually, by evaluating whether a straight line would fit through the confidence limits.
All hypothesis tests were two-tailed with a 0.05 Type I error rate.
Results
Patient characteristics
Table 1 summarizes the study population characteristics. Mean age was 60.1 ± 10.9 years and 38% were women. Coronary angiography revealed normal coronaries in 122 patients (24%), mild disease in 111 patients (22%), and one-, two-, and three-vessel disease in 85 (17%), 80 (16%), and 106 (21%) patients, respectively.
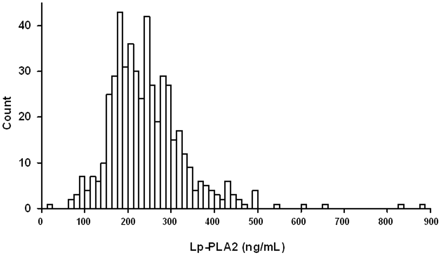
As expected, patients with significant CAD were more likely to be male, older, and to have a history of hyperlipidaemia, hypertension, or myocardial infarction (Table 1). Patients with significant CAD also had a higher mean creatinine, LDL cholesterol, fibrinogen, and Lp-PLA2, and lower HDL cholesterol. Mean Lp-PLA2 level was 245±91 ng/mL, and its distribution is shown in Figure 1.
Lp-PLA2 in acute coronary syndromes
Of the 504 patients, 169 had an acute coronary syndrome: 41 had an AMI and 128 had UA. Lp-PLA2 levels were similar in patients with or without an acute coronary syndrome (Table 2). In contrast, median CRP was significantly higher in AMI patients (Table 2). Therefore, analyses of the correlation between CRP and the extent of angiographic CAD were performed in only the 463 patients who did not have an AMI at the time of study enrolment (Tables 1, 3, and 4).
Associations of Lp-PLA2
Lp-PLA2 was significantly higher in men and was positively associated with creatinine, total and LDL cholesterol, and fibrinogen, and was negatively associated with HDL (Tables 3 and 4). It was not significantly associated with age, BMI, current smoking, hypertension, systolic or diastolic blood pressure, triglycerides, homocysteine, or CRP.
CRP correlated with age, gender (higher in women), history of hypertension, BMI, systolic blood pressure, total cholesterol, LDL cholesterol, triglycerides, and fibrinogen, but not with homocysteine or HDL cholesterol (Tables 3 and 4).
Lp-PLA2 and angiographic CAD
Lp-PLA2 levels were higher in patients with more extensive angiographic CAD, even when AMI patients were excluded (Table 1). However, after adjusting for clinical and lipid variables (age, gender, smoking, hypertension, total and HDL cholesterol, triglycerides, and CRP), Lp-PLA2 was not independently predictive of angiographic CAD. CRP levels did not have a statistically significant association with angiographic CAD on either univariate (Table 1) or multivariable analysis (OR=1.13 per standard deviation, P=0.16).
Lp-PLA2 and major adverse events
During a median follow-up of 4.0 years (interquartile range 3.9–4.2 years), 72 major adverse events occurred in 61 of 466 patients (the Kaplan–Meier estimated event rate was 3.2% at 1 year and 10% at 4 years): 20 patients died (6 cardiac deaths), 14 had a myocardial infarction, 26 underwent coronary revascularization (15 percutaneous intervention only, 9 coronary artery bypass surgery only, and 2 with both), and 10 had a stroke. Seven patients had two events and two had three (one patient had a myocardial infarction, percutaneous coronary intervention and coronary artery bypass grafting surgery, and one had a myocardial infarction, a stroke, and eventually died).
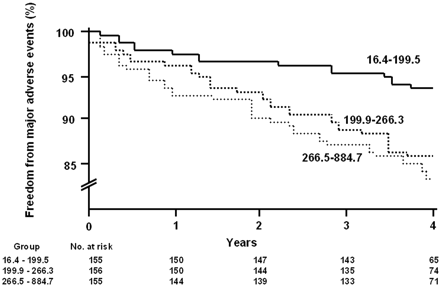
On univariate analysis, higher levels of both Lp-PLA2 and CRP (log-transformed) were associated with the higher incidence of events: the hazard ratio (HR) per standard deviation was 1.28 and 1.40, respectively (Table 5). Figure 2 depicts the incidence of major adverse events in the study population over time, classified according to Lp-PLA2 levels (tertiles), suggesting that individuals in the lowest tertile had fewer events during the follow-up period than those in the upper two tertiles.
Cox proportional hazard models were developed to examine the association of Lp-PLA2 with events. Those models included Lp-PLA2, age, gender, smoking history, hypertension, total and HDL cholesterol, triglycerides, and log-CRP. Both Lp-PLA2 and log-CRP were independent predictors of major adverse events (HR per standard deviation 1.30 and 1.34, respectively) (Table 5).
When LDL cholesterol was substituted for total cholesterol and fibrinogen was added to the model, the effect of Lp-PLA2 remained statistically significant (HR per standard deviation 1.25, 95% CI 1.02–1.52, P=0.03), whereas the effect of log-CRP became non-statistically significant (HR per standard deviation 1.10, 95% CI 0.79–1.51, P=0.58).
Discussion
Our study demonstrates that Lp-PLA2: (i) is associated with gender, total LDL, and HDL cholesterol, fibrinogen, and creatinine but not with other traditional or emerging CAD risk factors such as age, hypertension, obesity, and CRP; (ii) is not increased in AMI patients, in contrast to acute-phase reactants such as CRP and fibrinogen; (iii) is associated with angiographic CAD on univariate but not multivariable analysis; and (iv) is associated with the incidence of major adverse events at follow-up independently of clinical and lipid risk factors and CRP.
Associations of Lp-PLA2
Lp-PLA2 levels were measured in both men and women in this study; two previous such studies included either men (WOSCOPS sub-study),4 or women,5 but not both. In our study, Lp-PLA2 levels were higher in men, but the difference was no longer significant after adjusting for HDL, which was higher in women. The correlation between Lp-PLA2 and LDL observed in both the current and the WOSCOPS study was expected, since ∼80% of Lp-PLA2 is associated with LDL.2 Similar to the WOSCOPS study, Lp-PLA2 was not associated with age or BMI.
Both Lp-PLA2 and CRP were associated with lipid parameters (total cholesterol and LDL cholesterol; Tables 3 and 4). In contrast to what was reported in the WOSCOPS study where there was a very weak correlation, Lp-PLA2 had a significant negative association with HDL cholesterol in our study (Table 4). CRP was associated with obesity and AMI, whereas Lp-PLA2 was not. The difference in those associations suggests that Lp-PLA2 may act on the atherosclerotic process through different pathophysiological mechanisms than CRP. Lp-PLA2 cleaves oxidized phosphatidylcholine on LDL in the vessel wall to produce the bioactive lipids lysophosphatidylcholine and oxidatively modified non-esterified fatty acid, which may promote atherogenesis by functioning as monocyte chemoattractants and by inducing endothelial leukocyte adhesion molecules.7,8
Lp-PLA2 and angiographic CAD
Caslake et al.2 demonstrated that Lp-PLA2 levels were higher in 94 patients with CAD than in 54 controls. The association persisted after adjusting for LDL and HDL cholesterol, smoking, and systolic blood pressure. However, this study had several important limitations: it was relatively small, only men were included, and coronary angiography was performed in only one-third of the patients, so that a possible association between Lp-PLA2 levels and the severity of CAD could not be explored.
In our population, Lp-PLA2 was higher in patients with CAD than those without CAD, as also found by Caslake et al. However, in our study the association between Lp-PLA2 and CAD was not independent of other CAD risk factors. Compared with the study by Caslake et al.,2 our study differed in the following three ways: (i) a larger number of patients (504 vs. 148); (ii) data on the severity and the extent of CAD, rather than just the presence of CAD; and (iii) inclusion of a significant proportion of women (192 of 504, 38%).
In the current study CRP did not correlate with the angiographic extent of atherosclerosis. In the largest published study correlating CRP with the extent of angiographic CAD, the correlation was weak (Pearson's correlation coefficients 0.02–0.08), but reached statistical significance because of the large sample size (n=2554).9
Lp-PLA2 and major adverse events
In our study, higher Lp-PLA2 levels were associated with a higher incidence of major adverse events independently of traditional CAD risk factors and CRP, a finding that is consistent with the recently reported results from three other studies.4,10
In the WOSCOPS population (exclusively men), Lp-PLA2 was a predictor of coronary events independent of CRP, fibrinogen level, white cell count, age, systolic blood pressure, plasma triglycerides, HDL cholesterol, and LDL cholesterol levels (relative risk 1.18 per 1 standard deviation increase in Lp-PLA2, P=0.005).4
In the Atherosclerosis Risk in Communities (ARIC) study10 12 819 apparently healthy middle-aged men and women were followed for ∼6 years: 609 individuals developed CAD and were compared with 741 randomly selected controls. In subjects with LDL cholesterol below the median (130 mg/dL), Lp-PLA2 and CRP were both significantly and independently associated with CHD in fully adjusted models.
Similarly, in a post hoc analysis of the MONICA (MONItoring trends and determinants of CArdiovascular disease) Augsburg cohort, Lp-PLA2 levels were significantly higher in the 97 of the 934 apparently healthy men aged 45–64 who had suffered a coronary event during 14 years of follow-up (data presented at the 2003 American Heart Association Scientific Session in November 2003).
In contrast, Blake et al.5 did not find an independent association between Lp-PLA2 and cardiac events in a nested case–control study of apparently healthy women from the Women's Health study, with 123 cases, 40% of which were stroke.
Similar to the WOSCOPS, ARIC, and MONICA studies, in our study higher Lp-PLA2 levels were associated with a higher incidence of major adverse events independently of other CAD risk factors and CRP, suggesting that Lp-PLA2 may help in risk stratification of those patients. In contrast to the ARIC study, the predictive role of Lp-PLA2 appeared to be similar in patients with high or low LDL. The discordance between the weak association of Lp-PLA2 with angiographic CAD and the stronger association of Lp-PLA2 with major adverse events has also been observed with CRP, which was only weakly associated with coronary artery calcification but was strongly associated with clinical events in one study.11 As specific inhibitors of Lp-PLA2 have been developed and shown to be orally active in animal models,12 Lp-PLA2 has the potential to be a therapeutic target in patients with cardiovascular disease.11
Limitations
Measurements of Lp-PLA2 were performed on frozen rather than fresh plasma. We demonstrated that Lp-PLA2 is stable in samples stored at 4°C or −70°C for at least 7 days and that repeated freeze thaw cycles (three cycles) did not reduce Lp-PLA2 concentration. The effect of long-term storage is yet to be addressed. Patients in this study were all referred for cardiac catheterization; therefore, our control patients may not be representative of the population-based controls believed to be free of atherosclerosis. However, the use of patients with normal coronary arteries on angiography also has strengths over using population-based controls, because the presence of subclinical coronary disease can be excluded. Follow-up was obtained in 92.5% of patients and it is possible that some events were not detected. The incidence of major adverse events was low (13%), limiting the power of our study, but we were still able to detect a significant association between Lp-PLA2 and events.
Conclusions
Lp-PLA2 was associated with different risk factors for CAD than CRP. Higher Lp-PLA2 levels were associated with more severe angiographic CAD on univariate but not on multivariable analysis. Higher Lp-PLA2 levels were associated with a higher incidence of major adverse events during follow-up, independently of traditional CAD risk factors and CRP.
Acknowledgements
Supported in part by research grants from diaDexus Inc., South San Francisco, CA, USA and Interleukin Genetics, Waltham, MA, USA.
Figure 1 Distribution of Lp-PLA2 levels in the study population.
Figure 2 Incidence of major adverse events in the study population (n=466) classified according to Lp-PLA2 levels (tertiles).
Clinical characteristics of the study population, classified according to the extent of CAD at angiography
| . | All patients (n=504) . | No CAD(n=122) . | Mild CAD(n=111) . | One-vessel CAD(n=85) . | Two-vessel CAD(n=80) . | Three-vessel CAD(n=106) . | P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 60.1±10.9 | 53.7±11.3 | 61.9±9.5 | 59.9±11.1 | 61.2±10.4 | 64.7±8.7 | <0.001 |
| Female (%) | 192 (38) | 76 (62) | 55 (50) | 22 (26) | 18 (23) | 21 (20) | <0.001 |
| Hypertension (%) | 232 (46) | 39 (32) | 50 (45) | 42 (49) | 46 (58) | 55 (52) | <0.001 |
| Current smoking (%) | 40 (8) | 8 (7) | 9 (8) | 12 (14) | 7 (9) | 4 (4) | 0.58 |
| History of AMI (%) | 77 (15) | 3 (2) | 6 (5) | 16 (19) | 20 (25) | 32 (30) | <0.001 |
| History of CHF (%) | 59 (12) | 25 (20) | 19 (17) | 6 (7) | 3 (4) | 6 (6) | <0.001 |
| Family history of CAD (%) | 128 (25) | 27 (22) | 24 (22) | 20 (24) | 28 (35) | 29 (27) | 0.10 |
| History of hyperlipidaemia (%) | 286 (57) | 44 (36) | 52 (47) | 45 (53) | 66 (83) | 79 (75) | <0.001 |
| BMI, kg/m2 | 29.3±5.6 | 28.8±6.6 | 29.2±6.0 | 29.1±4.3 | 30.8±5.8 | 29.1±4.3 | 0.21 |
| Creatinine, µmol/La | 97 (88–115) | 97 (88–106) | 97 (80–106) | 106 (88–115) | 106 (88–115) | 106 (97–115) | <0.001 |
| Total cholesterol, mmol/L | 5.4±1.2 | 5.3±1.1 | 5.3±1.1 | 5.2±1.0 | 5.4±1.0 | 5.6±1.5 | 0.07 |
| Triglycerides, mmol/La | 1.7 (1.3–2.3) | 1.5 (1.1–2.2) | 1.8 (1.3–2.5) | 1.8 (1.3–2.5) | 1.7 (1.3–2.4) | 1.8 (1.3–2.5) | 0.010 |
| HDL cholesterol, mmol/L | 1.2±0.4 | 1.4±0.5 | 1.3±0.4 | 1.2±0.3 | 1.1±0.3 | 1.2±0.3 | <0.001 |
| LDL cholesterol, mmol/L | 3.2±0.9 | 3.0±0.9 | 3.1±0.9 | 3.2±0.8 | 3.4±1.0 | 3.5±1.1 | 0.001 |
| Homocysteine, µmol/La | 8.8 (7.5–10.7) | 8.6 (7.2–10.9) | 8.6 (7.5–10.3) | 8.7 (7.4–10.7) | 8.8 (7.4–10.4) | 9.6 (7.8–11.1) | 0.026 |
| Fibrinogen, mg/dL | 455±125 | 422±113 | 442±121 | 466±144 | 472±126 | 487±118 | <0.001 |
| CRP, mg/La | 2.9 (1.2–6.7) | 3.2 (1.1–6.7) | 2.5 (1.1–6.0) | 2.9 (1.4–8.0) | 2.9 (1.4–7.7) | 3.0 (1.2–7.6) | 0.27 |
| Lp-PLA2, ng/mL | 245±91 | 223±85 | 248±80 | 243±73 | 251±97 | 263±114 | 0.003 |
| . | All patients (n=504) . | No CAD(n=122) . | Mild CAD(n=111) . | One-vessel CAD(n=85) . | Two-vessel CAD(n=80) . | Three-vessel CAD(n=106) . | P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 60.1±10.9 | 53.7±11.3 | 61.9±9.5 | 59.9±11.1 | 61.2±10.4 | 64.7±8.7 | <0.001 |
| Female (%) | 192 (38) | 76 (62) | 55 (50) | 22 (26) | 18 (23) | 21 (20) | <0.001 |
| Hypertension (%) | 232 (46) | 39 (32) | 50 (45) | 42 (49) | 46 (58) | 55 (52) | <0.001 |
| Current smoking (%) | 40 (8) | 8 (7) | 9 (8) | 12 (14) | 7 (9) | 4 (4) | 0.58 |
| History of AMI (%) | 77 (15) | 3 (2) | 6 (5) | 16 (19) | 20 (25) | 32 (30) | <0.001 |
| History of CHF (%) | 59 (12) | 25 (20) | 19 (17) | 6 (7) | 3 (4) | 6 (6) | <0.001 |
| Family history of CAD (%) | 128 (25) | 27 (22) | 24 (22) | 20 (24) | 28 (35) | 29 (27) | 0.10 |
| History of hyperlipidaemia (%) | 286 (57) | 44 (36) | 52 (47) | 45 (53) | 66 (83) | 79 (75) | <0.001 |
| BMI, kg/m2 | 29.3±5.6 | 28.8±6.6 | 29.2±6.0 | 29.1±4.3 | 30.8±5.8 | 29.1±4.3 | 0.21 |
| Creatinine, µmol/La | 97 (88–115) | 97 (88–106) | 97 (80–106) | 106 (88–115) | 106 (88–115) | 106 (97–115) | <0.001 |
| Total cholesterol, mmol/L | 5.4±1.2 | 5.3±1.1 | 5.3±1.1 | 5.2±1.0 | 5.4±1.0 | 5.6±1.5 | 0.07 |
| Triglycerides, mmol/La | 1.7 (1.3–2.3) | 1.5 (1.1–2.2) | 1.8 (1.3–2.5) | 1.8 (1.3–2.5) | 1.7 (1.3–2.4) | 1.8 (1.3–2.5) | 0.010 |
| HDL cholesterol, mmol/L | 1.2±0.4 | 1.4±0.5 | 1.3±0.4 | 1.2±0.3 | 1.1±0.3 | 1.2±0.3 | <0.001 |
| LDL cholesterol, mmol/L | 3.2±0.9 | 3.0±0.9 | 3.1±0.9 | 3.2±0.8 | 3.4±1.0 | 3.5±1.1 | 0.001 |
| Homocysteine, µmol/La | 8.8 (7.5–10.7) | 8.6 (7.2–10.9) | 8.6 (7.5–10.3) | 8.7 (7.4–10.7) | 8.8 (7.4–10.4) | 9.6 (7.8–11.1) | 0.026 |
| Fibrinogen, mg/dL | 455±125 | 422±113 | 442±121 | 466±144 | 472±126 | 487±118 | <0.001 |
| CRP, mg/La | 2.9 (1.2–6.7) | 3.2 (1.1–6.7) | 2.5 (1.1–6.0) | 2.9 (1.4–8.0) | 2.9 (1.4–7.7) | 3.0 (1.2–7.6) | 0.27 |
| Lp-PLA2, ng/mL | 245±91 | 223±85 | 248±80 | 243±73 | 251±97 | 263±114 | 0.003 |
Plus–minus values are mean±standard deviation.
aMedian (interquartile range).
CHF, congestive heart failure.
Clinical characteristics of the study population, classified according to the extent of CAD at angiography
| . | All patients (n=504) . | No CAD(n=122) . | Mild CAD(n=111) . | One-vessel CAD(n=85) . | Two-vessel CAD(n=80) . | Three-vessel CAD(n=106) . | P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 60.1±10.9 | 53.7±11.3 | 61.9±9.5 | 59.9±11.1 | 61.2±10.4 | 64.7±8.7 | <0.001 |
| Female (%) | 192 (38) | 76 (62) | 55 (50) | 22 (26) | 18 (23) | 21 (20) | <0.001 |
| Hypertension (%) | 232 (46) | 39 (32) | 50 (45) | 42 (49) | 46 (58) | 55 (52) | <0.001 |
| Current smoking (%) | 40 (8) | 8 (7) | 9 (8) | 12 (14) | 7 (9) | 4 (4) | 0.58 |
| History of AMI (%) | 77 (15) | 3 (2) | 6 (5) | 16 (19) | 20 (25) | 32 (30) | <0.001 |
| History of CHF (%) | 59 (12) | 25 (20) | 19 (17) | 6 (7) | 3 (4) | 6 (6) | <0.001 |
| Family history of CAD (%) | 128 (25) | 27 (22) | 24 (22) | 20 (24) | 28 (35) | 29 (27) | 0.10 |
| History of hyperlipidaemia (%) | 286 (57) | 44 (36) | 52 (47) | 45 (53) | 66 (83) | 79 (75) | <0.001 |
| BMI, kg/m2 | 29.3±5.6 | 28.8±6.6 | 29.2±6.0 | 29.1±4.3 | 30.8±5.8 | 29.1±4.3 | 0.21 |
| Creatinine, µmol/La | 97 (88–115) | 97 (88–106) | 97 (80–106) | 106 (88–115) | 106 (88–115) | 106 (97–115) | <0.001 |
| Total cholesterol, mmol/L | 5.4±1.2 | 5.3±1.1 | 5.3±1.1 | 5.2±1.0 | 5.4±1.0 | 5.6±1.5 | 0.07 |
| Triglycerides, mmol/La | 1.7 (1.3–2.3) | 1.5 (1.1–2.2) | 1.8 (1.3–2.5) | 1.8 (1.3–2.5) | 1.7 (1.3–2.4) | 1.8 (1.3–2.5) | 0.010 |
| HDL cholesterol, mmol/L | 1.2±0.4 | 1.4±0.5 | 1.3±0.4 | 1.2±0.3 | 1.1±0.3 | 1.2±0.3 | <0.001 |
| LDL cholesterol, mmol/L | 3.2±0.9 | 3.0±0.9 | 3.1±0.9 | 3.2±0.8 | 3.4±1.0 | 3.5±1.1 | 0.001 |
| Homocysteine, µmol/La | 8.8 (7.5–10.7) | 8.6 (7.2–10.9) | 8.6 (7.5–10.3) | 8.7 (7.4–10.7) | 8.8 (7.4–10.4) | 9.6 (7.8–11.1) | 0.026 |
| Fibrinogen, mg/dL | 455±125 | 422±113 | 442±121 | 466±144 | 472±126 | 487±118 | <0.001 |
| CRP, mg/La | 2.9 (1.2–6.7) | 3.2 (1.1–6.7) | 2.5 (1.1–6.0) | 2.9 (1.4–8.0) | 2.9 (1.4–7.7) | 3.0 (1.2–7.6) | 0.27 |
| Lp-PLA2, ng/mL | 245±91 | 223±85 | 248±80 | 243±73 | 251±97 | 263±114 | 0.003 |
| . | All patients (n=504) . | No CAD(n=122) . | Mild CAD(n=111) . | One-vessel CAD(n=85) . | Two-vessel CAD(n=80) . | Three-vessel CAD(n=106) . | P-value . |
|---|---|---|---|---|---|---|---|
| Age, years | 60.1±10.9 | 53.7±11.3 | 61.9±9.5 | 59.9±11.1 | 61.2±10.4 | 64.7±8.7 | <0.001 |
| Female (%) | 192 (38) | 76 (62) | 55 (50) | 22 (26) | 18 (23) | 21 (20) | <0.001 |
| Hypertension (%) | 232 (46) | 39 (32) | 50 (45) | 42 (49) | 46 (58) | 55 (52) | <0.001 |
| Current smoking (%) | 40 (8) | 8 (7) | 9 (8) | 12 (14) | 7 (9) | 4 (4) | 0.58 |
| History of AMI (%) | 77 (15) | 3 (2) | 6 (5) | 16 (19) | 20 (25) | 32 (30) | <0.001 |
| History of CHF (%) | 59 (12) | 25 (20) | 19 (17) | 6 (7) | 3 (4) | 6 (6) | <0.001 |
| Family history of CAD (%) | 128 (25) | 27 (22) | 24 (22) | 20 (24) | 28 (35) | 29 (27) | 0.10 |
| History of hyperlipidaemia (%) | 286 (57) | 44 (36) | 52 (47) | 45 (53) | 66 (83) | 79 (75) | <0.001 |
| BMI, kg/m2 | 29.3±5.6 | 28.8±6.6 | 29.2±6.0 | 29.1±4.3 | 30.8±5.8 | 29.1±4.3 | 0.21 |
| Creatinine, µmol/La | 97 (88–115) | 97 (88–106) | 97 (80–106) | 106 (88–115) | 106 (88–115) | 106 (97–115) | <0.001 |
| Total cholesterol, mmol/L | 5.4±1.2 | 5.3±1.1 | 5.3±1.1 | 5.2±1.0 | 5.4±1.0 | 5.6±1.5 | 0.07 |
| Triglycerides, mmol/La | 1.7 (1.3–2.3) | 1.5 (1.1–2.2) | 1.8 (1.3–2.5) | 1.8 (1.3–2.5) | 1.7 (1.3–2.4) | 1.8 (1.3–2.5) | 0.010 |
| HDL cholesterol, mmol/L | 1.2±0.4 | 1.4±0.5 | 1.3±0.4 | 1.2±0.3 | 1.1±0.3 | 1.2±0.3 | <0.001 |
| LDL cholesterol, mmol/L | 3.2±0.9 | 3.0±0.9 | 3.1±0.9 | 3.2±0.8 | 3.4±1.0 | 3.5±1.1 | 0.001 |
| Homocysteine, µmol/La | 8.8 (7.5–10.7) | 8.6 (7.2–10.9) | 8.6 (7.5–10.3) | 8.7 (7.4–10.7) | 8.8 (7.4–10.4) | 9.6 (7.8–11.1) | 0.026 |
| Fibrinogen, mg/dL | 455±125 | 422±113 | 442±121 | 466±144 | 472±126 | 487±118 | <0.001 |
| CRP, mg/La | 2.9 (1.2–6.7) | 3.2 (1.1–6.7) | 2.5 (1.1–6.0) | 2.9 (1.4–8.0) | 2.9 (1.4–7.7) | 3.0 (1.2–7.6) | 0.27 |
| Lp-PLA2, ng/mL | 245±91 | 223±85 | 248±80 | 243±73 | 251±97 | 263±114 | 0.003 |
Plus–minus values are mean±standard deviation.
aMedian (interquartile range).
CHF, congestive heart failure.
Lp-PLA2, CRP, and fibrinogen levels in patients with and without an acute coronary syndrome
| . | AMI (n=41) . | UA (n=128) . | Non-ACS (n=335) . | P-value . |
|---|---|---|---|---|
| Lp-PLA2, ng/mL | 254±75 | 245±83 | 243±97 | 0.77 |
| CRP, mg/La | 13.4 (5.9–32.8) | 3.2 (1.3–6.6) | 2.2 (1.0–5.6) | <0.001 |
| Fibrinogen, mg/dL | 549±164 | 468±123 | 438±115 | <0.001 |
| Total cholesterol, mmol/L | 5.3±1.1 | 5.4±1.0 | 5.4±1.2 | 0.79 |
| Triglycerides, mmol/La | 1.7 (1.3–2.10) | 1.7 (1.3–2.6) | 1.7 (1.3–2.3) | 0.74 |
| HDL cholesterol, mmol/L | 1.1±0.3 | 1.2±0.4 | 1.3±0.4 | <0.001 |
| LDL cholesterol, mmol/L | 3.4±1.0 | 3.3±1.0 | 3.2±0.9 | 0.39 |
| . | AMI (n=41) . | UA (n=128) . | Non-ACS (n=335) . | P-value . |
|---|---|---|---|---|
| Lp-PLA2, ng/mL | 254±75 | 245±83 | 243±97 | 0.77 |
| CRP, mg/La | 13.4 (5.9–32.8) | 3.2 (1.3–6.6) | 2.2 (1.0–5.6) | <0.001 |
| Fibrinogen, mg/dL | 549±164 | 468±123 | 438±115 | <0.001 |
| Total cholesterol, mmol/L | 5.3±1.1 | 5.4±1.0 | 5.4±1.2 | 0.79 |
| Triglycerides, mmol/La | 1.7 (1.3–2.10) | 1.7 (1.3–2.6) | 1.7 (1.3–2.3) | 0.74 |
| HDL cholesterol, mmol/L | 1.1±0.3 | 1.2±0.4 | 1.3±0.4 | <0.001 |
| LDL cholesterol, mmol/L | 3.4±1.0 | 3.3±1.0 | 3.2±0.9 | 0.39 |
Plus–minus values are mean±standard deviation.
aMedian (interquartile range).
ACS, acute coronary syndrome. Other abbreviations as in text and Table 1.
Lp-PLA2, CRP, and fibrinogen levels in patients with and without an acute coronary syndrome
| . | AMI (n=41) . | UA (n=128) . | Non-ACS (n=335) . | P-value . |
|---|---|---|---|---|
| Lp-PLA2, ng/mL | 254±75 | 245±83 | 243±97 | 0.77 |
| CRP, mg/La | 13.4 (5.9–32.8) | 3.2 (1.3–6.6) | 2.2 (1.0–5.6) | <0.001 |
| Fibrinogen, mg/dL | 549±164 | 468±123 | 438±115 | <0.001 |
| Total cholesterol, mmol/L | 5.3±1.1 | 5.4±1.0 | 5.4±1.2 | 0.79 |
| Triglycerides, mmol/La | 1.7 (1.3–2.10) | 1.7 (1.3–2.6) | 1.7 (1.3–2.3) | 0.74 |
| HDL cholesterol, mmol/L | 1.1±0.3 | 1.2±0.4 | 1.3±0.4 | <0.001 |
| LDL cholesterol, mmol/L | 3.4±1.0 | 3.3±1.0 | 3.2±0.9 | 0.39 |
| . | AMI (n=41) . | UA (n=128) . | Non-ACS (n=335) . | P-value . |
|---|---|---|---|---|
| Lp-PLA2, ng/mL | 254±75 | 245±83 | 243±97 | 0.77 |
| CRP, mg/La | 13.4 (5.9–32.8) | 3.2 (1.3–6.6) | 2.2 (1.0–5.6) | <0.001 |
| Fibrinogen, mg/dL | 549±164 | 468±123 | 438±115 | <0.001 |
| Total cholesterol, mmol/L | 5.3±1.1 | 5.4±1.0 | 5.4±1.2 | 0.79 |
| Triglycerides, mmol/La | 1.7 (1.3–2.10) | 1.7 (1.3–2.6) | 1.7 (1.3–2.3) | 0.74 |
| HDL cholesterol, mmol/L | 1.1±0.3 | 1.2±0.4 | 1.3±0.4 | <0.001 |
| LDL cholesterol, mmol/L | 3.4±1.0 | 3.3±1.0 | 3.2±0.9 | 0.39 |
Plus–minus values are mean±standard deviation.
aMedian (interquartile range).
ACS, acute coronary syndrome. Other abbreviations as in text and Table 1.
Relationship between Lp-PLA2 and CRP levels with gender, current smoking, hypertension, and BMI
| . | Lp-PLA2a (n=504) . | Lp-PLA2a (n=463 without AMI) . | CRPb (n=463 without AMI) . |
|---|---|---|---|
| Men | 254±87 | 254±88 | 2.0 (0.9–4.5) |
| Women | 229±97 | 227±97 | 3.6 (1.4–7.3) |
| P | 0.003 | 0.002 | <0.001 |
| Current smoker | 264±73 | 270±76 | 2.4 (1.1–6.0) |
| Current non-smoker | 243±93 | 242±94 | 3.7 (1.7–4.7) |
| P | 0.16 | 0.089 | 0.37 |
| Hypertension | 240±99 | 240±99 | 3.1 (1.4–6.2) |
| No hypertension | 249±85 | 248±85 | 2.0 (0.9–5.5) |
| P | 0.28 | 0.26 | 0.015 |
| BMI<24.9 | 242±89 | 240±89 | 1.7 (0.7–4.9) |
| BMI 25–29.9 | 243±80 | 243±81 | 2.1 (1.0–4.8) |
| BMI≥30 | 248±103 | 247±106 | 3.5 (1.6–7.7) |
| P | 0.84 | 0.82 | <0.001 |
| . | Lp-PLA2a (n=504) . | Lp-PLA2a (n=463 without AMI) . | CRPb (n=463 without AMI) . |
|---|---|---|---|
| Men | 254±87 | 254±88 | 2.0 (0.9–4.5) |
| Women | 229±97 | 227±97 | 3.6 (1.4–7.3) |
| P | 0.003 | 0.002 | <0.001 |
| Current smoker | 264±73 | 270±76 | 2.4 (1.1–6.0) |
| Current non-smoker | 243±93 | 242±94 | 3.7 (1.7–4.7) |
| P | 0.16 | 0.089 | 0.37 |
| Hypertension | 240±99 | 240±99 | 3.1 (1.4–6.2) |
| No hypertension | 249±85 | 248±85 | 2.0 (0.9–5.5) |
| P | 0.28 | 0.26 | 0.015 |
| BMI<24.9 | 242±89 | 240±89 | 1.7 (0.7–4.9) |
| BMI 25–29.9 | 243±80 | 243±81 | 2.1 (1.0–4.8) |
| BMI≥30 | 248±103 | 247±106 | 3.5 (1.6–7.7) |
| P | 0.84 | 0.82 | <0.001 |
Relationship between Lp-PLA2 and CRP levels with gender, current smoking, hypertension, and BMI
| . | Lp-PLA2a (n=504) . | Lp-PLA2a (n=463 without AMI) . | CRPb (n=463 without AMI) . |
|---|---|---|---|
| Men | 254±87 | 254±88 | 2.0 (0.9–4.5) |
| Women | 229±97 | 227±97 | 3.6 (1.4–7.3) |
| P | 0.003 | 0.002 | <0.001 |
| Current smoker | 264±73 | 270±76 | 2.4 (1.1–6.0) |
| Current non-smoker | 243±93 | 242±94 | 3.7 (1.7–4.7) |
| P | 0.16 | 0.089 | 0.37 |
| Hypertension | 240±99 | 240±99 | 3.1 (1.4–6.2) |
| No hypertension | 249±85 | 248±85 | 2.0 (0.9–5.5) |
| P | 0.28 | 0.26 | 0.015 |
| BMI<24.9 | 242±89 | 240±89 | 1.7 (0.7–4.9) |
| BMI 25–29.9 | 243±80 | 243±81 | 2.1 (1.0–4.8) |
| BMI≥30 | 248±103 | 247±106 | 3.5 (1.6–7.7) |
| P | 0.84 | 0.82 | <0.001 |
| . | Lp-PLA2a (n=504) . | Lp-PLA2a (n=463 without AMI) . | CRPb (n=463 without AMI) . |
|---|---|---|---|
| Men | 254±87 | 254±88 | 2.0 (0.9–4.5) |
| Women | 229±97 | 227±97 | 3.6 (1.4–7.3) |
| P | 0.003 | 0.002 | <0.001 |
| Current smoker | 264±73 | 270±76 | 2.4 (1.1–6.0) |
| Current non-smoker | 243±93 | 242±94 | 3.7 (1.7–4.7) |
| P | 0.16 | 0.089 | 0.37 |
| Hypertension | 240±99 | 240±99 | 3.1 (1.4–6.2) |
| No hypertension | 249±85 | 248±85 | 2.0 (0.9–5.5) |
| P | 0.28 | 0.26 | 0.015 |
| BMI<24.9 | 242±89 | 240±89 | 1.7 (0.7–4.9) |
| BMI 25–29.9 | 243±80 | 243±81 | 2.1 (1.0–4.8) |
| BMI≥30 | 248±103 | 247±106 | 3.5 (1.6–7.7) |
| P | 0.84 | 0.82 | <0.001 |
Relationship between Lp-PLA2, CRP, and other CAD risk factors
| . | Lp-PLA2 (n=504) . | Lp-PLA2 (n=463 without AMI) . | CRP (n=463 without AMI) . | |||
|---|---|---|---|---|---|---|
| . | Spearman's rho . | P . | Spearman's rho . | P . | Spearman's rho . | P . |
| Age | 0.004 | 0.92 | 0.02 | 0.71 | 0.10 | 0.04 |
| BMI | −0.005 | 0.91 | −0.007 | 0.88 | 0.22 | <0.001 |
| Systolic blood pressure | −0.08 | 0.07 | −0.08 | 0.11 | 0.11 | 0.01 |
| Diastolic blood pressure | 0.04 | 0.37 | 0.05 | 0.33 | 0.02 | 0.60 |
| Creatinine | 0.13 | 0.004 | 0.13 | 0.007 | −0.10 | 0.03 |
| Total cholesterol | 0.25 | <0.001 | 0.24 | <0.001 | 0.11 | 0.02 |
| Triglycerides | 0.08 | 0.06 | 0.09 | 0.07 | 0.12 | 0.01 |
| HDL cholesterol | −0.26 | <0.001 | −0.26 | <0.001 | −0.01 | 0.76 |
| LDL cholesterol | 0.32 | <0.001 | 0.32 | <0.001 | 0.11 | 0.02 |
| Homocysteine | 0.08 | 0.09 | 0.08 | 0.10 | 0.08 | 0.07 |
| Fibrinogen | 0.12 | 0.007 | 0.12 | 0.01 | 0.49 | <0.001 |
| CRP | 0.01 | 0.83 | −0.02 | 0.62 | — | — |
| Lp-PLA2 | — | — | — | — | −0.02 | 0.62 |
| . | Lp-PLA2 (n=504) . | Lp-PLA2 (n=463 without AMI) . | CRP (n=463 without AMI) . | |||
|---|---|---|---|---|---|---|
| . | Spearman's rho . | P . | Spearman's rho . | P . | Spearman's rho . | P . |
| Age | 0.004 | 0.92 | 0.02 | 0.71 | 0.10 | 0.04 |
| BMI | −0.005 | 0.91 | −0.007 | 0.88 | 0.22 | <0.001 |
| Systolic blood pressure | −0.08 | 0.07 | −0.08 | 0.11 | 0.11 | 0.01 |
| Diastolic blood pressure | 0.04 | 0.37 | 0.05 | 0.33 | 0.02 | 0.60 |
| Creatinine | 0.13 | 0.004 | 0.13 | 0.007 | −0.10 | 0.03 |
| Total cholesterol | 0.25 | <0.001 | 0.24 | <0.001 | 0.11 | 0.02 |
| Triglycerides | 0.08 | 0.06 | 0.09 | 0.07 | 0.12 | 0.01 |
| HDL cholesterol | −0.26 | <0.001 | −0.26 | <0.001 | −0.01 | 0.76 |
| LDL cholesterol | 0.32 | <0.001 | 0.32 | <0.001 | 0.11 | 0.02 |
| Homocysteine | 0.08 | 0.09 | 0.08 | 0.10 | 0.08 | 0.07 |
| Fibrinogen | 0.12 | 0.007 | 0.12 | 0.01 | 0.49 | <0.001 |
| CRP | 0.01 | 0.83 | −0.02 | 0.62 | — | — |
| Lp-PLA2 | — | — | — | — | −0.02 | 0.62 |
Abbreviations as in text.
Relationship between Lp-PLA2, CRP, and other CAD risk factors
| . | Lp-PLA2 (n=504) . | Lp-PLA2 (n=463 without AMI) . | CRP (n=463 without AMI) . | |||
|---|---|---|---|---|---|---|
| . | Spearman's rho . | P . | Spearman's rho . | P . | Spearman's rho . | P . |
| Age | 0.004 | 0.92 | 0.02 | 0.71 | 0.10 | 0.04 |
| BMI | −0.005 | 0.91 | −0.007 | 0.88 | 0.22 | <0.001 |
| Systolic blood pressure | −0.08 | 0.07 | −0.08 | 0.11 | 0.11 | 0.01 |
| Diastolic blood pressure | 0.04 | 0.37 | 0.05 | 0.33 | 0.02 | 0.60 |
| Creatinine | 0.13 | 0.004 | 0.13 | 0.007 | −0.10 | 0.03 |
| Total cholesterol | 0.25 | <0.001 | 0.24 | <0.001 | 0.11 | 0.02 |
| Triglycerides | 0.08 | 0.06 | 0.09 | 0.07 | 0.12 | 0.01 |
| HDL cholesterol | −0.26 | <0.001 | −0.26 | <0.001 | −0.01 | 0.76 |
| LDL cholesterol | 0.32 | <0.001 | 0.32 | <0.001 | 0.11 | 0.02 |
| Homocysteine | 0.08 | 0.09 | 0.08 | 0.10 | 0.08 | 0.07 |
| Fibrinogen | 0.12 | 0.007 | 0.12 | 0.01 | 0.49 | <0.001 |
| CRP | 0.01 | 0.83 | −0.02 | 0.62 | — | — |
| Lp-PLA2 | — | — | — | — | −0.02 | 0.62 |
| . | Lp-PLA2 (n=504) . | Lp-PLA2 (n=463 without AMI) . | CRP (n=463 without AMI) . | |||
|---|---|---|---|---|---|---|
| . | Spearman's rho . | P . | Spearman's rho . | P . | Spearman's rho . | P . |
| Age | 0.004 | 0.92 | 0.02 | 0.71 | 0.10 | 0.04 |
| BMI | −0.005 | 0.91 | −0.007 | 0.88 | 0.22 | <0.001 |
| Systolic blood pressure | −0.08 | 0.07 | −0.08 | 0.11 | 0.11 | 0.01 |
| Diastolic blood pressure | 0.04 | 0.37 | 0.05 | 0.33 | 0.02 | 0.60 |
| Creatinine | 0.13 | 0.004 | 0.13 | 0.007 | −0.10 | 0.03 |
| Total cholesterol | 0.25 | <0.001 | 0.24 | <0.001 | 0.11 | 0.02 |
| Triglycerides | 0.08 | 0.06 | 0.09 | 0.07 | 0.12 | 0.01 |
| HDL cholesterol | −0.26 | <0.001 | −0.26 | <0.001 | −0.01 | 0.76 |
| LDL cholesterol | 0.32 | <0.001 | 0.32 | <0.001 | 0.11 | 0.02 |
| Homocysteine | 0.08 | 0.09 | 0.08 | 0.10 | 0.08 | 0.07 |
| Fibrinogen | 0.12 | 0.007 | 0.12 | 0.01 | 0.49 | <0.001 |
| CRP | 0.01 | 0.83 | −0.02 | 0.62 | — | — |
| Lp-PLA2 | — | — | — | — | −0.02 | 0.62 |
Abbreviations as in text.
Univariate and multivariable association between different baseline parameters and the incidence of major adverse events at follow-up
| Parameter (SD) . | Univariate HR (95% CI) . | P . | Multivariable HR (95% CI)a . | P . |
|---|---|---|---|---|
| Age (10.8 years) | 1.59 (1.19–2.13) | 0.002 | 1.55 (1.16–2.08) | 0.003 |
| Male gender | 1.18 (0.70–2.01) | 0.53 | 1.33 (0.70–2.54) | 0.38 |
| Smoking history | 1.22 (0.73–2.03) | 0.45 | 1.21 (0.70–2.09) | 0.49 |
| Hypertension | 1.49 (0.90–2.48) | 0.12 | 1.40 (0.84–2.35) | 0.20 |
| Total cholesterol (1.17 mmol/L) | 0.92 (0.70–1.19) | 0.52 | 0.75 (0.55–1.04) | 0.08 |
| HDL cholesterol (0.37 mmol/L) | 0.82 (0.62–1.07) | 0.15 | 1.05 (0.74–1.50) | 0.78 |
| log triglycerides (0.01 mmol/L) | 1.17 (0.91–1.51) | 0.22 | 1.32 (0.94–1.87) | 0.11 |
| log-CRP (1.32 mg/L) | 1.40 (1.10–1.79) | 0.006 | 1.34 (1.05–1.72) | 0.02 |
| Lp-PLA2 (92.8 ng/mL) | 1.28 (1.06–1.54) | 0.009 | 1.30 (1.06–1.59) | 0.01 |
| Parameter (SD) . | Univariate HR (95% CI) . | P . | Multivariable HR (95% CI)a . | P . |
|---|---|---|---|---|
| Age (10.8 years) | 1.59 (1.19–2.13) | 0.002 | 1.55 (1.16–2.08) | 0.003 |
| Male gender | 1.18 (0.70–2.01) | 0.53 | 1.33 (0.70–2.54) | 0.38 |
| Smoking history | 1.22 (0.73–2.03) | 0.45 | 1.21 (0.70–2.09) | 0.49 |
| Hypertension | 1.49 (0.90–2.48) | 0.12 | 1.40 (0.84–2.35) | 0.20 |
| Total cholesterol (1.17 mmol/L) | 0.92 (0.70–1.19) | 0.52 | 0.75 (0.55–1.04) | 0.08 |
| HDL cholesterol (0.37 mmol/L) | 0.82 (0.62–1.07) | 0.15 | 1.05 (0.74–1.50) | 0.78 |
| log triglycerides (0.01 mmol/L) | 1.17 (0.91–1.51) | 0.22 | 1.32 (0.94–1.87) | 0.11 |
| log-CRP (1.32 mg/L) | 1.40 (1.10–1.79) | 0.006 | 1.34 (1.05–1.72) | 0.02 |
| Lp-PLA2 (92.8 ng/mL) | 1.28 (1.06–1.54) | 0.009 | 1.30 (1.06–1.59) | 0.01 |
aHRs were adjusted for Lp-PLA2, age, gender, smoking history, hypertension, total and HDL cholesterol, triglycerides, and log-CRP.
CI, confidence intervals. Other abbreviations as in text.
Univariate and multivariable association between different baseline parameters and the incidence of major adverse events at follow-up
| Parameter (SD) . | Univariate HR (95% CI) . | P . | Multivariable HR (95% CI)a . | P . |
|---|---|---|---|---|
| Age (10.8 years) | 1.59 (1.19–2.13) | 0.002 | 1.55 (1.16–2.08) | 0.003 |
| Male gender | 1.18 (0.70–2.01) | 0.53 | 1.33 (0.70–2.54) | 0.38 |
| Smoking history | 1.22 (0.73–2.03) | 0.45 | 1.21 (0.70–2.09) | 0.49 |
| Hypertension | 1.49 (0.90–2.48) | 0.12 | 1.40 (0.84–2.35) | 0.20 |
| Total cholesterol (1.17 mmol/L) | 0.92 (0.70–1.19) | 0.52 | 0.75 (0.55–1.04) | 0.08 |
| HDL cholesterol (0.37 mmol/L) | 0.82 (0.62–1.07) | 0.15 | 1.05 (0.74–1.50) | 0.78 |
| log triglycerides (0.01 mmol/L) | 1.17 (0.91–1.51) | 0.22 | 1.32 (0.94–1.87) | 0.11 |
| log-CRP (1.32 mg/L) | 1.40 (1.10–1.79) | 0.006 | 1.34 (1.05–1.72) | 0.02 |
| Lp-PLA2 (92.8 ng/mL) | 1.28 (1.06–1.54) | 0.009 | 1.30 (1.06–1.59) | 0.01 |
| Parameter (SD) . | Univariate HR (95% CI) . | P . | Multivariable HR (95% CI)a . | P . |
|---|---|---|---|---|
| Age (10.8 years) | 1.59 (1.19–2.13) | 0.002 | 1.55 (1.16–2.08) | 0.003 |
| Male gender | 1.18 (0.70–2.01) | 0.53 | 1.33 (0.70–2.54) | 0.38 |
| Smoking history | 1.22 (0.73–2.03) | 0.45 | 1.21 (0.70–2.09) | 0.49 |
| Hypertension | 1.49 (0.90–2.48) | 0.12 | 1.40 (0.84–2.35) | 0.20 |
| Total cholesterol (1.17 mmol/L) | 0.92 (0.70–1.19) | 0.52 | 0.75 (0.55–1.04) | 0.08 |
| HDL cholesterol (0.37 mmol/L) | 0.82 (0.62–1.07) | 0.15 | 1.05 (0.74–1.50) | 0.78 |
| log triglycerides (0.01 mmol/L) | 1.17 (0.91–1.51) | 0.22 | 1.32 (0.94–1.87) | 0.11 |
| log-CRP (1.32 mg/L) | 1.40 (1.10–1.79) | 0.006 | 1.34 (1.05–1.72) | 0.02 |
| Lp-PLA2 (92.8 ng/mL) | 1.28 (1.06–1.54) | 0.009 | 1.30 (1.06–1.59) | 0.01 |
aHRs were adjusted for Lp-PLA2, age, gender, smoking history, hypertension, total and HDL cholesterol, triglycerides, and log-CRP.
CI, confidence intervals. Other abbreviations as in text.
Tew DG, Southan C, Rice SQ et al. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins.
Caslake MJ, Packard CJ, Suckling KE et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease.
Hakkinen T, Luoma JS, Hiltunen MO et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions.
Packard CJ, O'Reilly DS, Caslake MJ et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group.
Blake GJ, Dada N, Fox JC et al. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women.
Killip T. The National Heart, Lung, and Blood Institute Coronary Artery Surgery Study (CASS).
Tselepis AD, Chapman JM. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase.
MacPhee CH, Moores KE, Boyd HF et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor.
Zebrack JS, Muhlestein JB, Horne BD, Anderson JL. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina.
Ballantyne CM, Hoogeveen RC, Bang H et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study.
MacPhee CH. Lipoprotein-associated phospholipase A2: a potential new risk factor for coronary artery disease and a therapeutic target.
Author notes
Present address: University of Texas Southwestern Medical School, 5323 Harry Hines Blvd, Dallas, TX 75216, USA