-
PDF
- Split View
-
Views
-
Cite
Cite
Massimiliano Valeriani, Alfonso Sestito, Domenica Le Pera, Liala De Armas, Fabio Infusino, Toni Maiese, Gregory Angelo Sgueglia, Pietro Attilio Tonali, Filippo Crea, Domenico Restuccia, Gaetano Antonio Lanza, Abnormal cortical pain processing in patients with cardiac syndrome X, European Heart Journal, Volume 26, Issue 10, May 2005, Pages 975–982, https://doi.org/10.1093/eurheartj/ehi229
Close - Share Icon Share
Abstract
Aims Previous studies suggested that an enhanced pain sensitivity is present in patients with cardiac syndrome X (SX). We investigated whether SX patients present abnormalities in the electrical cerebral signals generated by pain stimuli.
Methods and results Cortical laser evoked potentials (LEPs) were recorded in 16 SX patients, in 10 patients with refractory angina due to obstructive coronary artery disease (CAD) and in 13 healthy controls. LEPs were recorded during stimulation of chest and right hand dorsum. Three sequences of painful stimuli were applied at each site. Subjective pain rating was assessed by a 0–100 mm visual analogic scale (VAS). Basal LEPs did not differ among groups and there were no differences for most LEP components across the repetitions of stimuli. However, the amplitude of the N2/P2 LEP component, specifically reflecting cortical pain processing, decreased across the three sequences of stimuli in controls and CAD patients, but not in SX patients. Compared with the first sequence, the N2/P2 amplitude during the third sequence of stimuli in the three groups was 77±16, 56±24, and 99±34%, respectively, for chest (P=0.001), and 63±31, 72±17, and 98±46%, respectively, for right hand (P=0.03) stimulation. The changes in VAS pain score across the three sequences paralleled those of N2/P2 amplitude.
Conclusion Our data show that in SX patients, central handling of painful stimuli is characterized by inadequate habituation, which might play a role in determining the peculiar clinical characteristics of anginal chest pain of these patients.
See page 946 for the editorial comment on this article (doi:10.1093/eurheartj/ehi242)
Introduction
Cardiac syndrome X (SX) is characterized by angina-like chest pain episodes, mainly related to exertion, ST-segment depression during spontaneous and/or provoked (e.g. by exercise stress test) angina pain, and normal coronary arteries at angiography. Myocardial ischaemia due to coronary microvascular dysfunction has been suggested to be responsible for this syndrome.1–5
However, in several SX patients, the severity and refractoriness of chest pain episodes usually contrast with the limited objective evidence of myocardial ischaemia on standard diagnostic techniques,6–9 suggesting that, in most cases, the latter may not be sufficient to account by itself for patient symptoms. Previous studies in SX patients consistently demonstrated increased painful sensitivity to cardiac stimuli which do not usually cause any sensation in healthy subjects,10–13 thus suggesting that an abnormal cardiac nociception might play a major role in the pathophysiology of the syndrome, allowing even to mild myocardial ischaemia to result in a relevant clinical syndrome.
In a recent study, Rosen et al.14 using positron emission tomography showed increased blood flow to the right insular cortex during dobutamine stress test in SX patients. The authors suggested that an increased activity of this cortical region, resulting in a facilitating top–down influence on pain transmission, might be a major mechanism of chest pain in SX patients. However, whether the right insular activation was a primary phenomenon or it was rather the effect of a more peripheral (e.g. cardiac, spinal, or thalamic) nociception abnormality could not be ascertained by that study.
In the present study, we investigated the function of the brain areas specifically devoted to nociception in SX patients, by assessing cortical laser evoked potentials (LEPs) in response to cutaneous CO2 laser pulses. These latter have indeed been shown to be suitable to study the nociceptive pathway function, as they are able to activate selectively the thin myelinated (Aδ) and unmyelinated (C) nociceptive fibres without any concurrent activation of the larger, non-nociceptive afferent fibres.15
Specifically, two main cortical LEP components are recorded during cutaneous CO2 laser stimulation, N1–P1 and N2/P2. The N1–P1 wave is believed to originate in the secondary somatosensory area (SII) and correlates with the sensory-discriminative aspect of pain.24 The N2/P2 response, on the other hand, is mostly generated by neurons in the cingulate cortex18,19,25–27 and is important for the emotional component of sensation.28,29
Methods
Subjects
We studied 16 patients with cardiac SX (age 59.9±8; eight men, Table 1). All patients had a history of effort angina, ST-segment depression associated with angina during exercise stress test, and totally smooth coronary arteries at angiography. All patients included in this study also had evidence of reversible myocardial perfusion defects on exercise. Coronary artery spasm was excluded according to intracoronary or systemic ergonovine test in all patients. A mild hypertension (blood pressure >140/90 but <160/100 mmHg) was present in six patients, but left ventricular hypertrophy was excluded by echocardiography in all of them. Other cardiac or systemic diseases were carefully excluded, according to clinical history, careful physical examination, routine laboratory exams, and two-dimensional and Doppler echocardiography. Patients with a history of headache or any chronic pain syndrome other than anginal chest pain were also excluded.
A group of 13 apparently healthy subjects (controls, age 54.6±10; seven men) served as a control group. These subjects were enrolled from the non-medical staff of our hospital and were selected to be comparable to SX patients as to age and gender. To select controls, every time we included a few SX patients, we identified apparently healthy subjects, which allowed us to maintain a mean age similar to that of SX patients, also maintaining a gender balance between the two groups. Potential controls had to deny any symptoms or disease and had to have normal echocardiographic examination and normal maximal exercise stress test.
In the attempt to limit the possibility that the detection of cortical LEP abnormalities in SX patients might be secondary to recurrent chest pain, rather than an expression of a potential pathogenetic mechanism of the syndrome, we also studied a group of 10 patients with documented severe coronary artery disease (CAD), who presented a chronic clinical pattern of stable angina episodes refractory to maximally tolerated drug therapy (refractory angina), caused by a CAD pattern judged unsuitable for both percutaneous and surgical coronary revascularization. These patients were older than SX patients and controls (age 65.8±10, P<0.01 vs. the other groups), but similar in gender (six men, four women; P=0.53 for comparisons among groups). Furthermore, the frequency of angina episodes in these patients was similar to that of patients with SX (Table 1). Patients with neurological or systemic diseases or with any other chronic painful condition other than angina pectoris were excluded from the study.
Laser stimulation and LEP recording
The study protocol was approved by the Ethics Committee of our Institution, and investigational procedures were performed after obtaining written informed consent to participate in the study. All neurophysiological investigation tests were performed by expert neurologists who were blinded to the clinical diagnosis of the subjects. The tests were always performed in the afternoon (from 3:00 to 6:00 PM), in standard conditions, with the subjects lying on a couch in a warm and semi-dark room. In SX and CAD patients, LEP recordings were performed no less than 24 h after the last angina attack. The stimulation site was visualized by a He–Ne laser beam. After each stimulus, the laser beam was slightly shifted to a nearby spot to avoid nociceptor sensitization and skin damage. Laser pulses (wavelength 10.6 µm, beam diameter 2 mm, duration 10 ms) were delivered by a CO2 Neurolas (Electronic Engineering, Florence, Italy).
The chest skin and the right hand dorsum were stimulated in all subjects. Chest was chosen because, according to the theory of referred visceral pain, the painful laser stimuli delivered on the chest skin are likely to be processed by the same dorsal horn neurons onto which the nociceptive stimuli coming from the heart converge.16 To make this assumption most likely, laser pulses were individualized being delivered on the chest area where each patient referred the pain during angina episodes. In contrast, the right hand was chosen because the nociceptive pathways from this hand are usually separated from those coming from the heart at both peripheral and spinal levels; in our patients, in particular, this was suggested by the fact that none of them had ever referred pain in the right arm.
For each subject, we first identified the sensory threshold (STh), defined as the lowest stimulus intensity [measured in watts (W)] required to elicit a distinct sensation, and determined by the method of limits in three series of increasing and decreasing stimulus intensities. Then, the stimulus intensity was set at 2.5×STh, felt as clearly painful by all our subjects and this recording intensity (RI) was used to record LEPs.
All subjects underwent an LEP recording session with two scalp electrodes placed along the midline in the frontal (Fz) and in the vertex (Cz) regions and one electrode in the left temporal region (T3). The electrode positions were defined according to the 10–20 International System.17 The reference electrode was placed at the nose and the ground on the forehead (Fpz). Eye movements and eye-blinks were monitored by an electroculography (EOG) derivation, obtained by referring an active electrode above the right eyebrow to the nose. Signals were amplified, filtered (bandpass 0.3–70 Hz), and stored for off-line average and analysis. The analysis time was 1000 ms with a bin width of 2 ms. An automatic artefact rejection system excluded all trials contaminated by transient signals exceeding the average value by ±65 µV at any recording channel, including EOG.
Experimental protocol
Three consecutive sequences of 30 stimuli, with interstimulus intervals varying randomly from 8 to 12 s, were delivered to the chest and to the right hand dorsum. In each site, the sequences were separated by a 5 min interval, and a 10 min interval elapsed after the stimulation site was changed. The sequence of the stimulation sites was randomly varied across subjects. At the end of each sequence of stimuli, patients were asked to score pain sensation induced by laser pulses according to a 0–100 point visual analogic scale (VAS), in which ‘0’ corresponded to ‘no pain’ and ‘100’ to the ‘highest imaginable pain’.
In all subjects, recordings from midline electrodes during peripheral laser stimulation showed a late, high-amplitude, biphasic (negative–positive) complex (N2/P2). The N2/P2 amplitude was measured from the negative to the positive peak of the biphasic complex. Besides this biphasic complex, a negative N1 potential in the temporal region and, at approximately the same latency, a positive P1 potential in the frontal region contralateral to the stimulation site were identifiable in all subjects. These potentials were shorter in latency and lower in amplitude than the N2/P2 responses. As the N1–P1 potentials are generated by the same dipolar source in the contralateral second somatosensory area,18,19 the N1–P1 amplitude was measured off-line by referring the temporal lead to Fz.20
Statistical analysis
Basal continuous clinical variables were compared by analysis of variance (ANOVA), whereas proportions were compared by χ2 test. LEP amplitudes and latencies and VAS pain scores recorded in the three groups during the three successive sequences of laser pulses at each stimulation site were compared by two-way ANOVA with a repeated measure design to assess whether changes were present throughout the three sequences of stimuli and whether the changes differ among groups (group–sequence interaction). F-test results were corrected for identity covariance matrix by the Greenhouse–Geisser method to take into account possible intragroup correlations.
Furthermore, as we were specifically interested in assessing between-group differences in the changes of LEP components and of pain rating during the third (final), when compared with the first (initial), sequence of stimuli, the measures of variables during the third repetition in each patient at each stimulation site were also expressed as a per cent of the measure during the first sequence and values were compared by ANOVA. For completeness of analysis, a comparison was also separately done for the changes observed during the second, when compared with the first, sequence of stimuli. Student's t-test with Bonferroni correction was used for multiple comparisons in case of global statistical significance by ANOVA. When significant, between-group differences were also adjusted for age and gender. Correlation analyses were done by Pearson's test. No prior sample size calculation was done for this study owing to the quite rare types of patients included. Data are reported as mean±SD. A two-sided P value <0.05 was always required for statistical significance. The software SPSS 12.0.2 (SPSS Inc., Florence, Italy) was used for statistical analyses.
Results
Chest stimulation
There were no differences among groups in RI values for stimulation of the chest skin (9.1±2.01, 10±2.04, and 9.6±1.93 W for SX, CAD patients, and controls, respectively; P=0.28). No significant differences among groups were also found in latency and amplitude of each LEP component during the first series of chest stimulation. Furthermore, the latency and the amplitude of the N1–P1 component and the latency of the N2/P2 component did not change significantly across the three sequences of stimuli in any group (Table 2).
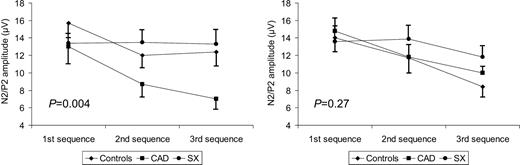
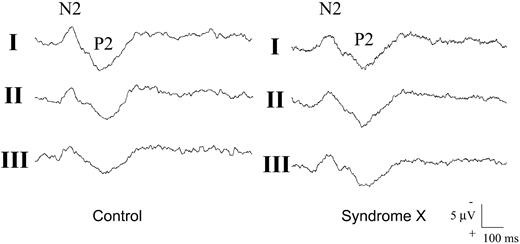
In contrast, there was a significant difference among groups in the response of N2/P2 amplitude to repeated sequences of laser stimuli (P=0.004; Figure 1). Indeed, the N2/P2 amplitude decreased progressively during the second and third sequences, when compared with the first one, in controls and in CAD patients (P<0.01 for both), but not in SX patients, in which it did not change significantly across the stimulation sequences (P=0.97). The difference among groups persisted significant after adjustment for age and gender (P<0.01). An example of the changes of N2/P2 amplitude through the three sequences of laser chest pain stimuli in a healthy subject and in an SX patient is shown in Figure 2.
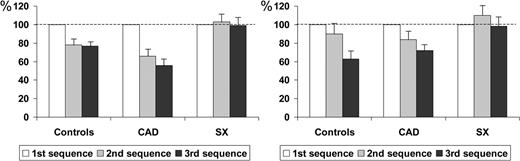
When compared with N2/P2 amplitude during the initial sequence, the N2/P2 amplitude in controls, CAD patients, and SX patients during the final sequence of stimuli was 77±16, 56±24, and 99±34%, respectively (P=0.001), whereas it was 78±22, 66±25, and 103±33%, respectively, during the second sequence of stimuli (P=0.005) (Figure 3).
VAS pain rating during the first sequence of CO2 laser chest stimuli seemed slightly higher in SX patients (Table 2), but there were no significant differences among groups. However, there was a significant difference among groups in the changes of VAS score following repeated sequences of laser stimuli (P=0.019). A significant decrease in VAS score across the three sequences of stimuli was indeed observed in controls and CAD patients (P=0.017 and P=0.019, respectively), but not in SX patients (P=0.20). The difference among groups persisted significantly after adjustment for age and gender (P<0.01).
When compared with pain rating during the initial sequence, the VAS score in controls, CAD patients, and SX patients was 66±30, 73±28, and 114±50%, respectively, during the final sequence of stimuli (P=0.004), whereas it was 103±39, 99±32, and 108±28%, respectively, during the second sequence of stimuli (P=0.81).
There was a significant correlation between the per cent changes in N2/P2 and VAS score during the third (r=0.47; P=0.002), but not during the second (r=0.24; P=0.14), sequence of stimuli.
Right hand stimulation
There were no differences among groups in RI values for stimulation of the right hand (10.6±1.71, 11.1±1.24, and 10.9±1.58 W for SX, CAD, and controls, respectively; P=0.43). Latency and amplitude of LEP components during the first series of stimuli were similar among groups, and the changes in latency and amplitude of the N1–P1 component and in latency of the N2/P2 component did not differ among groups across the three sequences of stimuli (Table 3).
Although there was a decrease in N2/P2 amplitude during the second and third sequences which appeared to be reduced in SX patients, two-way ANOVA did not show a statistical significant difference among curves. (Figure 1; P=0.27). However, when compared with N2/P2 amplitude during the first sequence, the N2/P2 amplitude during the final (third) sequence of stimuli was significantly reduced in controls and in CAD patients, but not in SX patients (63±31, 72±17, and 98±46%, respectively, P=0.03; Figure 3), a difference which persisted even after correction for age and gender (P=0.038). Similar differences in N2/P2 amplitude were present among groups during the second, when compared with the first, sequence of stimuli (90±43, 84±28, and 110±45%, respectively), but the differences were not significant (P=0.22).
VAS pain rating during the first sequence of CO2 laser chest stimuli was not significantly different among groups (Table 3). There was a significant difference among groups in the changes of VAS score following repeated sequences of laser stimuli (P=0.001). A significant decrease of VAS score across the three sequences of stimuli was indeed observed in controls and in CAD patients (P=0.003 and P=0.034), whereas VAS score paradoxically increased in SX patients (P=0.047).
When compared with pain rating during the first sequence, the VAS score during the final sequence was reduced in controls and in CAD patients, but not in SX patients (77±20, 82±19, and 110±50%, respectively, P=0.036; Figure 3), a difference persisting after correction for age and gender (P=0.04). No differences were present among groups during the second, when compared with the first, sequence of stimuli (103±27, 93±13, and 104±40%, respectively, P=0.64).
No significant correlation between the percent changes in N2/P2 and VAS score was observed during the third sequence (r=0.23; P=0.16), although there was a tendency for a mild correlation during the second sequence (r=0.30; P=0.06) of stimuli.
Discussion
Our study yielded two main results: (i) patients with cardiac SX did not show basal significant functional abnormalities of the systemic nociceptive pathways when compared with control subjects and to patients with refractory angina due to known severe CAD; (ii) SX patients, however, had a reduced habituation to repetitive noxious stimuli; the difference was more apparent for chest than for right hand stimulation.
Nociceptive function in SX patients
To assess the nociceptive pathway function, we examined latency and amplitude of all LEP components recorded during a basal sequence of cutaneous stimuli. LEP components recorded after skin stimulation at noxious intensities have been proved to be generated by inputs transmitted by Aδ fibres,15 and abnormalities in LEP latency and/or amplitude were demonstrated in diseases involving the peripheral Aδ afferents or the central spinothalamic pathway.21
In this study, LEP components were similar in SX patients, CAD patients, and control subjects, thus suggesting no basal abnormalities in peripheral pain threshold. Accordingly, RIs of both chest and right hand skin, and even subjective basal VAS pain rating, did not differ significantly among groups. These findings contrast with some previous uncontrolled data suggesting reduced systemic pain threshold in SX patients.22,23 However, in agreement with our data, the only study which used a controlled blind protocol to assess dental pulp pain threshold failed to find evidence of generalized enhanced pain perception in these patients.11
It remains to be ascertained whether the reduced pain threshold for myocardial stimuli demonstrated in our previous controlled study13 is associated with abnormalities of electrical cortical activity.
Repetitive noxious stimuli and cerebral cortex excitability
Two main cortical LEP components were recorded during CO2 laser stimulation, N1–P1 and N2/P2. Previous studies showed that the middle-latency N1–P1 wave is probably generated in the secondary somatosensory area (SII) and may represent the neurophysiologic correlatation of the sensory-discriminative aspect of pain.24 The N2/P2 response, on the other hand, is mostly generated by neurons in the cingulate cortex,18,19,25–27 which is part of the limbic system and is important for the emotional component of sensation.28,29
In this study, in agreement with previous data,30,31 healthy controls showed a significant reduction in amplitude of the vertex LEP components N2/P2 during repetitive sequence of stimuli. Although all previous neurophysiological studies suggested that the SII area and the cingulate cortex are sequentially activated after pain stimulation,15 the N2/P2 habituation, in spite of the unmodified N1–P1 amplitude during repetitive painful stimuli, likely represents a phenomenon occurring within the cortical areas generating the N2/P2 LEP components.
In patients with cardiac SX, the habituation of the N2/P2 complex was virtually absent, thus suggesting that some cortical areas devoted to pain processing, namely, those generating the N2/P2 potentials, have an abnormal excitability in SX patients.
The dysfunction seems to involve not only the cortical projections coming from the chest, but also those from the right hand, the nociceptive pathways of which are likely separated from those coming from the heart both at peripheral and at spinal levels, thus suggesting a generalized abnormal pain processing occurring at cortical level. However, it is worth noting that differences among groups were more apparent during chest stimulation than during right hand stimulation, which might suggest preferential abnormalities of cortical processing of stimuli coming from specific sites of the body.
Independent of the mechanisms, the cortical LEP abnormalities in SX patients cannot be interpreted as a secondary response to recurrent angina or an unspecific effect of pain as CAD patients with refractory angina showed normal habituation to pain.
The neurophysiological results in our groups were paralleled by similar changes in subjective pain rating, a finding also shown in previous reports.30,31 This finding confirms the strict relation of N2/P2 amplitude with pain perception, also suggesting that pain rating by VAS can be sufficiently reliable, although the poor correlation between N2/P2 amplitude and VAS score suggests that the neural mechanisms underlying these two parameters are, at least in part, different.
Abnormal cortical excitability and pathophysiology of SX
Two major pathophysiological components have been suggested to significantly contribute to cardiac SX, i.e. coronary microvascular dysfunction1–5 and an abnormal pain perception of cardiac stimuli.10–13 The relationship between these two pathophysiological components has not been clarified yet, with hypotheses including independent occurrence in a same patient, common neuro-mediated pathogenetic mechanisms, or even afferent cardiac nerve fibre abnormalities secondary to microvascular dysfunction and scattered ischaemia.32
In the present study, we aimed at obtaining further insights on pain function and processing in SX patients in whom the relevance of microvascular dysfunction was suggested by both exercise-induced ST-segment depression on the ECG and reversible perfusion defects on 201Tl myocardial scintigraphy. Our results tend to deny that this abnormal finding can be related to a reduced central pain threshold, which is in agreement with our demonstration of preferential ventricular issues in abnormal cardiac pain perception in these patients.13 Furthermore, in cardiac SX, the detection of pronounced functional abnormalities of efferent cardiac adrenergic nerve fibres33 suggests the possibility of an impairment of afferent cardiac nerve fibre function,32 as also suggested by the beneficial effects on angina symptoms of spinal cord stimulation, which is believed to mainly act through an enhancement of pain gate control in dorsal horns.34,35
On the other hand, the lack of habituation to pain seems typical of SX patients and might contribute to define some peculiar characteristics of angina, such as prolonged duration, frequent persistence after effort interruption, and recurrence, although a relationship with these features needs to be established.
Therefore, it is conceivable that in SX patients, an abnormal cardiac nociception, possibly due to local vascular or nervous autonomic factors, interacts with an abnormal excitability of the cortical areas devoted to pain processing, to result in the appearance and clinical feature of the disease.
It is worth noting, indeed, that the phenomenon of reduced habituation of vertex LEPs found in cardiac SX is similar to that shown in patients with migraine,36 another chronic painful condition also involving the peripheral vascular system.
Clinical implications
Although clinical prognosis quoad vitam is usually excellent in SX patients,37,38 angina attacks in some cases become frequent, disabling and refractory to maximal standard drug therapy, and this may be more frequent among patients showing impaired coronary endothelial function.38,39 The demonstration of abnormal brain excitability in SX patients may suggest that the favourable effects of β-blockers40 in SX patients might be, at least in part, mediated by the effect on cortical excitability, as also suggested for their benefits in migraine.
Figure 1 Amplitude of N2/P2 complex of LEPs (average, SEM) during three sequences of stimuli applied to the chest skin (left) and to the right hand skin (right) in the three groups of subjects. P-values are for group–sequence interaction.
Figure 2 Examples of the change in N2/P2 amplitude during the three sequences of laser stimuli applied to the chest skin in a healthy control subject and in a SX patient. The N2/P2 potential amplitude decreases progressively from the first (I) to the third (III) sequence of stimuli in the control subject, but remains unchanged in the SX patient.
Figure 3 N2/P2 amplitude of LEPs during three sequences of stimuli applied to the chest skin (left) and to the right hand skin (right) in the three groups of subjects. Values of the variables during the second and the third sequences of stimuli are expressed as per cent of those obtained in the first sequence, considered as 100%. Bars express SEM.
Main clinical characteristics of subjects studied
| . | SX (n=16) . | CAD (n=10) . | Controls (n=13) . |
|---|---|---|---|
| Age (years) | 59.9±8 | 65.8±10 | 54.6±10 |
| Sex (M : F) | 8 : 8 | 6 : 4 | 7 : 6 |
| Previous myocardial infarction, n (%) | — | 5 (50) | 0 ( |
| Coronary angiography, n (%) | |||
| 0-vessel | 16 (100) | — | — |
| 1-vessel | — | 2 (20) | — |
| 2-vessel | — | 4 (40) | — |
| 3-vessel | — | 4 (40) | — |
| Risk factors, n (%) | |||
| Family history | 2 (12) | 3 (30) | 2 (15) |
| Smoking history | 4 (25) | 5 (50) | 3 (23) |
| Hypertension | 6 (38) | 8 (80) | — |
| Dyslipidemia | 9 (56) | 7 (70) | 3 (23) |
| Diabetes mellitus | — | 2 (20) | — |
| Frequency of angina episodes*, n (%) | |||
| 1–3 per week | 5 (31) | 1 (10) | — |
| 4–7 per week | 8 (50) | 7 (70) | — |
| >7 per week | 3 (19) | 2 (20) | — |
| Therapy, n (%) | |||
| ACE-inhibitors | 6 (38) | 8 (80) | — |
| Beta-blockers | 10 (62) | 8 (80) | — |
| Ca(2+)-channel blockers | 12 (75) | 5 (50) | — |
| Nitrates | 9 (56) | 6 (60) | — |
| Antiplatelet agents | 5 (31) | 10 (100) | — |
| Statins | 7 (44) | 7 (70) | — |
| . | SX (n=16) . | CAD (n=10) . | Controls (n=13) . |
|---|---|---|---|
| Age (years) | 59.9±8 | 65.8±10 | 54.6±10 |
| Sex (M : F) | 8 : 8 | 6 : 4 | 7 : 6 |
| Previous myocardial infarction, n (%) | — | 5 (50) | 0 ( |
| Coronary angiography, n (%) | |||
| 0-vessel | 16 (100) | — | — |
| 1-vessel | — | 2 (20) | — |
| 2-vessel | — | 4 (40) | — |
| 3-vessel | — | 4 (40) | — |
| Risk factors, n (%) | |||
| Family history | 2 (12) | 3 (30) | 2 (15) |
| Smoking history | 4 (25) | 5 (50) | 3 (23) |
| Hypertension | 6 (38) | 8 (80) | — |
| Dyslipidemia | 9 (56) | 7 (70) | 3 (23) |
| Diabetes mellitus | — | 2 (20) | — |
| Frequency of angina episodes*, n (%) | |||
| 1–3 per week | 5 (31) | 1 (10) | — |
| 4–7 per week | 8 (50) | 7 (70) | — |
| >7 per week | 3 (19) | 2 (20) | — |
| Therapy, n (%) | |||
| ACE-inhibitors | 6 (38) | 8 (80) | — |
| Beta-blockers | 10 (62) | 8 (80) | — |
| Ca(2+)-channel blockers | 12 (75) | 5 (50) | — |
| Nitrates | 9 (56) | 6 (60) | — |
| Antiplatelet agents | 5 (31) | 10 (100) | — |
| Statins | 7 (44) | 7 (70) | — |
*P=0.44 between SX and CAD groups.
Main clinical characteristics of subjects studied
| . | SX (n=16) . | CAD (n=10) . | Controls (n=13) . |
|---|---|---|---|
| Age (years) | 59.9±8 | 65.8±10 | 54.6±10 |
| Sex (M : F) | 8 : 8 | 6 : 4 | 7 : 6 |
| Previous myocardial infarction, n (%) | — | 5 (50) | 0 ( |
| Coronary angiography, n (%) | |||
| 0-vessel | 16 (100) | — | — |
| 1-vessel | — | 2 (20) | — |
| 2-vessel | — | 4 (40) | — |
| 3-vessel | — | 4 (40) | — |
| Risk factors, n (%) | |||
| Family history | 2 (12) | 3 (30) | 2 (15) |
| Smoking history | 4 (25) | 5 (50) | 3 (23) |
| Hypertension | 6 (38) | 8 (80) | — |
| Dyslipidemia | 9 (56) | 7 (70) | 3 (23) |
| Diabetes mellitus | — | 2 (20) | — |
| Frequency of angina episodes*, n (%) | |||
| 1–3 per week | 5 (31) | 1 (10) | — |
| 4–7 per week | 8 (50) | 7 (70) | — |
| >7 per week | 3 (19) | 2 (20) | — |
| Therapy, n (%) | |||
| ACE-inhibitors | 6 (38) | 8 (80) | — |
| Beta-blockers | 10 (62) | 8 (80) | — |
| Ca(2+)-channel blockers | 12 (75) | 5 (50) | — |
| Nitrates | 9 (56) | 6 (60) | — |
| Antiplatelet agents | 5 (31) | 10 (100) | — |
| Statins | 7 (44) | 7 (70) | — |
| . | SX (n=16) . | CAD (n=10) . | Controls (n=13) . |
|---|---|---|---|
| Age (years) | 59.9±8 | 65.8±10 | 54.6±10 |
| Sex (M : F) | 8 : 8 | 6 : 4 | 7 : 6 |
| Previous myocardial infarction, n (%) | — | 5 (50) | 0 ( |
| Coronary angiography, n (%) | |||
| 0-vessel | 16 (100) | — | — |
| 1-vessel | — | 2 (20) | — |
| 2-vessel | — | 4 (40) | — |
| 3-vessel | — | 4 (40) | — |
| Risk factors, n (%) | |||
| Family history | 2 (12) | 3 (30) | 2 (15) |
| Smoking history | 4 (25) | 5 (50) | 3 (23) |
| Hypertension | 6 (38) | 8 (80) | — |
| Dyslipidemia | 9 (56) | 7 (70) | 3 (23) |
| Diabetes mellitus | — | 2 (20) | — |
| Frequency of angina episodes*, n (%) | |||
| 1–3 per week | 5 (31) | 1 (10) | — |
| 4–7 per week | 8 (50) | 7 (70) | — |
| >7 per week | 3 (19) | 2 (20) | — |
| Therapy, n (%) | |||
| ACE-inhibitors | 6 (38) | 8 (80) | — |
| Beta-blockers | 10 (62) | 8 (80) | — |
| Ca(2+)-channel blockers | 12 (75) | 5 (50) | — |
| Nitrates | 9 (56) | 6 (60) | — |
| Antiplatelet agents | 5 (31) | 10 (100) | — |
| Statins | 7 (44) | 7 (70) | — |
*P=0.44 between SX and CAD groups.
LEPs and pain rating by VAS during repetitive chest skin stimulation
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 4.0±2.8 | 3.9±2.4 | 6.2±3.4 | |
| 2nd sequence | 4.6±1.3 | 4.2±2.6 | 4.3±1.7 | 0.27 |
| 3rd sequence | 4.4±2.3 | 4.5±2.9 | 4.6±2.6 | |
| N2/P2 | ||||
| 1st sequence | 13.4±4.9 | 13.0±7.3 | 15.7±6.6 | |
| 2nd sequence | 13.5±5.7 | 8.7±5.3(†) | 12.0±6.0(‡) | 0.004 |
| 3rd sequence | 13.3±6.4 | 7.0±4.1(‡) | 12.4±7.0(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 134.5±21.9 | 144.0±20.7 | 133.4±12.4 | |
| 2nd sequence | 130.7±15.7 | 165.2±32.1 | 130.9±16.1 | 0.28 |
| 3rd sequence | 136.2±26.9 | 157.3±26.7 | 145.4±23.1 | |
| N2 | ||||
| 1st sequence | 203.2±38.9 | 221.8±38.3 | 182.7±36.4 | |
| 2nd sequence | 202.3±42.5 | 227.9±35.9 | 189.2±43.5 | 0.31 |
| 3rd sequence | 202.3±54.4 | 209.5±47.5 | 190.4±41.3 | |
| P2 | ||||
| 1st sequence | 340.2±46.5 | 338.8±38.1 | 302.0±53.8 | |
| 2nd sequence | 323.9±42.9 | 353.0±60.6 | 304.9±52.2 | 0.34 |
| 3rd sequence | 331.5±49.0 | 365.8±68.7 | 326.8±52.6 | |
| VAS score | ||||
| 1st sequence | 40±23 | 27±15 | 22±18 | |
| 2nd sequence | 43±26 | 28±18 | 24±20 | 0.014 |
| 3rd sequence | 46±29 | 20±17(†) | 17±17(‡) |
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 4.0±2.8 | 3.9±2.4 | 6.2±3.4 | |
| 2nd sequence | 4.6±1.3 | 4.2±2.6 | 4.3±1.7 | 0.27 |
| 3rd sequence | 4.4±2.3 | 4.5±2.9 | 4.6±2.6 | |
| N2/P2 | ||||
| 1st sequence | 13.4±4.9 | 13.0±7.3 | 15.7±6.6 | |
| 2nd sequence | 13.5±5.7 | 8.7±5.3(†) | 12.0±6.0(‡) | 0.004 |
| 3rd sequence | 13.3±6.4 | 7.0±4.1(‡) | 12.4±7.0(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 134.5±21.9 | 144.0±20.7 | 133.4±12.4 | |
| 2nd sequence | 130.7±15.7 | 165.2±32.1 | 130.9±16.1 | 0.28 |
| 3rd sequence | 136.2±26.9 | 157.3±26.7 | 145.4±23.1 | |
| N2 | ||||
| 1st sequence | 203.2±38.9 | 221.8±38.3 | 182.7±36.4 | |
| 2nd sequence | 202.3±42.5 | 227.9±35.9 | 189.2±43.5 | 0.31 |
| 3rd sequence | 202.3±54.4 | 209.5±47.5 | 190.4±41.3 | |
| P2 | ||||
| 1st sequence | 340.2±46.5 | 338.8±38.1 | 302.0±53.8 | |
| 2nd sequence | 323.9±42.9 | 353.0±60.6 | 304.9±52.2 | 0.34 |
| 3rd sequence | 331.5±49.0 | 365.8±68.7 | 326.8±52.6 | |
| VAS score | ||||
| 1st sequence | 40±23 | 27±15 | 22±18 | |
| 2nd sequence | 43±26 | 28±18 | 24±20 | 0.014 |
| 3rd sequence | 46±29 | 20±17(†) | 17±17(‡) |
*P-value for group–sequence interaction.
(†)P<0.05 vs. first sequence.
(‡)P<0.01 vs. first sequence.
LEPs and pain rating by VAS during repetitive chest skin stimulation
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 4.0±2.8 | 3.9±2.4 | 6.2±3.4 | |
| 2nd sequence | 4.6±1.3 | 4.2±2.6 | 4.3±1.7 | 0.27 |
| 3rd sequence | 4.4±2.3 | 4.5±2.9 | 4.6±2.6 | |
| N2/P2 | ||||
| 1st sequence | 13.4±4.9 | 13.0±7.3 | 15.7±6.6 | |
| 2nd sequence | 13.5±5.7 | 8.7±5.3(†) | 12.0±6.0(‡) | 0.004 |
| 3rd sequence | 13.3±6.4 | 7.0±4.1(‡) | 12.4±7.0(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 134.5±21.9 | 144.0±20.7 | 133.4±12.4 | |
| 2nd sequence | 130.7±15.7 | 165.2±32.1 | 130.9±16.1 | 0.28 |
| 3rd sequence | 136.2±26.9 | 157.3±26.7 | 145.4±23.1 | |
| N2 | ||||
| 1st sequence | 203.2±38.9 | 221.8±38.3 | 182.7±36.4 | |
| 2nd sequence | 202.3±42.5 | 227.9±35.9 | 189.2±43.5 | 0.31 |
| 3rd sequence | 202.3±54.4 | 209.5±47.5 | 190.4±41.3 | |
| P2 | ||||
| 1st sequence | 340.2±46.5 | 338.8±38.1 | 302.0±53.8 | |
| 2nd sequence | 323.9±42.9 | 353.0±60.6 | 304.9±52.2 | 0.34 |
| 3rd sequence | 331.5±49.0 | 365.8±68.7 | 326.8±52.6 | |
| VAS score | ||||
| 1st sequence | 40±23 | 27±15 | 22±18 | |
| 2nd sequence | 43±26 | 28±18 | 24±20 | 0.014 |
| 3rd sequence | 46±29 | 20±17(†) | 17±17(‡) |
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 4.0±2.8 | 3.9±2.4 | 6.2±3.4 | |
| 2nd sequence | 4.6±1.3 | 4.2±2.6 | 4.3±1.7 | 0.27 |
| 3rd sequence | 4.4±2.3 | 4.5±2.9 | 4.6±2.6 | |
| N2/P2 | ||||
| 1st sequence | 13.4±4.9 | 13.0±7.3 | 15.7±6.6 | |
| 2nd sequence | 13.5±5.7 | 8.7±5.3(†) | 12.0±6.0(‡) | 0.004 |
| 3rd sequence | 13.3±6.4 | 7.0±4.1(‡) | 12.4±7.0(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 134.5±21.9 | 144.0±20.7 | 133.4±12.4 | |
| 2nd sequence | 130.7±15.7 | 165.2±32.1 | 130.9±16.1 | 0.28 |
| 3rd sequence | 136.2±26.9 | 157.3±26.7 | 145.4±23.1 | |
| N2 | ||||
| 1st sequence | 203.2±38.9 | 221.8±38.3 | 182.7±36.4 | |
| 2nd sequence | 202.3±42.5 | 227.9±35.9 | 189.2±43.5 | 0.31 |
| 3rd sequence | 202.3±54.4 | 209.5±47.5 | 190.4±41.3 | |
| P2 | ||||
| 1st sequence | 340.2±46.5 | 338.8±38.1 | 302.0±53.8 | |
| 2nd sequence | 323.9±42.9 | 353.0±60.6 | 304.9±52.2 | 0.34 |
| 3rd sequence | 331.5±49.0 | 365.8±68.7 | 326.8±52.6 | |
| VAS score | ||||
| 1st sequence | 40±23 | 27±15 | 22±18 | |
| 2nd sequence | 43±26 | 28±18 | 24±20 | 0.014 |
| 3rd sequence | 46±29 | 20±17(†) | 17±17(‡) |
*P-value for group–sequence interaction.
(†)P<0.05 vs. first sequence.
(‡)P<0.01 vs. first sequence.
LEPs and pain rating by VAS during repetitive right hand skin stimulation
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 5.9±2.3 | 4.4±2.0 | 5.5±2.1 | |
| 2nd sequence | 5.4±3.1 | 3.4±1.2 | 4.3±1.4 | 0.48 |
| 3rd sequence | 4.9±2.2 | 4.4±2.1 | 3.8±1.8 | |
| N2/P2 | ||||
| 1st sequence | 13.6±6.9 | 14.8±5.5 | 14.0±6.8 | |
| 2nd sequence | 13.9±6.1 | 11.8±4.9 | 11.7±6.5 | 0.27 |
| 3rd sequence | 11.8±4.7 | 10.0±2.3(†) | 8.4±4.7(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 157.8±21.9 | 160.5±28.9 | 149.7±19.1 | |
| 2nd sequence | 158.5±27.1 | 161.8±34.5 | 160.7±26.8 | 0.97 |
| 3rd sequence | 156.4±22.4 | 161.5±30.4 | 162.7±20.9 | |
| N2 | ||||
| 1st sequence | 234.7±40.1 | 215.2±26.5 | 206.7±38.3 | |
| 2nd sequence | 235.3±41.3 | 244.2±53.6 | 211.2±36.8 | 0.14 |
| 3rd sequence | 229.1±34.6 | 246.7±32.9 | 225.8±46.2 | |
| P2 | ||||
| 1st sequence | 369.4±46.9 | 366.8±40.5 | 341.6±47.2 | |
| 2nd sequence | 376.4±48.8 | 360.3±34.1 | 337.8±47.2 | 0.17 |
| 3rd sequence | 358.3±61.9 | 386.9±28.7 | 349.4±72.5 | |
| VAS score | ||||
| 1st sequence | 27±14 | 34±13 | 22±6 | |
| 2nd sequence | 31±22 | 31±11 | 23±11 | 0.0007 |
| 3rd sequence | 34±25 | 30±14(†) | 17±7(‡) |
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 5.9±2.3 | 4.4±2.0 | 5.5±2.1 | |
| 2nd sequence | 5.4±3.1 | 3.4±1.2 | 4.3±1.4 | 0.48 |
| 3rd sequence | 4.9±2.2 | 4.4±2.1 | 3.8±1.8 | |
| N2/P2 | ||||
| 1st sequence | 13.6±6.9 | 14.8±5.5 | 14.0±6.8 | |
| 2nd sequence | 13.9±6.1 | 11.8±4.9 | 11.7±6.5 | 0.27 |
| 3rd sequence | 11.8±4.7 | 10.0±2.3(†) | 8.4±4.7(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 157.8±21.9 | 160.5±28.9 | 149.7±19.1 | |
| 2nd sequence | 158.5±27.1 | 161.8±34.5 | 160.7±26.8 | 0.97 |
| 3rd sequence | 156.4±22.4 | 161.5±30.4 | 162.7±20.9 | |
| N2 | ||||
| 1st sequence | 234.7±40.1 | 215.2±26.5 | 206.7±38.3 | |
| 2nd sequence | 235.3±41.3 | 244.2±53.6 | 211.2±36.8 | 0.14 |
| 3rd sequence | 229.1±34.6 | 246.7±32.9 | 225.8±46.2 | |
| P2 | ||||
| 1st sequence | 369.4±46.9 | 366.8±40.5 | 341.6±47.2 | |
| 2nd sequence | 376.4±48.8 | 360.3±34.1 | 337.8±47.2 | 0.17 |
| 3rd sequence | 358.3±61.9 | 386.9±28.7 | 349.4±72.5 | |
| VAS score | ||||
| 1st sequence | 27±14 | 34±13 | 22±6 | |
| 2nd sequence | 31±22 | 31±11 | 23±11 | 0.0007 |
| 3rd sequence | 34±25 | 30±14(†) | 17±7(‡) |
*P-value for group–sequence interaction.
(†)P<0.01 vs. first sequence.
(‡)P<0.001 vs. first sequence.
LEPs and pain rating by VAS during repetitive right hand skin stimulation
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 5.9±2.3 | 4.4±2.0 | 5.5±2.1 | |
| 2nd sequence | 5.4±3.1 | 3.4±1.2 | 4.3±1.4 | 0.48 |
| 3rd sequence | 4.9±2.2 | 4.4±2.1 | 3.8±1.8 | |
| N2/P2 | ||||
| 1st sequence | 13.6±6.9 | 14.8±5.5 | 14.0±6.8 | |
| 2nd sequence | 13.9±6.1 | 11.8±4.9 | 11.7±6.5 | 0.27 |
| 3rd sequence | 11.8±4.7 | 10.0±2.3(†) | 8.4±4.7(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 157.8±21.9 | 160.5±28.9 | 149.7±19.1 | |
| 2nd sequence | 158.5±27.1 | 161.8±34.5 | 160.7±26.8 | 0.97 |
| 3rd sequence | 156.4±22.4 | 161.5±30.4 | 162.7±20.9 | |
| N2 | ||||
| 1st sequence | 234.7±40.1 | 215.2±26.5 | 206.7±38.3 | |
| 2nd sequence | 235.3±41.3 | 244.2±53.6 | 211.2±36.8 | 0.14 |
| 3rd sequence | 229.1±34.6 | 246.7±32.9 | 225.8±46.2 | |
| P2 | ||||
| 1st sequence | 369.4±46.9 | 366.8±40.5 | 341.6±47.2 | |
| 2nd sequence | 376.4±48.8 | 360.3±34.1 | 337.8±47.2 | 0.17 |
| 3rd sequence | 358.3±61.9 | 386.9±28.7 | 349.4±72.5 | |
| VAS score | ||||
| 1st sequence | 27±14 | 34±13 | 22±6 | |
| 2nd sequence | 31±22 | 31±11 | 23±11 | 0.0007 |
| 3rd sequence | 34±25 | 30±14(†) | 17±7(‡) |
| . | SX . | CAD . | Controls . | P-value* . |
|---|---|---|---|---|
| LEP amplitude | ||||
| N1–P1 | ||||
| 1st sequence | 5.9±2.3 | 4.4±2.0 | 5.5±2.1 | |
| 2nd sequence | 5.4±3.1 | 3.4±1.2 | 4.3±1.4 | 0.48 |
| 3rd sequence | 4.9±2.2 | 4.4±2.1 | 3.8±1.8 | |
| N2/P2 | ||||
| 1st sequence | 13.6±6.9 | 14.8±5.5 | 14.0±6.8 | |
| 2nd sequence | 13.9±6.1 | 11.8±4.9 | 11.7±6.5 | 0.27 |
| 3rd sequence | 11.8±4.7 | 10.0±2.3(†) | 8.4±4.7(‡) | |
| LEP latency | ||||
| N1–P1 | ||||
| 1st sequence | 157.8±21.9 | 160.5±28.9 | 149.7±19.1 | |
| 2nd sequence | 158.5±27.1 | 161.8±34.5 | 160.7±26.8 | 0.97 |
| 3rd sequence | 156.4±22.4 | 161.5±30.4 | 162.7±20.9 | |
| N2 | ||||
| 1st sequence | 234.7±40.1 | 215.2±26.5 | 206.7±38.3 | |
| 2nd sequence | 235.3±41.3 | 244.2±53.6 | 211.2±36.8 | 0.14 |
| 3rd sequence | 229.1±34.6 | 246.7±32.9 | 225.8±46.2 | |
| P2 | ||||
| 1st sequence | 369.4±46.9 | 366.8±40.5 | 341.6±47.2 | |
| 2nd sequence | 376.4±48.8 | 360.3±34.1 | 337.8±47.2 | 0.17 |
| 3rd sequence | 358.3±61.9 | 386.9±28.7 | 349.4±72.5 | |
| VAS score | ||||
| 1st sequence | 27±14 | 34±13 | 22±6 | |
| 2nd sequence | 31±22 | 31±11 | 23±11 | 0.0007 |
| 3rd sequence | 34±25 | 30±14(†) | 17±7(‡) |
*P-value for group–sequence interaction.
(†)P<0.01 vs. first sequence.
(‡)P<0.001 vs. first sequence.
References
Opherk D, Zebe H, Weihe E et al. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms.
Cannon RO III, Watson RM, Rosing DR et al. Angina caused by reduced vasodilator reserve of the small coronary arteries.
Egashira K, Inou T, Hirooka Y et al. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms.
Chauhan A, Mullins PA, Taylor G et al. Both endothelium-dependent and endothelium-independent function is impaired in patients with angina pectoris and normal coronary angiograms.
Buffon A, Rigattieri S, Santini SA et al. Myocardial ischaemia-reperfusion damage after pacing-induced tachycardia in patients with cardiac syndrome X.
Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. Comparison with patients having significant coronary artery disease.
Nihoyannopoulos P, Kaski JC, Crake T et al. Absence of myocardial dysfunction during stress in patients with syndrome X.
Camici PG, Marraccini P, Lorenzoni R et al. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: response to pacing stress.
Panza JA, Laurienzo JM, Curiel RV et al. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography.
Shapiro LM, Crake T, Poole-Wilson PA. Is altered cardiac sensation responsible for chest pain in patients with normal coronary arteries? Clinical observations during cardiac catheterization.
Cannon RO III, Quyyumi AA, Schenke WH et al. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries.
Chauhan A, Mullins PA, Thuraisingham SI et al. Abnormal cardiac pain perception in syndrome X.
Pasceri V, Lanza GA, Buffon A et al. Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X.
Rosen SD, Paulesu E, Wise RJ et al. Central neural contribution to the perception of chest pain in cardiac syndrome X.
Bromm B, Lorenz J. Neurophysiological evaluation of pain.
Arendt-Nielsen L, Laursen RJ, Drewes AM. Referred pain as an indicator for neural plasticity.
Klem GH, Luders HO, Jasper HH et al. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology.
Valeriani M, Rambaud L, Mauguière F. Scalp topography and dipolar source modelling of potentials evoked by CO2 laser stimulation of the hand.
Valeriani M, Restuccia D, Barba C et al. Sources of cortical responses to painful CO(2) laser skin stimulation of the hand and foot in the human brain.
Kunde V, Treede RD. Topography of middle-latency somatosensory evoked potentials following painful laser stimuli and non-painful electrical stimuli.
Kakigi R, Watanabe S, Yamasaki H. Pain-Related somatosensory evoked potentials.
Turiel M, Galassi AR, Glazier JJ et al. Pain threshold and tolerance in women with syndrome X and women with stable angina pectoris.
Lanza GA, Pasceri V, Colonna G et al. Cardiac autonomic function and sensitivity to pain in postmenopausal women with angina and normal coronary arteries.
Frot M, Rambaud L, Guenot M et al. Related articles, links intracortical recordings of early pain-related CO2-laser evoked potentials in the human second somatosensory (SII) area.
Tarkka IM, Treede RD. Equivalent electrical source analysis of pain-related somatosensory evoked potentials elicited by a CO2 laser.
Bromm B, Chen AC. Brain electrical source analysis of laser evoked potentials in response to painful trigeminal nerve stimulation.
Lenz FA, Rios M, Zirh A et al. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus.
Derbyshire SW, Jones AK, Gyulai F et al. Pain processing during three levels of noxious stimulation produces differential patterns of central activity.
Buchel C, Bornhovd K, Quante M et al. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study.
Arendt-Nielsen L, Bjerring P. Sensory and pain threshold characteristics to laser stimuli.
Weiss T, Kumpf K, Ehrhardt J et al. A bioadaptive approach for experimental pain research in humans using laser-evoked brain potentials.
Lanza GA, Crea F. The complex link between brain and heart in cardiac syndrome X.
Lanza GA, Giordano A, Pristipino C et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy.
Lanza GA, Sestito A, Sandric S et al. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries.
Lanza GA, Sestito A, Sgueglia GA et al. Effect of spinal cord stimulation on spontaneous and stress-induced angina episodes and ‘ischemia-like’ ST-segment depression in patients with cardiac syndrome X.
Valeriani M, de Tommaso M, Restuccia D et al. Reduced habituation to experimental pain in migraine patients: a CO(2) laser evoked potential study.
Kaski JC, Rosano GMC, Collins P et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study.
Bugiardini R, Manfrini O, Pizzi C et al. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms.
Johnson BD, Shaw LJ, Buchthal SD et al. Prognosis in women with myocardial ischaemia in the absence of obstructive coronary disease: Results from the National Institutes of Health–National Heart, Lung, and Blood Institute–Sponsored Women's Ischaemia Syndrome Evaluation (WISE).