-
PDF
- Split View
-
Views
-
Cite
Cite
Yasuhide Asaumi, Satoshi Yasuda, Isao Morii, Hiroyuki Kakuchi, Yoritaka Otsuka, Atsushi Kawamura, Yoshikado Sasako, Takeshi Nakatani, Hiroshi Nonogi, Shunichi Miyazaki, Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation, European Heart Journal, Volume 26, Issue 20, October 2005, Pages 2185–2192, https://doi.org/10.1093/eurheartj/ehi411
Close - Share Icon Share
Abstract
Aims The clinical outcome of severe acute myocarditis patients with cardiogenic shock who require circulatory support devices is not well known. We studied the survival and clinical courses of patients with fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation (ECMO) and compared them with those of patients with acute non-fulminant myocarditis.
Methods and results Patients with acute myocarditis were divided into the following two groups. Fourteen patients who required ECMO for cardiogenic shock were defined as having fulminant myocarditis (F group), whereas 13 patients who had an acute onset of symptoms, but did not have compromised, were defined as having acute non-fulminant myocarditis (NF group). In the F group, 10 patients were weaned successfully from percutaneous ECMO. Therefore, the overall acute survival rate was 71%. Patients who were not weaned from ECMO showed smaller left ventricular end-diastolic and end-systolic dimensions, thicker left ventricular wall, and higher creatine phosphokinase MB isoform levels than those who were weaned from ECMO. When compared with patients in the NF group, the fractional shortening in the F group was more severely decreased in the acute phase [F: 10±4 vs. NF: 23±8% (mean±SD), P<0.001], but recovered in the chronic phase (F: 33±7 vs. NF: 34±6%). The prevalence of adverse clinical events in both groups was similar during the follow-up period of 50 months.
Conclusion In patients with fulminant myocarditis, percutaneous ECMO is a highly effective form of a haemodynamic support. Once a patient recovers from inflammatory myocardial damage, the subsequent clinical outcome is favourable, similar to that observed in patients with acute non-fulminant myocarditis.
Introduction
Myocarditis is defined as an inflammation of the myocardium caused by viral, rickettsial, bacterial or protozoal infections, or drug toxicity.1–3 Its clinical features vary, ranging from asymptomatic secondary to focal inflammation to fulminant fatal congestive heart failure. Moreover, there is a possibility that viral myocarditis may lead to dilated cardiomyopathy, presumably as a consequence of a late immunological response.2 Patients with fulminant myocarditis often present with cardiogenic shock due to a severe left ventricular dysfunction.
Critically ill patients often require mechanical circulatory support such as a percutaneous extracorporeal membrane oxygenation (ECMO) with a cardiopulmonary bypass. Some studies showed that mechanical circulatory support is effective and can eliminate the need for cardiac transplantation in patients with cardiogenic shock secondary to fulminant myocarditis. These studies further showed an overall survival rate range of 50–70%, in the case of using mechanical circulatory support, is possible either by cardiac recovery or by transplantation.4–7 These studies showed that the survival rate in the case of using percutaneous ECMO is higher than that in using a ventricular assist device.6 This result may be due to the quick and easy application of percutaneous ECMO preventing multiple organ failure secondary to haemodynamic deterioration, when compared with a ventricular assist device. McCarthy et al.8 demonstrated that patients with lymphocytic fulminant myocarditis have a better prognosis than those with acute non-fulminant myocarditis, providing important information for a better understanding of the pathophysiology of myocarditis. However, in their study, only two of 15 patients with fulminant myocarditis were treated with mechanical circulatory support. The clinical outcome of critical myocarditis patients with cardiogenic shock who require circulatory support devices is not well known. Thus, in the present study, we have focused on the survival and clinical courses of severely ill patients who are under mechanical circulatory support with percutaneous ECMO and compared them with those of patients with acute non-fulminant myocarditis.
Methods
Clinical classification
The diagnosis of myocarditis was made on the basis of the following findings: (i) a recent medical history consistent with the occurrence of a viral infection, (ii) positive findings of inflammation (high fever and increased white blood cell count and C-reactive protein level), (iii) evidence of myocardial damage [significant changes in electrocardiographic and echocardiographic features and elevations of serum creatine phosphokinase (CPK) and its MB isoform (CK-MB) level], and (iv) signs of a recent onset of cardiac dysfunction that were not due to myocardial ischaemia (determined by coronary angiography). Patients who had signs of myocarditis associated with other systemic diseases, such as immunodeficiency, sarcoidosis, collagen diseases, endocrine diseases, drug-induced toxicity, or alcoholism, were excluded. Cardiogenic shock was defined on the basis of the criteria set by the Myocardial Infarction Research Units of the National Heart and Lung Institute.9
In the present study, patients with fulminant myocarditis were defined as those who require percutaneous ECMO or a ventricular assist device for cardiogenic shock and do not respond to intensive medical treatments like high doses of intravenous catecholamines or for refractory ventricular tachyarrhythmia. Patients with acute non-fulminant myocarditis were defined as those who had an acute onset of symptoms but did not have compromised haemodynamics following conventional medical treatment.
Details of percutaneous ECMO system
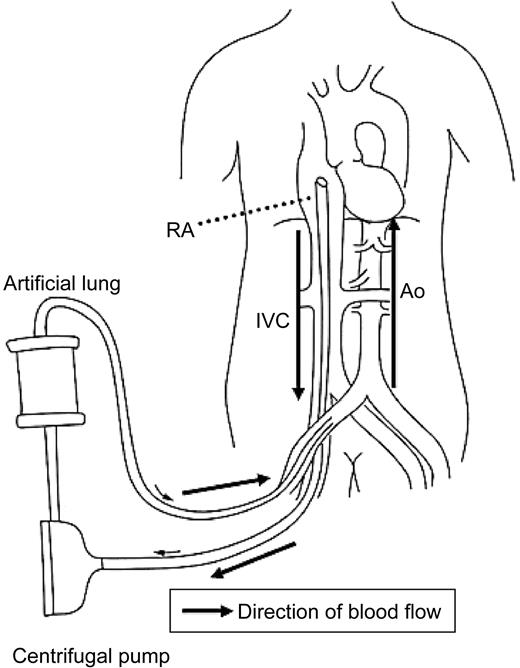
A percutaneous ECMO system is basically a femoro-femora bypass without a reservoir (Figure 1). This system is completely pre-connected to a compact integrated cardiopulmonary bypass unit consisting of an artificial lung (Kurare Menox EL-4000) and a Sarns Delphin pump (Sarns 3M Healthcare, Ann Arbor, MI, USA). An oxygenator and a centrifugal pump are placed in the body of the compact integrated cardiopulmonary bypass unit as reported previously.10 Heparin was used for anticoagulation and activated clotting time was maintained between 200 and 300 s.
Study patients
Between January 1993 and December 2001, 27 patients were diagnosed as having acute myocarditis at the National Cardiovascular Centre (Japan). All patients except one had clinical symptoms and signs of acute myocarditis with a distinct onset (from days 2 to 28). The first application of percutaneous ECMO for patients with fulminant myocarditis was in June 1996. The distribution of year when the enrolled patients were admitted was as follows: F group: before 1995, n=0; 1996–98, n=5; 1999–2001, n=9; NF group: before 1995, n=2; 1996–98, n=5; 1999–2001, n=6.
Fourteen patients, whose systemic blood pressure was low [74±15 (mean±SD) mmHg] and heart rate was high (134±21 b.p.m.; excluding two patients with cardiac arrest and using temporary right ventricular pacemaker) even after an intensive treatment with inotropic or vasopressor drugs, required percutaneous ECMO (F group) (male, seven; female, seven; mean age, 38±15). The remaining 13 patients whose blood pressure and heart rate were maintained at 118±17 mmHg and 86±21 b.p.m., respectively, were not treated with percutaneous ECMO (NF group) (male, 12; female, one; mean age, 33±18).
Laboratory examination
On admission, blood samples were obtained every 3 h until the peak CPK and CK-MB levels were determined; thereafter, at least every 24 h until the patients recovered. Inflammation indexes (white blood cell count and C-reactive protein level), liver function (total bilirubin, aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels), and renal function [blood urea nitrogen (BUN) and serum creatinine levels] were also analysed.
Echocardiographic and haemodynamic measurements
Standard two-dimensional echocardiography (SONOS 5500, Phillips) was performed to assess the existence of pericardial effusion and to determine left ventricular end-diastolic dimension (LVDd), end-systolic dimension (LVDs), and wall thickness. These parameters of LV function were measured in the M-mode from the parasternal short-axis view using the leading-edge-to-leading-edge method. Fractional shortening (FS) was also calculated by a standard method.11 Inferior venacava (IVC) diameters were measured from the long-axis two-dimensional subxiphoid views with the patients in a supine position to 30° upright position.12 Flow across the valves was assessed by colour Doppler to grade the degree of mitral and tricuspid regurgitation. A 7.5 F Swan–Ganz thermodilution catheter (model: T-157A; Goodtech Inc.) was inserted through the internal jugular vein or the femoral vein to measure cardiac index, pulmonary capillary wedge, and right atrial pressures.
On admission, the data were obtained every 24 h until the patients were weaned successfully from the percutaneous ECMO system. During the period of using ECMO, we also measured LV ejection time corrected for √RR (LVETc). When LVETc improved to >200 m s, ECMO flow rate was gradually decreased to 1.5 L/min, and ECMO was then discontinued if haemodynamics did not deteriorate.13
Endomyocardial biopsy and postmortem autopsy
Endomyocardial biopsies were performed via the right internal jugular or femoral veins using disposable bioptomes in surviving patients in stable conditions. Postmortem examination was also performed. At least four specimens were obtained from the right ventricular septum and immediately immersed in 10% formalin, embedded in paraffin, sectioned, stained with haematoxylin and eosin, and examined by a pathologist to determine whether myocarditis was present on the basis of the Dallas criteria.14
Follow-up
After discharge, patients visited the hospital every 3–6 months. In the chronic phase (∼6–12 months), echocardiography was performed to reassess LV function following myocarditis. The median period of chronic echocardiography was 12 months. Thereafter, follow-up data regarding death and cardiovascular events (e.g. rehospitalization due to congestive heart failure) were obtained from the medical records or telephone interviews of all patients.
Statistical analyses
The values are presented as the mean±standard deviation (SD) or median (25–75%). The normality of distribution was assessed using the Kolmogorov–Smirnov test. Echocardiography and laboratory findings were compared between the two groups using the Student's t-test for normally distributed variables or the Mann–Whitney U test for other variables. To compare the proportions of patients, Fisher's exact test was performed. Comparisons of data using all these statistical tests were performed using Sigma Stat version 3.0 (SPSS, Chicago, IL, USA). All statistical tests were two-sided and significance was defined as P<0.05.
Results
Comparisons between patients who were weaned and those who were not weaned from ECMO in the F group
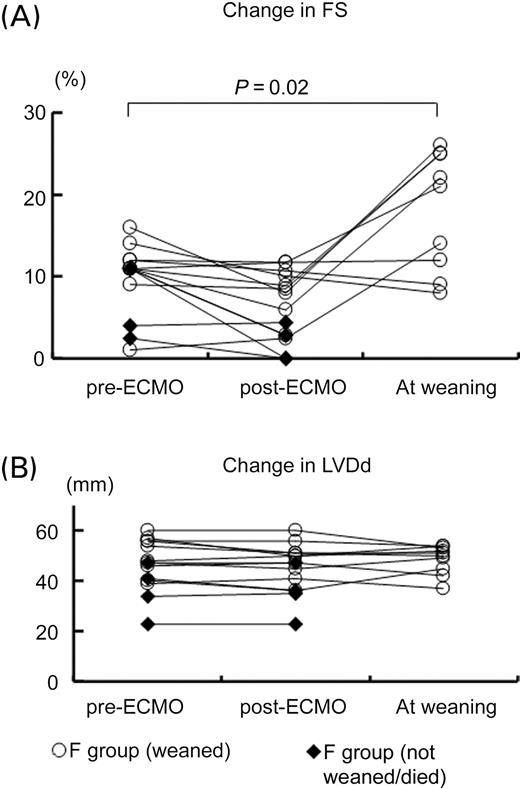
Table 1 shows the summary of the patients' characteristics in the F group. The median time interval to ECMO application from the onset of heart failure was 15 (12–20) h (range: 7–36 h). Among the 14 patients in the F group (on ECMO support), a temporary right ventricular pacemaker was used in four patients (29%). In six patients (43%), intraaortic balloon pumping (IABP) had already been inserted because they had been transferred from other hospitals. Between patients with and without IABP, systolic blood pressure (75±17 vs. 73±15 mmHg), own heart rate (127±17 vs. 138±22 b.p.m.), LVDd (47±8 vs. 46±12 mm), and FS (10±3 vs. 10±5%) were similar before ECMO application. Figure 2 shows acute changes in LV function before and immediately after the support and at the time of weaning from ECMO. Neither LVDd nor FS changed immediately after the ECMO support.
The median support time for percutaneous ECMO in the F group was 130 (42–171) h (maximum support time, 12 days). Four patients were not weaned from mechanical support and died. In one of them, the support system was changed to a left ventricular assist device (the Toyobo-NCVC-type pump)15 because of the development of multiple organ failure despite ECMO support. Therefore, the acute survival rate was 71% in the F group.
We then compared the clinical characteristics between patients who were weaned and those who were not weaned from ECMO (Table 2). Although systemic inflammation indexes (white blood cell count and C-reactive protein level) and liver function were similar, the peaks of CK-MB level and BUN level differed significantly between patients who were weaned and those who were not weaned from ECMO.
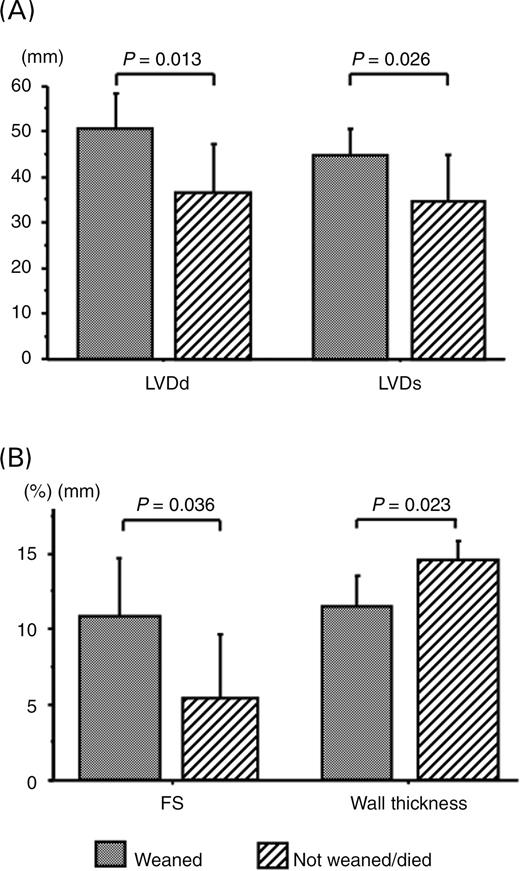
Figure 3 shows echocardiographic measurements for patients in the F group. Patients who were not weaned from ECMO had smaller LVDd (36±10 vs. 50±7 mm, P=0.013) and LVDs (34±10 vs. 45±6 mm, P=0.026) and thicker ventricular wall (15±1 vs. 11±2 mm, P=0.023) than those who were weaned from ECMO. The left ventricular systolic function in patients who were not weaned from ECMO was more depressed than those who were weaned successfully, as shown by the difference in FS (5±4 vs. 11±4%, P=0.036).
Comparison between F group and NF group
All the 13 patients in the NF group survived after the onset of acute myocarditis. IABP was used in one patient in the NF group. Inotropic agents were used under haemodynamic monitoring in five of 13 patients in the NF group and in 14 of 14 patients in the F group (P<0.05). The median doses of dopamine [NF: 0 (0–3.25) vs. F: 5.5 (3–15) µg/kg body weight/min, P=0.002] and dobutamine [NF: 0 (0–3) vs. F: 3 (3–10) µg/kg body weight/min, P=0.017] used were significantly lower for patients in the NF group than for those in the F group. There were significant differences in stroke volume index (NF: 29±12 vs. F: 19±8 mL/beat/m2, P=0.048), pulmonary capillary wedge (NF: 15±6 vs. F: 23±5 mmHg, P=0.013), and right atrial pressure (NF: 8±4 vs. F: 14±6 mmHg, P=0.026) between these two groups. FS assessed by echocardiography on admission was moderately depressed in patients in the NF group when compared with that in those in the F group (23±8 vs. 10±4%, P<0.001), although peak CK-MB levels and systemic inflammation indexes (e.g. white blood cell count and C-reactive protein level) were similar between these two groups (Table 3). Liver and renal functions were preserved in patients in the NF group, whereas these were impaired in patients in the F group.
Follow-up study and clinical course
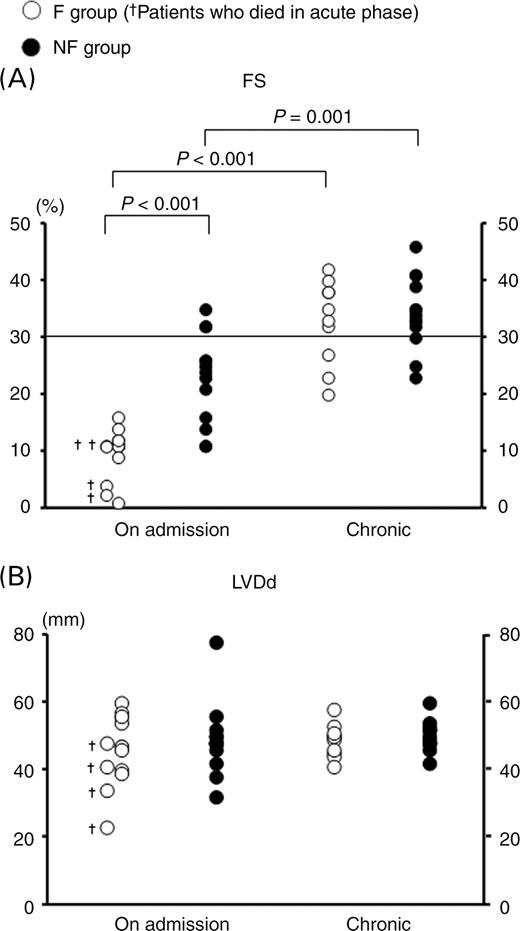
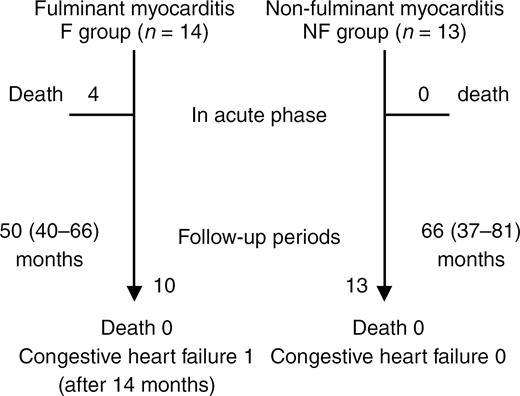
Endomyocardial biopsy or postmortem examination was performed at 25 (5.75–36.5) days in nine of 14 patients in the F group and at 14.5 (8.5–25.5) days in 12 of 13 patients in the NF group. The percentages of patients positive for myocardial infiltration by inflammatory cells were 78% (seven of nine patients) for the F group and 58% (seven of 12 patients) for the NF group. Moreover, as shown in Figure 4, echocardiography performed at the chronic stage (6–12 months) demonstrated that FS reversed dramatically in the F group reaching a similar level to that in the NF group (F: 33±7%, NF: 34±6%), although LVDd did not change throughout the study.
Figure 5 shows the summary of the clinical course. The follow-up period was 50 (40–66) months for the F group and 66 (37–81) months for the NF group. Only one patient in the F group had congestive heart failure 14 months after the onset of acute myocarditis. None of the patients died or received cardiac transplantation in both groups.
Discussion
This study demonstrated that ∼70% of patients with fulminant myocarditis supported by percutaneous ECMO could be saved. Cardiac function was severely depressed in the acute phase but improved markedly in the chronic phase. The clinical course in the chronic phase in the patients with fulminant myocarditis who were weaned from ECMO was similar to that in patients with non-fulminant myocarditis.
Survival and percutaneous ECMO
McCarthy et al.8 reported that fulminant myocarditis is a distinct clinical entity with an excellent long-term prognosis. However, there were few patients requiring circulatory supports in their reports, and the clinical outcome of those patients remains undetermined. In our series of patients, even though cardiac function was severely depressed in the acute phase reaching zero myocardial FS, haemodynamic volume support by percutaneous ECMO could effectively prevent the development of multiple organ failure. When compared with left ventricular assist devices, which we used to treat 106 patients with deteriorated haemodynamics since 1982, percutaneous ECMO has an advantage in terms of its quick, easy, and less invasive application,4–6 which may help in overcoming potential complications such as stroke, peripheral arterial ischaemia, haemorrhage, and infections.16 If there is no improvement in cardiac function, the patients should be bridged from ECMO to ventricular assist devices. The present study includes only one bridged patient. The results derived from other studies of fulminant myocarditis showed a survival rate of 40–50% for patients supported with ventricular assist devices.
Patients with fulminant myocarditis may be better managed by maintaining circulatory support than pursuing transplantation. As reported previously, the survival rate of patients with post-cardiotomy shock who required ECMO but had already suffered from irreversible myocardial damage was 20–40%.17 However, the present study demonstrated that many of these patients (≈70%) have a reasonable chance for full cardiac recovery and benefit from several days or weeks of circulatory support using ECMO, without undergoing transplantation. Particularly for children in whom transplantation is certainly not encouraging, ECMO is useful in delaying transplantation by providing support sufficiently long to determine whether cardiac function may improve.18
Temporary myocardial damage in patients with fulminant myocarditis
In the present study of fulminant myocarditis, patients who were not weaned from ECMO and died exhibited a higher peak CK-MB level and a more depressed systolic function (lower FS) than those who were weaned from ECMO. Interestingly, despite similar peak CK-MB levels, there was a significant difference in FS between patients with fulminant myocarditis who were weaned from ECMO and those with non-fulminant myocarditis. These findings indicate that the extent of myocardial dysfunction and necrosis caused by inflammatory responses may determine the acute outcome in myocarditis patients. Moreover, it is speculated that the echocardiographic finding of less dilatation may be related to a severe infectious insult with myocardial oedema. In light of accumulating evidence, myocardial dysfunction is associated with cardiodepressant mediators including free radicals and inflammatory cytokines.19,20 From the current data shown in Figure 2, percutaneous ECMO does not appear to directly promote functional recovery. However, it may be useful in supporting a compromised heart until the inflammatory storm in the myocardium has subsided. Potential therapies specific for the pathophysiological process of acute myocarditis include immunomodulation (i.e. immunoglobulin and interferon)21–23 and vaccination,24,25 the use of which may provide new insights into the treatment of this disease. Duncan et al.7 reported that mechanical circulatory support in combination with immunotherapy (intravenous administration of gamma globulin and/or steroids) results in 60% of the acute survival of children with fulminant myocarditis.
Recovery of ventricular function and long-term outcome
In patients with fulminant myocarditis who survived, FS improved in the chronic phase to a level similar to that in patients with acute non-fulminant myocarditis. The present results were different from those reported previously by Felker et al.26 They reported a significant improvement in FS in patients with fulminant myocarditis (from 19±4 to 30±8%), whereas no improvement was observed in those with acute myocarditis (from 17±7 to 19±7%). We also noted that the percentages of adverse clinical events were similar between the two groups during the follow-up period. Only one patient in the present study group was rehospitalized due to heart failure. However, the previous studies showed that the long-term outcome of patients with acute myocarditis was poor, that is, 50–60% of patients had a 5-year survival rate, compared with those with fulminant myocarditis.8,27,28 This difference may be due to the differences in patients' clinical backgrounds. In the present study, all the 14 patients with fulminant myocarditis and 12 of 13 patients with non-fulminant acute myocarditis had a distinct onset of cardiac symptoms within a short duration from flu-like symptoms and had no recurrence of myocarditis. The myocarditis cases observed in the present study appear to be more acute than those reported by others.8,28 In the previous studies, designed on the basis of the classification of Lieberman's report,29 enrolled patients with acute non-fulminant myocarditis had heart failure without a distinct onset of cardiac symptoms, which lasted for a period of weeks to months. The timing of cardiac symptom presentation may be associated with the pathophysiology and/or the state of myocarditis. Patients in the previous studies may have included those with acute myocarditis without distinct onset and/or chronic (active or persistent) myocarditis. Kodama et al.30 showed the long-term favourable outcome of acute myocarditis patients with a distinct onset classified by clinical subtypes, compared with those without a distinct onset. Patients with myocarditis without a distinct onset may have already undergone the remodelling process following a viral infection, leading to dilated cardiomyopathy. Thus, the clinical presentation may play an important role in the prognosis of this particular disease.31
Study limitations
This study has a few potential limitations. First, this is a retrospective cohort study performed at one centre. The number of patients was too small to permit multivariate analysis with adjustment for underlying confounders. However, the clinical relevance of the findings regarding such a rare but life threatening disease allows the present comparison. Secondly, endomyocardial biopsy was not performed in all the patients. Endomyocardial biopsy is of value in evaluating the activity of inflammation and identifying infiltrating cells. However, Dec et al.32 demonstrated that the combination of the clinical features of viral myocarditis and subsequent substantial improvement in the left ventricular function suggest the clinical diagnosis of active myocarditis, even when supportive biopsy evidence is lacking. In the present study, as shown in Figure 4, left ventricular function recovered to almost normal in the chronic phase and was not accompanied by cardiac dilatation or remodelling. Thus, biopsy was deemed unnecessary; in some cases, it is difficult to obtain informed consent from the patients of this study.
In conclusion, percutaneous ECMO is a highly effective form of haemodynamic support for patients with fulminant myocarditis. Once a patient recovers from inflammatory myocardial damage, the subsequent clinical outcome is favourable, similar to that observed in patients with acute non-fulminant myocarditis. A further study is required to determine the potential trigger promoting the remodelling process following viral myocarditis.
Figure 1 Illustration of ECMO system. RA, right atrium; IVC, inferior venacava; Ao, aorta.
Figure 2 Acute changes in left ventricular function before and immediately after the support and at weaning from ECMO in patients with fulminant myocarditis (F group). (A) FS and (B) LVDd. Open circles indicate patients who were weaned from ECMO and closed diamonds indicate F patients who were not weaned from ECMO and died.
Figure 3 Comparison of echocardiographic data between patients who were weaned and those who were not weaned from ECMO in F group. All these data were gathered on admission. (A) LVDd and LVDs. (B) FS and ventricular wall thickness.
Figure 4 Changes in FS (A) and LVDd (B) (determined by echocardiography) on admission, in the chronic phase (∼6–12 months after). Open circles indicate F group, closed circles indicate non-fulminant acute myocarditis (NF) group, and crosses indicate patients who died.
Figure 5 Clinical events in follow-up period.
The characteristics of patients with fulminant myocarditis using ECMO (F group)
| Age, gender . | Time interval to application (h) . | Inotropic agents before support . | Indication for support . | Haemodynamics . | Echocardiography . | IABP . | Pacing . | Outcomes . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SBP (mmHg) . | HR (b.p.m) . | RA (mmHg) . | PCW (mmHg) . | Cl (L/min/m2) . | MR . | TR . | IVCd (mm) . | Pericardial effusion . | . | . | . |
| 67 years, F | 20 | DA16, DB8, NE0.2, E0.14 | Hypotension | 80 | 111 | 12 | 24 | 1.4 | − | − | 18 | + | Y | N | Dead |
| 59 years, M | 18 | DA6, DB10 | Hypotension | 94 | 136 | 10 | 16 | 3.1 | − | − | 21 | + | Y | N | Weaned |
| 22 years, F | 12 | DA5, DB10 | VT/VF | 86 | 120 | 12 | 18 | 1.9 | + | − | 14 | + | Y | N | Weaned |
| 37 years, M | 36 | DA11, DB6, NE0.3 | Hypotension | 64 | 152 | 17 | 29 | 2.7 | + | − | 29 | + | Y | N | Weaned |
| 32 years, F | 26 | DA3, DB3 | VT/VF | 76 | 126 | 13 | 16 | 2.1 | + | + | 21 | + | N | Y | Weaned |
| 53 years, F | 26 | DA20, DB20 | Cardiac arrest | NM | NP | 15 | + | N | N | Dead | |||||
| 24 years, M | 7 | DB3 | Hypotension | 84 | 122 | 10 | 25 | 1.4 | + | − | 20 | + | N | N | Weaned |
| 29 years, M | 14 | DA5 | VT/VF | 60 | 180 | 20 | 25 | 4.3 | − | − | 18 | + | N | Y | Weaned |
| 16 years, M | 11 | DA11 | Hypotension | 80 | 117 | 17 | 21 | 2.0 | − | − | 19 | + | Y | N | Dead |
| 54 years, F | 8 | DA3, DB3 | VT/VF | 90 | 156 | 17 | 28 | 2.9 | + | − | 22 | + | N | Y | Weaned |
| 49 years, F | 15 | DB3 | Hypotension | 60 | 110 | 16 | 19 | 1.7 | + | − | 15 | + | N | N | Weaned |
| 22 years, F | 18 | DA27, DB27 | Hypotension | 53 | 150 | 15 | 25 | 1.9 | − | − | 19 | + | N | N | Weaned |
| 31 years, M | 12 | DA10, DB15, NE0.5 | Hypotension | 88 | 132 | 2 | 21 | 2.2 | ++ | + | 19 | + | N | N | Weaned |
| 42 years, M | 15 | DA5 | Hypotension | 47 | 70a | 15 | 30 | 1.4 | + | − | 17 | + | Y | Y | Dead |
| Age, gender . | Time interval to application (h) . | Inotropic agents before support . | Indication for support . | Haemodynamics . | Echocardiography . | IABP . | Pacing . | Outcomes . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SBP (mmHg) . | HR (b.p.m) . | RA (mmHg) . | PCW (mmHg) . | Cl (L/min/m2) . | MR . | TR . | IVCd (mm) . | Pericardial effusion . | . | . | . |
| 67 years, F | 20 | DA16, DB8, NE0.2, E0.14 | Hypotension | 80 | 111 | 12 | 24 | 1.4 | − | − | 18 | + | Y | N | Dead |
| 59 years, M | 18 | DA6, DB10 | Hypotension | 94 | 136 | 10 | 16 | 3.1 | − | − | 21 | + | Y | N | Weaned |
| 22 years, F | 12 | DA5, DB10 | VT/VF | 86 | 120 | 12 | 18 | 1.9 | + | − | 14 | + | Y | N | Weaned |
| 37 years, M | 36 | DA11, DB6, NE0.3 | Hypotension | 64 | 152 | 17 | 29 | 2.7 | + | − | 29 | + | Y | N | Weaned |
| 32 years, F | 26 | DA3, DB3 | VT/VF | 76 | 126 | 13 | 16 | 2.1 | + | + | 21 | + | N | Y | Weaned |
| 53 years, F | 26 | DA20, DB20 | Cardiac arrest | NM | NP | 15 | + | N | N | Dead | |||||
| 24 years, M | 7 | DB3 | Hypotension | 84 | 122 | 10 | 25 | 1.4 | + | − | 20 | + | N | N | Weaned |
| 29 years, M | 14 | DA5 | VT/VF | 60 | 180 | 20 | 25 | 4.3 | − | − | 18 | + | N | Y | Weaned |
| 16 years, M | 11 | DA11 | Hypotension | 80 | 117 | 17 | 21 | 2.0 | − | − | 19 | + | Y | N | Dead |
| 54 years, F | 8 | DA3, DB3 | VT/VF | 90 | 156 | 17 | 28 | 2.9 | + | − | 22 | + | N | Y | Weaned |
| 49 years, F | 15 | DB3 | Hypotension | 60 | 110 | 16 | 19 | 1.7 | + | − | 15 | + | N | N | Weaned |
| 22 years, F | 18 | DA27, DB27 | Hypotension | 53 | 150 | 15 | 25 | 1.9 | − | − | 19 | + | N | N | Weaned |
| 31 years, M | 12 | DA10, DB15, NE0.5 | Hypotension | 88 | 132 | 2 | 21 | 2.2 | ++ | + | 19 | + | N | N | Weaned |
| 42 years, M | 15 | DA5 | Hypotension | 47 | 70a | 15 | 30 | 1.4 | + | − | 17 | + | Y | Y | Dead |
SBP, systolic blood pressure; HR, heart rate; RA, right atrial pressure; PCW, pulmonary capillary wedge pressure; CI, cardiac index; MR, mitral valve regurgitation; TR, tricuspid valve regurgitation; IVCd, inferior vena cava dimension size; DA, dopamine; DB, dobutamine; NE, norepinephrine; E, epinephrine; VT/VF means the existence of ventricular tachycardia or ventricular fibrillation; NM, not measured; NP, not palpable.
aHeart rate by temporary right ventricular pacing.
The characteristics of patients with fulminant myocarditis using ECMO (F group)
| Age, gender . | Time interval to application (h) . | Inotropic agents before support . | Indication for support . | Haemodynamics . | Echocardiography . | IABP . | Pacing . | Outcomes . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SBP (mmHg) . | HR (b.p.m) . | RA (mmHg) . | PCW (mmHg) . | Cl (L/min/m2) . | MR . | TR . | IVCd (mm) . | Pericardial effusion . | . | . | . |
| 67 years, F | 20 | DA16, DB8, NE0.2, E0.14 | Hypotension | 80 | 111 | 12 | 24 | 1.4 | − | − | 18 | + | Y | N | Dead |
| 59 years, M | 18 | DA6, DB10 | Hypotension | 94 | 136 | 10 | 16 | 3.1 | − | − | 21 | + | Y | N | Weaned |
| 22 years, F | 12 | DA5, DB10 | VT/VF | 86 | 120 | 12 | 18 | 1.9 | + | − | 14 | + | Y | N | Weaned |
| 37 years, M | 36 | DA11, DB6, NE0.3 | Hypotension | 64 | 152 | 17 | 29 | 2.7 | + | − | 29 | + | Y | N | Weaned |
| 32 years, F | 26 | DA3, DB3 | VT/VF | 76 | 126 | 13 | 16 | 2.1 | + | + | 21 | + | N | Y | Weaned |
| 53 years, F | 26 | DA20, DB20 | Cardiac arrest | NM | NP | 15 | + | N | N | Dead | |||||
| 24 years, M | 7 | DB3 | Hypotension | 84 | 122 | 10 | 25 | 1.4 | + | − | 20 | + | N | N | Weaned |
| 29 years, M | 14 | DA5 | VT/VF | 60 | 180 | 20 | 25 | 4.3 | − | − | 18 | + | N | Y | Weaned |
| 16 years, M | 11 | DA11 | Hypotension | 80 | 117 | 17 | 21 | 2.0 | − | − | 19 | + | Y | N | Dead |
| 54 years, F | 8 | DA3, DB3 | VT/VF | 90 | 156 | 17 | 28 | 2.9 | + | − | 22 | + | N | Y | Weaned |
| 49 years, F | 15 | DB3 | Hypotension | 60 | 110 | 16 | 19 | 1.7 | + | − | 15 | + | N | N | Weaned |
| 22 years, F | 18 | DA27, DB27 | Hypotension | 53 | 150 | 15 | 25 | 1.9 | − | − | 19 | + | N | N | Weaned |
| 31 years, M | 12 | DA10, DB15, NE0.5 | Hypotension | 88 | 132 | 2 | 21 | 2.2 | ++ | + | 19 | + | N | N | Weaned |
| 42 years, M | 15 | DA5 | Hypotension | 47 | 70a | 15 | 30 | 1.4 | + | − | 17 | + | Y | Y | Dead |
| Age, gender . | Time interval to application (h) . | Inotropic agents before support . | Indication for support . | Haemodynamics . | Echocardiography . | IABP . | Pacing . | Outcomes . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SBP (mmHg) . | HR (b.p.m) . | RA (mmHg) . | PCW (mmHg) . | Cl (L/min/m2) . | MR . | TR . | IVCd (mm) . | Pericardial effusion . | . | . | . |
| 67 years, F | 20 | DA16, DB8, NE0.2, E0.14 | Hypotension | 80 | 111 | 12 | 24 | 1.4 | − | − | 18 | + | Y | N | Dead |
| 59 years, M | 18 | DA6, DB10 | Hypotension | 94 | 136 | 10 | 16 | 3.1 | − | − | 21 | + | Y | N | Weaned |
| 22 years, F | 12 | DA5, DB10 | VT/VF | 86 | 120 | 12 | 18 | 1.9 | + | − | 14 | + | Y | N | Weaned |
| 37 years, M | 36 | DA11, DB6, NE0.3 | Hypotension | 64 | 152 | 17 | 29 | 2.7 | + | − | 29 | + | Y | N | Weaned |
| 32 years, F | 26 | DA3, DB3 | VT/VF | 76 | 126 | 13 | 16 | 2.1 | + | + | 21 | + | N | Y | Weaned |
| 53 years, F | 26 | DA20, DB20 | Cardiac arrest | NM | NP | 15 | + | N | N | Dead | |||||
| 24 years, M | 7 | DB3 | Hypotension | 84 | 122 | 10 | 25 | 1.4 | + | − | 20 | + | N | N | Weaned |
| 29 years, M | 14 | DA5 | VT/VF | 60 | 180 | 20 | 25 | 4.3 | − | − | 18 | + | N | Y | Weaned |
| 16 years, M | 11 | DA11 | Hypotension | 80 | 117 | 17 | 21 | 2.0 | − | − | 19 | + | Y | N | Dead |
| 54 years, F | 8 | DA3, DB3 | VT/VF | 90 | 156 | 17 | 28 | 2.9 | + | − | 22 | + | N | Y | Weaned |
| 49 years, F | 15 | DB3 | Hypotension | 60 | 110 | 16 | 19 | 1.7 | + | − | 15 | + | N | N | Weaned |
| 22 years, F | 18 | DA27, DB27 | Hypotension | 53 | 150 | 15 | 25 | 1.9 | − | − | 19 | + | N | N | Weaned |
| 31 years, M | 12 | DA10, DB15, NE0.5 | Hypotension | 88 | 132 | 2 | 21 | 2.2 | ++ | + | 19 | + | N | N | Weaned |
| 42 years, M | 15 | DA5 | Hypotension | 47 | 70a | 15 | 30 | 1.4 | + | − | 17 | + | Y | Y | Dead |
SBP, systolic blood pressure; HR, heart rate; RA, right atrial pressure; PCW, pulmonary capillary wedge pressure; CI, cardiac index; MR, mitral valve regurgitation; TR, tricuspid valve regurgitation; IVCd, inferior vena cava dimension size; DA, dopamine; DB, dobutamine; NE, norepinephrine; E, epinephrine; VT/VF means the existence of ventricular tachycardia or ventricular fibrillation; NM, not measured; NP, not palpable.
aHeart rate by temporary right ventricular pacing.
Comparison between patients who were weaned and those who were not weaned from ECMO in the F group
| . | Patients who were weaned (n=10) . | Patients who were not weaned/died (n=4) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 145 (108–381) | 280 (208–3775) | 0.138 |
| Alanine aminotransferase (IU/L) | 70 (54–358) | 81 (60–2123) | 0.524 |
| Lactate dehydrogenase (IU/L) | 635 (475–1229) | 1222 (630–6301) | 0.358 |
| Peak CPK (IU/L) | 3860 (1097–6168) | 12005 (7167–16117) | 0.138 |
| Peak CK-MB (IU/L) | 102 (16–134) | 229 (200–538) | 0.042 |
| Blood urine nitrogen (mg/dL) | 18.5 (15–26) | 34.5 (30–38.5) | 0.004 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.1) | 2.4 (1.55–2.6) | 0.179 |
| White blood cell count (/µL) | 11635 (9230–12200) | 8535 (6400–12885) | 0.289 |
| C-reactive protein (mg/dL) | 10.2 (6.6–12.4) | 7.9 (4.5–15.1) | 0.832 |
| . | Patients who were weaned (n=10) . | Patients who were not weaned/died (n=4) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 145 (108–381) | 280 (208–3775) | 0.138 |
| Alanine aminotransferase (IU/L) | 70 (54–358) | 81 (60–2123) | 0.524 |
| Lactate dehydrogenase (IU/L) | 635 (475–1229) | 1222 (630–6301) | 0.358 |
| Peak CPK (IU/L) | 3860 (1097–6168) | 12005 (7167–16117) | 0.138 |
| Peak CK-MB (IU/L) | 102 (16–134) | 229 (200–538) | 0.042 |
| Blood urine nitrogen (mg/dL) | 18.5 (15–26) | 34.5 (30–38.5) | 0.004 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.1) | 2.4 (1.55–2.6) | 0.179 |
| White blood cell count (/µL) | 11635 (9230–12200) | 8535 (6400–12885) | 0.289 |
| C-reactive protein (mg/dL) | 10.2 (6.6–12.4) | 7.9 (4.5–15.1) | 0.832 |
The median (25–75%) data. All data except peak creatine phosphokinase (CPK) and its isoform (CK-MB) are presented as baseline (measured on admission).
Comparison between patients who were weaned and those who were not weaned from ECMO in the F group
| . | Patients who were weaned (n=10) . | Patients who were not weaned/died (n=4) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 145 (108–381) | 280 (208–3775) | 0.138 |
| Alanine aminotransferase (IU/L) | 70 (54–358) | 81 (60–2123) | 0.524 |
| Lactate dehydrogenase (IU/L) | 635 (475–1229) | 1222 (630–6301) | 0.358 |
| Peak CPK (IU/L) | 3860 (1097–6168) | 12005 (7167–16117) | 0.138 |
| Peak CK-MB (IU/L) | 102 (16–134) | 229 (200–538) | 0.042 |
| Blood urine nitrogen (mg/dL) | 18.5 (15–26) | 34.5 (30–38.5) | 0.004 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.1) | 2.4 (1.55–2.6) | 0.179 |
| White blood cell count (/µL) | 11635 (9230–12200) | 8535 (6400–12885) | 0.289 |
| C-reactive protein (mg/dL) | 10.2 (6.6–12.4) | 7.9 (4.5–15.1) | 0.832 |
| . | Patients who were weaned (n=10) . | Patients who were not weaned/died (n=4) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 145 (108–381) | 280 (208–3775) | 0.138 |
| Alanine aminotransferase (IU/L) | 70 (54–358) | 81 (60–2123) | 0.524 |
| Lactate dehydrogenase (IU/L) | 635 (475–1229) | 1222 (630–6301) | 0.358 |
| Peak CPK (IU/L) | 3860 (1097–6168) | 12005 (7167–16117) | 0.138 |
| Peak CK-MB (IU/L) | 102 (16–134) | 229 (200–538) | 0.042 |
| Blood urine nitrogen (mg/dL) | 18.5 (15–26) | 34.5 (30–38.5) | 0.004 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.1) | 2.4 (1.55–2.6) | 0.179 |
| White blood cell count (/µL) | 11635 (9230–12200) | 8535 (6400–12885) | 0.289 |
| C-reactive protein (mg/dL) | 10.2 (6.6–12.4) | 7.9 (4.5–15.1) | 0.832 |
The median (25–75%) data. All data except peak creatine phosphokinase (CPK) and its isoform (CK-MB) are presented as baseline (measured on admission).
Comparison with laboratory data between F groups and NF (non-fulminant acute myocarditis) groups
| . | F group (n=14) . | NF group (n=13) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 188 (108–381) | 46 (39–127) | 0.006 |
| Alanine aminotransferase (IU/L) | 70 (57–358) | 42 (27–69) | 0.051 |
| Lactate dehydrogenase (IU/L) | 711 (477–1229) | 361 (175–491) | 0.004 |
| Peak CPK (IU/L) | 3903 (1765–11667) | 529 (253–1042) | <0.001 |
| Peak CK-MB (IU/L) | 117 (67–210) | 98 (67–124) | 0.447 |
| Blood urine nitrogen (mg/dL) | 24 (16–32) | 11 (9–19) | 0.003 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.6) | 0.75 (0.6–0.85) | 0.004 |
| White blood cell count (/µL) | 11385 (9049–12200) | 9030 (7550–9918) | 0.099 |
| C-reactive protein (mg/dL | 9.9 (5.4–12.4) | 3.6 (2.6–12.3) | 0.201 |
| . | F group (n=14) . | NF group (n=13) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 188 (108–381) | 46 (39–127) | 0.006 |
| Alanine aminotransferase (IU/L) | 70 (57–358) | 42 (27–69) | 0.051 |
| Lactate dehydrogenase (IU/L) | 711 (477–1229) | 361 (175–491) | 0.004 |
| Peak CPK (IU/L) | 3903 (1765–11667) | 529 (253–1042) | <0.001 |
| Peak CK-MB (IU/L) | 117 (67–210) | 98 (67–124) | 0.447 |
| Blood urine nitrogen (mg/dL) | 24 (16–32) | 11 (9–19) | 0.003 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.6) | 0.75 (0.6–0.85) | 0.004 |
| White blood cell count (/µL) | 11385 (9049–12200) | 9030 (7550–9918) | 0.099 |
| C-reactive protein (mg/dL | 9.9 (5.4–12.4) | 3.6 (2.6–12.3) | 0.201 |
The median (25–75%) data. All data except peak creatine phosphokinase (CK) and its isoform (CK-MB) are presented as baseline (measured on admission).
Comparison with laboratory data between F groups and NF (non-fulminant acute myocarditis) groups
| . | F group (n=14) . | NF group (n=13) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 188 (108–381) | 46 (39–127) | 0.006 |
| Alanine aminotransferase (IU/L) | 70 (57–358) | 42 (27–69) | 0.051 |
| Lactate dehydrogenase (IU/L) | 711 (477–1229) | 361 (175–491) | 0.004 |
| Peak CPK (IU/L) | 3903 (1765–11667) | 529 (253–1042) | <0.001 |
| Peak CK-MB (IU/L) | 117 (67–210) | 98 (67–124) | 0.447 |
| Blood urine nitrogen (mg/dL) | 24 (16–32) | 11 (9–19) | 0.003 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.6) | 0.75 (0.6–0.85) | 0.004 |
| White blood cell count (/µL) | 11385 (9049–12200) | 9030 (7550–9918) | 0.099 |
| C-reactive protein (mg/dL | 9.9 (5.4–12.4) | 3.6 (2.6–12.3) | 0.201 |
| . | F group (n=14) . | NF group (n=13) . | P-value . |
|---|---|---|---|
| Aspartate aminotransferase (IU/L) | 188 (108–381) | 46 (39–127) | 0.006 |
| Alanine aminotransferase (IU/L) | 70 (57–358) | 42 (27–69) | 0.051 |
| Lactate dehydrogenase (IU/L) | 711 (477–1229) | 361 (175–491) | 0.004 |
| Peak CPK (IU/L) | 3903 (1765–11667) | 529 (253–1042) | <0.001 |
| Peak CK-MB (IU/L) | 117 (67–210) | 98 (67–124) | 0.447 |
| Blood urine nitrogen (mg/dL) | 24 (16–32) | 11 (9–19) | 0.003 |
| Serum creatinine (mg/dL) | 1.0 (0.8–1.6) | 0.75 (0.6–0.85) | 0.004 |
| White blood cell count (/µL) | 11385 (9049–12200) | 9030 (7550–9918) | 0.099 |
| C-reactive protein (mg/dL | 9.9 (5.4–12.4) | 3.6 (2.6–12.3) | 0.201 |
The median (25–75%) data. All data except peak creatine phosphokinase (CK) and its isoform (CK-MB) are presented as baseline (measured on admission).
References
Kato S, Morimoto S, Hiramatsu S, Nomura M, Ito T, Hishida H. Use of percutaneous cardiopulmonary support of patients with fulminant myocarditis and cardiogenic shock for improving prognosis.
Chen JM, Spanier TB, Gonzalez JJ, Marelli D, Flannery MA, Tector KA, Cullinane S, Oz MC. Improved survival using external pulsatile mechanical ventricular assistance.
Acker MA. Mechanical circulatory support for patients with acute-fulminant myocarditis.
Duncan BW, Bohn DJ, Atz AM, French JW, Laussen PC, Wessel DL. Mechanical circulatory support for treatment of children with acute fulminant myocarditis.
McCarthy RE III, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis.
Swan HJC, Forrester JS, Diamond G, Chatterjee K, Parmley WW. Hemodynamic spectrum of myocardial infarction and cardiogenic shock.
Sasako Y, Nakatani T, Nonogi H, Miyazaki S, Kito Y, Takano H, Kawashima Y. Clinical experience of percutaneous cardiopulmonary support.
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendation for quantification of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms.
Kircher BJ, Himelman RB, Shiller NB. Non invasive estimation of right atrial pressure from the respiratory collapse of the inferior vena cava.
Nakatani T, Takano H, Beppu S, Noda H, Taenaka Y, Kumon K, Kito Y, Fujita T, Kawashima Y. Practical assessment of natural heart function using echocardiography in mechanically assisted patients.
Takano H, Nakatani T. Ventricular assist systems: experience in Japan with Toyobo pump and Zeon pump.
Pagani FD, Aaronson KD, Swaniker F, Bartlett RH. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device.
Magovern GJ, Kathleen A, Simpson KA. Extracorporeal membrane oxygenation for adult cardiac support: the Allegheny experience.
Pennington DG, Smedira NG, Samuels LE, Acker MA, Curtis JJ, Pagani FD. Mechanical circulatory support for acute heart failure.
Sasayama S, Matsumori A, Kihara Y. New insights into the pathophysiological role for cytokines in heart failure.
Mann DL. Inflammatory mediator and failing heart: past, present and foreseeable future.
Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, Baker AL, Perez-Atayde AR, Newburger JW. Gamma-globulin treatment of acute myocarditis in the pediatric population.
McNamara DM, Rosenblum WD, Janosko KM, Trost MK, Villaneuva FS, Demetris AJ, Murali S, Feldman AM. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy.
Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss HP. Interferon-beta treatment eliminates cardiotropic viruses and improvement left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction.
Kishimoto C, Takada H, Hiraoka Y, Shinohara H, Kitazawa M. Protection against murine Coxackievirus B3 myocarditis by T cell vaccination.
Matsumoto Y, Jee Y, Sugisaki M. Successful TCR-based immunotherapy for autoimmune myocarditis with DNA vaccines after rapid identification of pathogenic TCR.
Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM. Echocardiographic findings in fulminant and acute myocarditis.
Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators.
Grogan M, Redfield MM, Bailey KR, Reeder GS, Gersh BJ, Edwards WD, Rodeheffer RJ. Long-term outcome of patients with biopsy-proved myocarditis: comparison with idiopathic dilated cardiomyopathy.
Lieberman EB, Hutchins GM, Herskowitz A, Rose NR, Baughman KL. Clinicopathologic description of myocarditis.
Kodama M, Oda H, Okabe M, Aizawa Y, Izumi T. Early and long-term mortality of the clinical subtypes of myocarditis.
D'Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F, Di Sciascio G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review.