-
PDF
- Split View
-
Views
-
Cite
Cite
Chris J. Malkin, Peter J. Pugh, John N. West, Edwin J.R. van Beek, T. Hugh Jones, Kevin S. Channer, Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial, European Heart Journal, Volume 27, Issue 1, January 2006, Pages 57–64, https://doi.org/10.1093/eurheartj/ehi443
Close - Share Icon Share
Abstract
Aims Chronic heart failure is associated with maladaptive and prolonged neurohormonal and pro-inflammatory cytokine activation causing a metabolic shift favouring catabolism, vasodilator incapacity, and loss of skeletal muscle bulk and function. In men, androgens are important determinants of anabolic function and physical strength and also possess anti-inflammatory and vasodilatory properties.
Methods and results We conducted a randomized, double-blind, placebo-controlled parallel trial of testosterone replacement therapy (5 mg Androderm®) at physiological doses in 76 men (mean±SD, age 64±9.9) with heart failure (ejection fraction 32.5±11%) over a maximum follow-up period of 12 months. The primary endpoint was functional capacity as assessed by the incremental shuttle walk test (ISWT). At baseline, 18 (24%) had serum testosterone below the normal range and bioavailable testosterone correlated with distance walked on the initial ISWT (r=0.3, P=0.01). Exercise capacity significantly improved with testosterone therapy compared with placebo over the full study period (mean change +25±15 m) corresponding to a 15±11% improvement from baseline (P=0.006 ANOVA). Symptoms improved by at least one functional class on testosterone in 13 (35%) vs. 3 (8%) on placebo (P=0.01). No significant changes were found in handgrip strength, skeletal muscle bulk by cross-sectional computed tomography, or in tumour necrosis factor levels. Testosterone therapy was safe with no excess of adverse events although the patch preparation was not well tolerated by the study patients.
Conclusion Testosterone replacement therapy improves functional capacity and symptoms in men with moderately severe heart failure.
See page 10 for the editorial comment on this article (doi:10.1093/eurheartj/ehi653)
Introduction
Chronic heart failure (CHF) is a major health problem throughout the world. In the UK, the prevalence of heart failure is around 1% and the financial impact of delivering medical support to this population consumes ∼2% of the total health care budget.1 CHF has a very poor prognosis and registry data have suggested that the 1-year mortality after hospital discharge for heart failure is still as high as 30%.2 In addition, the symptoms associated with heart failure have a significant impact on the quality of life, with unplanned hospital admissions, reduced functional capacity, and low mood increasingly common and severe with disease progression. There is evidence that in the past decade the prognosis of heart failure has improved, an effect that at least in part is due to the development and widespread use of medical therapies.3 The only effective medical treatments that improve the symptoms and prognosis of CHF act by inhibition of maladaptive neuro-endocrine responses, but currently these are limited to inhibition of the renin–angiotensin–aldosterone system and sympathetic blockade. Other metabolic derangements in CHF include chronic anaemia and enhanced immune activation and these pathophysiological changes have been the targets for therapeutic intervention with variable success.4–6 The excess of catabolic hormones and a relative deficiency of anabolic hormones have been well documented,7,8 but until recently restoration of this hormone balance has been relatively ignored as a potential therapy.
Previous studies have shown testosterone deficiency in men with CHF,9 and levels of the weaker adrenal androgen dehydroepiandrosterone and its sulfate are consistently reported to be low in proportion to heart failure severity.7,10 This deficiency may be responsible for some of the features of advanced heart failure such as reduced mass of skeletal muscle, abnormal energy handling, cachexia, depression, and fatigue.
Testosterone is a hormone that may have therapeutic benefit in CHF for a number of reasons. It has vasodilatory properties and acute administration lowers peripheral vascular resistance, reduces cardiac afterload, and increases cardiac index.11 Testosterone modulates the immune response and replacement therapy reduces the pro-inflammatory cytokines TNFα and interleukin 1β while increasing the anti-inflammatory cytokine interleukin 10,12 which could counteract the cytokine imbalance in CHF. Both CHF and androgen deficiency are characterized by low mood and depression,13,14 and testosterone replacement therapy improves mood and depressive symptoms in testosterone deficient subjects,15 and is likely to be beneficial given the morbidity of CHF.
There have been few long-term studies on the effect of androgens in CHF. A single animal study reported prolonged survival following treatment with nandrolone decanoate in hamsters with inherited cardiomyopathy.16 In an unblinded descriptive study on 12 patients with CHF, oxymethalone reduced left ventricular mass and brain natriuretic peptide.17 In a short-term randomized, placebo controlled trial in men with CHF, Sustanon® (intramuscular depot testosterone) treatment was associated with a significant increase in effort tolerance and improved symptoms.18
Although testosterone treatment in very high supra-physiological doses causes myocardial hypertrophy and stiffening,19,20 we hypothesized that low dose physiological replacement therapy may help redress the anabolic/catabolic imbalance of established CHF and improve functional capacity and symptoms.
Methods
Subjects
The local research ethics committee approved the study protocol and all patients provided written informed consent. Ambulant male patients with stable CHF of at least 6 months duration were recruited if they were over 18 years of age and had impaired exercise tolerance, limited by fatigue or dyspnoea deemed to be of cardiac origin. All patients had at least moderate left ventricular systolic dysfunction on 2D trans-thoracic echocardiography. Exclusion criteria were use of sex hormone manipulating therapy, prostate specific antigen (PSA) level above the age adjusted normal range, and exercise limitation due to a non-cardiac cause or malignancy.
Study design and treatment
The study was a randomized, double-blind, placebo-controlled parallel trial of physiological testosterone replacement therapy vs. placebo with blinded treatment for a 12-month trial period. Drug was supplied as an adhesive skin patch preparation (Androderm®, Watson Laboratories, Salt Lake City, USA) or identical placebo patches. Subjects applied a single 5 mg patch at night, replaced every 24 h. Patients were instructed to site the patch on the trunk or upper limbs avoiding joints and bony prominences and to rotate the sites allowing at least a 7-day rest period before using a site for a further 24 h. This 5 mg dose of testosterone has been shown to raise levels of serum testosterone to the normal range in 93% of androgen deficient men and to preserve the normal diurnal variation of hormone levels.21
Randomization
Patients were screened and randomized between November 2001 and February 2003, and the final patient completed the study in February 2004. Patients were randomized after recording baseline data and were reviewed at 3, 6, and 12 months. The randomization incorporated a single stratification of heart failure due to ischaemic aetiology or non-ischaemic aetiology, as testosterone has been shown to improve mood and ischaemic threshold in patients with angina pectoris.22
Data collected
At baseline, subjects completed a medical questionnaire, detailing medical history and concomitant medications. Resting pulse, blood pressure, height, and weight were recorded, and body mass index (BMI) calculated [weight (kg)/height2 (m2)].
Exercise capacity was assessed using the incremental shuttle walk test (ISWT). Two tests were performed prior to starting treatment, one at the screening visit and the second before randomization. This second test result was used as the baseline in subsequent analysis. The ISWT is a symptom-limited exercise test with a progressive increase in workload designed to allow subjects to achieve maximum effort tolerance. Subjects walk back and forth along a horizontal 10 m course, marked out by two cones and must complete the shuttle before a pre-recorded signal from a cassette player, which shortens incrementally after each shuttle. The endpoint (distance walked in metres) is reached when the subject fails to complete the shuttle before the signal. The ISWT has been evaluated in patients with CHF, as an alternative to cardiopulmonary exercise testing and the traditional 6 min walk test. It is highly reproducible, preferred by patients and correlates strongly with peak VO2, in multivariate analysis the ISWT was found to be the most significant independent predictor of peak VO223,24 and, after 17 months follow-up, predicted event free survival, whereas the 6 min walk test did not.25
Left ventricular ejection fraction was determined by trans-thoracic echocardiography from 2D apical four-chamber images using Simpson's rule. Left ventricular end-diastolic diameter and cross-sectional area were measured from M-mode images taken from the left parasternal view. Left ventricular mass was calculated using the American Society of Echocardiography simplified cubed equation, LVM (g)=0.8×[1.05×{(EDD×IVST×PWT)3−(EDD)3}], where EDD, end-diastolic diameter; IVST, interventricular wall thickness; PWT, posterior wall thickness. Body surface area (BSA) was calculated by the formula BSA=0.0001×71.84×(weight)0.425×(height)0.725. LV mass was divided by BSA to derive the LV mass index (g/m2). Transverse computed tomogram (CT) images of the mid-thigh and mid-calf of both legs were used to estimate skeletal muscle bulk by computer-assisted planimetry of the cross-sectional area of the skeletal muscle at these points. Handgrip strength was measured using a hand held dynamometer, which was performed three times in both hands and the mean value used for analysis. Full blood count, PSA, serum electrolytes, brain natriuretic peptide (BNP; Biosite Diagnostics Inc., San Diego, CA, USA), and sex hormones were measured from early morning blood samples. Total testosterone and sex hormone binding globulin (SHBG) were measured by ELISA, inter and intra-assay coefficients of the variance were <14 and <10%, respectively. Bio-available testosterone (comprising free testosterone and that component of total testosterone loosely bound to albumen) was determined by a modification of the method described by Tremblay and Dube,26 inter and intra-assay coefficients of variance for this technique were <14 and <12%, respectively. Bio-available testosterone level provides a more precise measure of androgen status, being unaffected by the level of SHBG. Tumour necrosis factor (TNFα) was measured from venous blood samples drawn at baseline and at 3 months.
Subjects completed the Minnesota Living with Heart Failure questionnaire (MLHF) to assess disease specific symptoms and quality of life, and the Beck Depression Inventory (BDI) to give a standardized assessment of mood and depressive symptoms.
Statistical analysis and power calculation
The power calculation was based on the results of our pilot study,18 in which 3 months of intra-muscular testosterone therapy improved distance achieved in an ISWT. The mean±SD improvement on testosterone was 65±79 m. In order to detect an improvement in exercise capacity similar to that seen in the pilot study, with 90% power and 5% significance, we calculated the need to recruit a total of 66 subjects. We aimed to recruit 15–20% more patients to allow for patient withdrawals giving a final target of 80 subjects.
All data were compared with the normal distribution using the Kolmogorov–Smirnov test. Raw data are presented as mean±SD throughout unless specified and analysis was by intention-to-treat. The intention-to-treat analysis confined the patients to their randomization group and data were carried over from previous visits in the case of patient withdrawal. Parameters that were only sampled twice (muscle size by CT scanning, serum TNF) were analysed by unpaired t-tests and ordinal data (NYHA class) by the Mann–Whitney U-test. Correlations were tested with Pearson correlation coefficient for normally distributed data or Spearman's rank correlation for non-normally distributed data and ordinal data (NYHA class, ejection fraction, and mood scores). Because a large number of analyses were performed significance at the 1% (P=0.01) level was sought.
Pre-specified primary endpoint
The pre-specified primary endpoint was the treatment effect of testosterone vs. placebo on the ISWT distance in metres at 12 months, we have also presented the data as percentage change from baseline. In cases of patient withdrawal, data were carried forward for the purpose of analysis. The treatment effect is calculated by comparing the change from baseline effect of testosterone with the change from baseline effect of placebo using univariate analysis of the variance. In this analysis, the dependent variable was the change from baseline and the two fixed class variables were: the treatment group (placebo or testosterone) and the time of sampling (3, 6, or 12 months), the random variable was the patient, each patient was uniquely coded (1–76). This method permits complete intention-to-treat analysis of the data without excluding data and controlling for within patient correlations. Secondary/exploratory endpoints were calculated in the same way in each case to determine the treatment effect against placebo such that no within group analyses were performed. A single secondary post hoc t-test was performed on the incremental shuttle walk data to estimate the absolute improvement of exercise capacity with confidence intervals. This analysis was performed on the 6-month data as this included the majority of the data, analysis using this method at 12 months would have excluded nearly 45% of the data (because of patient withdrawals due to skin side-effects) with a consequent risk of bias.
Results
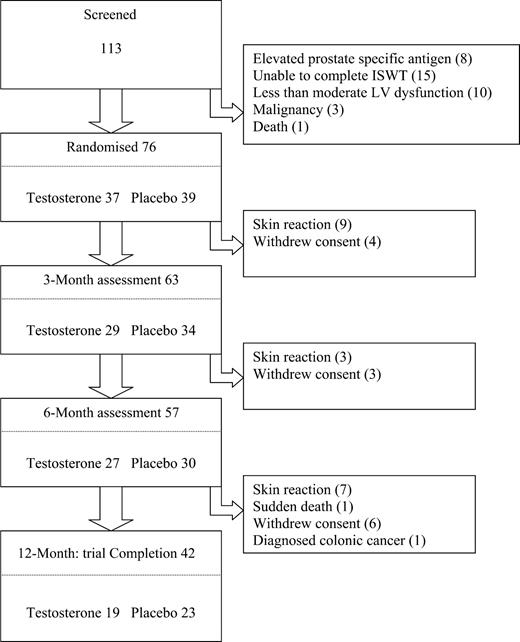
One hundred and thirteen patients were screened and 76 patients were eventually randomized (Figure 1). There were a number of patient withdrawals during the study and only 42 patients of the initial cohort remained on therapy for the planned 12 months. The summary baseline data are shown in Table 1, overall the groups were well matched except for a lower resting pulse rate in the placebo group (68.3±11.3 vs. 75±11.3 b.p.m., P=0.05), a difference that only approached significance.
Functional capacity
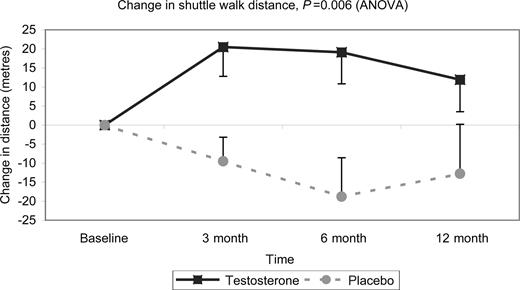
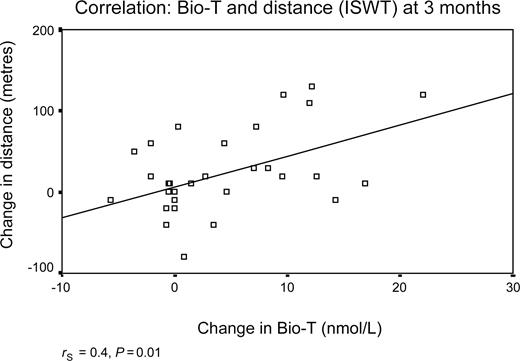
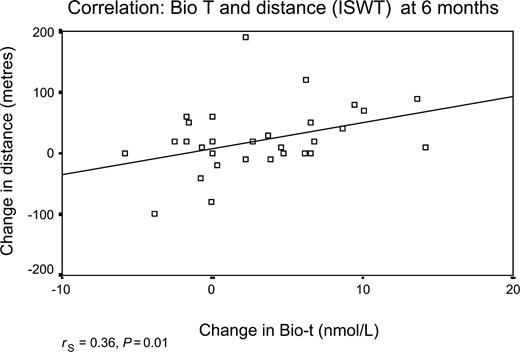
Walking distance as assessed with the ISWT improved significantly in the patients treated with testosterone compared with those on placebo (P=0.006 ANOVA; Figure 2). The mean change in shuttle walk distances (95% confidence intervals) at 12 months was +25±15 m (7–56 m) corresponding to a 15±11% improvement from baseline; at 6 months the difference between testosterone and placebo was +38±13 m (11.6–64 m), corresponding to a 18±7% improvement from baseline. In the treatment group, the change in walking distance was positively correlated with the change in the serum bioavailable testosterone at 3 months (rS=0.4, P=0.01) and at 6 months (rS=0.36, P=0.01), but was lost at 12 months (rS=0.3, P=0.04) (Figures 3 and 4). Dominant handgrip strength improved with testosterone treatment (P=0.04). There was no significant change in BMI or in cross-sectional areas of thigh and calf muscle in either group (Table 2).
Hormones and serum parameters
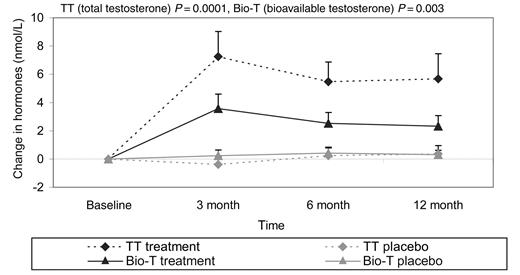
At baseline, serum testosterone levels were low and 18 men (24%) had levels below the normal range (total testosterone <7.5 nmol/L or bioavailable testosterone <2.5 nmol/L). Baseline bioavailable testosterone (but not total testosterone) correlated with both distance reached in the ISWT (r=0.3, P=0.01) and handgrip strength [r=0.3 (dominant); r=0.4 (non-dominant), both P=0.002]. Total testosterone and bioavailable testosterone both increased with treatment (Figure 5) but remained within the normal physiological range (7.5–30 nmol/L). There were no significant changes in serum electrolytes, BNP, haematological parameters, or TNFα (Table 2).
Haemodynamic and echocardiographic parameters
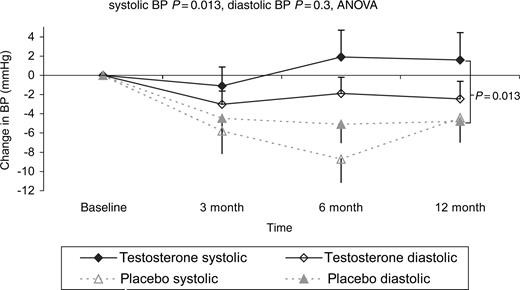
On serial echocardiography, there was a statistically significant increase in left ventricular cavity length seen in patients treated with testosterone of 8.4±0.28 mm compared with placebo (P=0.0001), but a trend to a reduction in left ventricular mass index of −13.7 g/m2 (P=0.055). There were no other changes in cardiac morphology or ejection fraction (Table 2). Mean systolic blood pressure remained stable over the follow-up period in those on testosterone but fell in those on placebo (difference P=0.013) (Table 2 and Figure 6).
Self reported mood and symptoms
No significant differences were found in the questionnaire scores for the MLHF or BDI over the follow-up period. New York Heart Association (NYHA) heart failure class at baseline and at study completion improved by least one functional class in 13 (35%) patients in the testosterone group compared with three (8%) on placebo (P=0.01). The treatment effect of testosterone vs. placebo was statistically significant (mean improvement NYHA class −0.32±0.85 vs. 0.13±0.7, P=0.01).
Safety and tolerability
There was no excess of adverse events in patients on testosterone; a single sudden death was reported (on placebo). In the testosterone arm five patients required an unplanned hospital admission and one patient suffered a small anterior circulation stroke affecting right upper limb function. A summary of serious adverse events is shown in Table 3. Over the study follow-up period, there was no significant change in either haematocrit or PSA (Table 3).
Skin reactions necessitated withdrawal of study therapy in 19 patients but minor skin reactions were reported by several patients who continued in the study as planned. In total 42 patients (55%) experienced skin reactions.
Discussion
In this study we have shown that physiological testosterone replacement therapy improves functional capacity and NYHA class compared with placebo. Moreover, there was a significant correlation between the increase in bioavailable testosterone with treatment and the increase in the ISWT distance. There were no significant changes in any serum parameters except for the testosterone levels. Over the study period testosterone therapy appeared to be safe, with no excess of serious adverse events. The Androderm® delivery system was not well tolerated, with over half of the patients developing at least minor skin reactions and 19 (nine on testosterone) withdrew prematurely because of these problems.
In our pilot study,18 we examined the effect of intra-muscular testosterone therapy using identical methodology and showed an improvement in mood and self-reported symptoms of heart failure in addition to a significant absolute increase in distance walked in the ISWT. The patients in the pilot study achieved a greater increase in ISWT distance after 3 months therapy than the patients in the present study at the same time point (65±24.9 vs. 30±9.9 m). We believe the reason for this difference is the higher post-treatment testosterone levels achieved using the intra-muscular preparation (total testosterone 38.4±4.1 nmol/L vs. 22.8±2.5 nmol/L). This inference is supported by our current data that show a significant correlation between the absolute increase in serum bioavailable testosterone level and the increase in walking distance. Mood and self-reported symptoms of heart failure improved in the pilot study but did not improve significantly in this study, which may also be accounted for by the lower level of serum testosterone achieved.
The mechanism of benefit in this study is not clear. We speculated in the pilot study that improved mood as assessed with the BDI may result in better performance in the ISWT; however, there was no improvement in mood recorded in the present study and this hypothesis now seems unlikely. There was some evidence of increased physical strength in the treatment group, although this only approached the stringent levels of significance chosen for this study, we were unable to detect any change in muscle mass using cross-sectional computed tomography. The relationship between maximal voluntary strength and functional capacity is uncertain, numerous trials have shown that androgen therapy increases maximal muscle strength (reviewed in 27), few trials have tested functional capacity or endurance and those that have reported conflicting results.28–30 There is evidence in animal studies that anabolic androgens attenuate muscle fatigue in response to exercise, although the mechanism has not been identified.31,32
There were no changes in the objective assessments of left ventricular function but a change in cardiac morphology was seen with a small increase in internal left ventricular length. High dose testosterone therapy (almost exclusively in athletes 19,33,34) has been shown to induce myocardial hypertrophy. In the present study, LV mass and thus LV wall thickness did not increase on testosterone: an important safety feature. We cannot easily explain the increase in LV cavity length, this is likely to represent ventricular remodelling but whether this is beneficial is currently not known and is the subject of further work. The difference in the blood pressures between the treatment and placebo groups is unexpected as testosterone is an acute vasodilator11 and it might be expected to lower blood pressure.35 The difference between the groups may reflect the natural history of progressive heart failure which is characterized by increasing left ventricular mass and falling blood pressure over time, this effect being attenuated by testosterone treatment. Greater improvements in exercise capacity were seen in the pilot study in which larger doses of testosterone were administered; this suggests that there may be a dose-response relationship. However, only one dose of testosterone was used in the present study and we therefore cannot confirm a dose-response relationship. These data do suggest that the biological effects of testosterone on functional exercise capacity are related to the serum levels reached in vivo as increased functional exercise capacity correlated with the increase in serum testosterone.
This trial was confined to male patients; the issue of testosterone therapy in women is highly contentious. Testosterone therapy in both sexes augments anabolic function leading to increased muscle mass and physical strength. The acute vascular effects of testosterone in women have never been assessed; in men testosterone increases cardiac output because of peripheral vasodilatation,11 an effect mediated by an L-type calcium channel.36 This calcium channel is not sex-specific and therefore any beneficial effects of testosterone on haemodynamics in men should be matched in women. However, even low dose testosterone therapy is likely to virilize female patients and may lead to sex-specific deleterious effects on insulin sensitivity and lipid profiles.37 The possible use of SARMS (selective androgen receptor modulators) is inviting as these drugs can be designed to be non-virilizing, but these remain untested.38 At this stage, androgen therapy in women is potentially deleterious and any effects observed in men cannot be assumed to be the same in women.
Limitations
We are unable to confirm the mechanism by which testosterone improves functional capacity and symptoms. Indeed, it may be multi-factorial and may vary between patients such that in subjects with very low testosterone levels effects on mood and muscle strength may be more relevant than effects on ventricular remodelling or blood pressure. The testosterone replacement preparation that we used was poorly tolerated by patients, so fewer patients than originally planned stayed on treatment for 12 months.
Fortunately there are new and better-tolerated preparations now available including gels, bioadhesive buccal tablets, and depot testosterone injections, which provide reliable testosterone delivery. This trial does not provide any data on the effects of testosterone replacement therapy on endpoints including hospitalization, deterioration in CHF, and death; longer, larger trials are needed.
Conclusions
This trial is the largest prospective study of testosterone replacement therapy in men with CHF. We have demonstrated a significant benefit in functional capacity by raising the serum levels of testosterone by about 40% and well within the normal physiological range. The delivery of the drug by the Androderm® system was disappointing with 55% of patients reporting skin reactions which was the most common reason for withdrawal also reported in other studies with patch systems.39 The correction of the metabolic maladaptations that characterize CHF by drug therapies has proven efficacy. A feature of the CHF syndrome is a relative androgen deficiency. This study suggests that testosterone replacement therapy to within the physiological range is another avenue of potential treatment for this group of patients.
Acknowledgements
Jo Hall, Katherine Kerry, and Jo Nettleship performed many of the hormone analyses. D Sugden performed the planimetry of the CT scans. The study was supported by a project grant from The National Heart Research Fund. Active and placebo testosterone patches (Androderm®) were supplied by Watson Pharmaceuticals Salt Lake City, USA. We thank Dr Norman Mazer for his help with this study.
Figure 1 Trial progression.
Figure 2 Change in distance walked on the ISWT. The treatment effect of testosterone compared with placebo over the 12-month treatment period. The mean change in distance walked was significantly greater in those patients taking testosterone compared with placebo (P=0.006, ANOVA).
Figure 3 Shows the correlation between the change in distance walked on the ISWT and the change in bio-available testosterone level at 3 months.
Figure 4 Shows the correlation between the change in distance walked on the ISWT and the change in bioavailable testosterone level at 6 months.
Figure 5 Shows the changes in serum testosterone levels over the 12-month study period.
Figure 6 Shows the changes in systolic and diastolic blood pressure over the 12-month study period.
Baseline data (mean±SD)
| Parameter . | Active group (n=37) . | Placebo group (n=39) . | P-valuea . |
|---|---|---|---|
| Age (years) | 63.1±10.7 | 64.9±9.3 | 0.4 |
| BMI (kg/m)2 | 27.9±4.08 | 27.7±4.1 | 0.8 |
| ISWT (m) | 280±162.9 | 298±158.9 | 0.6 |
| Dominant grip (kg) | 40.6±11.1 | 38.6±8.6 | 0.4 |
| Non-dominant grip (kg) | 37.6±10 | 35.9±8.2 | 0.4 |
| Resting pulse (min−1) | 75±14.1 | 68±11.3 | 0.04 |
| Systolic BP (mmHg) | 129±17.8 | 131±15.8 | 0.6 |
| Diastolic BP (mmHg) | 78±9.1 | 77±11.2 | 0.8 |
| EDD (mm) | 68.4±9.9 | 67.5±11.9 | 0.8 |
| Ejection fraction (%) | 33.8±10.4 | 33.1±11.8 | 0.6 |
| MLHF score | 39.9±22.2 | 32.8±25.9 | 0.2 |
| BDI score | 11.1±8.3 | 10±11.2 | 0.7 |
| BNP (pg/mL) | 206±226.2 | 161±143 | 0.3 |
| Haematocrit (L/L) | 0.43±0.04 | 0.43±0.04 | 0.5 |
| Creatinine (mmol/L) | 113.8±35.2 | 107.8±29.9 | 0.4 |
| PSA (µg/L) | 1.7±1.4 | 1.5±1.2 | 0.5 |
| FSH (nmol/L) | 5.32±4.5 | 6.9±9.8 | 0.4 |
| LH (nmol/L) | 5.1±4.5 | 7.6±14.6 | 0.3 |
| Total testosterone (nmol/L) | 13.9±5.3 | 12.1±5.4 | 0.16 |
| Bioavailable testosterone (nmol/L) | 4.7±1.9 | 4.6±2.6 | 0.9 |
| Aetiology CHF: IS/NIS (n) | 19/18 | 22/17 | 0.7b/0.7b |
| Cigarette smoker (n) | 10 | 6 | 0.2b |
| ACE/ARB (n) | 32/4 | 32/9 | 0.6b/0.2b |
| β-blocker (n) | 16 | 18 | 0.8b |
| Digoxin (n) | 19 | 16 | 0.4b |
| Diuretic (n) | 27 | 29 | 0.9b |
| NYHA class (II/III/IV) | 21/14/2 | 24/13/2 | 0.4b |
| Diabetes mellitus (n) | 5 | 8 | 0.4b |
| Parameter . | Active group (n=37) . | Placebo group (n=39) . | P-valuea . |
|---|---|---|---|
| Age (years) | 63.1±10.7 | 64.9±9.3 | 0.4 |
| BMI (kg/m)2 | 27.9±4.08 | 27.7±4.1 | 0.8 |
| ISWT (m) | 280±162.9 | 298±158.9 | 0.6 |
| Dominant grip (kg) | 40.6±11.1 | 38.6±8.6 | 0.4 |
| Non-dominant grip (kg) | 37.6±10 | 35.9±8.2 | 0.4 |
| Resting pulse (min−1) | 75±14.1 | 68±11.3 | 0.04 |
| Systolic BP (mmHg) | 129±17.8 | 131±15.8 | 0.6 |
| Diastolic BP (mmHg) | 78±9.1 | 77±11.2 | 0.8 |
| EDD (mm) | 68.4±9.9 | 67.5±11.9 | 0.8 |
| Ejection fraction (%) | 33.8±10.4 | 33.1±11.8 | 0.6 |
| MLHF score | 39.9±22.2 | 32.8±25.9 | 0.2 |
| BDI score | 11.1±8.3 | 10±11.2 | 0.7 |
| BNP (pg/mL) | 206±226.2 | 161±143 | 0.3 |
| Haematocrit (L/L) | 0.43±0.04 | 0.43±0.04 | 0.5 |
| Creatinine (mmol/L) | 113.8±35.2 | 107.8±29.9 | 0.4 |
| PSA (µg/L) | 1.7±1.4 | 1.5±1.2 | 0.5 |
| FSH (nmol/L) | 5.32±4.5 | 6.9±9.8 | 0.4 |
| LH (nmol/L) | 5.1±4.5 | 7.6±14.6 | 0.3 |
| Total testosterone (nmol/L) | 13.9±5.3 | 12.1±5.4 | 0.16 |
| Bioavailable testosterone (nmol/L) | 4.7±1.9 | 4.6±2.6 | 0.9 |
| Aetiology CHF: IS/NIS (n) | 19/18 | 22/17 | 0.7b/0.7b |
| Cigarette smoker (n) | 10 | 6 | 0.2b |
| ACE/ARB (n) | 32/4 | 32/9 | 0.6b/0.2b |
| β-blocker (n) | 16 | 18 | 0.8b |
| Digoxin (n) | 19 | 16 | 0.4b |
| Diuretic (n) | 27 | 29 | 0.9b |
| NYHA class (II/III/IV) | 21/14/2 | 24/13/2 | 0.4b |
| Diabetes mellitus (n) | 5 | 8 | 0.4b |
aComparison by unpaired t-test unless marked.
bComparison by chi-squared statistic with 2×2 table.
BP, blood pressure; FSH, follicle stimulating hormone; LH, luteinizing hormone; IS, ischaemic; NIS, non-ischaemic; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Baseline data (mean±SD)
| Parameter . | Active group (n=37) . | Placebo group (n=39) . | P-valuea . |
|---|---|---|---|
| Age (years) | 63.1±10.7 | 64.9±9.3 | 0.4 |
| BMI (kg/m)2 | 27.9±4.08 | 27.7±4.1 | 0.8 |
| ISWT (m) | 280±162.9 | 298±158.9 | 0.6 |
| Dominant grip (kg) | 40.6±11.1 | 38.6±8.6 | 0.4 |
| Non-dominant grip (kg) | 37.6±10 | 35.9±8.2 | 0.4 |
| Resting pulse (min−1) | 75±14.1 | 68±11.3 | 0.04 |
| Systolic BP (mmHg) | 129±17.8 | 131±15.8 | 0.6 |
| Diastolic BP (mmHg) | 78±9.1 | 77±11.2 | 0.8 |
| EDD (mm) | 68.4±9.9 | 67.5±11.9 | 0.8 |
| Ejection fraction (%) | 33.8±10.4 | 33.1±11.8 | 0.6 |
| MLHF score | 39.9±22.2 | 32.8±25.9 | 0.2 |
| BDI score | 11.1±8.3 | 10±11.2 | 0.7 |
| BNP (pg/mL) | 206±226.2 | 161±143 | 0.3 |
| Haematocrit (L/L) | 0.43±0.04 | 0.43±0.04 | 0.5 |
| Creatinine (mmol/L) | 113.8±35.2 | 107.8±29.9 | 0.4 |
| PSA (µg/L) | 1.7±1.4 | 1.5±1.2 | 0.5 |
| FSH (nmol/L) | 5.32±4.5 | 6.9±9.8 | 0.4 |
| LH (nmol/L) | 5.1±4.5 | 7.6±14.6 | 0.3 |
| Total testosterone (nmol/L) | 13.9±5.3 | 12.1±5.4 | 0.16 |
| Bioavailable testosterone (nmol/L) | 4.7±1.9 | 4.6±2.6 | 0.9 |
| Aetiology CHF: IS/NIS (n) | 19/18 | 22/17 | 0.7b/0.7b |
| Cigarette smoker (n) | 10 | 6 | 0.2b |
| ACE/ARB (n) | 32/4 | 32/9 | 0.6b/0.2b |
| β-blocker (n) | 16 | 18 | 0.8b |
| Digoxin (n) | 19 | 16 | 0.4b |
| Diuretic (n) | 27 | 29 | 0.9b |
| NYHA class (II/III/IV) | 21/14/2 | 24/13/2 | 0.4b |
| Diabetes mellitus (n) | 5 | 8 | 0.4b |
| Parameter . | Active group (n=37) . | Placebo group (n=39) . | P-valuea . |
|---|---|---|---|
| Age (years) | 63.1±10.7 | 64.9±9.3 | 0.4 |
| BMI (kg/m)2 | 27.9±4.08 | 27.7±4.1 | 0.8 |
| ISWT (m) | 280±162.9 | 298±158.9 | 0.6 |
| Dominant grip (kg) | 40.6±11.1 | 38.6±8.6 | 0.4 |
| Non-dominant grip (kg) | 37.6±10 | 35.9±8.2 | 0.4 |
| Resting pulse (min−1) | 75±14.1 | 68±11.3 | 0.04 |
| Systolic BP (mmHg) | 129±17.8 | 131±15.8 | 0.6 |
| Diastolic BP (mmHg) | 78±9.1 | 77±11.2 | 0.8 |
| EDD (mm) | 68.4±9.9 | 67.5±11.9 | 0.8 |
| Ejection fraction (%) | 33.8±10.4 | 33.1±11.8 | 0.6 |
| MLHF score | 39.9±22.2 | 32.8±25.9 | 0.2 |
| BDI score | 11.1±8.3 | 10±11.2 | 0.7 |
| BNP (pg/mL) | 206±226.2 | 161±143 | 0.3 |
| Haematocrit (L/L) | 0.43±0.04 | 0.43±0.04 | 0.5 |
| Creatinine (mmol/L) | 113.8±35.2 | 107.8±29.9 | 0.4 |
| PSA (µg/L) | 1.7±1.4 | 1.5±1.2 | 0.5 |
| FSH (nmol/L) | 5.32±4.5 | 6.9±9.8 | 0.4 |
| LH (nmol/L) | 5.1±4.5 | 7.6±14.6 | 0.3 |
| Total testosterone (nmol/L) | 13.9±5.3 | 12.1±5.4 | 0.16 |
| Bioavailable testosterone (nmol/L) | 4.7±1.9 | 4.6±2.6 | 0.9 |
| Aetiology CHF: IS/NIS (n) | 19/18 | 22/17 | 0.7b/0.7b |
| Cigarette smoker (n) | 10 | 6 | 0.2b |
| ACE/ARB (n) | 32/4 | 32/9 | 0.6b/0.2b |
| β-blocker (n) | 16 | 18 | 0.8b |
| Digoxin (n) | 19 | 16 | 0.4b |
| Diuretic (n) | 27 | 29 | 0.9b |
| NYHA class (II/III/IV) | 21/14/2 | 24/13/2 | 0.4b |
| Diabetes mellitus (n) | 5 | 8 | 0.4b |
aComparison by unpaired t-test unless marked.
bComparison by chi-squared statistic with 2×2 table.
BP, blood pressure; FSH, follicle stimulating hormone; LH, luteinizing hormone; IS, ischaemic; NIS, non-ischaemic; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Treatment effects of testosterone
| Parameter . | Placebo baseline . | Change from baseline: effect of placebo at 12 months . | Testosterone baseline . | Change from baseline: effect of testosterone at 12 months . | Treatment effect: Δ testosterone vs Δ placebo at 12 months (mean±standard error of the difference) . | P-value (ANOVA at 12 months) . | |
|---|---|---|---|---|---|---|---|
| Anabolic | |||||||
| BMI (kg/m2) | 27.7±4.1 | +3.8±3.1 | 27.9±4.0 | −0.09±1.7 | −0.46±0.6 | 0.5 | |
| Dominant handgrip strength (kg) | 38.6±8.6 | −0.6±5.3 | 40.6±11.1 | +1.0±4.0 | + 1.6±1.1 | 0.04 | |
| Non-dominant handgrip strength (kg) | 35.9±8.2 | −0.56±7.8 | 37.6±10 | 38.1±1.65 | + 1.1±1.5 | 0.27 | |
| Cross-sectional area thigh (cm2) | 6555±1501 | +52.6±720 | 6532±995 | +11.5±642 | −12±183a | 0.9a | |
| Cross-sectional area of calf (cm2) | 6582±1515 | −158.6±973 | 7034±1379 | +41.8±542.7 | + 71±139a | 0.6a | |
| Echocardiography | |||||||
| Ejection fraction (%) | 33.1±11.8 | +0.95±13.6 | 31.8±10.7 | +1.8±13.1 | +0.86±3.1 | 0.7 | |
| EDD (mm) | 67.5±11.9 | −3.3±13 | 68.4±9.9 | −1.9±5.9 | +0.7±2.9 | 0.48 | |
| Cross-sectional LV area (1) (mm) | 54.7±11.9 | −0.4±14.3 | 56.4±12.6 | +1.4±14.4 | +1.1±3.2 | 0.9 | |
| Cross sectional LV area (2) (mm) | 32.5±11.4 | +8.1±46.5 | 35.2±11.5 | −0.8±12.9 | −8.9±8.0 | 0.2 | |
| LV length (mm) | 9.0±1.08 | −0.58±1.6 | 8.5±1.3 | +0.26±0.7 | + 8.4±0.28 | 0.0001 | |
| LV mass index (g/m2) | 146±42.7 | −1.5±35.8 | 162±33.8 | 146±28.8 | −12.7±7.4 | 0.055 | |
| Bloods | |||||||
| Total testosterone (nmol/L) | 12.1±5.4 | +0.4±3.6 | 13.9±5.3 | +5.7±10.8 | + 5.3±1.82 | 0.0001 | |
| Bio-available testosterone (nmol/L) | 4.6±2.6 | +0.±2.0 | 4.7±1.9 | +2.32±4.6 | + 2.02±0.82 | 0.003 | |
| BNP (pg/mL) | 161.4±143 | +71.7±205 | 205.8±226.2 | +43.3±273.3 | −28.3±55.1 | 0.65 | |
| Creatinine (mmol/L) | 107.8±29.9 | −0.6±16.1 | 113.8±35.2 | +8.6±30.1 | +9.2±5.5 | 0.16 | |
| Haematocrit (L/L) | 0.43±0.04 | −0.005±0.003 | 0.43±0.04 | +0.009±0.3 | +0.014±0.007 | 0.03 | |
| PSA (ng/mL) | 1.5±1.2 | −0.08±0.5 | 1.7±1.42 | +0.08±0.7 | +0.088±0.13 | 0.19 | |
| Glucose (mmol/L) | 6.3±2.9 | +0.003±1.9 | 5.9±2.95 | −0.4±3.4 | −0.4±0.6 | 0.06 | |
| TNFα (pg/mL) | 3.13±8.99 | 1.91±2.19 | 3.29±9.69 | 2.53±3.55 | −0.76±10.4 | 0.85a | |
| Haemodynamic | |||||||
| Systolic BP (mmHg) | 131±15.8 | −4.4±13.9 | 129±17.8 | +1.6±17.4 | +6±3.6 | 0.013 | |
| Diastolic BP (mmHg) | 77±11.2 | −4.8±13.5 | 78±9.1 | −2.5±11.2 | +2.3±2.85 | 0.3 | |
| Pulse (min−1) | 68.3±11.3 | −0.3±10 | 75±14.1 | −2.1±10.5 | −1.8±2.35 | 0.38 | |
| Parameter . | Placebo baseline . | Change from baseline: effect of placebo at 12 months . | Testosterone baseline . | Change from baseline: effect of testosterone at 12 months . | Treatment effect: Δ testosterone vs Δ placebo at 12 months (mean±standard error of the difference) . | P-value (ANOVA at 12 months) . | |
|---|---|---|---|---|---|---|---|
| Anabolic | |||||||
| BMI (kg/m2) | 27.7±4.1 | +3.8±3.1 | 27.9±4.0 | −0.09±1.7 | −0.46±0.6 | 0.5 | |
| Dominant handgrip strength (kg) | 38.6±8.6 | −0.6±5.3 | 40.6±11.1 | +1.0±4.0 | + 1.6±1.1 | 0.04 | |
| Non-dominant handgrip strength (kg) | 35.9±8.2 | −0.56±7.8 | 37.6±10 | 38.1±1.65 | + 1.1±1.5 | 0.27 | |
| Cross-sectional area thigh (cm2) | 6555±1501 | +52.6±720 | 6532±995 | +11.5±642 | −12±183a | 0.9a | |
| Cross-sectional area of calf (cm2) | 6582±1515 | −158.6±973 | 7034±1379 | +41.8±542.7 | + 71±139a | 0.6a | |
| Echocardiography | |||||||
| Ejection fraction (%) | 33.1±11.8 | +0.95±13.6 | 31.8±10.7 | +1.8±13.1 | +0.86±3.1 | 0.7 | |
| EDD (mm) | 67.5±11.9 | −3.3±13 | 68.4±9.9 | −1.9±5.9 | +0.7±2.9 | 0.48 | |
| Cross-sectional LV area (1) (mm) | 54.7±11.9 | −0.4±14.3 | 56.4±12.6 | +1.4±14.4 | +1.1±3.2 | 0.9 | |
| Cross sectional LV area (2) (mm) | 32.5±11.4 | +8.1±46.5 | 35.2±11.5 | −0.8±12.9 | −8.9±8.0 | 0.2 | |
| LV length (mm) | 9.0±1.08 | −0.58±1.6 | 8.5±1.3 | +0.26±0.7 | + 8.4±0.28 | 0.0001 | |
| LV mass index (g/m2) | 146±42.7 | −1.5±35.8 | 162±33.8 | 146±28.8 | −12.7±7.4 | 0.055 | |
| Bloods | |||||||
| Total testosterone (nmol/L) | 12.1±5.4 | +0.4±3.6 | 13.9±5.3 | +5.7±10.8 | + 5.3±1.82 | 0.0001 | |
| Bio-available testosterone (nmol/L) | 4.6±2.6 | +0.±2.0 | 4.7±1.9 | +2.32±4.6 | + 2.02±0.82 | 0.003 | |
| BNP (pg/mL) | 161.4±143 | +71.7±205 | 205.8±226.2 | +43.3±273.3 | −28.3±55.1 | 0.65 | |
| Creatinine (mmol/L) | 107.8±29.9 | −0.6±16.1 | 113.8±35.2 | +8.6±30.1 | +9.2±5.5 | 0.16 | |
| Haematocrit (L/L) | 0.43±0.04 | −0.005±0.003 | 0.43±0.04 | +0.009±0.3 | +0.014±0.007 | 0.03 | |
| PSA (ng/mL) | 1.5±1.2 | −0.08±0.5 | 1.7±1.42 | +0.08±0.7 | +0.088±0.13 | 0.19 | |
| Glucose (mmol/L) | 6.3±2.9 | +0.003±1.9 | 5.9±2.95 | −0.4±3.4 | −0.4±0.6 | 0.06 | |
| TNFα (pg/mL) | 3.13±8.99 | 1.91±2.19 | 3.29±9.69 | 2.53±3.55 | −0.76±10.4 | 0.85a | |
| Haemodynamic | |||||||
| Systolic BP (mmHg) | 131±15.8 | −4.4±13.9 | 129±17.8 | +1.6±17.4 | +6±3.6 | 0.013 | |
| Diastolic BP (mmHg) | 77±11.2 | −4.8±13.5 | 78±9.1 | −2.5±11.2 | +2.3±2.85 | 0.3 | |
| Pulse (min−1) | 68.3±11.3 | −0.3±10 | 75±14.1 | −2.1±10.5 | −1.8±2.35 | 0.38 | |
LV, left ventricle; LV mass index, LV mass in grammes/body surface area in gm/m2.
aAnalysis by unpaired t-tests at six months.
Treatment effects of testosterone
| Parameter . | Placebo baseline . | Change from baseline: effect of placebo at 12 months . | Testosterone baseline . | Change from baseline: effect of testosterone at 12 months . | Treatment effect: Δ testosterone vs Δ placebo at 12 months (mean±standard error of the difference) . | P-value (ANOVA at 12 months) . | |
|---|---|---|---|---|---|---|---|
| Anabolic | |||||||
| BMI (kg/m2) | 27.7±4.1 | +3.8±3.1 | 27.9±4.0 | −0.09±1.7 | −0.46±0.6 | 0.5 | |
| Dominant handgrip strength (kg) | 38.6±8.6 | −0.6±5.3 | 40.6±11.1 | +1.0±4.0 | + 1.6±1.1 | 0.04 | |
| Non-dominant handgrip strength (kg) | 35.9±8.2 | −0.56±7.8 | 37.6±10 | 38.1±1.65 | + 1.1±1.5 | 0.27 | |
| Cross-sectional area thigh (cm2) | 6555±1501 | +52.6±720 | 6532±995 | +11.5±642 | −12±183a | 0.9a | |
| Cross-sectional area of calf (cm2) | 6582±1515 | −158.6±973 | 7034±1379 | +41.8±542.7 | + 71±139a | 0.6a | |
| Echocardiography | |||||||
| Ejection fraction (%) | 33.1±11.8 | +0.95±13.6 | 31.8±10.7 | +1.8±13.1 | +0.86±3.1 | 0.7 | |
| EDD (mm) | 67.5±11.9 | −3.3±13 | 68.4±9.9 | −1.9±5.9 | +0.7±2.9 | 0.48 | |
| Cross-sectional LV area (1) (mm) | 54.7±11.9 | −0.4±14.3 | 56.4±12.6 | +1.4±14.4 | +1.1±3.2 | 0.9 | |
| Cross sectional LV area (2) (mm) | 32.5±11.4 | +8.1±46.5 | 35.2±11.5 | −0.8±12.9 | −8.9±8.0 | 0.2 | |
| LV length (mm) | 9.0±1.08 | −0.58±1.6 | 8.5±1.3 | +0.26±0.7 | + 8.4±0.28 | 0.0001 | |
| LV mass index (g/m2) | 146±42.7 | −1.5±35.8 | 162±33.8 | 146±28.8 | −12.7±7.4 | 0.055 | |
| Bloods | |||||||
| Total testosterone (nmol/L) | 12.1±5.4 | +0.4±3.6 | 13.9±5.3 | +5.7±10.8 | + 5.3±1.82 | 0.0001 | |
| Bio-available testosterone (nmol/L) | 4.6±2.6 | +0.±2.0 | 4.7±1.9 | +2.32±4.6 | + 2.02±0.82 | 0.003 | |
| BNP (pg/mL) | 161.4±143 | +71.7±205 | 205.8±226.2 | +43.3±273.3 | −28.3±55.1 | 0.65 | |
| Creatinine (mmol/L) | 107.8±29.9 | −0.6±16.1 | 113.8±35.2 | +8.6±30.1 | +9.2±5.5 | 0.16 | |
| Haematocrit (L/L) | 0.43±0.04 | −0.005±0.003 | 0.43±0.04 | +0.009±0.3 | +0.014±0.007 | 0.03 | |
| PSA (ng/mL) | 1.5±1.2 | −0.08±0.5 | 1.7±1.42 | +0.08±0.7 | +0.088±0.13 | 0.19 | |
| Glucose (mmol/L) | 6.3±2.9 | +0.003±1.9 | 5.9±2.95 | −0.4±3.4 | −0.4±0.6 | 0.06 | |
| TNFα (pg/mL) | 3.13±8.99 | 1.91±2.19 | 3.29±9.69 | 2.53±3.55 | −0.76±10.4 | 0.85a | |
| Haemodynamic | |||||||
| Systolic BP (mmHg) | 131±15.8 | −4.4±13.9 | 129±17.8 | +1.6±17.4 | +6±3.6 | 0.013 | |
| Diastolic BP (mmHg) | 77±11.2 | −4.8±13.5 | 78±9.1 | −2.5±11.2 | +2.3±2.85 | 0.3 | |
| Pulse (min−1) | 68.3±11.3 | −0.3±10 | 75±14.1 | −2.1±10.5 | −1.8±2.35 | 0.38 | |
| Parameter . | Placebo baseline . | Change from baseline: effect of placebo at 12 months . | Testosterone baseline . | Change from baseline: effect of testosterone at 12 months . | Treatment effect: Δ testosterone vs Δ placebo at 12 months (mean±standard error of the difference) . | P-value (ANOVA at 12 months) . | |
|---|---|---|---|---|---|---|---|
| Anabolic | |||||||
| BMI (kg/m2) | 27.7±4.1 | +3.8±3.1 | 27.9±4.0 | −0.09±1.7 | −0.46±0.6 | 0.5 | |
| Dominant handgrip strength (kg) | 38.6±8.6 | −0.6±5.3 | 40.6±11.1 | +1.0±4.0 | + 1.6±1.1 | 0.04 | |
| Non-dominant handgrip strength (kg) | 35.9±8.2 | −0.56±7.8 | 37.6±10 | 38.1±1.65 | + 1.1±1.5 | 0.27 | |
| Cross-sectional area thigh (cm2) | 6555±1501 | +52.6±720 | 6532±995 | +11.5±642 | −12±183a | 0.9a | |
| Cross-sectional area of calf (cm2) | 6582±1515 | −158.6±973 | 7034±1379 | +41.8±542.7 | + 71±139a | 0.6a | |
| Echocardiography | |||||||
| Ejection fraction (%) | 33.1±11.8 | +0.95±13.6 | 31.8±10.7 | +1.8±13.1 | +0.86±3.1 | 0.7 | |
| EDD (mm) | 67.5±11.9 | −3.3±13 | 68.4±9.9 | −1.9±5.9 | +0.7±2.9 | 0.48 | |
| Cross-sectional LV area (1) (mm) | 54.7±11.9 | −0.4±14.3 | 56.4±12.6 | +1.4±14.4 | +1.1±3.2 | 0.9 | |
| Cross sectional LV area (2) (mm) | 32.5±11.4 | +8.1±46.5 | 35.2±11.5 | −0.8±12.9 | −8.9±8.0 | 0.2 | |
| LV length (mm) | 9.0±1.08 | −0.58±1.6 | 8.5±1.3 | +0.26±0.7 | + 8.4±0.28 | 0.0001 | |
| LV mass index (g/m2) | 146±42.7 | −1.5±35.8 | 162±33.8 | 146±28.8 | −12.7±7.4 | 0.055 | |
| Bloods | |||||||
| Total testosterone (nmol/L) | 12.1±5.4 | +0.4±3.6 | 13.9±5.3 | +5.7±10.8 | + 5.3±1.82 | 0.0001 | |
| Bio-available testosterone (nmol/L) | 4.6±2.6 | +0.±2.0 | 4.7±1.9 | +2.32±4.6 | + 2.02±0.82 | 0.003 | |
| BNP (pg/mL) | 161.4±143 | +71.7±205 | 205.8±226.2 | +43.3±273.3 | −28.3±55.1 | 0.65 | |
| Creatinine (mmol/L) | 107.8±29.9 | −0.6±16.1 | 113.8±35.2 | +8.6±30.1 | +9.2±5.5 | 0.16 | |
| Haematocrit (L/L) | 0.43±0.04 | −0.005±0.003 | 0.43±0.04 | +0.009±0.3 | +0.014±0.007 | 0.03 | |
| PSA (ng/mL) | 1.5±1.2 | −0.08±0.5 | 1.7±1.42 | +0.08±0.7 | +0.088±0.13 | 0.19 | |
| Glucose (mmol/L) | 6.3±2.9 | +0.003±1.9 | 5.9±2.95 | −0.4±3.4 | −0.4±0.6 | 0.06 | |
| TNFα (pg/mL) | 3.13±8.99 | 1.91±2.19 | 3.29±9.69 | 2.53±3.55 | −0.76±10.4 | 0.85a | |
| Haemodynamic | |||||||
| Systolic BP (mmHg) | 131±15.8 | −4.4±13.9 | 129±17.8 | +1.6±17.4 | +6±3.6 | 0.013 | |
| Diastolic BP (mmHg) | 77±11.2 | −4.8±13.5 | 78±9.1 | −2.5±11.2 | +2.3±2.85 | 0.3 | |
| Pulse (min−1) | 68.3±11.3 | −0.3±10 | 75±14.1 | −2.1±10.5 | −1.8±2.35 | 0.38 | |
LV, left ventricle; LV mass index, LV mass in grammes/body surface area in gm/m2.
aAnalysis by unpaired t-tests at six months.
Serious adverse events
| Events . | Testosterone . | Placebo . |
|---|---|---|
| New diagnosis arrhythmia (AF) | 2 | |
| New diagnosis diabetes mellitus | 2 | |
| Sudden death | 1 | |
| Stroke | 1 | |
| Diagnosed colonic carcinoma | 1 | |
| Skin reactions | 10 | 9 |
| Hospital admission due to | ||
| Unstable angina pectoris | 1 | |
| Presyncope | 1 | 1 |
| Lower respiratory tract infection | 1 | 1 |
| Exacerbation of heart failure | 2 | 2 |
| Events . | Testosterone . | Placebo . |
|---|---|---|
| New diagnosis arrhythmia (AF) | 2 | |
| New diagnosis diabetes mellitus | 2 | |
| Sudden death | 1 | |
| Stroke | 1 | |
| Diagnosed colonic carcinoma | 1 | |
| Skin reactions | 10 | 9 |
| Hospital admission due to | ||
| Unstable angina pectoris | 1 | |
| Presyncope | 1 | 1 |
| Lower respiratory tract infection | 1 | 1 |
| Exacerbation of heart failure | 2 | 2 |
Serious adverse events
| Events . | Testosterone . | Placebo . |
|---|---|---|
| New diagnosis arrhythmia (AF) | 2 | |
| New diagnosis diabetes mellitus | 2 | |
| Sudden death | 1 | |
| Stroke | 1 | |
| Diagnosed colonic carcinoma | 1 | |
| Skin reactions | 10 | 9 |
| Hospital admission due to | ||
| Unstable angina pectoris | 1 | |
| Presyncope | 1 | 1 |
| Lower respiratory tract infection | 1 | 1 |
| Exacerbation of heart failure | 2 | 2 |
| Events . | Testosterone . | Placebo . |
|---|---|---|
| New diagnosis arrhythmia (AF) | 2 | |
| New diagnosis diabetes mellitus | 2 | |
| Sudden death | 1 | |
| Stroke | 1 | |
| Diagnosed colonic carcinoma | 1 | |
| Skin reactions | 10 | 9 |
| Hospital admission due to | ||
| Unstable angina pectoris | 1 | |
| Presyncope | 1 | 1 |
| Lower respiratory tract infection | 1 | 1 |
| Exacerbation of heart failure | 2 | 2 |
References
McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure.
Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; data from the Swedish Hospital Discharge Registry 1988 to 2000.
Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure.
Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin.
Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH.
Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, Lien E, Froland SS, Aukrust P. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure.
Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia.
Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure.
Kontoleon PE, Anastasiou-Nana MI, Papapetrou PD, Alexopoulos G, Ktenas V, Rapti AC, Tsagalou EP, Nanas JN. Hormonal profile in patients with congestive heart failure.
Moriyama Y, Yasue H, Yoshimura M, Mizuno Y, Nishiyama K, Tsunoda R, Kawano H, Kugiyama K, Ogawa H, Saito Y, Nakao K. The plasma levels of dehydroepiandrosterone sulfate are decreased in patients with chronic heart failure in proportion to the severity.
Pugh PJ, Jones TH, Channer KS. Acute haemodynamic effects of testosterone in men with chronic heart failure.
Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men.
Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study.
Rumsfeld JS, Havranek E, Masoudi FA, Peterson ED, Jones P, Tooley JF, Krumholz HM, Spertus JA. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure.
Wang C, Swedloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group.
Davis WM, Long SF, Lin TL. Nandrolone decanoate reduces the premature mortality of cardiomyopathic hamsters.
Tomoda H. Effect of oxymetholone on left ventricular dimensions in heart failure secondary to idiopathic dilated cardiomyopathy or to mitral or aortic regurgitation.
Pugh PJ, Jones RD, West JN, Jones TH, Channer KS. Testosterone treatment for men with chronic heart failure.
Karila TA, Karjalainen JE, Mantysaari MJ, Viitasalo MT, Seppala TA. Anabolic androgenic steroids produce dose-dependant increase in left ventricular mass in power atheletes, and this effect is potentiated by concomitant use of growth hormone.
De Piccoli B, Giada F, Benettin A, Sartori F, Piccolo E. Anabolic steroid use in body builders: an echocardiographic study of left ventricle morphology and function.
Arver S, Dobs AS, Meikle AW, Caramelli KE, Rajaram L, Sanders SW, Mazer NA. Long-term efficacy and safety of a permeation-enhanced testosterone transdermal system in hypogonadal men.
English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study.
Morales FJ, Martinez A, Mendez M, Agarrado A, Ortega F, Fernandez-Guerra J, Montemayor T, Burgos J. A shuttle walk test for assessment of functional capacity in chronic heart failure.
Lewis ME, Newall C, Townend JN, Hill SL, Bonser RS. Incremental shuttle walk test in the assessment of patients for heart transplantation.
Morales FJ, Montemayor T, Martinez A. Shuttle versus six-minute walk test in the prediction of outcome in chronic heart failure.
Tremblay RR, Dube JY. Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives.
Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle.
Grinspoon S, Corcoran C, Askari H, Schoenfeld D, Wolf L, Burrows B, Walsh M, Hayden D, Parlman K, Anderson E, Basgoz N, Klibanski A. Effects of androgen administration in men with the AIDS wasting syndrome. A randomized, double-blind, placebo-controlled trial.
Strawford A, Barbieri T, Neese R, Van Loan M, Christiansen M, Hoh R, Sathyan G, Skowronski R, King J, Hellerstein M. Effects of nandrolone decanoate therapy in borderline hypogonadal men with HIV-associated weight loss.
Ferreira IM, Verreschi IT, Nery LE, Goldstein RS, Zamel N, Brooks D, Jardim JR. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients.
Tamaki T, Uchiyama S, Uchiyama Y, Akatsuka A, Roy RR, Edgerton VR. Anabolic steroids increase exercise tolerance.
Van Zyl CG, Noakes TD, Lambert MI. Anabolic-androgenic steroid increases running endurance in rats.
Di Bello V, Giorgi D, Bianchi M, Bertini A, Caputo MT, Valenti G, Furioso O, Alessandri L, Paterni M, Giusti C. Effects of anabolic-androgenic steroids on weight-lifters' myocardium: an ultrasonic videodensitometric study.
Dickerman RD, Schaller F, Zachariah NY, McConathy WJ. Left ventricular size and function in elite bodybuilders using anabolic steroids.
Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis-effects of 6 months of treatment on bone mineral density and cardiovascular risk factors.
Scragg JL, Jones RD, Channer KS, Jones TH, Peers C. Testosterone is a potent inhibitor of L-type Ca2+ channels.
Holmang A, Svedberg J, Jennische E, Bjorntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats.
Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium.
Wang C, Eyre DR, Clark R, Kleinberg D, Newman C, Iranmanesh A, Veldhuis J, Dudley RE, Berman N, Davidson T, Barstow TJ, Sinow R, Alexander G, Swerdloff RS. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—a clinical research center study.