-
PDF
- Split View
-
Views
-
Cite
Cite
David J. Farwell, Nick Freemantle, Neil Sulke, The clinical impact of implantable loop recorders in patients with syncope, European Heart Journal, Volume 27, Issue 3, February 2006, Pages 351–356, https://doi.org/10.1093/eurheartj/ehi602
Close - Share Icon Share
Abstract
Aims Implantable loop recorders (ILR) provide an opportunity to record ECG data from a spontaneous syncopal event. We conducted a randomized study to investigate the impact of the Reveal Plus ILR on an unselected population of patients with recurrent syncope. Initial follow-up (at least 6 months) did not demonstrate a reduction in syncopal events or an improvement in quality of life. We report the planned extension of follow-up to 18 months.
Methods and results All patients presenting acutely with recurrent unexplained syncope over a 16-month period, following a basic clinical work-up, were randomized to receive the ILR or conventional investigation and management. A total of 421 patients presented, 201 were eligible, median age 74, (IQ range 61–81) 54% female, with median syncopes 3 (IQ range 2–6). Median follow-up 17 months (IQ range 9–23). 42 (43%) of ILR patients and 8 (6%) of conventional patients received an ECG diagnosis (hazard ratio 6.53, 95% CI 3.73–11.4, P<0.001). Time to second syncope was significantly longer for ILR patients, although of borderline significance (P=0.04). A greater variety of diagnoses and treatments were seen in ILR patients. ILR patients had fewer post-randomization investigations and fewer days in hospital; however, cost savings were not statistically significant. There was improved quality of life in the ILR group (visual analogue scales, P=0.03) for general wellbeing. Overall mortality was 12% with no difference between the two groups.
Conclusion Investigation by the ILR significantly increases the diagnostic rate and ECG directed treatments in a typical unselected syncopal population. Long-term follow-up has demonstrated a significant subsequent reduction in syncopal events with improved quality of life.
Introduction
Syncope is a common disorder with an annual incidence of 1.3–2.7 episodes per thousand of population per annum.1,2 Retrospective studies suggest that up to 40% of the general population have experienced an episode of syncope in their lifetime.3–5 It is the cause of 6% of all acute medical admissions and in 3% of emergency room consultations.6–9
Determination of the cause of syncope is often made on the basis of history and examination alone. However, the gold standard of diagnosis remains the recording of physical observations during a spontaneous event.10 Syncope occurs at random, unpredictably and can be infrequent though disabling.11 Owing to the transitory nature of these symptoms, the opportunity to record such data seldom arises. Investigations such as head up tilt testing, carotid sinus massage and electrophysiological studies attempt to provoke syncopal symptoms. As with any provocative test, the specificity and clinical relevance of such investigations are hard to evaluate.
The Reveal Plus (Medtronic USA) implantable loop recorder (ILR) is small, requires no intracardiac electrodes and can be implanted, quickly and easily, subcutaneously under local anaesthetic. It records a single channel ECG and can be manually or automatically triggered following a syncopal episode. Improved diagnostic rates in selected populations of undiagnosed, highly symptomatic syncopal patients have been reported.12–14
Previously reported15 6-month follow-up of a cohort of patients with ILR has demonstrated greatly improved diagnostic rates. We planned to extend follow-up by a further 12 months to observe the clinical rather than mechanistic effects of ILR use.
Methods
Objectives
The purpose of the study was to determine the clinical and cost effectiveness of an ILR in the management of recurrent syncope in an unselected patient population with recurrent syncope.
Patients
From 1 September 2000 to 31 December 2001 (16 months), every patient presenting to our institution with syncope was assessed by the investigators. Consecutive patients with recurrent syncope and without a definite diagnosis following initial clinical work-up were recruited into the Eastbourne Syncope Assessment Study (EaSyAS) and randomly assigned to investigation by ILR or conventional means.
Interventions
Initial patient evaluation is described elsewhere.15 The ILR was sited, in left pectoral region and gain and sensitivity programmed, according to the manufacturer's instructions. It was set to record three patient activations and five automatic activations. Automatic activation was programmed for a ventricular pause of >3 s, a ventricular rate of <40 bpm, or a ventricular rate >165 bpm for more than 16 beats. Patients were taught to use the device and were required to activate it once, by themselves, prior to discharge post-implantation.
Outcomes
The study of primary, secondary, and Tertiary outcome measures are described subsequently. Primary: Time to ECG diagnosis. Secondary: (1) Time to first recurrence of syncope following study induction. (2) Time to second recurrence of syncope following study induction. (3) Time to the introduction of ECG guided therapy. Tertiary: (1) Quality of life. Measured by SF-12 questionnaire and visual analogue scales (VAS) at induction, 6, 12, and 18 months post-enrolment. (2) Cost effectiveness. Costs incurred by further hospital admission and investigations for syncope, calculated from time of device implantation to censorship, were based on local National Health Service (NHS) costs.16
Sample size
A sample size of 200 was considered appropriate to detect an 18% improvement in ECG diagnosis, assuming constant proportional hazards, 90 power (1-beta) and a conventional alpha value of 0.05 (twin tailed—equivalent to 0.025 single tailed).
Statistical methods
Randomization was by random number tables. Patients were allocated by sealed envelopes held in the study centre. Analysis was by intention to treat. Time to event outcomes were described using Kaplan Meier curves, and hazard ratios determined using Cox proportional hazards models, in which treatment group was the only factor included, which were also used to create prognostic models, predicting outcome using baseline characteristics of patients.17 Difference in mean cost was described, and 95% confidence intervals for costs were derived using non-parametric bootstrap methods.18 All analyses were undertaken using SAS 8.1 (SAS Institute, Cary, NC, USA). Quality of life data were analysed between groups by Student's t-test (normal distribution confirmed by the Shapiro–Wilk W test19).
Ethics
All patients gave written informed consent. The study was approved by the local Ethics Committee.
Results
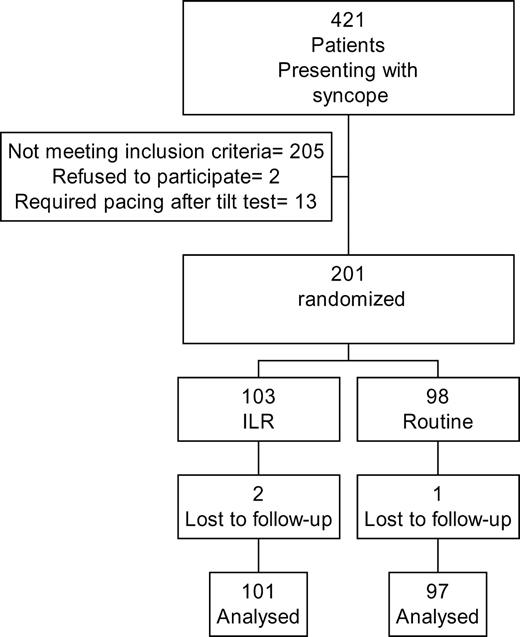
A total of 421 syncopal patients presented from 1 September 2000 to 31 December 2001 (16 months) (Figure 1). 205 patients failed to meet inclusion criteria (Figure 2). Twenty-two patients achieved a diagnosis by initial evaluation by history and examination alone, 67 were diagnosed as having a neurological cause for their syncope, and 19 were diagnosed by 24 h holter. Of the remaining 313, 97 had no previous history of syncope. This left 216 patients fulfilling enrolment criteria. Two refused study enrolment and 13 required cardiac pacing after tilt test and CSM according to ESC and ACC/AHA guidelines. 201 patients were randomized, 103 received ILR, and 98 conventional investigation and management. Two patients who received ILR and one conventional management were lost to follow-up.
Baseline clinical characteristics were similar for both groups (Table 1). Mean event rate prior to enrolment was 1.50 syncopal episodes per year.
By study final census (30 June 2003), median follow-up was 17 months (IQ range 9–23). Eighty-five patients had a further syncopal event, 48 with ILR, and 37 without. Mean syncopal event rate approximately halved during follow-up (0.60 syncopes/year).
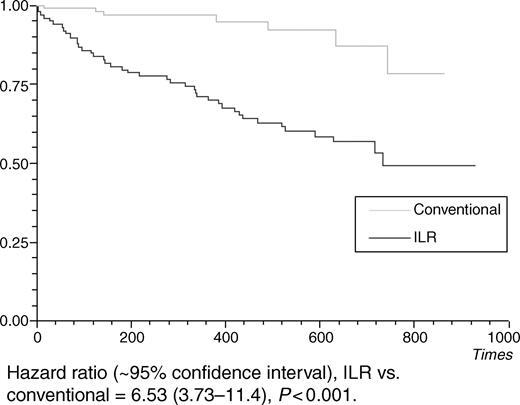
At censorship, 43 patients in the ILR group had an ECG diagnosis compared with seven in the non-ILR group. Hazard ratio for time to ECG diagnosis was 6.53 (95% CI 3.73–11.4, P<0.0001) (Figure 3). Twenty-seven of the 43 (63%) ILR diagnoses were made at the first syncope post-induction, 12 diagnoses were made at the second syncope, and the remaining four diagnoses required more than two syncopes to be achieved. Failure to diagnose an episode occurred because the patient or spouse had failed to activate the device and the data in the auto-holters at device interrogation failed to cover the time period of the syncope, usually due to ‘over-writing’ of the index event by subsequent non-syncopal episodes. Eight (19%) ILR diagnoses were made with data from the auto-activation feature of the device. Of the 50 patients who received an ECG diagnosis, 15 were bradycardic, three had SVT, and three had VT (Table 2).
ECG recording of an event demonstrated sinus rhythm in 26 syncopal patients. This data with concurrent clinical data enabled this group to be further subdivided into 17 patients with vaso-vagal syncope, five with epilepsy, and three with hyperventilation. One patient was diagnosed having reactive hypoglycaemia following an abnormal prolonged glucose tolerance test. Sixteen patients with ILR went on to receive pacemakers (15 bradycardic and one vaso-vagal), whereas four non-ILR patients received device therapy (three pacemakers and one ICD). Forty-three patients with ILR were commenced on ECG guided therapies by census compared with seven in the conventional group (Table 3). Time to ECG directed therapy was quicker for the ILR group (hazard ratio 6.53, 95% CI 3.73–11.4, P<0.001).
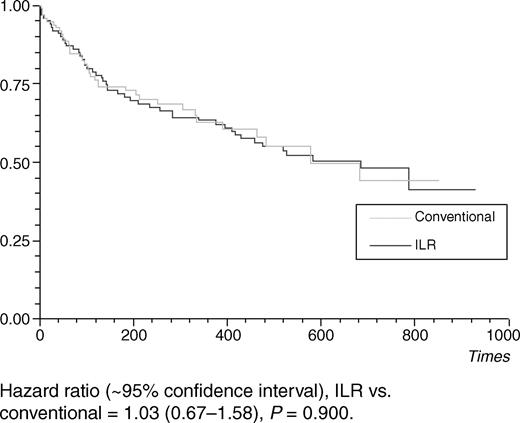
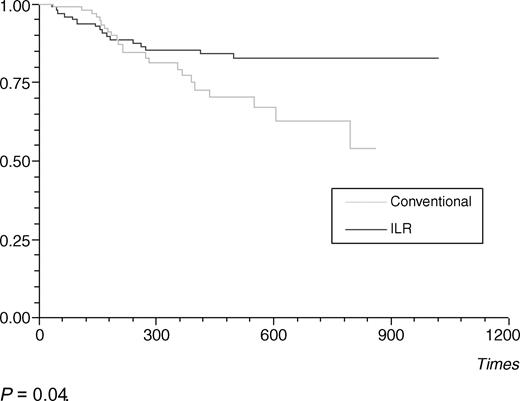
Time to first syncope recurrence was similar to patients in both study groups (Figure 4). Thirty-nine patients had a second syncope during follow-up, 16 with ILR, and 23 without ILR. Further syncope was predicted by a greater number syncopal episodes prior to enrolment (P=0.002) and the presence of any ECG conduction defect (P=0.003). Time to second syncope was significantly longer for the ILR group (P=0.04) (Figure 5). There was a trend towards improved quality of life in the ILR group compared with that of controls with significant increases observed in VAS of general wellbeing (P=0.03) at 18 months, no change was noted in SF-12 scores.
A highly significant reduction in subsequent investigational costs £37.9 (0/0/100 lower quartile/median/upper quartile) vs. £108 (0/100/200 lower quartile/median/upper quartile) (mean difference £70.1, 95% CI £40.3–99.3, P<0.001) was observed. Overall costs (including hospital stay) incurred post-randomization, not including ILR (list price £1350), were lower in the ILR group, £820 (0/0/200 lower quartile/median/upper quartile) vs. £1380 (0/100/800 lower quartile/median/upper quartile) but not significantly so (mean difference £555, 95% CI £252–£1990, P=0.28).
There were no device related adverse events. There were 17 deaths (8% mortality) at censorship, eight in the ILR, and nine in the conventional group. Two of the deaths (non-ILR) were sudden and may have been consistent with sudden cardiac death.
Discussion
This study investigated a typical unselected western population suffering from recurrent syncope and shows that the use of the ILR substantially improves diagnostic rates. The study population underwent a pre-randomization basic clinical work-up only. All patients with any cardiac abnormality (history of heart disease, abnormal cardiac examination, or 12 lead ECG) were initially investigated by 24 h holter (and echocardiogram when indicated). Only those without diagnosis at this stage were subsequently enrolled into the randomized trial. This reflects European practice and furthermore is encouraged by the UK guidelines for the use of implantable cardioverter defibrillators.20 Only one study patient underwent diagnostic electrophysiological testing (in the non-ILR group). EP studies for syncope diagnosis alone are used much less frequently in the Europe than in the United States. Our investigation differs from all other studies using the Reveal device, which have used diagnostic EP testing prior to ILRl implantation in patients with impaired LV function or ECG evidence of abnormal intracardiac conduction.13,21
Time to second syncope
We have previously demonstrated that the ILR is highly effective in the diagnosis of syncope this in this patient population. However, initial follow-up failed to demonstrate a reduction in further syncopal events or an improvement in quality of life.15 With extended follow-up, ILR patients achieved a longer time to second syncope, suggesting more appropriate treatment. Figure 5 demonstrates similar rates of second syncopes (between groups) up to about 300 days from randomization. At this point, the curves diverge with a marked reduction in the rate of further events in the ILR group. We believe that the initial period is mainly due to the occurrence of non-arrhythmic syncopes. Furthermore, many of these non-arrhythmic syncopes were due to vaso-vagal episodes (65%) for which there may be no effective therapy. Of the treatments prescribed to treat diagnosed syncope, only cardiac pacing has consistently been shown to improve symptoms.22,23 Therefore, it is not until later in follow-up that treatable causes of syncope are diagnosed thus delaying the observed effect of the ILR.15
Improved diagnosis rate
Previous data would suggest an actuarial risk of further syncope occurring in the study group of 29%.24 Forty-seven per cent of ILR patients went on to have at least one further episode of syncope, higher than expected. The ILR diagnostic rate was 42% and would have been higher if all syncopal events had been captured by the ILR.25 Achieving a diagnosis at the first event may have been hampered by the fact that our population was older and less symptomatic than previous groups studied.14,17,26 Older patients tended to find it more difficult to activate the ILR after syncope and were less able to present rapidly following a syncopal event thus losing valuable auto-holter data.
Does the ILR make a diagnosis every time syncope occurs?
More than a third of patients (37%) failed to capture their first syncopal event with the ILR. This was due to a failure to activate the ILR or to a delay between the syncopal event and subsequent device interrogation allowing the automatic holters covering the time of the event to be overwritten by subsequently captured data. However, with long-term follow-up, only 5% of ILR patients (who had a further syncope) failed to achieve a diagnosis. This is a slight improvement to other studies, which report a failure rate of 9–20%.13,27 The diagnostic rate of the ILR was notably enhanced by the use of automatic holters (19% of all ILR diagnoses). We recommend regular follow-up of patients with an ILR to
Interrogate the device.
‘Fine-tune’ device sensitivity for auto-activation.
Re-educate patients about the technique of manual activation.
Encourage early presentation after any syncopal event to prevent overwriting of the auto-holters and the loss of diagnostic data.
Diagnoses achieved
The types and frequency of diagnoses achieved are similar to those observed by other investigators.25,27 Forty-nine per cent of study patients were syncopal due to an arrhythmia by accepted diagnostic criteria.28 Other studies have demonstrated greater frequencies of arrhythmias,13 but they have selected a patient population specifically with confirmed heart disease. Fifty-one per cent of patients demonstrated sinus rhythm during an episode of syncope. On the basis of concurrent clinical findings, a more precise diagnosis was possible, enabling the distinction between neurological seizures, vaso-vagal episodes and episodes of hyperventilation and thus the use of a greater variety of appropriate therapies in the patients investigated with the ILR.
ECG directed treatment
As a result of more rapid diagnosis, ECG directed therapy was commenced significantly earlier in the ILR group and a greater variety of therapies were commenced. All therapies were based on AHA and ESC Class I guidelines.28,29 An increase time to second syncope suggests that therapies commenced upon ILR data were appropriate.
Adverse events
Other studies have reported few complications from ILR insertion.27 We report no major complications. There was no difference in mortality between groups. The mortality rate of 8% is similar to other studies have reported.30–32 Age at enrolment was the only baseline characteristic which predicted mortality.
Costs
There is a high initial cost for the ILR (UK list price £1350). We have demonstrated a small significant reduction in costs of further investigations with the use of ILR. However, no significant reduction in overall subsequent costs was observed.
Absence of device placebo effect
Syncope may be precipitated by abrupt physical or psycho-social stress. An open label trial could be confounded by a placebo effect.33 The presence of an ILR made no difference to the time to first recurrence of syncope. This observation would suggest that ILR does not have an important placebo effect in the prevention of syncope.
Quality of life
Patients with recurrent syncope have a greatly reduced quality of life.11 This impact is mainly due to fear of having another syncopal episode.34 We have noted an improvement in general wellbeing after 18 months of follow-up in the ILR group. This may reflect improved quality of life due to the earlier use of appropriate therapeutic interventions significantly decreasing subsequent syncope.
Study limitations
Quality of life
Assessment of QOL in a syncopal population is complicated by the usually rare and random nature of the symptom. This decreases the sensitivity of the SF-12 and VAS questionnaires.
Conclusion
Use of the ILR has led to significantly more diagnoses of the cause of syncope being achieved, more rapid introduction of therapy and a greater variety of therapies being introduced. Use of the ILR also allows appropriate therapies for syncope to be selected. This results in a significant increase in the time to recurrent syncopal episodes and improved general wellbeing in an unselected population with syncope of unknown cause. Baseline clinical characteristics are poor predictors of mechanism of syncope.
Acknowledgements
Russell Canavan and Janet Large, for their invaluable work on data collection and patient identification. All the Doctors and Nurses of Eastbourne General Hospital, especially those of the Medical and Accident and Emergency Directorates. This study was partly supported by grants from the Medtronic UK.
Conflict of interest: N. Sulke and N. Freemantle have received funding for research, consulting and speaking from Medtronic, Inc.
Figure 1 Patient recruitment and randomization.
Figure 2 Inclusion criteria.
Figure 3 Time to ECG diagnosis.
Figure 4 Time to first syncope recurrence.
Figure 5 Time to second syncope recurrence.
Baseline characteristics
| . | ILRl 103 patients . | Conventional 98 patients . | Not enrolled 220 . |
|---|---|---|---|
| Gender (female) | 57 (55.3%) | 52 (53.1%) | 119 (54%) |
| Prior IHD | 49 (47.6%) | 51 (52.0%) | 108 (49%) |
| Age (median, interquartile range) | 73.9 (61.6–80.7) | 74.1 (59.1–81.0) | 76 (63–84) |
| Duration of symptoms (median, interquartile range) | 12 (6–36) | 18 (5–48) | 0 (0–0) |
| Previous episodes (median, interquartile range) | 3 (2–6) | 3 (2–6) | 1 (1–2) |
| . | ILRl 103 patients . | Conventional 98 patients . | Not enrolled 220 . |
|---|---|---|---|
| Gender (female) | 57 (55.3%) | 52 (53.1%) | 119 (54%) |
| Prior IHD | 49 (47.6%) | 51 (52.0%) | 108 (49%) |
| Age (median, interquartile range) | 73.9 (61.6–80.7) | 74.1 (59.1–81.0) | 76 (63–84) |
| Duration of symptoms (median, interquartile range) | 12 (6–36) | 18 (5–48) | 0 (0–0) |
| Previous episodes (median, interquartile range) | 3 (2–6) | 3 (2–6) | 1 (1–2) |
Baseline characteristics
| . | ILRl 103 patients . | Conventional 98 patients . | Not enrolled 220 . |
|---|---|---|---|
| Gender (female) | 57 (55.3%) | 52 (53.1%) | 119 (54%) |
| Prior IHD | 49 (47.6%) | 51 (52.0%) | 108 (49%) |
| Age (median, interquartile range) | 73.9 (61.6–80.7) | 74.1 (59.1–81.0) | 76 (63–84) |
| Duration of symptoms (median, interquartile range) | 12 (6–36) | 18 (5–48) | 0 (0–0) |
| Previous episodes (median, interquartile range) | 3 (2–6) | 3 (2–6) | 1 (1–2) |
| . | ILRl 103 patients . | Conventional 98 patients . | Not enrolled 220 . |
|---|---|---|---|
| Gender (female) | 57 (55.3%) | 52 (53.1%) | 119 (54%) |
| Prior IHD | 49 (47.6%) | 51 (52.0%) | 108 (49%) |
| Age (median, interquartile range) | 73.9 (61.6–80.7) | 74.1 (59.1–81.0) | 76 (63–84) |
| Duration of symptoms (median, interquartile range) | 12 (6–36) | 18 (5–48) | 0 (0–0) |
| Previous episodes (median, interquartile range) | 3 (2–6) | 3 (2–6) | 1 (1–2) |
Diagnoses achieved
| ECG diagnosis . | ILR . | Conventional . | Total . | % of diagnosed patients . |
|---|---|---|---|---|
| Bradycardia | 15 | 3 | 18 | 37 |
| VT | 2 | 1 | 3 | 6 |
| SVT | 3 | 0 | 3 | 6 |
| SR | 51 | |||
| Vaso-vagal | 16 | 1 | 17 | 35 |
| Hyperventilation | 3 | 0 | 3 | 4 |
| Epilepsy | 4 | 1 | 5 | 10 |
| Reactive hypoglycaemia | 0 | 1 | 1 | 1 |
| Total | 43 | 7 | 50 | 100 |
| ECG diagnosis . | ILR . | Conventional . | Total . | % of diagnosed patients . |
|---|---|---|---|---|
| Bradycardia | 15 | 3 | 18 | 37 |
| VT | 2 | 1 | 3 | 6 |
| SVT | 3 | 0 | 3 | 6 |
| SR | 51 | |||
| Vaso-vagal | 16 | 1 | 17 | 35 |
| Hyperventilation | 3 | 0 | 3 | 4 |
| Epilepsy | 4 | 1 | 5 | 10 |
| Reactive hypoglycaemia | 0 | 1 | 1 | 1 |
| Total | 43 | 7 | 50 | 100 |
Diagnoses achieved
| ECG diagnosis . | ILR . | Conventional . | Total . | % of diagnosed patients . |
|---|---|---|---|---|
| Bradycardia | 15 | 3 | 18 | 37 |
| VT | 2 | 1 | 3 | 6 |
| SVT | 3 | 0 | 3 | 6 |
| SR | 51 | |||
| Vaso-vagal | 16 | 1 | 17 | 35 |
| Hyperventilation | 3 | 0 | 3 | 4 |
| Epilepsy | 4 | 1 | 5 | 10 |
| Reactive hypoglycaemia | 0 | 1 | 1 | 1 |
| Total | 43 | 7 | 50 | 100 |
| ECG diagnosis . | ILR . | Conventional . | Total . | % of diagnosed patients . |
|---|---|---|---|---|
| Bradycardia | 15 | 3 | 18 | 37 |
| VT | 2 | 1 | 3 | 6 |
| SVT | 3 | 0 | 3 | 6 |
| SR | 51 | |||
| Vaso-vagal | 16 | 1 | 17 | 35 |
| Hyperventilation | 3 | 0 | 3 | 4 |
| Epilepsy | 4 | 1 | 5 | 10 |
| Reactive hypoglycaemia | 0 | 1 | 1 | 1 |
| Total | 43 | 7 | 50 | 100 |
ECG directed therapies introduced by study census
| . | ILR . | Conventional . | Total . |
|---|---|---|---|
| Pacemaker | 16 | 3 | 19 |
| Lifestyle modification | 12 | 1 | 13 |
| Drug therapy | 8 | 1 | 9 |
| Drug cessation | 2 | 1 | 3 |
| Awaiting therapy | 2 | 0 | 2 |
| RF ablation | 1 | 0 | 1 |
| ICD | 0 | 1 | 1 |
| Tilt training | 1 | 0 | 1 |
| Psychiatry reference | 1 | 0 | 1 |
| Total | 43 | 7 | 50 |
| . | ILR . | Conventional . | Total . |
|---|---|---|---|
| Pacemaker | 16 | 3 | 19 |
| Lifestyle modification | 12 | 1 | 13 |
| Drug therapy | 8 | 1 | 9 |
| Drug cessation | 2 | 1 | 3 |
| Awaiting therapy | 2 | 0 | 2 |
| RF ablation | 1 | 0 | 1 |
| ICD | 0 | 1 | 1 |
| Tilt training | 1 | 0 | 1 |
| Psychiatry reference | 1 | 0 | 1 |
| Total | 43 | 7 | 50 |
ECG directed therapies introduced by study census
| . | ILR . | Conventional . | Total . |
|---|---|---|---|
| Pacemaker | 16 | 3 | 19 |
| Lifestyle modification | 12 | 1 | 13 |
| Drug therapy | 8 | 1 | 9 |
| Drug cessation | 2 | 1 | 3 |
| Awaiting therapy | 2 | 0 | 2 |
| RF ablation | 1 | 0 | 1 |
| ICD | 0 | 1 | 1 |
| Tilt training | 1 | 0 | 1 |
| Psychiatry reference | 1 | 0 | 1 |
| Total | 43 | 7 | 50 |
| . | ILR . | Conventional . | Total . |
|---|---|---|---|
| Pacemaker | 16 | 3 | 19 |
| Lifestyle modification | 12 | 1 | 13 |
| Drug therapy | 8 | 1 | 9 |
| Drug cessation | 2 | 1 | 3 |
| Awaiting therapy | 2 | 0 | 2 |
| RF ablation | 1 | 0 | 1 |
| ICD | 0 | 1 | 1 |
| Tilt training | 1 | 0 | 1 |
| Psychiatry reference | 1 | 0 | 1 |
| Total | 43 | 7 | 50 |
References
Savage DD, Corwin L, McGee DL, Kannel WB, Wolf PA. Epidemiologic features of isolated syncope: the Framingham Study.
Chen L, Chen MH, Larson MG, Evans J, Benjamin EJ, Levy D. Risk factors for syncope in a community-based sample (the Framingham Heart Study).
Murdoch BD. Loss of consciousness in healthy South African men: Incidence causes and EEG abnormality.
Farwell DJ, Sulke AN. Does the use of a syncope diagnostic protocol improve the investigation and management of syncope?
Day SC, Cook EF, Funkenstein H, Goldman L. Evaluation and outcome of emergency room patients with transient loss of consciousness.
Gendelman HE, Linzer M, Gabelman M, Smoller S, Scheuer J. Syncope in a general hospital patient population. Usefulness of the radionuclide brain scan, electroencephalogram, and 24-hour Holter monitor.
Brignole M, Menozzi C, Moya A, Garcia-Civera R. Implantable loop recorder: towards a gold standard for the diagnosis of syncope?
Linzer M, Pontinen M, Gold DT, Divine GW, Felder A, Brooks WB. Impairment of physical and psychosocial function in recurrent syncope.
Sanatani S, Peirone A, Chiu C, Human DG, Gross GJ, Hamilton RM. Use of an implantable loop recorder in the evaluation of children with congenital heart disease.
Brignole M, Menozzi C, Moya A, Garcia-Civera R, Mont L, Alvarez M, Errazquin F, Beiras J, Bottoni N, Donateo P. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test.
Moya A, Brignole M, Menozzi C, Garcia-Civera R, Tognarini S, Mont L, Botto G, Giada F, Cornacchia D. Mechanism of syncope in patients with isolated syncope and in patients with tilt-positive syncope.
Farwell D, Freemantle N, Sulke AN. The use of implantable loop recorders in the diagnosis and management of syncope.
Farwell D, Sulke N. How do we diagnose syncope?
Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy.
Lakehurst R, Barnett D, Berry C, Bird S, Buxton M, Carter Y. Guidance on the use of implantable cardioverter defibrillators for arrhythmias. 1 September
Chettaoui R, Kouakam C, Klug D, Marquie C, Lacroix D, Kacet S. Contribution of the implantable ECG monitor in the etiologic diagnosis of syncope and unexplained recurrent syncopal attacks. Initial experience with 32 patients.
Edhag O, Swahn A. Prognosis of patients with complete heart block or arrhythmic syncope who were not treated with artificial pacemakers. A long-term follow-up study of 101 patients.
Michaelsson M, Riesenfeld T, Jonzon A. Natural history of congenital complete atrioventricular block.
Rose MS, Koshman ML, Spreng S, Sheldon R. The relationship between health-related quality of life and frequency of spells in patients with syncope.
Krahn AD, Klein GJ, Fitzpatrick A, Seidl K, Zaidi A, Skanes A, Yee R. Predicting the outcome of patients with unexplained syncope undergoing prolonged monitoring.
Nierop PR, van Mechelen R, van Elsacker A, Luijten RH, Elhendy A. Heart rhythm during syncope and presyncope: results of implantable loop recorders.
Krahn AD, Klein GJ, Yee R, Takle-Newhouse T, Norris C. Use of an extended monitoring strategy in patients with problematic syncope. Reveal Investigators.
Brignole M, Alboni P, Benditt D, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, Fitzpatrick A, Hohnloser S, Kapoor W, Kenny RA, Theodorakis G, Kulakowski P, Moya A, Raviele A, Sutton R, Wieling W, Janousek J, van Dijk G. Task force on syncope, European Society of Cardiology. Part 2. Diagnostic tests and treatment: summary of recommendations.
Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, Kerber RE, Naccarelli GV, Schoenfeld MH, Silka MJ, Winters SL, Gibbons RJ, Antman EM, Alpert JS, Gregoratos G, Hiratzka LF, Faxon DP, Jacobs AK, Fuster V, Smith SC Jr. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines).
Denniss AR, Ross DL, Richards DA, Uther JB. Electrophysiologic studies in patients with unexplained syncope.
Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope.
Racco F, Sconocchini C, Alesi C, Zappelli L, Pratillo G. Long-term follow-up after syncope. A group of 183 patients observed for 5 years.