-
PDF
- Split View
-
Views
-
Cite
Cite
Wojciech Wojakowski, Michał Tendera, Anna Zebzda, Anna Michałowska, Marcin Majka, Magdalena Kucia, Katarzyna Maślankiewicz, Rafał Wyderka, Marek Król, Andrzej Ochała, Krystyna Kozakiewicz, Mariusz Z. Ratajczak, Mobilization of CD34+, CD117+, CXCR4+, c-met+ stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction, European Heart Journal, Volume 27, Issue 3, February 2006, Pages 283–289, https://doi.org/10.1093/eurheartj/ehi628
Close - Share Icon Share
Abstract
Aims The aim of the study was to assess the correlation between the number of CD34+, CD117+, c-met+, CXCR4+ stem cells mobilized into peripheral blood, left ventricular ejection fraction (LVEF), NT-proBNP levels, and myocardial necrosis markers in patients with acute myocardial infarction (AMI).
Methods and results 43 patients with STEMI were enrolled. Stem cells number was measured using flow-cytometer and concentrations of NT-proBNP, SDF-1, G-CSF, VEGF, IL-6, and HGF were measured using ELISA kits. The number of stem cells mobilized early (<12 h) in AMI was significantly, positively correlated with LVEF: r=0.49 (P=0.0012) for CD34+ cells, r=0.48 (P=0.0018) for CXCR4+ cells, r=0.45 (P=0.0043) for CD117+ cells, and r=0.41 (P=0.01) for c-met+ cells and negatively correlated with NT-proBNP levels on admission r=−0.35 (P=0.024) for CD34+ cells, r=−0.42 (P=0.007) for CXCR4+ cells, r=−0.33 (P=0.04). In patients with LVEF ≤40%, the peak number of CD34+, CXCR4+, CD117+, and c-met+ stem cells was significantly lower when compared patients with LVEF >40%. The number of CXCR4+ cells on admission and after 24 h was negatively correlated with respective cardiac Troponin I levels (r=−0.37; P=0.029 and r=−0.45, P=0.02) and maximum activity of CK-MB (r=−0.37; P=0.021). No significant correlations between levels of haematopoietic cytokines and LVEF were found.
Conclusion The mobilization of CD34+, CD117+, CXCR4+, c-met+ stem cells into peripheral blood early in STEMI is positively correlated with LVEF and negatively correlated with NT-proBNP levels and myocardial necrosis markers.
Introduction
We recently demonstrated a significant mobilization of stem cells expressing CD34, CD117 (c-kit), c-met, and CXCR4 antigens along with increase of inflammatory and haematopoietic cytokines [stromal cell-derived factor 1 (SDF-1), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF)] early in ST-elevation acute myocardial infarction (AMI). The number of stem cells in peripheral blood in AMI increases early within 12 h after symptoms onset and remains significantly higher when compared with patient's stable angina and healthy subjects. Moreover, synchronously with the rise of CD34+/CD117+ and CD34+/CXCR4+ cells number, there is also a marked increase of mRNA expression for cardiac (Nkx2.5/Csx, GATA-4, MEF2C), endothelial (VE-cadherin, von Willebrand), and muscle (Myf5, MyoD, myogenin) markers in peripheral blood mononuclear cells.1 Similar findings were also reported by Massa et al.2 with regard to mobilization of CD34+ haematopoietic stem cells (HSC) and endothelial progenitor cells (EPC) which occurred within 6 h after the infarction, persisted up to 7 days and normalized in 60 days. The mobilization of populations other than HSC (EPC) in patients with AMI was showed also by Shintani et al.3
In animal models of myocardial infarction mobilized HSC and mesenchymal stem cells showed the capability to differentiate into cardiomyocytes and take part in myocardial regeneration probably by increased neovascularization and paracrine modulation of myocardial remodelling; however, no direct evidence of such reparatory mechanisms are available for AMI in humans.4,5
Reduced left ventricular ejection fraction (LVEF) and elevation of plasma natriuretic peptide NT-proBNP levels are major clinical issues and predictors of mortality in the setting of AMI.6 Clinical trials, such as the pioneering TOPCARE-AMI, demonstrated that intracoronary transfusion of either bone marrow or peripheral blood-derived progenitor cells leads to the improvement of the LVEF in patients with AMI.7 If the mobilization of endogenous HSC is a reparatory mechanism during myocardial infarction, there is also a possibility that in patients with low LVEF, the increase of stem cell number may be compromised when compared patients with less evident post-infarction impairment of left ventricular function. Important data recently published by Leone et al.8 showed that the number of circulating CD34+ stem cells significantly correlated with the indices of left ventricular remodelling [LVEF, wall motion score index (WMSI), left ventricular end-diastolic, and left ventricular end-systolic volume] 1 year after AMI.
The aim of this study is to investigate whether the early mobilization of CD34+, CD117+, CXCR4+, and c-met+ stem cells as well as the levels of haematopoietic cytokines are correlated with LVEF, plasma levels of NT-proBNP, and myocardial necrosis markers in patients with AMI treated with primary percutaneous coronary intervention.
Methods
Study population
The study population consisted of 43 patients with AMI admitted within 12 h after the chest pain onset referred for coronary angiography. All enrolled patients were subsequently successfully treated with primary percutaneous angioplasty (PTCA) with final TIMI 3 flow in the infarct-related artery. Consecutive patients with AMI referred for primary PTCA were screened for enrolment (n=77). Patients with systemic inflammatory process, cancer, active infection, or febrile status were excluded (n=2), as well as patients with acute coronary syndromes other than ST-elevation AMI (n=8). Subsequently, patients with time-to-onset of the symptoms >12 h (n=11) and with prior AMI (n=13) were excluded. Table 1 shows the characteristics of the study group. All of the enrolled patients completed the follow-up at 7 days. The study protocol complies with the Declaration of Helsinki and was approved by the institutional Ethics Committees. All patients gave written informed consents.
Laboratory measurements
Circulating stem cells
Peripheral blood samples were drawn into EDTA-tubes on admission prior to PTCA, after 24 h and 7 days. Samples for the assessment of stem cells number were processed within 12 h after drawing. The whole blood samples (100 µL) were stained with phycoerythrin (PE)-conjugated (5 µL) anti-CD34, anti-CD-117, anti-CXCR4 (Becton Dickinson), and anti-c-met (Sigma) monoclonal antibodies for 30 min at 40°C. Cells were subsequently lysed for 15 min, centrifuged, washed twice, and re-suspended in PBS and analyzed using FACSCalibur flow-cytometer (Becton Dickinson). Isotype-matched PE-conjugated antibodies were used as controls (Becton Dickinson). The number of early tissue-committed progenitor cells was calculated on the basis of absolute leukocyte count×percentage (%) of gated CD34+ events and expressed as the absolute number of cells per 1 µL of whole blood.
Plasma concentrations of inflammatory and haematopoietic cytokines
Plasma samples were immediately frozen and stored at −40°C. The concentrations of NT-proBNP, SDF-1, granulocyte colony-stimulating factor (G-CSF), VEGF, interleukin-6 (IL-6), and HGF were measured using high-sensitivity ELISA kits (Bender MedSystems, R&D, Promedica). SDF-1 levels were assayed in platelet-depleted samples centrifuged at 11 000 g for 10 min.
Left ventricular ejection fraction
Echocardiographic evaluations of LVEF were carried out on admission prior to cardiac catheterization by an experienced echocardiographer. LVEF was assessed according to the recommendations of the American Society of Echocardiography with Simpson rule.7 Mean LVEF was 42.1±5.5% (range, 28–65%). In 40% of patients the baseline LVEF was ≤40%.9
Statistical analysis
Data are expressed as median±range. As the distribution of all parameters was skewed, the non-parametric tests (Mann–Whitney U test and Wilcoxon test) were used. The correlations between stem cells number, levels of cytokines, cardiac necrosis markers, and LVEF were assessed with Spearman rank correlation test; P-value <0.05 was considered significant. Analyses were done using Statistica 6.0 PL for Windows package. All tests were two-sided and no adjustments were made to the significance level. Calculation of the sample size was based on the data showing that stem cell numbers in healthy subjects and patients with stable angina (expected average number of CD34+ cells of 2.0±3.0 per µL) are not significantly different but significantly increased (at least two-fold) in AMI. We calculated the sample size to allow detection of 50% increase (detection of at least of 4.0 CD34+ cells per µL) with 85% power at the P<0.05 in AMI patients.
Results
Number of circulating stem cells and LVEF
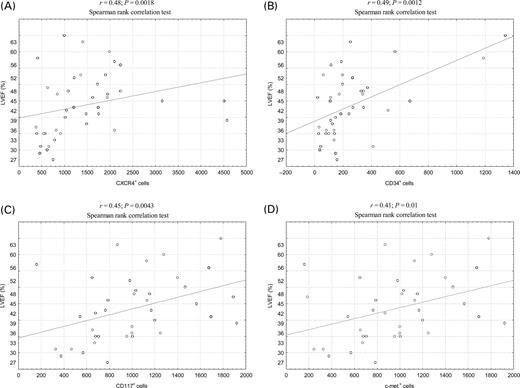
The absolute number of CXCR4+, CD34+, CD117+, and c-met+ cells at admission was significantly higher in AMI patients when compared patients with stable angina and healthy controls as previously described. Number of stem cells remained significantly higher after 24 h and 7 days but did not show further increase in comparison to baseline, so the maximum mobilization occurred early (≤12 h) in the course of acute myocardial ischaemia.1 Relative changes in stem cells numbers as well as haematopoietic cytokines are shown in Table 2. The correlation analyses (Spearman rank correlation test) revealed the significant positive correlations between the maximum number of stem cells (stem cell numbers were highest on admission) and LVEF: r=0.49 (P=0.0012) for CD34+ cells, r=0.48 (P=0.0018) for CXCR4+ cells, r=0.45 (P=0.0043) for CD117+ cells, and r=0.41 (P=0.01) for c-met+ cells (Figure 1A–D).
In patients with LVEF ≤40% on admission, the baseline number of CXCR4+, CD34+, and c-met+ stem cells was significantly lower in comparison to patients with LVEF >40% (data not shown).
Stem cells number NT-proBNP levels and myocardial necrosis markers
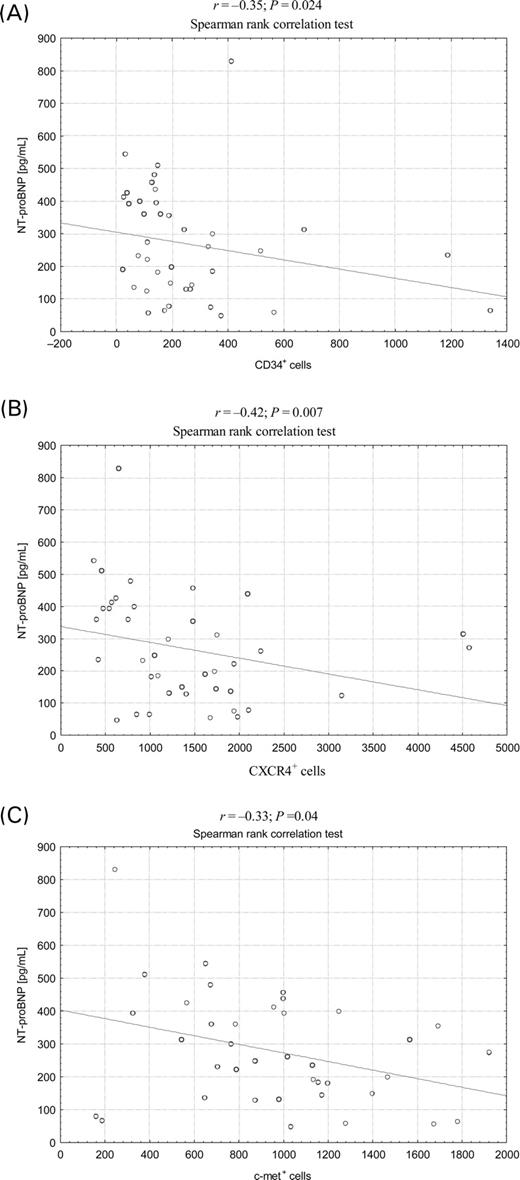
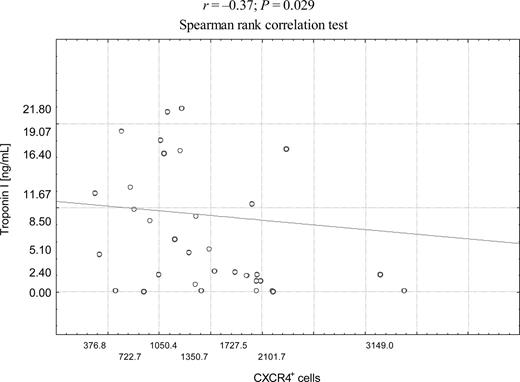
In AMI patients, the median (range) levels of NT-proBNP were 240 (46.4–829) pg/mL. The maximum numbers of stem cells were negatively correlated with NT-proBNP levels on admission r=−0.35; P=0.024 for CD34+ cells, r=−0.42; P=0.007 for CXCR4+ cells, and r=−0.33; P=0.04 for c-met+ cells (Figure 2A–C); however, no significant correlation was found in regard to number of CD117+ cells (r=−0.1; P=0.1). The number of CXCR4+ cells on admission and after 24 h was negatively correlated with respective cardiac Troponin I levels (r=−0.37; P=0.029 and r=−0.45, P=0.02) (Figure 3). In addition, number of CXCR4+ cells on admission was negatively correlated with maximum activity of CK-MB (r=−0.37; P=0.021) (data not shown).
Plasma concentrations of inflammatory and haematopoietic cytokines
As previously described, the levels of IL-6, G-CSF, VEGF, and HGF were significantly higher, whereas SDF-1 was lower in STEMI patients than in controls and stable angina (Table 2).1 Multiple significant correlations between levels of haematopoietic cytokines and stem cells number were showed; however, no significant correlations between cytokine levels and LVEF were found (Table 3).
Discussion
We demonstrated that in patients with AMI treated with primary PTCA, the mobilization of CD34+, CXCR4+, CD117+, and c-met+ stem cells is significantly correlated to important predictors of clinical outcome: LVEF and NT-proBNP levels. There is a positive correlation between the number of mobilized stem cells and LVEF and negative correlation with NT-proBNP levels. The increase in stem cells number is significantly lower in patients with significantly depressed LVEF (≤40%) in comparison to patients with lesser degree of left ventricular contractile dysfunction (LVEF >40%). Moreover, the number of mobilized stem cells was negatively correlated with cardiac necrosis markers (Troponin I, CK-MB).
Similar to our findings, Massa et al.2 reported a significant, median 5.8-fold increase of CD34+ cells and CD34+/CD45+ HSC and EPC number within 6 h and lasting up to 60 days after the chest pain onset in 26 patients with STEMI. Among various cell subpopulations measured, they reported a significantly increased absolute number of CD34+/CD117+ at admission in STEMI patients. In addition, similar to our findings, the HSC number was highest at admission with subsequent decrease during follow-up. The analysis of correlations between HSC and clinical parameters in study of Massa et al.2 revealed, however, no significant association between the CD34+ HSC number and myocardial necrosis markers (peak CK, peak CK-MB, and enzyme AUC) and echocardiographic parameters of the left ventricular function (LVEF and WMSI 24 hours and 1 year after STEMI), gender, age cardiovascular risk factors. Important new findings of Leone et al.8 showed that the number of circulating CD34+ stem cells 1 year after AMI is significantly correlated to a degree of left ventricular improvement, evidenced by change of LVEF, WMSI, LVEDD, and LVESD. Moreover, they showed that patients with greater cell mobilization early in AMI and higher number of circulating stem cells after 1 year, there is a trend for better improvement of LVEF. These data as well as ours are different from previously published data by Massa, which showed no correlations between stem cells and LVEF. The study of Leone et al.8 also showed a different time-course of mobilization with relatively late peak of stem cells number on the day 5 in comparison to described by Massa et al.2 as well as our team (6–12 h). All patients in our study and those of Massa et al.2 were treated with primary PTCA, whereas the group from study of Leone included patients treated with fibrynolysis, as well as patients who received no reperfusion therapy at all because of long duration of symptoms (12–24 h) and patients with prior AMI. Both the differences of the treatment and duration of symptoms may influence the results given the rapid peak in stem cell mobilization.1,8
Tomoda and Aoki10 showed for the first time that the stimulation of the bone marrow in AMI evidenced by the increased number of circulating immature blood cells such as myelocytes, promyelocytes, and myeloblasts is associated with significantly higher LVEF after 6 months when compared with patients without increase of immature blood cells in acute phase of AMI. Interestingly, there were no differences between both groups in baseline LVEF measured by contrast ventriculography in acute phase of AMI. The comparison of these findings to studies in which specific subpopulations of cells were measured by FACS is, however, difficult. Leone et al.8 showed that >95% of mobilized cells co-expressed CD34 and CD45 and were negative for KDR, which suggests that these cells originate in bone marrow and are not EPC. Interestingly, the animal studies showed that putative bone marrow-derived cells associated with myocardial repair are predominantly non-haematopoietic (CXCR4+/Sca-1+/lin-/CD45− in mice and CXCR4+/CD34+/AC133+/CD45− in humans).11
Most of the studies investigating the mobilization of stem cells involved the population of endothelial progenitors, which have different mobilization signals, e.g. VEGF as opposed to SDF-1 binding to CXCR4 on haematopoietic progenitors. Shintani et al.3 also documented in the setting of STEMI a significant mobilization of EPC (KDR+, VE-cadherin+, CD31+, Dil-acLDL uptake+) peaking after 7 days; however, this study did not investigate other subsets of progenitor cells or the clinical data.
The depletion of CXCR4+ cells may be particularly important in terms of myocardial salvage. As showed by our group in experimental models of AMI, this subpopulation of cells contains tissue-committed stem cells expressing mRNA specific for early cardiac markers (Nkx2.5/Csx, GATA-4, MEF2C). These non-haematopoietic cells mobilized from bone marrow are strongly chemoattracted by SDF-1/CXCR4, HGF/c-met, and leukaemia inhibitory factor (LIF)/LIF-receptor axes.11–14 This concept seems valid because as shown by TOPCARE-AMI Investigators the migratory capacity of infused CXCR4+ progenitors cells induced by SDF-1 was the strongest independent predictor of the reduction of the infarction size assessed in contrast-enhanced MRI. This finding supports the concept of the pivotal role of CXCR4/SDF-1 axis in mobilization and engraftment of progenitor cells to the infarcted myocardium.15
The stem cell-associated post-infarct improvement in myocardial function is probably multifactorial, as the transdifferentiation of mobilized stem cells into functional cardiomyocytes is controversial at best, and other mechanisms, such as improved neovascularization and enhanced production of cytokines that may modulate the survival of border-zone cardiomyocytes are probably involved. In stable CAD patients with inadequate collateral support, the number of circulating endothelial cells (EC) is significantly lower. Moreover, the chemotactic and angiogenic function of CEC is impaired.15–18 In addition, the bone marrow-derived stem cells are not the only population of cells that can play a role in myocardial repair, other sources such as population of multipotent cardiac-stem cells residing in the heart as described by group of Anversa and mesenchymal stem cells identified in adipose tissue and liver must be taken into consideration.5
No significant correlation between haematopoietic cytokines levels and LVEF was found, however, in patients with LVEF ≤40% the plasma VEGF levels were significantly higher than in subjects with LVEF >40%. This finding is in concordance with other studies, which showed up-regulation of VEGF gene expression, release of VEGF protein in the ischaemic myocardium and higher plasma VEGF levels in patients with AMI.3,19,20 We found also that in this group of patients plasma VEGF level is not an independent predictor of the CD34+/CXCR4+ and CD34+/CD117+ cells mobilization, and the only cytokine independently associated with significant increase of CD34+ cells in STEMI is SDF-1.1 In murine model of AMI, the SDF-1 mRNA and protein synthesis were significantly up-regulated in the infarct and border zone and myocardial ischaemia was associated with lower plasma levels of SDF-1 [11]. In our study, there was a trend towards lower plasma SDF-1 levels in patients with LVEF ≤40% in comparison to subjects with LVEF >40%, although this difference did not reach statistical significance (P=0.57, data not shown). The assessment of transcardiac gradient of SDF-1 with coronary sinus sampling might help to differentiate the subjects with low SDF-1 production which might be associated with impaired mobilization, as the ischaemia-induced SDF-1 release may be more important in progenitor cell mobilization and homing than the absolute levels of SDF-1 in particular subjects, which are dependent on other factors such as the activity of matrix metaloproteinases, levels of G-CSF, duration of ischaemia, local synthesis in arterial wall, and binding via SDF-1 receptor CXCR4 on lymphocytes, monocytes, and platelets. However, SDF-1 plays a crucial role in post-infarction recruitment of stem cell into the myocardium and its release may influence left ventricular function via the modulation of stem cell mobilization and engraftment in humans.12–14 Interestingly, the study of Leone et al.8 showed that anterior AMI was associated with significantly higher number of mobilized CD34+ which could suggest that larger area of infarction could be a stronger stimulus for stem cell mobilization.
We found that another pool of cells showing blunted mobilization in STEMI patients with low LVEF and high NT-proBNP levels are c-met+ cells. C-met is a receptor mediating cellular effect of HGF.21,22 The plasma levels of HGF were comparable in patients with LVEF ≤40% and >40%; therefore, the impaired mobilization of c-met+ cells seems to have other cause than low levels of HGF, e.g. down- regulation of c-met synthesis in the already circulating progenitor cells. Sato et al.22 showed that in the infarct border, the expression of c-met was significantly higher in comparison to subjects without AMI and that HGF is an important factor mediating stem cell mobilization in AMI.
Available data suggest that statins can stimulate the mobilization and modulate the function of EPC in patients with CHD.23,24 We found no significant differences between patients treated with statins before admission (42%) and those who were not with regard to early stem cell mobilization in STEMI. Similar to our findings, Massa et al.2 reported no differences in number of mobilized CD34+ HSC when comparing STEMI patients with or without statin treatment on admission. In addition, primary PCI may have influenced cell mobilization via induction of cytokine release. Conversely, Leone et al.8 found that statin use prior to AMI is associated with significantly greater mobilization of CD34+ stem cells.
The major challenge is to investigate the causal link between the impaired function of the left ventricle and poorer stem cell mobilization. One might speculate that impaired early mobilization of stem cells, which is a reparatory mechanism, results in compromised salvage of ischaemic but still viable myocardium in peri-infarct zone the first hours of STEMI. The blunted increase of stem cell number in patients with more extensively damaged myocardium (higher troponin levels, higher maximum and peak CK-MB activity, history of previous AMI) may be the result of lower SDF-1 release, its increased enzymatic degradation (metaloproteinase MMP-9) or from properties of the bone marrow-peripheral blood barrier such as expression of adhesion molecules or altered reactivity of stem cells. Nevertheless, another mechanism is also possible. In the setting of extensive infarction, the SDF-1 or HGF synthesis and release in peri-infarct myocardium may be actually higher and induce the more effective cell homing and engraftment leading to lower number of cells measured in peripheral blood in comparison to less extensive myocardial infarcts.4,12–14,25 We found no significant differences and correlations between specific haematopoietic cytokines and LVEF, however, the cytokine release in AMI was measured in peripheral blood. Coronary sinus sampling and assessment of transcardiac gradient of stem cell-mobilizing cytokines (SDF-1, G-CSF, HGF, LIF) may add valuable data in this matter.
The limitation of this study is the lack of explanation of the mechanisms of impaired stem cell mobilization. In addition, so far there is no direct evidence that mobilized endogenous progenitor cells contribute to the hemodynamically relevant improvement in cardiac function. The sample was too small to assess the prognostic significance of the stem cell mobilization in risk prediction in AMI patients. So far the number of circulating EC (CD146+, eNOS+, CD45−, CD34−, CD36−, CD31−) measured within 48 h after ACS in 156 consecutive patients was shown to be a predictor of outcomes in 30-days and 1-year follow-up.26 Thus, the clinical relevance of the study conclusion are speculative, as so far no study demonstrated that autogenous bone marrow cells mobilized in AMI contribute to myocardial repair.
Our study demonstrates that the mobilization of CD34+, CXCR4+, CD117+, and c-met+ stem cells in STEMI is significantly correlated with LVEF, plasma NT-proBNP levels and myocardial necrosis markers. In patients with significantly impaired LVEF and high concentrations of NT-proBNP, the mobilization of stem cells is significantly compromised in comparison to patients with higher LVEF and low levels of NT-proBNP.
Acknowledgement
This study was supported by grant PBZ-KBN-099/P05/2003 by Polish Ministry of Science and Informatics.
Conflict of interest: none declared.
Figure 1 Correlations between baseline absolute numbers of stem cells subpopulations and LVEF in AMI patients: (A) CXCR4+ stem cells; (B) CD34+ stem cells; (C) CD117+ stem cells; (D) c-met+ stem cells.
Figure 2 Correlations between baseline absolute numbers of stem cells subpopulations and NT-proBNP levels in AMI patients: (A) CD34+ stem cells; (B) CXCR4+ stem cells; (C) c-met+ stem cells.
Figure 3 Correlations between baseline absolute numbers of CXCR4+ stem cells and Troponin I levels in AMI patients.
Characteristics of AMI patients
| Age (years) (mean±SD) | 60.3±10 |
| Men/women | 25/18 |
| Hypertension, n (%) | 28 (65) |
| Hypercholesterolemia, n (%) | 35 (60) |
| Diabetes mellitus, n (%) | 11 (27) |
| Smoking, n (%) | 30 (69) |
| Family history of CAD, n (%) | 20 (47) |
| Statins, n (%) | 19 (44) |
| ACE inhibitors, n (%) | 17 (41) |
| Aspirin, n (%) | 24 (58) |
| Baseline CKMB (U/L)a | 36.2 (4–356) |
| Baseline Troponin I (ng/mL)a | 1.8 (0.02–100) |
| CKMB (24 h) (U/L)a | 67 (6–584) |
| Troponin I (24 h) (ng/mL)a | 6.4 (0–100) |
| Peak CKMB (U/L)a | 89 (8–572) |
| Peak Troponin I (ng/mL)a | 6.4 (0.1–100) |
| Baseline white blood cells, median (range) | 15.7×109/L (5.7–19.4) |
| Anterior STEMI, n (%) | 23 (54) |
| Mean LVEF, % (range) | 42.1±5.5% (28–65%) |
| Baseline LVEF≤40%, n (%) | 16 (40) |
| Age (years) (mean±SD) | 60.3±10 |
| Men/women | 25/18 |
| Hypertension, n (%) | 28 (65) |
| Hypercholesterolemia, n (%) | 35 (60) |
| Diabetes mellitus, n (%) | 11 (27) |
| Smoking, n (%) | 30 (69) |
| Family history of CAD, n (%) | 20 (47) |
| Statins, n (%) | 19 (44) |
| ACE inhibitors, n (%) | 17 (41) |
| Aspirin, n (%) | 24 (58) |
| Baseline CKMB (U/L)a | 36.2 (4–356) |
| Baseline Troponin I (ng/mL)a | 1.8 (0.02–100) |
| CKMB (24 h) (U/L)a | 67 (6–584) |
| Troponin I (24 h) (ng/mL)a | 6.4 (0–100) |
| Peak CKMB (U/L)a | 89 (8–572) |
| Peak Troponin I (ng/mL)a | 6.4 (0.1–100) |
| Baseline white blood cells, median (range) | 15.7×109/L (5.7–19.4) |
| Anterior STEMI, n (%) | 23 (54) |
| Mean LVEF, % (range) | 42.1±5.5% (28–65%) |
| Baseline LVEF≤40%, n (%) | 16 (40) |
aValues of CK-MB and Troponin I are expressed as median and range.
Characteristics of AMI patients
| Age (years) (mean±SD) | 60.3±10 |
| Men/women | 25/18 |
| Hypertension, n (%) | 28 (65) |
| Hypercholesterolemia, n (%) | 35 (60) |
| Diabetes mellitus, n (%) | 11 (27) |
| Smoking, n (%) | 30 (69) |
| Family history of CAD, n (%) | 20 (47) |
| Statins, n (%) | 19 (44) |
| ACE inhibitors, n (%) | 17 (41) |
| Aspirin, n (%) | 24 (58) |
| Baseline CKMB (U/L)a | 36.2 (4–356) |
| Baseline Troponin I (ng/mL)a | 1.8 (0.02–100) |
| CKMB (24 h) (U/L)a | 67 (6–584) |
| Troponin I (24 h) (ng/mL)a | 6.4 (0–100) |
| Peak CKMB (U/L)a | 89 (8–572) |
| Peak Troponin I (ng/mL)a | 6.4 (0.1–100) |
| Baseline white blood cells, median (range) | 15.7×109/L (5.7–19.4) |
| Anterior STEMI, n (%) | 23 (54) |
| Mean LVEF, % (range) | 42.1±5.5% (28–65%) |
| Baseline LVEF≤40%, n (%) | 16 (40) |
| Age (years) (mean±SD) | 60.3±10 |
| Men/women | 25/18 |
| Hypertension, n (%) | 28 (65) |
| Hypercholesterolemia, n (%) | 35 (60) |
| Diabetes mellitus, n (%) | 11 (27) |
| Smoking, n (%) | 30 (69) |
| Family history of CAD, n (%) | 20 (47) |
| Statins, n (%) | 19 (44) |
| ACE inhibitors, n (%) | 17 (41) |
| Aspirin, n (%) | 24 (58) |
| Baseline CKMB (U/L)a | 36.2 (4–356) |
| Baseline Troponin I (ng/mL)a | 1.8 (0.02–100) |
| CKMB (24 h) (U/L)a | 67 (6–584) |
| Troponin I (24 h) (ng/mL)a | 6.4 (0–100) |
| Peak CKMB (U/L)a | 89 (8–572) |
| Peak Troponin I (ng/mL)a | 6.4 (0.1–100) |
| Baseline white blood cells, median (range) | 15.7×109/L (5.7–19.4) |
| Anterior STEMI, n (%) | 23 (54) |
| Mean LVEF, % (range) | 42.1±5.5% (28–65%) |
| Baseline LVEF≤40%, n (%) | 16 (40) |
aValues of CK-MB and Troponin I are expressed as median and range.
Relative changes of stem cells numbers and concentrations of haematopoietic cytokines in AMI in comparison with healthy subjects
| . | STEMI baseline . | P-value vs. control . | STEMI 24 h . | P-value vs. baseline . | STEMI day 7 . | P-value vs. baseline . |
|---|---|---|---|---|---|---|
| Cells | ||||||
| CD34+ | ↑1.9 (1–2.7) | 0.0005 | ↑3.2 (0.9–3.9) | 0.065 | ↑2.3 (0.5–3.99) | 0.1 |
| CD117+ | ↑2.9 (0.8–3.7) | 0.04 | ↑2 (0.8–2.6) | 0.09 | ↑2.5 (0.4–2.9) | 0.12 |
| CXCR4+ | ↑2.0 (0.9–2.6) | 0.0023 | ↑1.98 (1–2.7) | 0.23 | ↑1.3 (0.6–2.0) | 0.2 |
| c-met+ | ↑7.6 (1.1–9.2) | 0.00008 | ↑6.5 (0.9–8.3) | 0.089 | ↑4.3 (1.8–5.9) | 0.06 |
| Cytokines | ||||||
| G-CSF | ↑1.44 (0.9–1.9) | 0.02 | ↑1.78 (0.2–2.4) | 0.0006 | ↑1.4 (1–1.89) | 0.54 |
| IL-6 | ↑3.0 (1.5–4.2) | 0.002 | ↑9.1 (2.0–11.1) | 0.01 | ↑3.3 (0.9–4.0) | 0.07 |
| HGF | ↑2.55 (0.9–3.2) | 0.001 | ↑3.57 (1.7–4.8) | 0.00001 | ↑2.07 (1.1–2.98) | 0.058 |
| VEGF | ↑1.58 (0.8–2.6) | 0.00002 | ↑1.55 (1–2.1) | 0.0002 | ↑2.58 (1.4–3.76) | 0.00001 |
| SDF-1 | ↓ 3.87 (0.2–3.4) | 0.00001 | ↓ 3.62 (0.1–2) | 0.05 | ↓ 2.14 (0.3–3.1) | 0.00001 |
| . | STEMI baseline . | P-value vs. control . | STEMI 24 h . | P-value vs. baseline . | STEMI day 7 . | P-value vs. baseline . |
|---|---|---|---|---|---|---|
| Cells | ||||||
| CD34+ | ↑1.9 (1–2.7) | 0.0005 | ↑3.2 (0.9–3.9) | 0.065 | ↑2.3 (0.5–3.99) | 0.1 |
| CD117+ | ↑2.9 (0.8–3.7) | 0.04 | ↑2 (0.8–2.6) | 0.09 | ↑2.5 (0.4–2.9) | 0.12 |
| CXCR4+ | ↑2.0 (0.9–2.6) | 0.0023 | ↑1.98 (1–2.7) | 0.23 | ↑1.3 (0.6–2.0) | 0.2 |
| c-met+ | ↑7.6 (1.1–9.2) | 0.00008 | ↑6.5 (0.9–8.3) | 0.089 | ↑4.3 (1.8–5.9) | 0.06 |
| Cytokines | ||||||
| G-CSF | ↑1.44 (0.9–1.9) | 0.02 | ↑1.78 (0.2–2.4) | 0.0006 | ↑1.4 (1–1.89) | 0.54 |
| IL-6 | ↑3.0 (1.5–4.2) | 0.002 | ↑9.1 (2.0–11.1) | 0.01 | ↑3.3 (0.9–4.0) | 0.07 |
| HGF | ↑2.55 (0.9–3.2) | 0.001 | ↑3.57 (1.7–4.8) | 0.00001 | ↑2.07 (1.1–2.98) | 0.058 |
| VEGF | ↑1.58 (0.8–2.6) | 0.00002 | ↑1.55 (1–2.1) | 0.0002 | ↑2.58 (1.4–3.76) | 0.00001 |
| SDF-1 | ↓ 3.87 (0.2–3.4) | 0.00001 | ↓ 3.62 (0.1–2) | 0.05 | ↓ 2.14 (0.3–3.1) | 0.00001 |
Data expressed as fold of difference compared with control. Description of the matched control group was published previously.1 Mann–Whitney U test and Wilcoxon test were used for comparisons; P<0.05 was considered statistically significant. The fold of difference is expressing the ratios of the median value and range.
Relative changes of stem cells numbers and concentrations of haematopoietic cytokines in AMI in comparison with healthy subjects
| . | STEMI baseline . | P-value vs. control . | STEMI 24 h . | P-value vs. baseline . | STEMI day 7 . | P-value vs. baseline . |
|---|---|---|---|---|---|---|
| Cells | ||||||
| CD34+ | ↑1.9 (1–2.7) | 0.0005 | ↑3.2 (0.9–3.9) | 0.065 | ↑2.3 (0.5–3.99) | 0.1 |
| CD117+ | ↑2.9 (0.8–3.7) | 0.04 | ↑2 (0.8–2.6) | 0.09 | ↑2.5 (0.4–2.9) | 0.12 |
| CXCR4+ | ↑2.0 (0.9–2.6) | 0.0023 | ↑1.98 (1–2.7) | 0.23 | ↑1.3 (0.6–2.0) | 0.2 |
| c-met+ | ↑7.6 (1.1–9.2) | 0.00008 | ↑6.5 (0.9–8.3) | 0.089 | ↑4.3 (1.8–5.9) | 0.06 |
| Cytokines | ||||||
| G-CSF | ↑1.44 (0.9–1.9) | 0.02 | ↑1.78 (0.2–2.4) | 0.0006 | ↑1.4 (1–1.89) | 0.54 |
| IL-6 | ↑3.0 (1.5–4.2) | 0.002 | ↑9.1 (2.0–11.1) | 0.01 | ↑3.3 (0.9–4.0) | 0.07 |
| HGF | ↑2.55 (0.9–3.2) | 0.001 | ↑3.57 (1.7–4.8) | 0.00001 | ↑2.07 (1.1–2.98) | 0.058 |
| VEGF | ↑1.58 (0.8–2.6) | 0.00002 | ↑1.55 (1–2.1) | 0.0002 | ↑2.58 (1.4–3.76) | 0.00001 |
| SDF-1 | ↓ 3.87 (0.2–3.4) | 0.00001 | ↓ 3.62 (0.1–2) | 0.05 | ↓ 2.14 (0.3–3.1) | 0.00001 |
| . | STEMI baseline . | P-value vs. control . | STEMI 24 h . | P-value vs. baseline . | STEMI day 7 . | P-value vs. baseline . |
|---|---|---|---|---|---|---|
| Cells | ||||||
| CD34+ | ↑1.9 (1–2.7) | 0.0005 | ↑3.2 (0.9–3.9) | 0.065 | ↑2.3 (0.5–3.99) | 0.1 |
| CD117+ | ↑2.9 (0.8–3.7) | 0.04 | ↑2 (0.8–2.6) | 0.09 | ↑2.5 (0.4–2.9) | 0.12 |
| CXCR4+ | ↑2.0 (0.9–2.6) | 0.0023 | ↑1.98 (1–2.7) | 0.23 | ↑1.3 (0.6–2.0) | 0.2 |
| c-met+ | ↑7.6 (1.1–9.2) | 0.00008 | ↑6.5 (0.9–8.3) | 0.089 | ↑4.3 (1.8–5.9) | 0.06 |
| Cytokines | ||||||
| G-CSF | ↑1.44 (0.9–1.9) | 0.02 | ↑1.78 (0.2–2.4) | 0.0006 | ↑1.4 (1–1.89) | 0.54 |
| IL-6 | ↑3.0 (1.5–4.2) | 0.002 | ↑9.1 (2.0–11.1) | 0.01 | ↑3.3 (0.9–4.0) | 0.07 |
| HGF | ↑2.55 (0.9–3.2) | 0.001 | ↑3.57 (1.7–4.8) | 0.00001 | ↑2.07 (1.1–2.98) | 0.058 |
| VEGF | ↑1.58 (0.8–2.6) | 0.00002 | ↑1.55 (1–2.1) | 0.0002 | ↑2.58 (1.4–3.76) | 0.00001 |
| SDF-1 | ↓ 3.87 (0.2–3.4) | 0.00001 | ↓ 3.62 (0.1–2) | 0.05 | ↓ 2.14 (0.3–3.1) | 0.00001 |
Data expressed as fold of difference compared with control. Description of the matched control group was published previously.1 Mann–Whitney U test and Wilcoxon test were used for comparisons; P<0.05 was considered statistically significant. The fold of difference is expressing the ratios of the median value and range.
Correlations between the levels of haematopoietic cytokines, LVEF, and NT-proBNP (Spearman rank correlation test)
| . | Spearman rank correlation . | P-value . |
|---|---|---|
| Cytokines and LVEF | ||
| G-CSF | 0.03 | 0.81 |
| IL-6 | 0.09 | 0.7 |
| HGF | −0.17 | 0.22 |
| VEGF | 0.16 | 0.25 |
| SDF-1 | −0.07 | 0.63 |
| Cytokines and NT-proBNP | ||
| G-CSF | −0.02 | 0.71 |
| IL-6 | 0.06 | 0.52 |
| HGF | −0.11 | 0. 23 |
| VEGF | 0.0 8 | 0.61 |
| SDF-1 | −0.03 | 0.74 |
| . | Spearman rank correlation . | P-value . |
|---|---|---|
| Cytokines and LVEF | ||
| G-CSF | 0.03 | 0.81 |
| IL-6 | 0.09 | 0.7 |
| HGF | −0.17 | 0.22 |
| VEGF | 0.16 | 0.25 |
| SDF-1 | −0.07 | 0.63 |
| Cytokines and NT-proBNP | ||
| G-CSF | −0.02 | 0.71 |
| IL-6 | 0.06 | 0.52 |
| HGF | −0.11 | 0. 23 |
| VEGF | 0.0 8 | 0.61 |
| SDF-1 | −0.03 | 0.74 |
NT-proBNP, N-terminal pro-brain natriuretic peptide.
Correlations between the levels of haematopoietic cytokines, LVEF, and NT-proBNP (Spearman rank correlation test)
| . | Spearman rank correlation . | P-value . |
|---|---|---|
| Cytokines and LVEF | ||
| G-CSF | 0.03 | 0.81 |
| IL-6 | 0.09 | 0.7 |
| HGF | −0.17 | 0.22 |
| VEGF | 0.16 | 0.25 |
| SDF-1 | −0.07 | 0.63 |
| Cytokines and NT-proBNP | ||
| G-CSF | −0.02 | 0.71 |
| IL-6 | 0.06 | 0.52 |
| HGF | −0.11 | 0. 23 |
| VEGF | 0.0 8 | 0.61 |
| SDF-1 | −0.03 | 0.74 |
| . | Spearman rank correlation . | P-value . |
|---|---|---|
| Cytokines and LVEF | ||
| G-CSF | 0.03 | 0.81 |
| IL-6 | 0.09 | 0.7 |
| HGF | −0.17 | 0.22 |
| VEGF | 0.16 | 0.25 |
| SDF-1 | −0.07 | 0.63 |
| Cytokines and NT-proBNP | ||
| G-CSF | −0.02 | 0.71 |
| IL-6 | 0.06 | 0.52 |
| HGF | −0.11 | 0. 23 |
| VEGF | 0.0 8 | 0.61 |
| SDF-1 | −0.03 | 0.74 |
NT-proBNP, N-terminal pro-brain natriuretic peptide.
References
Wojakowski W, Tendera M, Michałowska A, Majka M, Kucia M, Maślankiewicz K, Wyderka R, Ochała A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction.
Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction.
Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction.
Forrester JS, Price MJ, Makkar RR. Stem cell repair of infracted myocardium. An overview for clinicians.
Muller P, Beltrami AP, Cesselli D, Pfeiffer P, Kazakov A, Bohm M. Myocardial regeneration by endogenous adult progenitor cells.
Suzuki S, Yoshimura M, Nakayama M, Mizuno Y, Harada E, Ito T, Nakamura S, Abe K, Yamamuro M, Sakamoto T, Saito Y, Nakao K, Yasue H, Ogawa H. Plasma Level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: a long-term follow-up analysis.
Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI).
Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function.
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I for the American Society of Echocardiography Committee on Standards Subcommittee on Quantitation of Two-Dimensional Echocardiograms. Recomendations for quantification of the left ventricle by two-dimensional echocardiography.
Tomoda H, Aoki N. Bone marrow stimulation and left ventricular function in acute myocardial infarction.
Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ilstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction.
Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue committed stem cells (TCSC).
Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow.
Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles.
Britten MB, Abolmaali ND, Assmus B, Lehman R, Honold J, Schmitt J, Vogl TJ, Martin H, Schaechinger V, Dimmeler S, Zeiher AM. Infarct Remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI). Mechanistic insights from serial contrast-enhanced magnetic resonance imaging.
Lambiase PD, Edwards RJ, Anthopoulos P, Salman R, Mane GY, Bucknall CA, Redwood SR, Pearson JD, Marber MS. Circulating humoral factors and endothelial progenitor cells in patients with differing collateral support.
Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury.
Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Hematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts.
Wojakowski W, Maslankiewicz K, Ochala A, Wyderka R, Zuk-Popiolek I, Flak Z, Mroz I, Tendera M. The pro- and antiinflammatory cytokines (VEGF, interleukin-10) in patients with acute myocardial infarction.
Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction.
Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle.
Sato T, Tani Y, Murao S, Fujieda H, Sato H, Matsumoto M, Takeuchi T, Ohtsuki Y. Focal enhancement of expression of c-met/hepatocyte growth factor receptor in the myocardium in human myocardial infarction.
Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in endothelial progenitor cells by statin therapy in patients with stable coronary artery disease.
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway.
Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells.