-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Duerschmied, Lisa Olson, Manfred Olschewski, Alexandra Rossknecht, Gabriele Freund, Christoph Bode, Christoph Hehrlein, Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method, European Heart Journal, Volume 27, Issue 3, February 2006, Pages 310–315, https://doi.org/10.1093/eurheartj/ehi636
Close - Share Icon Share
Abstract
Aims The purpose of this study was to establish contrast-enhanced ultrasound perfusion imaging (CUPI) of the lower extremities as a novel non-invasive diagnostic tool for patients with peripheral arterial disease (PAD).
Methods and results Ultrasound contrast agent (SonoVue™) was injected into a peripheral vein of 16 control subjects and 16 PAD patients and its appearance in the calf muscle was detected by low-energy harmonic ultrasound. Analysis of the wash-in curves revealed that PAD patients had a significantly longer time to peak intensity (TTP), i.e. duration of maximum contrast perfusion [37 s (19–79 s) in control subjects vs. 56 s (32–104 s) in PAD patients at rest, age-adjusted P=0.002]. Exercise stress test of the calf muscle resulted in a decrease of the TTP, maintaining the significant difference in TTP between the groups [19 s (8–37 s) in control subjects vs. 32 s (18–48 s) in PAD patients after exercise, age-adjusted P=0.004]. Neither ankle-brachial index and TTP nor age and TTP showed a significant correlation.
Conclusion CUPI reflects the regional blood circulation of the calf muscle. In this pilot study, PAD patients show a significantly longer TTP than control subjects. The clinical relevance of CUPI is topic of ongoing studies.
Introduction
Peripheral arterial disease (PAD) is a common manifestation of disseminated atherothrombotic disease causing critical public health problems.1 It affects almost one-fifth of over 65-year-olds and is associated with a survival rate that is worse than the outcome for breast cancer and Hodgkin disease, but still remains largely underdiagnosed and undertreated.2–5 It is estimated that ∼15–20% of patients with PAD will progress to critical limb ischaemia, which is associated with a 1-year mortality rate of about 20%.6–8 Technical diagnostic tools that are commonly used include the determination of the ankle-brachial index (ABI), exercise exams such as treadmill and oscillography, colour-guided duplex ultrasound, and radiological exams such as magnetic resonance angiography or conventional angiography.
Except for the screening test ABI and the exercise exams, these tests focus on the detection of stenoses or occlusions. A possible approach to evaluate the end organ itself could be the measurement of the perfusion of the affected muscle. The aim of this pilot study was to establish contrast-enhanced ultrasound perfusion imaging (CUPI) of the lower extremities. Perfusion sonography was introduced in the early 1990s in echocardiography and liver sonography and in 1998 in neurological sonography of the brain.9–12 It is based on harmonic frequencies, which are reflected by contrast agent microbubbles.
In this pilot study, PAD patients with intermittent claudication and control subjects were examined following standard guidelines, including further radiological diagnostic procedures if needed. CUPI was performed additionally.
Methods
Enrolment of study participants
Study participants were consecutively enrolled from the beginning of September 2004 to the end of January 2005. Patients with PAD had to present with symptomatic intermittent claudication (Fontaine stages IIa and IIb or Rutherford classes I–III). Patients with pain at rest or ischaemic manifestations (Fontaine stages III and IV) were not included in this study. Persons with chronic heart failure NYHA III and IV, with acute coronary syndrome or severe pulmonary hypertension were excluded. Control subjects had to present without any muscle ischaemic symptoms, whereas other diseases apart from PAD were not an exclusion criterion. Mobility had to be unimpaired and an intermediate grade of physical activity had to be reported in both groups. All participants had to give informed consent to the study protocol which was approved by the Ethics Committee of the University Hospital of Freiburg, Germany. Baseline data were recorded to investigate the comparability of the two groups. Initially, 20 control subjects and 20 PAD patients were assessed for eligibility, all of them either patients or employees of the University Hospital of Freiburg. One control subject and one PAD patient did not give informed consent, one control subject did not show a normal ABI, two control subjects showed microbubble persistence, one PAD patient was not able to perform the standardized exercise test, and the data of one PAD patient were lost due to technical problems. Four out of the 16 enrolled control subjects were otherwise healthy, three had minor health problems, and nine had been hospitalized in the cardiologic department for health problems other than PAD (pneumonia, arrhythmia, and valvular heart disease). None of the included control subjects suffered from PAD or other significant manifestations of atherosclerosis at that time.
Assessment of PAD
History of intermittent claudication was assessed and all patients and control subjects underwent basic angiological testing: auscultation, palpation of pulses, and Doppler pressure measurement were performed in brachial, radial, ulnar, femoral, popliteal as well as in posterior and anterior tibial position. Doppler ABI, which can be calculated by dividing the ankle systolic pressure at the malleolar level by the higher of the two brachial pressures,13 was obtained bilaterally. The leg with the lower ABI was selected for perfusion imaging. Oscillographic tests were run on thighs, proximal and distal calves, and on dorsum and sole of foot at rest and after calf exercise (30 ankle extensions, i.e. getting on tiptoes 30 times), respectively. A constant workload treadmill test (3.5 km/h=2.2 mph; 12.5% grade) was performed to assess the maximum walking distance.14 Control subjects had to present with normal test results or were otherwise excluded from the study.
Exact location and grade of stenosis in PAD patients were then determined by colour-guided duplex ultrasound (peak velocity ratio intrastenotic/prestenotic >3.4 corresponding to >70% stenosis) and, if necessary, confirmed by further radiological investigation, such as conventional angiography or magnetic resonance angiography. All patients with a pain-limited walking distance of less than 300 m and an ABI<0.9 who suffered from stenoses >70% or occlusions of iliac or superficial femoral arteries were included in the patient group.
Contrast-enhanced ultrasound perfusion imaging
Two-dimensional sonography was performed using the LOGIQ 9 ultrasound system (GE Healthcare Technologies, Ultrasound, Milwaukee, WI, USA). A 3–7 MHz wide band linear transducer (7L-probe, GE Healthcare Technologies, Ultrasound) was used, applying the following settings in all cases: Coded Phase Inversion Technology with low mechanical index (MI) of 0.19 (acoustic output 7%), depth 4–5 cm, one focus zone at 2.75 cm, dynamic range 72, pulse repetition interval 1.25, grey map K, B (brightness-)-mode amplification 30. The ultrasound pulses were triggered with 1 pulse/s. A specific area of the calf muscle (M. gastrocnemius and M. soleus) not showing any bone tissue was designated for analysis. An optimized manual technique was applied to minimize movement artefacts.
A bolus of 2.4 mL SonoVue™ solution (sulphur hexafluoride, Bracco International B.V., Amsterdam, the Netherlands) was injected into a peripheral vein using a 20 gauge venous catheter. As preliminary experiments showed no obvious difference between the injection of 5 and 2.4 mL of SonoVue, the standard dose in this study had been defined as 2.4 mL. The SonoVue injection was followed immediately by a 5 mL saline bolus to flush the injection line. Two measurements were conducted. After 10 min at rest in a supine position (at rest—AR), the first injection was performed following a 2 min baseline acquisition of the calf muscle without any contrast agent. Another 4 min after injection was recorded. The second injection was performed after calf exercise (AE), consisting of 30 ankle extensions in a standardized way (participants had to tiptoe to maximal extension while holding a stabilizing bar)—this time preceded only by a 30 s baseline acquisition and followed again by an acquisition of 4 min. Thus, the total acquisition time was 6 min AR and 4 min 30 s AE. Liver and portal vein were examined in B-mode and colour-guided duplex ultrasound after the first measurement so as to exclude rare cases of SonoVue microbubble persistence15 from this study.
Image analysis
Image analysis was performed using the time intensity curve (TIC) analysis provided by GE Healthcare Technologies, Ultrasound and implemented into the LOGIQ 9 ultrasound system. Using the ultrasound raw data, the image displays the acoustic intensity (in dB) during acquisition time in a manually defined region of interest (ROI). The analysis was carried out in a blinded way. An ROI in the shape of a circle (diameter 4 cm) was placed randomly in the exam area (containing parts of M. gastrocnemius and M. soleus—but no bone tissue) and the wash-in curve was assessed, subsequently. In several preliminary experiments, it became evident that size and position of the ROI had an important impact. Small ROIs (3–10 mm diameter) showed important changes in the shape of the wash-in curve when being moved (data not shown). A circle-shaped ROI with a constant diameter of 4 cm provided the most stable results and was used in all further experiments. The determination of the wash-in curve was verified interactively using a scrolling feature that enabled the simultaneous review of the B-mode cine-loop. Curve morphology and time to peak intensity (TTP) were assessed. TTP was defined as the duration from the beginning of the contrast increase until its peak, i.e. the duration of the wash-in. A three times averaging was applied to minimize artefacts.
Starting and endpoint of the wash-in curve were defined manually. In preliminary experiments, the contrast agent was injected immediately after starting data acquisition (recorded in a cine-loop). Further analysis revealed that it was not always evident at what point the wash-in curve actually started, i.e. at what time the starting point should be determined. For that reason, a 2 min baseline acquisition time without contrast agent was introduced at rest. In order not to miss important effects of the calf exercise, the baseline acquisition time after exercise was 30 s. Analysis of the wash-out curves revealed a high inconsistency and it was not possible to properly define the endpoint of the wash-out. For that reason, the analysis of the wash-out was abandoned.
Statistical analysis
Patient characteristics are presented as median and range for quantitative data and as absolute frequencies for qualitative data. Mann–Whitney U test or Fisher's exact test were used to compare baseline variables between the study groups. The comparison of outcome variables between the study groups was performed with adjustment for age in an ANCOVA model. To assess the correlation between age, ABI, and TTP, scatter diagrams were plotted and the partial correlation coefficient was calculated taking into account whether a subject was a PAD patient or control subject. A conservative P value of <0.01 was interpreted to denote statistical significance to account for the pilot character of the study. All statistical tests were two-sided. Statistical analysis was performed using the SPSS Software, Version 13.0, LEAD Technologies, Inc., Chicago, IL, USA.
Results
Patient characteristics
Twelve male and four female volunteers at the median age of 53 (21–82) years showed normal test results and were included as control subjects (Table 1). The walking distance was not limited, there was no history of claudication, and the median ABI was 1.0 (1.0–1.3). Oscillography revealed normal curves at rest and amplitudes recovered immediately, i.e. 0 min (0–1 min) after calf exercise. The risk profile of the control subjects, including patients hospitalized in the cardiologic department for other reasons, displayed nine cases of arterial hypertension, four cases of dyslipidaemia, and 10 former or current smokers. There were no cases of diabetes in the control group. In the PAD group, 12 male and four female patients, 68 (58–75) years old, were included. Three had diabetes, 11 were current or former smokers, 11 were hypertonic, and 10 had dyslipidaemia. The patients presented with a history of typical claudication each and had to abandon the treadmill test after a maximum walking distance of 91 m (10–300 m). The median ABI was 0.5 (0.3–0.7). Oscillographic amplitudes were visibly diminished in the affected leg, according to the estimated approximate localization of the lesion. After calf exercise, it took 4.2 min (2.0–6.0 min) for the amplitudes to recover. Colour-guided duplex ultrasound and—in certain cases—further radiological investigation revealed that three patients had stenoses and one patient an occlusion of the iliac artery as leading lesion. Three patients had stenoses and nine patients had occlusions of the superficial femoral artery as leading lesion. Two control subjects showed SonoVue microbubble persistence in liver and portal vein and were excluded from the study. The persistence was evident for more than 30 min but less than 1 day and was not accompanied by any subjective complaints.
Contrast-enhanced ultrasound perfusion imaging
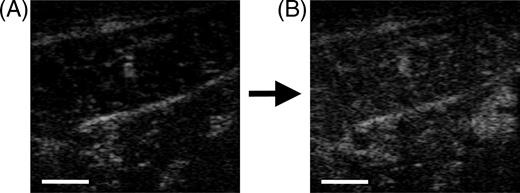
The total exam duration was 20–30 min in control subjects and PAD patients. Injection of 2.4 mL of SonoVue resulted in visible contrast enhancement in low-MI contrast-enhanced sonography of the calf muscle (Figure 1). None of the study participants reported any side effects.
Image analysis
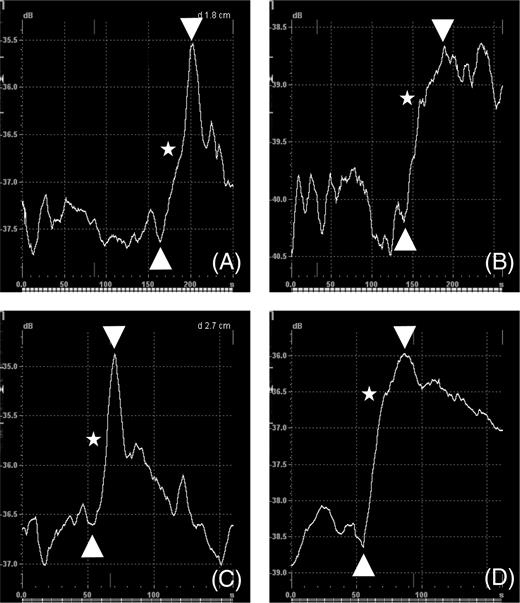
Wash-in started 15–20 s after injection (∼140/50 s after cine-loop start AR/AE; Figure 2). The typical wash-in curve morphology revealed a steep ascent in two phases (Figure 2). A higher gradient phase, which was correlated with the appearance of the contrast agent in visible small vessels, presumably small arteries and arterioles. This correlation was discovered by interactive scrolling through the TIC analysis graph and the B-mode cine-loop simultaneously. A lower gradient phase followed the first phase after a short decline in most cases and was correlated with further diffuse contrast enhancement in the muscle tissue, presumably the capillary bed, venoles, and veins. The interactive control also allowed confirming the definition of the wash-in curve. The wash-in curve geometry was visibly more homogeneous and distinct after exercise than at rest (Figure 2).
TTP was defined as the duration of the wash-in curve. At rest, i.e. after 10 min at rest in a supine position, the median TTP was 37 s (19–79s) in control subjects and 56 s (32–104s) in PAD patients (Figure 3). After calf exercise, control subjects showed a median TTP of 19 s (8–37s) vs. 32 s (18–48 s) in PAD patients. The difference of TTP between PAD patients and control subjects at rest was significant (age-adjusted P=0.002). After exercise, PAD patients still showed a significantly longer TTP (age-adjusted P=0.004). Calf exercise resulted in a decrease of the TTP in both groups.
Partial correlation analysis
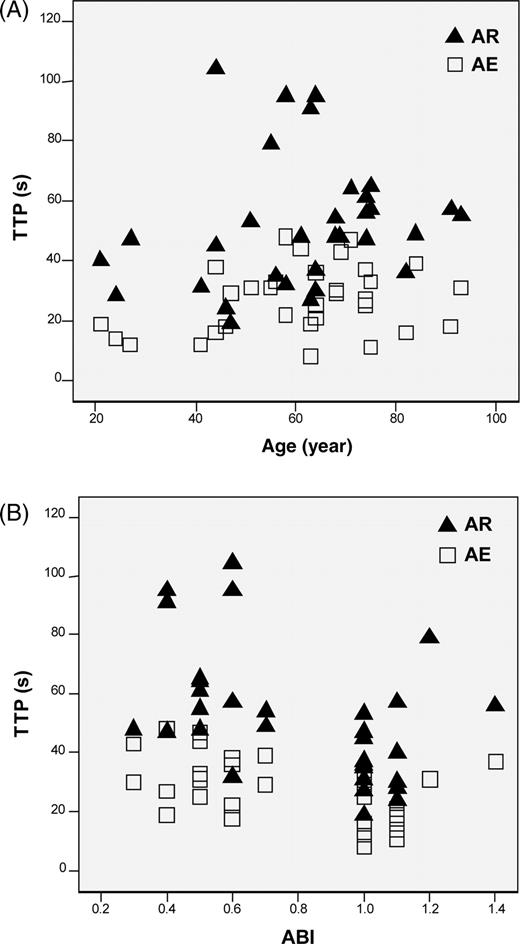
There was no significant partial correlation between age and TTP at rest (r=−0.115; P=0.536) and after exercise (r=0.08; P=0.967; Figure 4). Accordingly, there was no significant partial correlation between ABI and TTP at rest (r=0.192; P=0.301) and after exercise (r=0.126; P=0.498).
Discussion
The Doppler ABI is an excellent screening method to identify patients with PAD, providing high validity (an ABI<0.9 is 90%/95% sensitive/specific for PAD).3,5,16 ABI measurements are cost-effective and can be obtained with a standardized blood pressure cuff and a hand-held Doppler device in ∼10 min. However, some limitations of the ABI have to be considered. First, microcirculation cannot be evaluated with the ABI. Second, there are no reliable guidelines available that correlate the ABI values with an indication for invasive treatment of PAD. Third, its values in incompressible arteries with medial calcification are falsely high.
To our knowledge, a reliable and practical method does not yet exist to assess perfusion in the musculature of extremities using ultrasound contrast agents. The purpose of our study was to establish CUPI as a novel diagnostic method for patients with PAD. In this pilot study, we wanted to examine whether impaired blood perfusion of the lower extremities in patients with intermittent claudication can be assessed by CUPI. The single exam takes half an hour and has to be performed by an experienced examiner. Prior to analysis, intravenous administration of ultrasound contrast agent is necessary. The standard dose of SonoVue in this study was defined as 2.4 mL. If this will be sufficient for further studies, if performed in patients with more severe disease, remains to be shown. The pivotal parameter chosen in this pilot study was the TTP. It proved to be the most reliable parameter in perfusion studies in brain tissue comparing area under the curve, peak intensity, and TTP.17,18 Our study revealed a significant difference in TTP between the PAD patients and study controls at rest (P=0.002) and after exercise (P=0.004).
Potential limitations of this study merit consideration. In order to be able to perform a more concise follow-up study, we recruited a rather small sample size within a short time period. Additionally, although we used consecutively available patients and controls, we avoided formal procedures to ensure optimal comparability. Therefore, our results have to be regarded as preliminary at this stage. Owing to the favourable early results of our pilot study, however, we are currently in the process of planning a larger study to confirm these results.
The significant difference in TTP between the two study groups might have been in part due to a difference in the distribution of age that existed between the groups. To address this problem, the comparison had been adjusted for age—and still showed a significant difference. Additionally, a partial correlation analysis was performed, revealing that TTP and age were not correlated. To compare the study groups properly, the study design guaranteed that the examined condition—the PAD—was absent in the control group. The correlation analysis did not show any significant correlation between TTP and ABI. This finding might lead to the assumption that CUPI does not only assess the perfusion of the macrovessels, but integrates information about the perfusion of the muscle, including the microcirculation. It is possible that the TTP changes with alterations of the microcirculation—which requires further investigation. Changes in microcirculation are most likely linked to the progression of atherosclerotic large vessel disease and should be considered in PAD.19 Given that TTP reflects muscle perfusion, the decrease of TTP after calf exercise observed in both groups would have to be interpreted as reactive global hyperperfusion of the calf muscles. Patients with intermittent claudication showed a comparable degree of reactive hyperperfusion to that of control subjects. Changes of the collateral blood flow pattern or alterations of the peripheral vascular resistance could explain this finding.
Alternative methods to evaluate limb perfusion include 99mTc-tetrofosmin perfusion scintigraphy, which should be compared with CUPI in future studies.20 Capillaroscopy, laser Doppler fluxmetry, and transcutaneous measurement of oxygen tension (tcPO2) are used to evaluate microcirculation21 but have gained controversial clinical relevance in patients with intermittent claudication.22–24 Moreover, tcPO2 detects perfusion of the skin exclusively but not muscle perfusion. The future areas of application of CUPI could include the observation of statin effects,25 the influence of interventions with growth factors,26 or stem cells20 in the therapy of symptomatic PAD. Tyrosine phosphatase inhibition seems to augment collateral blood flow27 which might also be examined by CUPI. Pharmacological treatment strategies, including pentoxifylline,28 cilostazol,29 and prostaglandins30 may potentially be evaluated by CUPI.
We believe that CUPI may provide additional relevant information in the diagnosis of PAD. This pilot study was able to establish a novel method; further evidence by performing studies with larger patient numbers is now needed.
Acknowledgements
The authors thank Anja Becherer, Elisabeth Rink, Dorothee Harder, and Renate Haas from the Angiology Laboratory of the University Hospital, Freiburg, Germany. This work was supported by Dr. Christian Greis from Bracco International.
Conflict of interest: none declared.
Figure 1 Low-MI B-mode sonography of the calf muscle before (A) and 30 s after infusion of SonoVue (B). Increase of echogenity in muscle tissue is visible but not very distinct. Bar represents 1 cm.
Figure 2 TIC analysis: wash-in curve (between arrows) in calf muscle of control subjects (A, C) and in patients with PAD (B, D) at rest (A, B) and after calf exercise (C, D). Stars depict the transition from the early arterial/arteriolar to the subsequent tissue-enhancing phase. In A and B, the first 250 s and in C and D the first 160 s of acquisition time are shown. X-axis: time (s); Y-axis: acoustic intensity (dB).
Figure 3 Boxplot: TTP of the contrast agent wash-in curve in calf muscle of control subjects (white) and patients with PAD (grey) at rest and after calf exercise. PAD patients showed a significantly higher TTP at rest [56 s (32–104), age-adjusted P=0.002] as well as after exercise [32 s (18–48), age-adjusted P=0.004] than control subjects [37 s (19–79) at rest and 19 s (8–37) after exercise]. Horizontal bars—medians, whiskers—ranges.
Figure 4 Scatter diagrams of TTP and age (A) as well as TTP and ABI (B) in both study groups (control subjects and patients with PAD). Black triangles depict TTP values at rest (AR) and empty squares the corresponding values after exercise (AE). (A) There was no significant partial correlation age/TTP at rest (r=−0.115; P=0.536) and after exercise (r=0.08; P=0.967). (B) There was no significant partial correlation between ABI and TTP at rest (r=0.192; P=0.301) and after exercise (r=0.126; P=0.498). r denotes the correlation coefficient.
Baseline clinical variables and diagnostic parameters for PAD
| Variable/parameter . | Control subjects (n=16) . | PAD patients (n=16) . | P . |
|---|---|---|---|
| Age (year) | 53 (21–82) | 68 (58–75) | 0.008 |
| Sex—female/male | 4/12 | 4/12 | 1.000 |
| Diabetes (no.) | 0 | 3 | 0.226 |
| Hypertension (no.) | 9 | 11 | 0.716 |
| Dyslipidaemia (no.) | 4 | 10 | 0.073 |
| Smoking (no.) | 10 | 11 | 1.000 |
| Maximum walking distance (m) | Not limited | 91 (10–300) | |
| ABI | 1.0 (1.0–1.3) | 0.5 (0.3–0.7) | <0.001 |
| Recompensation time in oscillography (min) | 0.0 (0.0–1.0) | 4.2 (2.0–6.0) | <0.001 |
| Occlusion of iliac artery (no.) | 0 | 1 | |
| Stenosis of iliac artery (no.) | 0 | 3 | |
| Occlusion of SFA (no.) | 0 | 9 | |
| Stenosis of SFA (no.) | 0 | 3 |
| Variable/parameter . | Control subjects (n=16) . | PAD patients (n=16) . | P . |
|---|---|---|---|
| Age (year) | 53 (21–82) | 68 (58–75) | 0.008 |
| Sex—female/male | 4/12 | 4/12 | 1.000 |
| Diabetes (no.) | 0 | 3 | 0.226 |
| Hypertension (no.) | 9 | 11 | 0.716 |
| Dyslipidaemia (no.) | 4 | 10 | 0.073 |
| Smoking (no.) | 10 | 11 | 1.000 |
| Maximum walking distance (m) | Not limited | 91 (10–300) | |
| ABI | 1.0 (1.0–1.3) | 0.5 (0.3–0.7) | <0.001 |
| Recompensation time in oscillography (min) | 0.0 (0.0–1.0) | 4.2 (2.0–6.0) | <0.001 |
| Occlusion of iliac artery (no.) | 0 | 1 | |
| Stenosis of iliac artery (no.) | 0 | 3 | |
| Occlusion of SFA (no.) | 0 | 9 | |
| Stenosis of SFA (no.) | 0 | 3 |
Values are median and range or absolute frequencies. SFA denotes superficial femoral artery. Definitive diagnoses are provided by colour-guided duplex ultrasound, conventional angiography, or magnetic resonance angiography.
Baseline clinical variables and diagnostic parameters for PAD
| Variable/parameter . | Control subjects (n=16) . | PAD patients (n=16) . | P . |
|---|---|---|---|
| Age (year) | 53 (21–82) | 68 (58–75) | 0.008 |
| Sex—female/male | 4/12 | 4/12 | 1.000 |
| Diabetes (no.) | 0 | 3 | 0.226 |
| Hypertension (no.) | 9 | 11 | 0.716 |
| Dyslipidaemia (no.) | 4 | 10 | 0.073 |
| Smoking (no.) | 10 | 11 | 1.000 |
| Maximum walking distance (m) | Not limited | 91 (10–300) | |
| ABI | 1.0 (1.0–1.3) | 0.5 (0.3–0.7) | <0.001 |
| Recompensation time in oscillography (min) | 0.0 (0.0–1.0) | 4.2 (2.0–6.0) | <0.001 |
| Occlusion of iliac artery (no.) | 0 | 1 | |
| Stenosis of iliac artery (no.) | 0 | 3 | |
| Occlusion of SFA (no.) | 0 | 9 | |
| Stenosis of SFA (no.) | 0 | 3 |
| Variable/parameter . | Control subjects (n=16) . | PAD patients (n=16) . | P . |
|---|---|---|---|
| Age (year) | 53 (21–82) | 68 (58–75) | 0.008 |
| Sex—female/male | 4/12 | 4/12 | 1.000 |
| Diabetes (no.) | 0 | 3 | 0.226 |
| Hypertension (no.) | 9 | 11 | 0.716 |
| Dyslipidaemia (no.) | 4 | 10 | 0.073 |
| Smoking (no.) | 10 | 11 | 1.000 |
| Maximum walking distance (m) | Not limited | 91 (10–300) | |
| ABI | 1.0 (1.0–1.3) | 0.5 (0.3–0.7) | <0.001 |
| Recompensation time in oscillography (min) | 0.0 (0.0–1.0) | 4.2 (2.0–6.0) | <0.001 |
| Occlusion of iliac artery (no.) | 0 | 1 | |
| Stenosis of iliac artery (no.) | 0 | 3 | |
| Occlusion of SFA (no.) | 0 | 9 | |
| Stenosis of SFA (no.) | 0 | 3 |
Values are median and range or absolute frequencies. SFA denotes superficial femoral artery. Definitive diagnoses are provided by colour-guided duplex ultrasound, conventional angiography, or magnetic resonance angiography.
References
Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE, Jr, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review.
Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, Von Stritzky B, Tepohl G, Trampisch HJ. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study.
Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR III, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA. Critical issues in peripheral arterial disease detection and management: a call to action.
Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease.
Faxon DP, Creager MA, Smith SC, Jr, Pasternak RC, Olin JW, Bettmann MA, Criqui MH, Milani RV, Loscalzo J, Kaufman JA, Jones DW, Pearce WH. Atherosclerotic Vascular Disease Conference Proceeding for healthcare professionals from a special writing group of the American Heart Association.
Jelnes R, Gaardsting O, Hougaard JK, Baekgaard N, Tonnesen KH, Schroeder T. Fate in intermittent claudication: outcome and risk factors.
Dormandy J, Mahir M, Ascady G, Balsano F, De Leeuw P, Blombery P, Bousser MG, Clement D, Coffman J, Deutshinoff A. Fate of the patient with chronic leg ischaemia: a review article.
Muro T, Hozumi T, Watanabe H, Yamagishi H, Yoshiyama M, Takeuchi K, Yoshikawa J. Assessment of myocardial perfusion abnormalities by intravenous myocardial contrast echocardiography with harmonic power Doppler imaging: comparison with positron emission tomography.
Schrope BA, Newhouse VL. Second harmonic ultrasonic blood perfusion measurement.
Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound.
McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease.
Labs KH, Nehler MR, Roessner M, Jaeger KA, Hiatt WR. Reliability of treadmill testing in peripheral arterial disease: a comparison of a constant load with a graded load treadmill protocol.
Okada M, Albrecht T, Blomley MJ, Heckemann RA, Cosgrove DO, Wolf KJ. Heterogeneous delayed enhancement of the liver after ultrasound contrast agent injection—a normal variant.
Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression.
Harrer JU, Klotzsch C, Stracke CP, Moller-Hartmann W. Cerebral perfusion sonography in comparison with perfusion MRT: a study with healthy volunteers.
Meves SH, Wilkening W, Thies T, Eyding J, Holscher T, Finger M, Schmid G, Ermert H, Postert T. Comparison between echo contrast agent-specific imaging modes and perfusion-weighted magnetic resonance imaging for the assessment of brain perfusion.
Stokes KY, Granger DN. The microcirculation: a motor for the systemic inflammatory response and large vessel disease induced by hypercholesterolaemia?
Miyamoto M, Yasutake M, Takano H, Takagi H, Takagi G, Mizuno H, Kumita S, Takano T. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy.
Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease.
Bouye P, Picquet J, Jaquinandi V, Enon B, Leftheriotis G, Saumet JL, Abraham P. Reproducibility of proximal and distal transcutaneous oxygen pressure measurements during exercise in stage 2 arterial claudication.
Abraham P, Picquet J, Vielle B, Sigaudo-Roussel D, Paisant-Thouveny F, Enon B, Saumet JL. Transcutaneous oxygen pressure measurements on the buttocks during exercise to detect proximal arterial ischemia: comparison with arteriography.
Mouren X, Caillard P, Massonneau M, Thebault B. TcPo2 measurement reproducibility during stress in stage II obliterative arterial disease.
Mohler ER, III, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease.
Rajagopalan S, Mohler ER, III, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication.
Carr AN, Davis MG, Eby-Wilkens E, Howard BW, Towne BA, Dufresne TE, Peters KG. Tyrosine phosphatase inhibition augments collateral blood flow in a rat model of peripheral vascular disease.
Porter JM, Cutler BS, Lee BY, Reich T, Reichle FA, Scogin JT, Strandness DE. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients.
Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE, Jr, Bortey EB, Forbes WP. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial.



![Figure 3 Boxplot: TTP of the contrast agent wash-in curve in calf muscle of control subjects (white) and patients with PAD (grey) at rest and after calf exercise. PAD patients showed a significantly higher TTP at rest [56 s (32–104), age-adjusted P=0.002] as well as after exercise [32 s (18–48), age-adjusted P=0.004] than control subjects [37 s (19–79) at rest and 19 s (8–37) after exercise]. Horizontal bars—medians, whiskers—ranges.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/27/3/10.1093_eurheartj_ehi636/3/m_ehi63603.jpeg?Expires=1716427347&Signature=x~7cjDz1XdIanCVaeQM4IbfhCzU28lYubS99ZvFS-CfHiPYp3ONSvhy-MlUeTymcgAW-svxh70T13THG~33rHJVERsakMAa2zX89jK4ZIivrUWXqp3y7QJtM-WcTQj5XlUeynUwFYDFtOeGx3pyhefCOLhVM23CUu9Zq4oau1BUTxe9U9bMByG-hgkY81~r5x2kei2~c4SE7POmREj5YnUdrvVB83DE7eqtCEuhbw70pUlW4iUjw4AKRj8D9aXDer7bA~i5p-bPaJgGUnjK1o4LiylpLQXdGqe6VUWKuJp9j8QqlT2R5FOOUZKHrNbJbGOzS07MFOFo7aMJnizbDnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)