-
PDF
- Split View
-
Views
-
Cite
Cite
Fausto Rigo, Sonia Gherardi, Maurizio Galderisi, Lorenza Pratali, Lauro Cortigiani, Rosa Sicari, Eugenio Picano, The prognostic impact of coronary flow-reserve assessed by Doppler echocardiography in non-ischaemic dilated cardiomyopathy, European Heart Journal, Volume 27, Issue 11, June 2006, Pages 1319–1323, https://doi.org/10.1093/eurheartj/ehi795
Close - Share Icon Share
Abstract
Aims Coronary flow-reserve (CFR) can be impaired in non-ischaemic dilated cardiomyopathy (DCM), unmasking a coronary microcirculatory dysfunction of potential prognostic impact. The aim of the present study is to evaluate the prognostic value of Doppler echocardiographic-derived CFR in patients with DCM.
Methods and results We evaluated 129 DCM patients (85 male; age 62±11) by transthoracic dipyridamole (0.84 mg/kg in 10 min) stress echocardiography. All patients had an ejection fraction <40% (mean 32±7) and angiographically normal coronary arteries with NYHA class≤3. CFR was assessed on left anterior descending artery using pulsed Doppler as the ratio of maximal peak vasodilation (dipyridamole) to rest diastolic flow velocity. All patients were followed-up for a median of 22 months. Mean CFR was 2.0±0.5. At individual patient analysis 46 patients had normal (CFR>2.0) and 83 had abnormal CFR. During follow-up, 18 patients died and 33 showed worsening of NYHA class. The worse event-free survival was observed in those patients with an abnormal CFR when compared with those having a normal CFR at high dose of dipyridamole (70 vs. 22%, at 75 months of follow-up, P<0.0001). In the multivariable analysis, severity of mitral insufficiency (HR=1.9, 95% CI=1.06–2.87), abnormal CFR (HR=4.0, 95% CI=1.1–15.6), resting wall motion score index (HR=6.9, 95% CI=1.5–30.7) were independent predictors of survival.
Conclusion In DCM patients, CFR is often impaired. A reduced CFR during vasodilator stress is an independent prognostic marker of bad prognosis.
Introduction
Dilated cardiomyopathy (DCM) is a condition that predominantly affects left ventricular (LV) systolic function.1 Nevertheless, echocardiographic parameters at rest are poorly correlated with disease severity and prognosis.2,3 In patients with non-ischaemic DCM, the presence of an inotropic response elicited by pharmacologic stress echocardiography (dipyridamole or dobutamine) identifies a subset of patients at lower risk of death.4–6 Also coronary flow-reserve (CFR) analysed by PET imaging can be reduced in DCM patients, this reduction being a marker of impaired coronary microcirculation.7 The abnormal CFR in DCM is related to an increased incidence of cardiac mortality, independent of the degree of LV functional impairment and the evidence of overt heart failure.7 Dipyridamole stress echocardiography (DSE) has been demonstrated to be a suitable tool for the assessment of LV wall motion and CFR calculated by pulsed Doppler echocardiography on the territory of the left anterior descending artery (LAD).8–10 In DCM, Doppler-derived CFR evaluated by pulsed Doppler echocardiography is reduced with inter-individual variability, and is not related to LV inotropic reserve during dipyridamole stress.11 No data are available on the prognostic impact of CFR measured by pulsed Doppler in patients with DCM. Therefore, the primary aim of the present study is to assess the prognostic value of CFR measured by pulsed Doppler echocardiography during high dose of dipyridamole in patients with DCM in a multicentre study. The secondary aim is to assess whether this value was additive over simple variables of recognized prognostic value which may be derived from a baseline echocardiogram, such as resting wall motion or diastolic dysfunction.
Methods
Patients
From 1 May 1997 to 31 December 2004, 137 patients were prospectively enrolled from the Cardiology Division, Mestre (n=52) and Cesena (n=44), the Institute of Clinical Physiology, National Council of Research (n=28), and Federico II, University of Naples (n=13). The study population consisted of patients with idiopathic DCM presenting with: (i) a global LV dysfunction (ejection fraction <40% by biplane area length method on resting echocardiogram) but no history of ischaemic heart disease and angiographically normal coronary arteries (coronary angiography performed prior to study enrolment); (ii) transthoracic echocardiogram adequate to assess resting regional wall function (the echocardiogram was considered adequate if ≥13 of the maximum 16 segments were visualized in at least one projection); (iii) enrolment in a follow-up program. Exclusion criteria were: (i) technically poor acoustic window precluding satisfactory imaging of left ventricle (for two-dimensional echo) or of LAD coronary artery flow Doppler (for CFR assessment); (ii) haemodynamic instability or NYHA class IV; (iii) documentation of life-threatening ventricular arrhythmias (sustained ventricular tachycardia or ventricular fibrillation), (iv) significant co-morbidity reducing life expectancy to <1 year; and (v) unwillingness to give informed consent. All patients with congenital, valvular, hypertrophic cardiomyopathy, myocarditis, pericarditis, and thyroid disease were also excluded. Given these inclusion/exclusion criteria, the population represented a consecutive cohort of patients presenting at each institution. Of the 137 patients initially selected for the study, eight patients were subsequently excluded—three patients were excluded for inadequate echocardiographic image quality during stress precluding satisfactory imaging of LAD flow (n=2) or inadequate wall motion analysis (n=1). Five further patients were lost to follow-up of which two had a CFR<2 and three had a CFR>2. Thus, 129 patients (87 males, mean age 62±11 years) represent the final study group. The study was approved by the institutional review board. All patients gave their written informed consent when they underwent stress echocardiography. When patients signed the written informed consent they also authorized physicians to use their clinical data according to the Italian Law. Stress echo data were collected and analysed by stress echocardiographers not involved in patient care.
All patients were followed-up for a median of 22 months (1st quartile 9.8, 3rd quartile 38), with a minimum pre-defined follow-up time of 6 months.
Resting and stress echocardiography
Transthoracic stress echocardiographic studies were performed with commercially available ultrasound machine (Sonos 5500-7500 Philips Ultrasound, Andover, Mass, Sequoia C256 Acuson Siemens Mountain View, CA, USA and Vivid System 7, GE/Vingmed, Milwaukee, WI, USA) equipped with 2.5–3.5 MHz phased-array sector scan probe (S3–S8 or V3–V7) and with second harmonic technology. All standard echocardiographic views were obtained when possible. LV end-diastolic and end-systolic diameters were measured from the M-mode trace obtained by parasternal long axis view. LV volumes were measured and ejection fraction obtained by two-dimensional and four-chamber view using biplane area-length method, according to the recommendations of the American Society of Echocardiography.12 LV mass was calculated by the Devereux formula13 indexed to body surface area. Mitral regurgitation was assessed semi-quantitatively (from 1 to 4) by colour flow Doppler. LV diastolic function was evaluated by recording mitral inflow pattern at the tips level and calculating E/A ratio and deceleration time (DT) of E velocity. On the basis of the Doppler measurements, two principal mitral flow velocity patterns were defined: (i) a likewise restrictive pattern, characterized by an E/A ratio >1.5–2 and a DT≤150 ms; and (ii) a likewise non-restrictive pattern, characterized by an E/A <1 (whatever the value of the DT) or if >1 with a DT>150 ms.14
Two-dimensional echocardiography and 12-lead electrocardiographic (ECG) monitoring were performed in combination with high dose of dipyridamole (up to 0.84 mg over 10 min) in accordance to well-established protocol.15 During the procedure, blood pressure and ECG were recorded each minute. The LV was divided into 16 segments as suggested by the American Society of Echocardiography.15 Segmental wall motion was graded as follows: normal, 1; hypokinetic, 2; akinetic, 3; and dyskinetic, 4. Wall Motion Score Index (WMSI) was derived by dividing the sum of individual visualized segment scores by the number of visualized segments.15
CFR was performed during the standard stress echo examination by a semi-simultaneous imaging of both wall motion and LAD flow.8 CFR was assessed using a broad-band high-frequency (5–12 MHz) transthoracic transducer (S12) or 3.5–7.0 MHz with second harmonic capability (S8). Coronary flow in the mid-distal portion of LAD was searched in the low parasternal long-axis cross-section under the guidance of colour Doppler flow mapping.16 If no colour-coded blood flow from the LAD was visualized at the baseline condition, the procedure was attempted a second time during contrast enhancement with Sonovue (Bracco-Byk-Gulden, Konstanz, Germany) in bolus (0.5 mL intravenously) in 33 patients (27% of the total population).
All studies were digitally stored to simplify off-line reviewing and measurements. Coronary flow parameters were analysed off-line using the built-in calculation package of the ultrasound unit. Flow velocities were measured at least twice for each study: at baseline and at peak stress (before aminophylline injection). At each time point, three optimal profiles of peak diastolic Doppler flow velocities were measured, and the results were averaged. Coronary blood flow velocity reserve was defined as the ratio between hyperaemic and basal peak diastolic coronary flow. CFR was considered normal when it was >2. All observers were trained by the same senior investigators (FR), who granted consistency in data acquisition storage and interpretation. The previously assessed intra- and inter-observer variability for measurements of Doppler recordings were <10%.17
Follow-up data
Follow-up data were obtained from at least one of four sources: review of the patient's hospital record, personal communication with the patient's physician and review of the patient's chart, a telephone interview with the patient conducted by trained personnel, a staff physician visiting the patients at regular intervals in the out-patient clinic. According to the study protocol, follow-up information were obtained every 6 months. By inclusion criteria, follow-up data were obtained in all patients. Events were defined as death for all causes, cardiac death, development or progression of heart failure. In patients who died in hospital or at home, the cause of death was elucidated from the medical record, the family and the local physician who signed the death certificate. The definition of cardiac death required documentation of significant arrhythmias or cardiac arrest, or both, or death attributable to congestive heart failure or myocardial infarction in the absence of any other precipitating factors. In case of deaths out of hospital for which no autopsy was performed, sudden unexpected death was attributed to a cardiac cause. The development or progression of heart failure was defined as at least one of the following: worsening of functional class to NYHA class III and IV, new hospitalization for heart failure, or heart transplantation. Therefore, the outcome events were all cause-death (defined as cardiac and non-cardiac death) for survival, and spontaneous events (death and the development or progression of heart failure for spontaneous event-free survival). When more than one of these events occurred, the patient was censored at the time of the most severe event.
Statistical analysis
Values are expressed as mean±SD. The sample size has been determined on the basis of the individual contribution of covariates to the model, as assessed from the significance test given with each coefficient in the main output. We used for power a value of 80%, while 5% was the choice for alpha.18
The individual effect of certain variables on event-free survival was evaluated with the use of the Cox regression model. The individual effect of certain variable on event-free survival was evaluated using the Cox regression model (SPSS statistical software, SPSS Inc., Chicago, IL, USA and S–plus 6.1). Univariate analysis was undertaken by assessing the weight of each single variable by itself. Afterwards, all the significant variables were entered in the model all together without employing a stepwise approach, hence to adjust for several risk factors, multivariable Cox analysis was performed with all the variables found to be significant at the univariable analysis entering in a single step. The proportional hazards assumptions of Cox’ model was verified with the linear correlation test.19 A significance of 0.05 was required for a variable to be included into the multivariable model, while 0.1 was the cut-off value for exclusion. Hazard ratios (HR) with the corresponding 95% confidence interval (CI) were estimated.
The following covariates were analysed: age, sex, hypertension, smoking habit, diabetes, alcohol consumption, left bundle branch block, presence of medical therapy at time of testing, different class of drugs at time of testing (ACE-inhibitors, beta-blockers, digitalis, amiodarone), mitral insufficiency severity, LV end-diastolic volume, LV end-diastolic diameter, ejection fraction, LV mass index, restrictive pattern, resting WMSI, CFR on the LAD artery. Differences between survival curves were compared with the log-rank test. Proportions were compared by χ2 statistic; a Fisher's exact test was used when appropriate.
Comparison of the baseline characteristics between the groups (with normal vs. abnormal CFR) was performed with an independent sample t-test. Kaplan–Meier life table estimates of spontaneously occurring event-free survival was used to summarize the follow-up experience in these patients and to clarify presentation. Differences in survival rates between groups were tested by the log-rank test. All tests were two-sided and a P-value below 0.05 was considered statistically significant.
Results
The main clinical and echocardiographic data are reported in Table 1.
Rest and stress findings in the study population
| . | CFR>2.0 . | CFR≤2.0 . | Sigma . |
|---|---|---|---|
| No. of patients | 46 | 83 | |
| Age (years) | 60±11 | 63±11 | 0.5 |
| Sex (male/female) | 30/16 | 57/26 | 0.69 |
| Smoking habit | 20 (43%) | 40 (48%) | 0.71 |
| Hypertension | 21 (46%) | 36 (43%) | 0.85 |
| Diabetes | 6 (13%) | 11 (13%) | 1.00 |
| Alcohol consumption | 7 (15%) | 13 (15%) | 1.00 |
| Left bundle branch block | 26 (56%) | 59 (71%) | 0.12 |
| Medical therapy | |||
| Diuretics | 33 (72%) | 77 (93%) | 0.003 |
| ACE-inhibitors | 45 (98%) | 78 (94%) | 0.42 |
| Digoxin | 17 (37%) | 50 (60%) | 0.016 |
| Beta-blockers | 32 (69%) | 56 (67%) | 0.69 |
| Amiodarone | 2 (4%) | 7 (8%) | 0.48 |
| Resting echocardiography | |||
| LV end-diastolic volume (mL) | 203±74 | 229±72 | 0.05 |
| LV end systolic volume (mL) | 128±53 | 161±57 | 0.002 |
| LV end-diastolic diameter (mm) | 61±15 | 70±7 | 0.000 |
| LV end-systolic diameter (mm) | 49±7 | 56±9 | 0.000 |
| Restrictive diastolic pattern | 11 (24%) | 30 (36%) | 0.17 |
| Ejection fraction (%) | 36±6 | 30±7 | 0.000 |
| LV mass index (gm/m2) | 147±36 | 172±42 | 0.001 |
| WMSI at rest | 1.9±0.3 | 2.1±0.2 | 0.000 |
| Stress echocardiography | |||
| WMSI at peak stress | 1.6±0.3 | 1.9±0.4 | 0.000 |
| Ejection fraction at peak stress (%) | 46±8 | 35±8 | 0.000 |
| . | CFR>2.0 . | CFR≤2.0 . | Sigma . |
|---|---|---|---|
| No. of patients | 46 | 83 | |
| Age (years) | 60±11 | 63±11 | 0.5 |
| Sex (male/female) | 30/16 | 57/26 | 0.69 |
| Smoking habit | 20 (43%) | 40 (48%) | 0.71 |
| Hypertension | 21 (46%) | 36 (43%) | 0.85 |
| Diabetes | 6 (13%) | 11 (13%) | 1.00 |
| Alcohol consumption | 7 (15%) | 13 (15%) | 1.00 |
| Left bundle branch block | 26 (56%) | 59 (71%) | 0.12 |
| Medical therapy | |||
| Diuretics | 33 (72%) | 77 (93%) | 0.003 |
| ACE-inhibitors | 45 (98%) | 78 (94%) | 0.42 |
| Digoxin | 17 (37%) | 50 (60%) | 0.016 |
| Beta-blockers | 32 (69%) | 56 (67%) | 0.69 |
| Amiodarone | 2 (4%) | 7 (8%) | 0.48 |
| Resting echocardiography | |||
| LV end-diastolic volume (mL) | 203±74 | 229±72 | 0.05 |
| LV end systolic volume (mL) | 128±53 | 161±57 | 0.002 |
| LV end-diastolic diameter (mm) | 61±15 | 70±7 | 0.000 |
| LV end-systolic diameter (mm) | 49±7 | 56±9 | 0.000 |
| Restrictive diastolic pattern | 11 (24%) | 30 (36%) | 0.17 |
| Ejection fraction (%) | 36±6 | 30±7 | 0.000 |
| LV mass index (gm/m2) | 147±36 | 172±42 | 0.001 |
| WMSI at rest | 1.9±0.3 | 2.1±0.2 | 0.000 |
| Stress echocardiography | |||
| WMSI at peak stress | 1.6±0.3 | 1.9±0.4 | 0.000 |
| Ejection fraction at peak stress (%) | 46±8 | 35±8 | 0.000 |
Rest and stress findings in the study population
| . | CFR>2.0 . | CFR≤2.0 . | Sigma . |
|---|---|---|---|
| No. of patients | 46 | 83 | |
| Age (years) | 60±11 | 63±11 | 0.5 |
| Sex (male/female) | 30/16 | 57/26 | 0.69 |
| Smoking habit | 20 (43%) | 40 (48%) | 0.71 |
| Hypertension | 21 (46%) | 36 (43%) | 0.85 |
| Diabetes | 6 (13%) | 11 (13%) | 1.00 |
| Alcohol consumption | 7 (15%) | 13 (15%) | 1.00 |
| Left bundle branch block | 26 (56%) | 59 (71%) | 0.12 |
| Medical therapy | |||
| Diuretics | 33 (72%) | 77 (93%) | 0.003 |
| ACE-inhibitors | 45 (98%) | 78 (94%) | 0.42 |
| Digoxin | 17 (37%) | 50 (60%) | 0.016 |
| Beta-blockers | 32 (69%) | 56 (67%) | 0.69 |
| Amiodarone | 2 (4%) | 7 (8%) | 0.48 |
| Resting echocardiography | |||
| LV end-diastolic volume (mL) | 203±74 | 229±72 | 0.05 |
| LV end systolic volume (mL) | 128±53 | 161±57 | 0.002 |
| LV end-diastolic diameter (mm) | 61±15 | 70±7 | 0.000 |
| LV end-systolic diameter (mm) | 49±7 | 56±9 | 0.000 |
| Restrictive diastolic pattern | 11 (24%) | 30 (36%) | 0.17 |
| Ejection fraction (%) | 36±6 | 30±7 | 0.000 |
| LV mass index (gm/m2) | 147±36 | 172±42 | 0.001 |
| WMSI at rest | 1.9±0.3 | 2.1±0.2 | 0.000 |
| Stress echocardiography | |||
| WMSI at peak stress | 1.6±0.3 | 1.9±0.4 | 0.000 |
| Ejection fraction at peak stress (%) | 46±8 | 35±8 | 0.000 |
| . | CFR>2.0 . | CFR≤2.0 . | Sigma . |
|---|---|---|---|
| No. of patients | 46 | 83 | |
| Age (years) | 60±11 | 63±11 | 0.5 |
| Sex (male/female) | 30/16 | 57/26 | 0.69 |
| Smoking habit | 20 (43%) | 40 (48%) | 0.71 |
| Hypertension | 21 (46%) | 36 (43%) | 0.85 |
| Diabetes | 6 (13%) | 11 (13%) | 1.00 |
| Alcohol consumption | 7 (15%) | 13 (15%) | 1.00 |
| Left bundle branch block | 26 (56%) | 59 (71%) | 0.12 |
| Medical therapy | |||
| Diuretics | 33 (72%) | 77 (93%) | 0.003 |
| ACE-inhibitors | 45 (98%) | 78 (94%) | 0.42 |
| Digoxin | 17 (37%) | 50 (60%) | 0.016 |
| Beta-blockers | 32 (69%) | 56 (67%) | 0.69 |
| Amiodarone | 2 (4%) | 7 (8%) | 0.48 |
| Resting echocardiography | |||
| LV end-diastolic volume (mL) | 203±74 | 229±72 | 0.05 |
| LV end systolic volume (mL) | 128±53 | 161±57 | 0.002 |
| LV end-diastolic diameter (mm) | 61±15 | 70±7 | 0.000 |
| LV end-systolic diameter (mm) | 49±7 | 56±9 | 0.000 |
| Restrictive diastolic pattern | 11 (24%) | 30 (36%) | 0.17 |
| Ejection fraction (%) | 36±6 | 30±7 | 0.000 |
| LV mass index (gm/m2) | 147±36 | 172±42 | 0.001 |
| WMSI at rest | 1.9±0.3 | 2.1±0.2 | 0.000 |
| Stress echocardiography | |||
| WMSI at peak stress | 1.6±0.3 | 1.9±0.4 | 0.000 |
| Ejection fraction at peak stress (%) | 46±8 | 35±8 | 0.000 |
Stress echocardiographic findings
Mean CFR value was 2.0±0.5. At individual patient analysis, 46 patients had normal CFR (>2.0) and 83 had abnormal CFR. Patients with an abnormal CFR had a higher WMSI at rest when compared with those having a normal CFR (2.1±0.2 vs. 1.9±0.3, P=0.001). Resting ejection fraction was 32±7 and increased to 39±10 at peak dipyridamole dose (P<0.001).
Follow-up data: spontaneous events
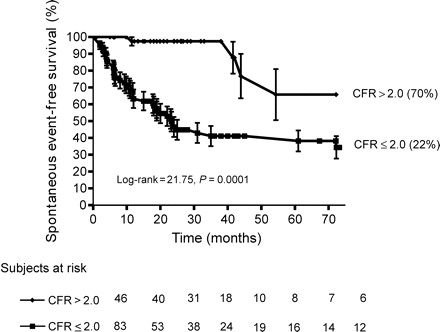
During a median follow-up of 22 months (1st quartile, 9.8; 3rd quartile, 38), a total of 51 (39%) events (18 deaths of which 16 were attributed to cardiac causes and 33 development or progression of heart failure) occurred. The distribution of events in relation to the presence/absence of normal CFR is reported in Table 2. The worse outcome was observed in those patients with an abnormal CFR when compared with those having a normal CFR (70 vs. 22%, at 75 months of follow-up, P<0.001) (Figure 1).
Kaplan–Meier survival curves (considering spontaneous events as an endpoint) in patients stratified according to normal (CFR>2) or abnormal (CFR≤2) CFR at Doppler echocardiography. The worst survival is observed in patients with abnormal CFR.
Event rate occurrence in relation with CFR
| . | CFR>2.0 (n=46) . | CFR<2.0 (n=83) . |
|---|---|---|
| Death (%) | 4 (9) | 14 (17) |
| Cardiac death (%) | 4 (9) | 12 (14) |
| Progression of heart failure (%) | 0 | 33 (49) |
| . | CFR>2.0 (n=46) . | CFR<2.0 (n=83) . |
|---|---|---|
| Death (%) | 4 (9) | 14 (17) |
| Cardiac death (%) | 4 (9) | 12 (14) |
| Progression of heart failure (%) | 0 | 33 (49) |
Event rate occurrence in relation with CFR
| . | CFR>2.0 (n=46) . | CFR<2.0 (n=83) . |
|---|---|---|
| Death (%) | 4 (9) | 14 (17) |
| Cardiac death (%) | 4 (9) | 12 (14) |
| Progression of heart failure (%) | 0 | 33 (49) |
| . | CFR>2.0 (n=46) . | CFR<2.0 (n=83) . |
|---|---|---|
| Death (%) | 4 (9) | 14 (17) |
| Cardiac death (%) | 4 (9) | 12 (14) |
| Progression of heart failure (%) | 0 | 33 (49) |
Univariate predictors of spontaneous events are reported in Table 3. In the multivariable analysis, severity of mitral insufficiency (HR=1.9, 95% CI=1.06–2.87), abnormal CFR (HR=4.0, 95% CI=1.1–15.6), resting WMSI (HR=6.9, 95% CI=1.5–30.7) were independent predictors of survival.
Univariate predictors of spontaneous events
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Alcohol use | 2.2 (1.13–4.4) | 0.020 |
| NYHA class | 3.8 (2.2–6.7) | 0.000 |
| Beta-blockers | 1.8 (0.9–3.5) | 0.07 |
| Amiodarone | 2.7 (1.14–6.4) | 0.024 |
| Mitral regurgitation severity | 3.4 (2.3–5.0) | 0.000 |
| End-diastolic diameter | 1.04 (1.01–1.08) | 0.011 |
| End-diastolic volume | 1.0 (1.003–1.010) | 0.001 |
| LV mass index | 1.0 (1.0–1.015) | 0.008 |
| Ejection fraction | 0.85 (0.8–0.89) | 0.000 |
| Rest WMSI | 12.8 (4.7–34.6) | 0.000 |
| Restrictive diastolic pattern | 2.0 (1.15–3.5) | 0.014 |
| Abnormal CFR | 7.7 (2.7–21.6) | 0.000 |
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Alcohol use | 2.2 (1.13–4.4) | 0.020 |
| NYHA class | 3.8 (2.2–6.7) | 0.000 |
| Beta-blockers | 1.8 (0.9–3.5) | 0.07 |
| Amiodarone | 2.7 (1.14–6.4) | 0.024 |
| Mitral regurgitation severity | 3.4 (2.3–5.0) | 0.000 |
| End-diastolic diameter | 1.04 (1.01–1.08) | 0.011 |
| End-diastolic volume | 1.0 (1.003–1.010) | 0.001 |
| LV mass index | 1.0 (1.0–1.015) | 0.008 |
| Ejection fraction | 0.85 (0.8–0.89) | 0.000 |
| Rest WMSI | 12.8 (4.7–34.6) | 0.000 |
| Restrictive diastolic pattern | 2.0 (1.15–3.5) | 0.014 |
| Abnormal CFR | 7.7 (2.7–21.6) | 0.000 |
Univariate predictors of spontaneous events
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Alcohol use | 2.2 (1.13–4.4) | 0.020 |
| NYHA class | 3.8 (2.2–6.7) | 0.000 |
| Beta-blockers | 1.8 (0.9–3.5) | 0.07 |
| Amiodarone | 2.7 (1.14–6.4) | 0.024 |
| Mitral regurgitation severity | 3.4 (2.3–5.0) | 0.000 |
| End-diastolic diameter | 1.04 (1.01–1.08) | 0.011 |
| End-diastolic volume | 1.0 (1.003–1.010) | 0.001 |
| LV mass index | 1.0 (1.0–1.015) | 0.008 |
| Ejection fraction | 0.85 (0.8–0.89) | 0.000 |
| Rest WMSI | 12.8 (4.7–34.6) | 0.000 |
| Restrictive diastolic pattern | 2.0 (1.15–3.5) | 0.014 |
| Abnormal CFR | 7.7 (2.7–21.6) | 0.000 |
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Alcohol use | 2.2 (1.13–4.4) | 0.020 |
| NYHA class | 3.8 (2.2–6.7) | 0.000 |
| Beta-blockers | 1.8 (0.9–3.5) | 0.07 |
| Amiodarone | 2.7 (1.14–6.4) | 0.024 |
| Mitral regurgitation severity | 3.4 (2.3–5.0) | 0.000 |
| End-diastolic diameter | 1.04 (1.01–1.08) | 0.011 |
| End-diastolic volume | 1.0 (1.003–1.010) | 0.001 |
| LV mass index | 1.0 (1.0–1.015) | 0.008 |
| Ejection fraction | 0.85 (0.8–0.89) | 0.000 |
| Rest WMSI | 12.8 (4.7–34.6) | 0.000 |
| Restrictive diastolic pattern | 2.0 (1.15–3.5) | 0.014 |
| Abnormal CFR | 7.7 (2.7–21.6) | 0.000 |
Discussion
In patients with DCM, an abnormal CFR detectable by Doppler echocardiography identifies a subset of patients at higher risk of spontaneous events (death and worsening of clinical status): the lower the CFR, the higher the incidence rate of spontaneous events.
Comparison with previous studies
The independent value of pulsed Doppler-derived CFR during dipyridamole stress echocardiography was already demonstrated in patients with known or suspected coronary artery disease.20 In a series of 327 patients with a negative high-dose dipyridamole stress echo, Rigo et al. showed that the presence of an abnormal CFR was related to a worse outcome.20 CFR is reduced in most patients with non-ischaemic DCM.11,21–24 It has already been demonstrated that the severity of this impairment is correlated with the clinical and/or haemodynamic severity of non-ischaemic DCM.11 In fact, higher NYHA functional class, lower EF, higher LV volumes, and restrictive filling pattern are associated with a reduced CFR.11 From the prognostic viewpoint, a normal CFR in non-ischaemic DCM identifies a subset of patients with a favourable outcome, although the data with this very demanding technique are limited. Neglia et al.7 demonstrated that an abnormal CFR assessed by PET imaging is related to a 3-fold increase in the relative risk of death and/or development or progression of heart failure. Our data confirm and expand previous studies, suggesting that CFR has a prognostic value when it is abnormally reduced in patients with idiopathic DCM.
Pathophysiologic mechanisms
Different mechanisms can be responsible for the CFR impairment in this set of patients: LV hypertrophy, increased LV end-diastolic pressure, and coronary small vessel disease. None of these factors can fully explain the impairment of CFR but all of them can be involved in the development of CFR reduction. Microvascular disease has been demonstrated to be present in non-ischaemic DCM25–27 at a very early stage21,28 and it might represent the triggering event leading to CFR impairment, which in its turn is responsible for the unfavourable clinical outcome. These results support the hypothesis that chronic myocardial hypoperfusion or repetitive myocardial ischaemia attributable to abnormal coronary microcirculatory flow could exert a detrimental role in the evolution of idiopathic LV dysfunction towards overt DCM. Cecchi et al.29 hypothesized that coronary microvascular dysfunction may represent a common pathway leading to a disease progression in different cardiomyopathies, including conditions such as aortic valve stenosis and hypertensive heart disease.
Study limitations
In the present study, we employed a high dose of dipyridamole to obtain a maximal vasodilation. Although the experience in CFR detection is wider with adenosine (140 µg/kg/min), high-dose dipyridamole (0.84 mg/kg) has been shown to exert a similar vasodilating effect.30 In this study, there was no central reading. Rest echocardiography and CFR measurement were interpreted in the peripheral centres and entered directly in the data bank. This system allowed substantial sparing of human and technologic resources but it was also the logical pre-requisite for a large-scale study, designed to represent the realistic performance of the test rather than the results of a single lab—or even a single person—working in a highly dedicated echo laboratory. Because the assessment of the echocardiograms was qualitative and subjective, variability in reading the echocardiograms might have modulated the results of individual centres,31 especially for some parameters, such as mitral insufficiency, which can be assessed with accuracy only with quantitative, relatively elaborated assessment.32 However, all our readers in individual centres had a long lasting experience in echocardiography, passed the quality control in stress echo reading as previously described,33 had extensive experience on execution and interpretation of CFR also through joint reading sessions. Data on resting and stress echo were directly entered in the data bank at the time of test execution. It is however, obvious that a core reading laboratory should have granted a higher robustness of echocardiographic measurements. By potentially limiting the impact of CFR index in the present study, this methodology provides however, some clinical implications on the role of this index in assessing prognosis in busy echo labs, thus allowing generalizability to the routine clinical setting.
Conclusions
In patients with idiopathic DCM, the prognostic role of impaired microvascular CFR has been shown to be unfavourable. The presence of an abnormal CFR during dipyridamole infusion allows the non-invasive identification of a subgroup of patients with LV dysfunction at high risk of developing progressive ventricular deterioration and heart failure.34 It is in this group of patients that an aggressive treatment is warranted. In contrast to other non-invasive tools as PET, very expensive and associated with radiation exposure, transthoracic Doppler-derived CFR is equally non-invasive but less expensive and more accessible.35
Conflict of interest: none declared.
References
- dipyridamole
- mitral valve insufficiency
- echocardiography
- coronary artery
- doppler pulsed
- anterior descending branch of left coronary artery
- doppler echocardiography
- stress echocardiography
- vasodilators
- follow-up
- patient prognosis
- stress
- vasodilation
- ejection fraction
- dilated cardiomyopathy, non-ischemic
- diastolic flow
- prognostic marker
- new york heart association classification
- fluid flow