-
PDF
- Split View
-
Views
-
Cite
Cite
Annabel A. Chen, Malissa J. Wood, Daniel G. Krauser, Aaron L. Baggish, Roderick Tung, Saif Anwaruddin, Michael H. Picard, James L. Januzzi, NT-proBNP levels, echocardiographic findings, and outcomes in breathless patients: results from the ProBNP Investigation of Dyspnoea in the Emergency Department (PRIDE) echocardiographic substudy, European Heart Journal, Volume 27, Issue 7, April 2006, Pages 839–845, https://doi.org/10.1093/eurheartj/ehi811
Close - Share Icon Share
Abstract
Aims The objective of this study was to determine the integrative utility of measuring plasma NT-proBNP levels with echocardiography in the evaluation of dyspnoeic patients.
Methods and results Of 599 emergency department patients enrolled in a clinical study of NT-proBNP at a tertiary-care hospital, 134 (22%) had echocardiographic results available for analysis. Echocardiographic parameters correlating with NT-proBNP levels were determined using multivariable linear-regression analysis. Independent predictors of 1-year mortality were determined using Cox-proportional hazard analysis. Independent relationships were found between NT-proBNP levels and ejection fraction (P=0.012), tissue Doppler early and late mitral annular diastolic velocities (P=0.007 and 0.018), right ventricular (RV) hypokinesis (P=0.006), and tricuspid regurgitation severity (P<0.001) and velocity (P=0.007). An NT-proBNP level <300 pg/mL had a negative predictive value of 91% for significant left ventricular systolic and diastolic dysfunction. Overall 1-year mortality was 20.1% and was independently predicted by NT-proBNP level [HR 8.65, 95% confidence interval (CI) 2.7–27.8, P=0.0003], ejection fraction (HR 0.95, 95% CI 0.91–0.99, P=0.009), RV dilation (HR 2.98, 95% CI 1.05–12.8, P=0.04), and systolic blood pressure (HR 0.97, 95% CI 0.96–0.99, P=0.01).
Conclusion NT-proBNP levels correlate with, and provide important prognostic information beyond, echocardiographic parameters of cardiac structure and function. Routine NT-proBNP testing may thus be useful to triage patients to more timely or deferred echocardiographic evaluation.
Introduction
The evaluation of patients with dyspnoea is often challenging because of the wide variety of cardiovascular and non-cardiovascular causes to be considered. With its ability to identify or exclude abnormalities in cardiac structure and function, echocardiography is a mainstay of the diagnostic work-up of dyspnoeic patients. In patients with heart failure (HF) symptoms, echocardiography often reveals a low left ventricular ejection fraction (LVEF). Up to 50% of symptomatic patients have a preserved LVEF, and abnormal diastolic function is assumed if no valvular lesions are identified1; in this regard, echocardiography may be useful in confirming diastolic dysfunction.2 However, echocardiography takes time, is expensive, requires specialized training to perform and interpret, and thus may not be optimal for regular use in some settings, such as the emergency department. A reliable, widely available, and more cost-effective test may help streamline the selection of patients for echocardiography.
B-type peptide (BNP) and its amino-terminal fragment (NT-proBNP) accurately identify HF in dyspnoeic patients.3–5 BNP correlates with echocardiographic indices of diastolic function and right ventricular (RV) systolic function,6–9 but the relationships between NT-proBNP and echocardiographic findings have not been established. Furthermore, the value of measuring natriuretic peptides for long-term prognostication above that of echocardiography is unclear. We performed the present investigation to better understand the integrative value of NT-proBNP testing with respect to the information gained from echocardiography in dyspnoeic emergency department patients.
Methods
Study population
This study was performed in compliance with the Declaration of Helsinki and with approval of the institutional review board of the Massachusetts General Hospital. All patients gave written informed consent. The design and results of the PRIDE study were recently described.3 Briefly, 599 patients presenting to the emergency department with dyspnoea were enrolled in a prospective trial examining the utility of NT-proBNP testing compared to clinical judgment for the diagnosis of acute HF. Adjudicated diagnosis of the cause of dyspnoea was determined by study physicians blinded to NT-proBNP results, using all hospital records from the first 60 days following presentation and a 60-day follow-up phone call. Diagnoses were grouped into three categories: (i) acute HF, (ii) non-cardiac dyspnoea in a patient with prior history of HF, or (iii) not acute HF.
NT-proBNP analysis
At enrolment, a blood sample was collected into EDTA-containing tubes, processed, and frozen at −80°C for later measurement of NT-proBNP using a commercially available automated immunoassay (Elecsys® proBNP, Roche Diagnostics, Indianapolis, IN, USA), performed on a Roche Elecsys® 1010 analyser. Treating clinicians were blinded to assay results.
Echocardiography
Of the 599 patients enrolled in the PRIDE study, echocardiography was determined to be indicated by the treating physicians and was performed in 135 as part of routine care. Hundred and thirty-four studies were of sufficient quality for inclusion in this analysis. Standard two-dimensional and colour Doppler imaging was performed. Off-line analysis was performed by observers blinded to the patients' clinical data and NT-proBNP levels. Measurements were averaged over three cycles (five if atrial fibrillation was present). Structural indices included: biplane LV end-diastolic and end-systolic volume indexed to body surface area (BSA); posterior wall thickness; LV mass by the modified American Society of Echocardiography formula indexed to BSA10; biplane LA volume indexed to BSA; and RV end-diastolic and end-systolic area measured in the apical four-chamber view. Left ventricular hypertrophy was defined as LV mass index >131 kg/m2 in men or 110 kg/m2 in women. LVEF was determined using biplane modified Simpson's measurements. Diastolic indices included: early and late transmitral diastolic velocities (E and A); early deceleration time (DT); pulmonary venous systolic and diastolic velocities (PV S and D); and early and late diastolic tissue Doppler velocities at the lateral mitral annulus (Ea and Aa). Overall diastolic stage was assigned as described by Troughton et al.7 except pulmonary venous atrial reversal velocity that was not included. RV indices included: RV fractional area change; the presence or absence of RV hypokinesis or dilation by qualitative visual assessment; and tricuspid regurgitation (TR) velocity. Mitral regurgitation (MR) and TR severity were graded from 0 (none) to 4 (severe) on the basis of visual assessment of structural and Doppler parameters.11 Significant systolic and diastolic dysfunctions were defined as: LVEF<50%; E/Ea>15; and diastolic dysfunction stage 1, 2, or 3.
Statistical analysis
NT-proBNP levels were log-transformed to achieve normality. Associations between NT-proBNP and echocardiographic indices were assessed by Spearman's correlation coefficient. Comparisons between groups were performed using the χ2 test for categorical variables and the Student's t-test or Wilcoxon rank-sum test for continuous variables.
Echocardiographic factors independently correlated with NT-proBNP levels were determined by multivariable linear-regression analysis with candidate variables added to a model containing NT-proBNP (as the dependent variable) and other non-echocardiographic covariates with previously reported relationships with NT-proBNP concentrations,12 including age, gender, body-mass index, acute or prior HF, prior cardiomyopathy, hypertension, atrial fibrillation, pulse (decile), and creatinine clearance (by the Modified Diet in Renal Disease Study Group formula). Assumptions of the multivariable linear-regression model were satisfied; plots of residuals confirmed normality, whereas constancy of variance was checked by plotting regression standardized residuals against predicted values. Standardized β coefficients were generated and presented. In order to avoid co-linearity, related indices such as measures of diastolic function and diastolic dysfunction stage were added individually.
In order to identify independent clinical and echocardiographic predictors of death at 1 year following presentation with dyspnoea, Cox models were first performed to examine variables of interest, with 1-year mortality as the dependent variable. For the purposes of analysing the relationship between NT-proBNP concentrations and mortality, patients were dichotomized as a function of being above or below the median for the group as a whole of 2430 pg/mL. All covariates associated with 1-year mortality with univariable P-values <0.05 were potentially eligible for inclusion in the final multivariable Cox model. Proportional hazard assumptions of the multivariable model were checked and were met. The results of the final multivariable model are presented. The variables in the final multivariable model were assessed in pairs for all possible first-order interactions and found to have none. For each significant predictor of death by 1 year, the hazard ratio with 95% confidence intervals (CIs) was generated. For all statistical analyses, P-values are two-sided, with a value <0.05 considered significant.
Results
Clinical characteristics
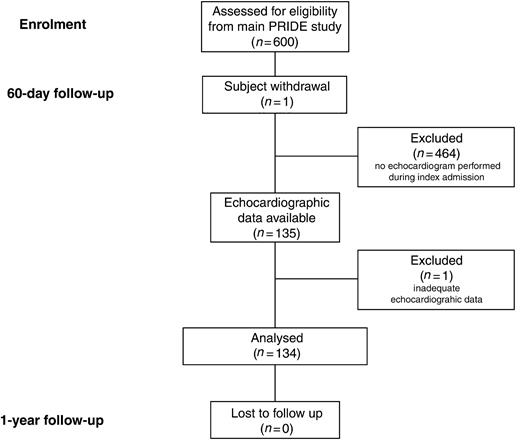
The description of patient selection for the present analysis is detailed in Figure 1. Among the 600 patients initially enrolled in the PRIDE study, one patient withdrew consent at 60-day follow-up, leaving 599 patients eligible for analysis. Of these, 135 (22%) underwent echocardiography, a median of 45 h [inter-quartile range (IQR) 23–73 h] after NT-proBNP measurement. One subject had insufficient echocardiographic data and was thus excluded from the analysis.
The clinical characteristics of patients with echocardiographic data compared with the rest of the PRIDE study population are shown in Table 1, demonstrating differences consistent with the standard of care prompting echocardiograms at our institution. Among those with echocardiographic data, we found more severe symptoms of dyspnoea, a higher prevalence of heart disease, more frequent medication use for heart disease, worse renal function, and higher NT-proBNP concentrations when compared with those without.
Notably, the NT-proBNP level in subjects with acute HF undergoing echocardiography was similar to others in the PRIDE study with acute HF (4488 vs. 4054 pg/mL, P=NS). Among those without HF, the NT-proBNP concentrations tended to be higher than in those who did not undergo echocardiography (390 pg/mL, IQR=149–1185 vs. 189 pg/mL, IQR=59–488 pg/mL; P=0.07 for difference).
NT-proBNP levels
The median NT-proBNP level among the 134 patients in this substudy was 2430 pg/mL (range 8–82200 pg/mL; IQR=637–7214 pg/mL). Ninety-one patients had NT-proBNP levels greater than the diagnostic threshold for acute HF (>450 pg/mL for patients <50 years old and >900 pg/mL for those ≥50 years old).3 Twenty-two patients had NT-proBNP levels <300 pg/mL, a cut-point we have previously shown to have a negative predictive value (NPV) of 99% for the clinical diagnosis of acute HF.3
Echocardiographic findings and NT-proBNP levels
A summary of the echocardiographic findings is presented in Table 2. The median NT-proBNP level was 1150 pg/mL (IQR 679–3070 pg/mL) in patients with LVEF ≥50% and 4686 pg/mL (IQR 1989–12796 pg/mL) in those with LVEF <50% (P=0.001).
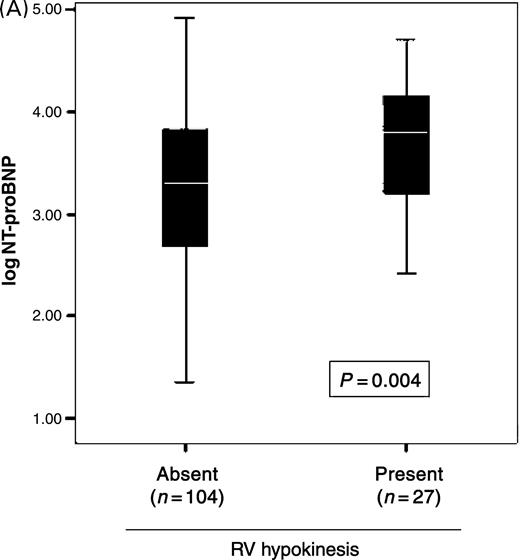
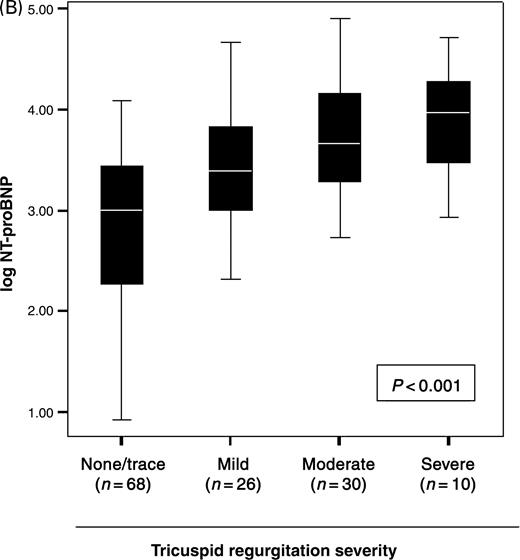
Univariable correlations between log NT-proBNP levels and echocardiographic indices are shown in Table 3. Several indices of diastolic function correlated with NT-proBNP levels, as did increasing severity of diastolic dysfunction, from normal (1703 pg/mL, IQR=476–6605 pg/mL) to stage 1 (2264 pg/mL, IQR=352–11862 pg/mL), stage 2 (2590 pg/mL, IQR=1874–8284 pg/mL), and stage 3 (7835 pg/mL, IQR=3684–16635 pg/mL; P=0.05 for trend across groups). RV hypokinesis correlated with NT-proBNP levels (Figure 2A), although RV dilation and RV fractional area change did not. NT-proBNP levels were higher with increasing TR (Figure 2B), but not MR severity. The associations between NT-proBNP levels and these echocardiographic indices were preserved whether or not patients had LVEF <50%.
As shown in Table 4, the echocardiographic indices that remained independent predictors of log NT-proBNP levels in multivariable analysis were LVEF, tissue Doppler Ea and Aa, the presence of RV hypokinesis, TR severity, and TR velocity.
NPV of NT-proBNP
An NT-proBNP level <300 pg/mL had a NPV of 91% for the exclusion of significant systolic or diastolic abnormalities: LVEF<50%; E/Ea>15; and diastolic dysfunction stage 1, 2, or 3. The NPV decreased to 68% with inclusion of the following factors: Ea<8 cm/s, LA volume index >32 mL/m2, regional wall motion abnormality, and LV mass index >131 g/m2 in men or >110 g/m2 in women. The addition of RV criteria of greater than mild TR, RVSP >40 mmHg, and the presence of RV hypokinesis or dilation reduced the NPV to 63%.
NT-proBNP levels, echocardiographic findings, and 1-year mortality
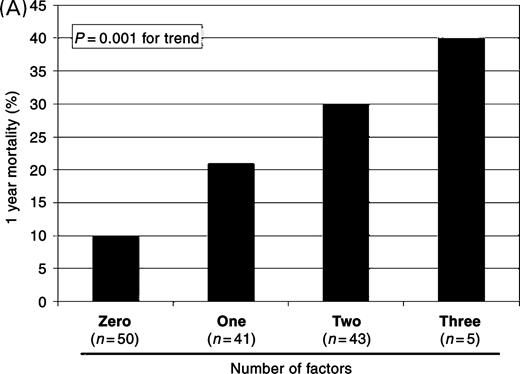
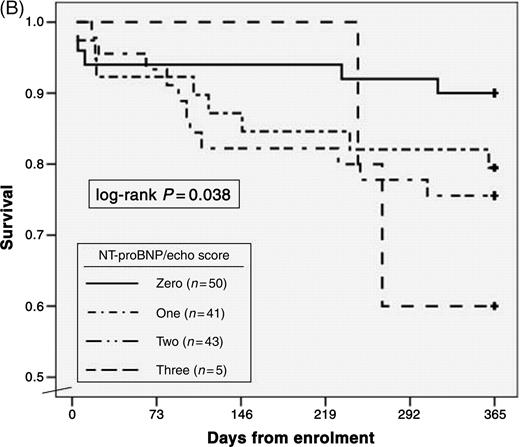
Overall mortality among the 134 patients was 20.1% at 1 year. As seen in Table 5, NT-proBNP levels in excess of the median value of 2430 pg/mL, LVEF, the presence of RV dilation, and systolic blood pressure were independent predictors of 1-year mortality. Considering NT-proBNP results in the context of echocardiographic results, patients with an NT-proBNP level above the median value, LVEF <50%, and/or the presence of RV dilation demonstrated incremental increases in the risk for mortality with the addition of each factor. As seen in Figure 3A, the average 1-year mortality for patients stratified by these factors was 10% if no factors present; 21% if one factor; 30% if two factors; 40% if three factors (P=0.001 for trend). This risk was sustained to the first year after enrolment (Figure 3B; log-rank P=0.038). Only two of the 22 subjects with an NT-proBNP level <300 pg/mL died by 1 year; accordingly, the NPV of an NT-proBNP level <300 pg/mL for 1-year mortality was 91%.
Discussion
Measurement of NT-proBNP and BNP levels is increasingly utilized in the evaluation of dyspnoeic patients with suspected HF.3,4 Natriuretic peptide levels can reflect LV systolic13,14 and diastolic function,7,15,16 RV function,17 and valvular heart disease,18 and understanding these complex relationships may elucidate the physiology of natriuretic peptide release and guide the management of dyspnoeic patients.
Our study demonstrates that in patients presenting to the emergency department with dyspnoea, elevated NT-proBNP levels not only correlate with the presence of echocardiographic abnormalities but also provide important prognostic information beyond such findings. Low NT-proBNP levels, in contrast, identify patients at very low risk for mortality and predict a low likelihood for clinically significant echocardiographic abnormalities such as impaired LV systolic or diastolic function. As a result, routine NT-proBNP testing may be valuable not only to diagnose or exclude HF but also to discriminate between patients in whom timelier echocardiographic evaluation would be indicated and those in whom echocardiography might be deferred or avoided altogether.
Many echocardiographic indices have been suggested to predict long-term mortality and morbidity. Although some have shown that LVEF predicts cardiovascular mortality,1,19 others suggest that the mortality risk of patients with HF symptoms and preserved LVEF approaches that of patients with impaired LVEF.20–22 Our data would support the latter hypothesis, as NT-proBNP levels—which are elevated in patients with HF and preserved LVEF—were the most powerful predictor of death in our cohort. Other echocardiographic indices that may predict outcome include LA and LV dimensions,23,24 transmitral filling patterns,23,25,26 tissue Doppler Ea,27 E/Ea,28 RV dilation and dysfunction,29 and TR.30 Although each of these in the proper context may be independently predictive of mortality, our study of echocardiography performed in a standard of care fashion demonstrates that the integration of simple, routinely assessed parameters (LVEF and RV dilation) with results from a widely available blood test (NT-proBNP) may stratify a dyspnoeic patient's risk for mortality.
Natriuretic peptides may be powerful predictors of mortality in diverse patient populations because the physiology of their release allows them to serve as a ‘global marker’ for abnormal cardiac structure and function. NT-proBNP and BNP predict mortality and cardiovascular events in population-based studies,28 in patients with chronic HF31,32 or hospitalized for HF,33,34 after acute coronary syndromes,35 and in those with hypertension and left ventricular hypertrophy.36 There have been fewer studies incorporating both natriuretic peptide levels and echocardiography in prognostication, but BNP and NT-proBNP may be superior to LVEF for predicting death in several cardiovascular disease states.37–39 In 116 patients hospitalized with HF who underwent BNP testing and echocardiography pre-discharge, BNP and diastolic function (E/Ea) were independent predictors for the combined endpoint of cardiac mortality and re-hospitalization for HF.40 In a study of 95 patients admitted to the intensive care unit with acute decompensated HF, BNP, a restrictive diastolic filling pattern, and pulmonary hypertension at the time of admission, but not echo indices obtained at 24 h and 7 days, correlated with prognosis.41 In contrast, our results indicate that LVEF and RV dilation, assessed an average of 2 days after presentation, may still have prognostic value.
Integrating echocardiography and natriuretic peptide testing
In patients hospitalized with dyspnoea and suspected HF, routine evaluation often includes echocardiography, which may not occur until days into hospitalization. Our findings suggest that natriuretic peptide testing may aid in targeting echocardiography for those patients more likely to have a cardiac abnormality, whereas avoiding an unnecessary test in others. Multiple studies have demonstrated the cost-effectiveness of natriuretic peptide measurement to screen for undiagnosed LV systolic dysfunction and to pre-select patients for echocardiography in the ambulatory setting,42,43 and our data suggest its potential benefit among dyspnoeic inpatients. As an example, using an NT-proBNP cutoff level of 300 pg/mL, a potential 22 echocardiograms in our substudy could have been avoided or deferred to the outpatient setting, with considerable cost-savings and potentially without risk of adverse consequences, as only two patients with NT-proBNP levels <300 pg/mL died by 1 year, and neither had acute HF or significant abnormalities on echocardiography. Prospective cost-effectiveness studies of natriuretic peptide-guided management in the inpatient setting are needed; preliminary data from our group suggest significant cost-savings with such an approach.
Limitations
The patients in this analysis represent a subset of the PRIDE study population, which may have introduced selection bias. It is worth noting that the managing clinicians were blinded to NT-proBNP results, which therefore did not influence the decision to pursue echocardiography. As well, our interpretation of the results is based on this inherent bias, namely, that NT-proBNP may be useful in the context of this standard of care. Another limitation is that NT-proBNP measurement and echocardiography were not performed simultaneously. The delay until echocardiography may be expected to influence our identification of correlations between NT-proBNP levels and echocardiographic parameters, and our observations may not apply to all situations. We believe our results remain informative, as they are representative of standard of care utilization of echocardiography in the inpatient setting. Our study was a retrospective analysis of subjects with echocardiographic data obtained as a function of standard of care, thus the sample size was not a priori defined, and as such, our power might not be adequate for more in-depth analyses; nonetheless, our results are robust and now have prospective validation in properly powered cohorts. Finally, the number of endpoints is lower than ideal for the number of variables examined in the multivariable analysis for predictors of 1-year mortality.
Conclusions
In a group of dyspnoeic patients in whom echocardiography was felt to be clinically indicated, we found that elevated NT-proBNP levels correlated with several important echocardiographic indices of cardiac structure and function and identified subjects with a high risk of adverse outcomes at 1 year. Conversely, a low NT-proBNP level excluded many significant echocardiographic abnormalities, particularly those involving the LV, and was associated with a very low risk of 1-year mortality. These findings extend the understanding of the relationship between NT-proBNP concentrations and findings on echocardiography and may be clinically useful in streamlining the diagnostic evaluation of dyspnoeic patients.
Acknowledgements
This study was supported in part by a research grant from Roche Diagnostics, Indianapolis, IN (approval of the manuscript only).
Conflict of interest: J.L. Januzzi reports having received research grants, speaking fees, and consulting honoraria from Roche Diagnostics, Inc.
Figure 1 Patient selection for the present analysis.
Figure 2 Relationship between NT-proBNP concentrations with right heart (A) function and (B) valvular regurgitation.
Figure 3 Relationship between the independent predictors of mortality and likelihood for death. With each added factor (NT-proBNP, LVEF <50%, and RV dilation), the percentage of subjects dying by 1 year rose (A), a risk that was sustained over the first year following enrolment (B).
Clinical characteristics of the study population
| Characteristic . | Echo (n=134) . | No echo (n=464) . | P-value . |
|---|---|---|---|
| Age (years), mean±standard deviation | 69±14 | 61±17 | 0.12 |
| Male (%) | 65 (49%) | 237 (51%) | 0.67 |
| Acute HF (%) | 89 (66%) | 116 (25%) | <0.001 |
| NYHA class at presentation (%) | 0.008 | ||
| I | 8 (6%) | 102 (22%) | |
| II | 28 (21%) | 84 (18%) | |
| III | 42 (31%) | 107 (23%) | |
| IV | 56 (42%) | 218 (37%) | |
| Past history (%) | |||
| HF or cardiomyopathy | 50 (37%) | 116 (25%) | 0.06 |
| HTN | 82 (61%) | 211 (45%) | 0.01 |
| CAD or prior MI | 41 (31%) | 148 (32%) | 0.88 |
| Aortic valve disease | 13 (10%) | 32 (7%) | 0.70 |
| Mitral valve disease | 14 (10%) | 51 (11%) | 0.35 |
| Arrhythmia | 32 (24%) | 70 (15%) | 0.20 |
| COPD or asthma | 36 (27%) | 176 (39%) | 0.008 |
| Medications (%) | |||
| No medications | 31 (23%) | 176 (38%) | 0.01 |
| Beta-blocker | 68 (51%) | 162 (35%) | 0.03 |
| Loop diuretic | 52 (39%) | 123 (26%) | 0.01 |
| ACE-inhibitor or ARB | 51 (38%) | 116 (25%) | 0.02 |
| Body-mass index (kg/m2) | 28±7 | 27±7 | 0.98 |
| Body-surface area (m2) | 1.9±0.3 | N/A | N/A |
| Pulse | 89±27 | 87±22 | 0.86 |
| Systolic blood pressure (mmHg) | 140±28 | 136±31 | 0.72 |
| Diastolic blood pressure (mmHg) | 77±18 | 77±16 | 0.83 |
| Creatinine clearance (mL/min/1.73 m2) | 66±26 | 76±30 | 0.02 |
| Median NT-proBNP (pg/mL; IQR) | 2430 (637–7214) | 260 (66–1634) | <0.001 |
| Characteristic . | Echo (n=134) . | No echo (n=464) . | P-value . |
|---|---|---|---|
| Age (years), mean±standard deviation | 69±14 | 61±17 | 0.12 |
| Male (%) | 65 (49%) | 237 (51%) | 0.67 |
| Acute HF (%) | 89 (66%) | 116 (25%) | <0.001 |
| NYHA class at presentation (%) | 0.008 | ||
| I | 8 (6%) | 102 (22%) | |
| II | 28 (21%) | 84 (18%) | |
| III | 42 (31%) | 107 (23%) | |
| IV | 56 (42%) | 218 (37%) | |
| Past history (%) | |||
| HF or cardiomyopathy | 50 (37%) | 116 (25%) | 0.06 |
| HTN | 82 (61%) | 211 (45%) | 0.01 |
| CAD or prior MI | 41 (31%) | 148 (32%) | 0.88 |
| Aortic valve disease | 13 (10%) | 32 (7%) | 0.70 |
| Mitral valve disease | 14 (10%) | 51 (11%) | 0.35 |
| Arrhythmia | 32 (24%) | 70 (15%) | 0.20 |
| COPD or asthma | 36 (27%) | 176 (39%) | 0.008 |
| Medications (%) | |||
| No medications | 31 (23%) | 176 (38%) | 0.01 |
| Beta-blocker | 68 (51%) | 162 (35%) | 0.03 |
| Loop diuretic | 52 (39%) | 123 (26%) | 0.01 |
| ACE-inhibitor or ARB | 51 (38%) | 116 (25%) | 0.02 |
| Body-mass index (kg/m2) | 28±7 | 27±7 | 0.98 |
| Body-surface area (m2) | 1.9±0.3 | N/A | N/A |
| Pulse | 89±27 | 87±22 | 0.86 |
| Systolic blood pressure (mmHg) | 140±28 | 136±31 | 0.72 |
| Diastolic blood pressure (mmHg) | 77±18 | 77±16 | 0.83 |
| Creatinine clearance (mL/min/1.73 m2) | 66±26 | 76±30 | 0.02 |
| Median NT-proBNP (pg/mL; IQR) | 2430 (637–7214) | 260 (66–1634) | <0.001 |
Clinical characteristics of the study population
| Characteristic . | Echo (n=134) . | No echo (n=464) . | P-value . |
|---|---|---|---|
| Age (years), mean±standard deviation | 69±14 | 61±17 | 0.12 |
| Male (%) | 65 (49%) | 237 (51%) | 0.67 |
| Acute HF (%) | 89 (66%) | 116 (25%) | <0.001 |
| NYHA class at presentation (%) | 0.008 | ||
| I | 8 (6%) | 102 (22%) | |
| II | 28 (21%) | 84 (18%) | |
| III | 42 (31%) | 107 (23%) | |
| IV | 56 (42%) | 218 (37%) | |
| Past history (%) | |||
| HF or cardiomyopathy | 50 (37%) | 116 (25%) | 0.06 |
| HTN | 82 (61%) | 211 (45%) | 0.01 |
| CAD or prior MI | 41 (31%) | 148 (32%) | 0.88 |
| Aortic valve disease | 13 (10%) | 32 (7%) | 0.70 |
| Mitral valve disease | 14 (10%) | 51 (11%) | 0.35 |
| Arrhythmia | 32 (24%) | 70 (15%) | 0.20 |
| COPD or asthma | 36 (27%) | 176 (39%) | 0.008 |
| Medications (%) | |||
| No medications | 31 (23%) | 176 (38%) | 0.01 |
| Beta-blocker | 68 (51%) | 162 (35%) | 0.03 |
| Loop diuretic | 52 (39%) | 123 (26%) | 0.01 |
| ACE-inhibitor or ARB | 51 (38%) | 116 (25%) | 0.02 |
| Body-mass index (kg/m2) | 28±7 | 27±7 | 0.98 |
| Body-surface area (m2) | 1.9±0.3 | N/A | N/A |
| Pulse | 89±27 | 87±22 | 0.86 |
| Systolic blood pressure (mmHg) | 140±28 | 136±31 | 0.72 |
| Diastolic blood pressure (mmHg) | 77±18 | 77±16 | 0.83 |
| Creatinine clearance (mL/min/1.73 m2) | 66±26 | 76±30 | 0.02 |
| Median NT-proBNP (pg/mL; IQR) | 2430 (637–7214) | 260 (66–1634) | <0.001 |
| Characteristic . | Echo (n=134) . | No echo (n=464) . | P-value . |
|---|---|---|---|
| Age (years), mean±standard deviation | 69±14 | 61±17 | 0.12 |
| Male (%) | 65 (49%) | 237 (51%) | 0.67 |
| Acute HF (%) | 89 (66%) | 116 (25%) | <0.001 |
| NYHA class at presentation (%) | 0.008 | ||
| I | 8 (6%) | 102 (22%) | |
| II | 28 (21%) | 84 (18%) | |
| III | 42 (31%) | 107 (23%) | |
| IV | 56 (42%) | 218 (37%) | |
| Past history (%) | |||
| HF or cardiomyopathy | 50 (37%) | 116 (25%) | 0.06 |
| HTN | 82 (61%) | 211 (45%) | 0.01 |
| CAD or prior MI | 41 (31%) | 148 (32%) | 0.88 |
| Aortic valve disease | 13 (10%) | 32 (7%) | 0.70 |
| Mitral valve disease | 14 (10%) | 51 (11%) | 0.35 |
| Arrhythmia | 32 (24%) | 70 (15%) | 0.20 |
| COPD or asthma | 36 (27%) | 176 (39%) | 0.008 |
| Medications (%) | |||
| No medications | 31 (23%) | 176 (38%) | 0.01 |
| Beta-blocker | 68 (51%) | 162 (35%) | 0.03 |
| Loop diuretic | 52 (39%) | 123 (26%) | 0.01 |
| ACE-inhibitor or ARB | 51 (38%) | 116 (25%) | 0.02 |
| Body-mass index (kg/m2) | 28±7 | 27±7 | 0.98 |
| Body-surface area (m2) | 1.9±0.3 | N/A | N/A |
| Pulse | 89±27 | 87±22 | 0.86 |
| Systolic blood pressure (mmHg) | 140±28 | 136±31 | 0.72 |
| Diastolic blood pressure (mmHg) | 77±18 | 77±16 | 0.83 |
| Creatinine clearance (mL/min/1.73 m2) | 66±26 | 76±30 | 0.02 |
| Median NT-proBNP (pg/mL; IQR) | 2430 (637–7214) | 260 (66–1634) | <0.001 |
Echocardiographic findings in the study population
| Characteristic . | 134 patients . |
|---|---|
| Chamber size and volume | |
| LV end-diastolic volume index (mL/m2) | 50±20 |
| LV end-systolic volume index (mL/m2) | 27±17 |
| Posterior wall thickness (mm) | 10.3±1.7 |
| LV mass index (g/m2) | 99±31 |
| LA volume index (mL/m2) | 36±20 |
| RV end-diastolic area (cm2) | 16±4 |
| RV end-systolic area (cm2) | 10±3 |
| Patients with RV dilation (%) | 19 (14) |
| LV systolic function | |
| LVEF (%) | 49±15 |
| Regional wall motion abnormality | 16 (12%) |
| LV diastolic function | |
| Transmitral flow | |
| E (cm/s) | 84±30 |
| A (cm/s) | 70±28 |
| E/A | 1.4±0.7 |
| DT (ms) | 175±62 |
| Pulmonary vein flow | |
| S (cm/s) | 44±18 |
| D (cm/s) | 53±20 |
| S/D | 0.95±0.4 |
| Tissue Doppler velocity | |
| Ea (cm/s) | 8.3±2.9 |
| Aa (cm/s) | 8.5±3.5 |
| E/Ea | 10.6±5.1 |
| Patients with diastolic dysfunction | |
| Stage 1 (impaired relaxation) | 7 (5%) |
| Stage 2 (pseudonormal) | 9 (7%) |
| Stage 3 (restrictive) | 13 (10%) |
| RV function | |
| Fractional area change (%) | 40±10 |
| Patients with RV hypokinesis (%) | 27 (20) |
| Valve disease | |
| TR severity (number of patients) | |
| Mild | 26 |
| Moderate | 30 |
| Severe | 10 |
| TR velocity (m/s) | 2.9±0.5 |
| MR severity (number of patients) | |
| Mild | 41 |
| Moderate | 40 |
| Severe | 8 |
| Characteristic . | 134 patients . |
|---|---|
| Chamber size and volume | |
| LV end-diastolic volume index (mL/m2) | 50±20 |
| LV end-systolic volume index (mL/m2) | 27±17 |
| Posterior wall thickness (mm) | 10.3±1.7 |
| LV mass index (g/m2) | 99±31 |
| LA volume index (mL/m2) | 36±20 |
| RV end-diastolic area (cm2) | 16±4 |
| RV end-systolic area (cm2) | 10±3 |
| Patients with RV dilation (%) | 19 (14) |
| LV systolic function | |
| LVEF (%) | 49±15 |
| Regional wall motion abnormality | 16 (12%) |
| LV diastolic function | |
| Transmitral flow | |
| E (cm/s) | 84±30 |
| A (cm/s) | 70±28 |
| E/A | 1.4±0.7 |
| DT (ms) | 175±62 |
| Pulmonary vein flow | |
| S (cm/s) | 44±18 |
| D (cm/s) | 53±20 |
| S/D | 0.95±0.4 |
| Tissue Doppler velocity | |
| Ea (cm/s) | 8.3±2.9 |
| Aa (cm/s) | 8.5±3.5 |
| E/Ea | 10.6±5.1 |
| Patients with diastolic dysfunction | |
| Stage 1 (impaired relaxation) | 7 (5%) |
| Stage 2 (pseudonormal) | 9 (7%) |
| Stage 3 (restrictive) | 13 (10%) |
| RV function | |
| Fractional area change (%) | 40±10 |
| Patients with RV hypokinesis (%) | 27 (20) |
| Valve disease | |
| TR severity (number of patients) | |
| Mild | 26 |
| Moderate | 30 |
| Severe | 10 |
| TR velocity (m/s) | 2.9±0.5 |
| MR severity (number of patients) | |
| Mild | 41 |
| Moderate | 40 |
| Severe | 8 |
Echocardiographic findings in the study population
| Characteristic . | 134 patients . |
|---|---|
| Chamber size and volume | |
| LV end-diastolic volume index (mL/m2) | 50±20 |
| LV end-systolic volume index (mL/m2) | 27±17 |
| Posterior wall thickness (mm) | 10.3±1.7 |
| LV mass index (g/m2) | 99±31 |
| LA volume index (mL/m2) | 36±20 |
| RV end-diastolic area (cm2) | 16±4 |
| RV end-systolic area (cm2) | 10±3 |
| Patients with RV dilation (%) | 19 (14) |
| LV systolic function | |
| LVEF (%) | 49±15 |
| Regional wall motion abnormality | 16 (12%) |
| LV diastolic function | |
| Transmitral flow | |
| E (cm/s) | 84±30 |
| A (cm/s) | 70±28 |
| E/A | 1.4±0.7 |
| DT (ms) | 175±62 |
| Pulmonary vein flow | |
| S (cm/s) | 44±18 |
| D (cm/s) | 53±20 |
| S/D | 0.95±0.4 |
| Tissue Doppler velocity | |
| Ea (cm/s) | 8.3±2.9 |
| Aa (cm/s) | 8.5±3.5 |
| E/Ea | 10.6±5.1 |
| Patients with diastolic dysfunction | |
| Stage 1 (impaired relaxation) | 7 (5%) |
| Stage 2 (pseudonormal) | 9 (7%) |
| Stage 3 (restrictive) | 13 (10%) |
| RV function | |
| Fractional area change (%) | 40±10 |
| Patients with RV hypokinesis (%) | 27 (20) |
| Valve disease | |
| TR severity (number of patients) | |
| Mild | 26 |
| Moderate | 30 |
| Severe | 10 |
| TR velocity (m/s) | 2.9±0.5 |
| MR severity (number of patients) | |
| Mild | 41 |
| Moderate | 40 |
| Severe | 8 |
| Characteristic . | 134 patients . |
|---|---|
| Chamber size and volume | |
| LV end-diastolic volume index (mL/m2) | 50±20 |
| LV end-systolic volume index (mL/m2) | 27±17 |
| Posterior wall thickness (mm) | 10.3±1.7 |
| LV mass index (g/m2) | 99±31 |
| LA volume index (mL/m2) | 36±20 |
| RV end-diastolic area (cm2) | 16±4 |
| RV end-systolic area (cm2) | 10±3 |
| Patients with RV dilation (%) | 19 (14) |
| LV systolic function | |
| LVEF (%) | 49±15 |
| Regional wall motion abnormality | 16 (12%) |
| LV diastolic function | |
| Transmitral flow | |
| E (cm/s) | 84±30 |
| A (cm/s) | 70±28 |
| E/A | 1.4±0.7 |
| DT (ms) | 175±62 |
| Pulmonary vein flow | |
| S (cm/s) | 44±18 |
| D (cm/s) | 53±20 |
| S/D | 0.95±0.4 |
| Tissue Doppler velocity | |
| Ea (cm/s) | 8.3±2.9 |
| Aa (cm/s) | 8.5±3.5 |
| E/Ea | 10.6±5.1 |
| Patients with diastolic dysfunction | |
| Stage 1 (impaired relaxation) | 7 (5%) |
| Stage 2 (pseudonormal) | 9 (7%) |
| Stage 3 (restrictive) | 13 (10%) |
| RV function | |
| Fractional area change (%) | 40±10 |
| Patients with RV hypokinesis (%) | 27 (20) |
| Valve disease | |
| TR severity (number of patients) | |
| Mild | 26 |
| Moderate | 30 |
| Severe | 10 |
| TR velocity (m/s) | 2.9±0.5 |
| MR severity (number of patients) | |
| Mild | 41 |
| Moderate | 40 |
| Severe | 8 |
Univariable correlations of echocardiographic indices with log NT-proBNP levels
| . | Spearman's correlation coefficient (ρ) . | P-value . |
|---|---|---|
| LV end-diastolic volume index | 0.216 | 0.015 |
| LV end-systolic volume index | 0.329 | <0.001 |
| Posterior wall thickness | −0.094 | 0.280 |
| LV mass index | 0.105 | 0.222 |
| LA volume index | 0.272 | 0.002 |
| RV end-diastolic area | 0.003 | 0.972 |
| RV end-systolic area | 0.083 | 0.345 |
| LVEF | −0.294 | 0.001 |
| E | 0.242 | 0.019 |
| A | −0.066 | 0.535 |
| E/A | 0.280 | 0.007 |
| DT | −0.980 | 0.284 |
| Pulmonary vein S | −0.302 | 0.002 |
| Pulmonary vein D | 0.301 | 0.002 |
| S/D | −0.463 | <0.001 |
| Tissue Doppler Ea velocity | −0.364 | 0.001 |
| Tissue Doppler Aa velocity | −0.456 | <0.001 |
| E/Ea | 0.345 | 0.003 |
| RV fractional area change | −0.169 | 0.06 |
| TR velocity | 0.460 | <0.001 |
| . | Spearman's correlation coefficient (ρ) . | P-value . |
|---|---|---|
| LV end-diastolic volume index | 0.216 | 0.015 |
| LV end-systolic volume index | 0.329 | <0.001 |
| Posterior wall thickness | −0.094 | 0.280 |
| LV mass index | 0.105 | 0.222 |
| LA volume index | 0.272 | 0.002 |
| RV end-diastolic area | 0.003 | 0.972 |
| RV end-systolic area | 0.083 | 0.345 |
| LVEF | −0.294 | 0.001 |
| E | 0.242 | 0.019 |
| A | −0.066 | 0.535 |
| E/A | 0.280 | 0.007 |
| DT | −0.980 | 0.284 |
| Pulmonary vein S | −0.302 | 0.002 |
| Pulmonary vein D | 0.301 | 0.002 |
| S/D | −0.463 | <0.001 |
| Tissue Doppler Ea velocity | −0.364 | 0.001 |
| Tissue Doppler Aa velocity | −0.456 | <0.001 |
| E/Ea | 0.345 | 0.003 |
| RV fractional area change | −0.169 | 0.06 |
| TR velocity | 0.460 | <0.001 |
Univariable correlations of echocardiographic indices with log NT-proBNP levels
| . | Spearman's correlation coefficient (ρ) . | P-value . |
|---|---|---|
| LV end-diastolic volume index | 0.216 | 0.015 |
| LV end-systolic volume index | 0.329 | <0.001 |
| Posterior wall thickness | −0.094 | 0.280 |
| LV mass index | 0.105 | 0.222 |
| LA volume index | 0.272 | 0.002 |
| RV end-diastolic area | 0.003 | 0.972 |
| RV end-systolic area | 0.083 | 0.345 |
| LVEF | −0.294 | 0.001 |
| E | 0.242 | 0.019 |
| A | −0.066 | 0.535 |
| E/A | 0.280 | 0.007 |
| DT | −0.980 | 0.284 |
| Pulmonary vein S | −0.302 | 0.002 |
| Pulmonary vein D | 0.301 | 0.002 |
| S/D | −0.463 | <0.001 |
| Tissue Doppler Ea velocity | −0.364 | 0.001 |
| Tissue Doppler Aa velocity | −0.456 | <0.001 |
| E/Ea | 0.345 | 0.003 |
| RV fractional area change | −0.169 | 0.06 |
| TR velocity | 0.460 | <0.001 |
| . | Spearman's correlation coefficient (ρ) . | P-value . |
|---|---|---|
| LV end-diastolic volume index | 0.216 | 0.015 |
| LV end-systolic volume index | 0.329 | <0.001 |
| Posterior wall thickness | −0.094 | 0.280 |
| LV mass index | 0.105 | 0.222 |
| LA volume index | 0.272 | 0.002 |
| RV end-diastolic area | 0.003 | 0.972 |
| RV end-systolic area | 0.083 | 0.345 |
| LVEF | −0.294 | 0.001 |
| E | 0.242 | 0.019 |
| A | −0.066 | 0.535 |
| E/A | 0.280 | 0.007 |
| DT | −0.980 | 0.284 |
| Pulmonary vein S | −0.302 | 0.002 |
| Pulmonary vein D | 0.301 | 0.002 |
| S/D | −0.463 | <0.001 |
| Tissue Doppler Ea velocity | −0.364 | 0.001 |
| Tissue Doppler Aa velocity | −0.456 | <0.001 |
| E/Ea | 0.345 | 0.003 |
| RV fractional area change | −0.169 | 0.06 |
| TR velocity | 0.460 | <0.001 |
Multivariable correlations of clinical and echocardiographic indices with log NT-proBNP levels
| . | Standardized β regression coefficient . | P-value . |
|---|---|---|
| Age | 0.144 | 0.029 |
| Prior HF | 0.159 | 0.015 |
| BMI | −0.144 | 0.024 |
| Creatinine clearance | −0.211 | <0.001 |
| Diagnosis of acute HF | 0.488 | <0.001 |
| Prior HF | 0.159 | 0.015 |
| LVEF | −0.166 | 0.012 |
| Tissue Doppler Ea velocity | −0.173 | 0.007 |
| Tissue Doppler Aa velocity | −0.159 | 0.018 |
| E/Ea | 0.087 | 0.188 |
| RV fractional area change | −0.120 | 0.06 |
| RV hypokinesis | 0.174 | 0.006 |
| TR severity | 0.277 | 0.001 |
| TR velocity | 0.181 | 0.007 |
| . | Standardized β regression coefficient . | P-value . |
|---|---|---|
| Age | 0.144 | 0.029 |
| Prior HF | 0.159 | 0.015 |
| BMI | −0.144 | 0.024 |
| Creatinine clearance | −0.211 | <0.001 |
| Diagnosis of acute HF | 0.488 | <0.001 |
| Prior HF | 0.159 | 0.015 |
| LVEF | −0.166 | 0.012 |
| Tissue Doppler Ea velocity | −0.173 | 0.007 |
| Tissue Doppler Aa velocity | −0.159 | 0.018 |
| E/Ea | 0.087 | 0.188 |
| RV fractional area change | −0.120 | 0.06 |
| RV hypokinesis | 0.174 | 0.006 |
| TR severity | 0.277 | 0.001 |
| TR velocity | 0.181 | 0.007 |
Multivariable correlations of clinical and echocardiographic indices with log NT-proBNP levels
| . | Standardized β regression coefficient . | P-value . |
|---|---|---|
| Age | 0.144 | 0.029 |
| Prior HF | 0.159 | 0.015 |
| BMI | −0.144 | 0.024 |
| Creatinine clearance | −0.211 | <0.001 |
| Diagnosis of acute HF | 0.488 | <0.001 |
| Prior HF | 0.159 | 0.015 |
| LVEF | −0.166 | 0.012 |
| Tissue Doppler Ea velocity | −0.173 | 0.007 |
| Tissue Doppler Aa velocity | −0.159 | 0.018 |
| E/Ea | 0.087 | 0.188 |
| RV fractional area change | −0.120 | 0.06 |
| RV hypokinesis | 0.174 | 0.006 |
| TR severity | 0.277 | 0.001 |
| TR velocity | 0.181 | 0.007 |
| . | Standardized β regression coefficient . | P-value . |
|---|---|---|
| Age | 0.144 | 0.029 |
| Prior HF | 0.159 | 0.015 |
| BMI | −0.144 | 0.024 |
| Creatinine clearance | −0.211 | <0.001 |
| Diagnosis of acute HF | 0.488 | <0.001 |
| Prior HF | 0.159 | 0.015 |
| LVEF | −0.166 | 0.012 |
| Tissue Doppler Ea velocity | −0.173 | 0.007 |
| Tissue Doppler Aa velocity | −0.159 | 0.018 |
| E/Ea | 0.087 | 0.188 |
| RV fractional area change | −0.120 | 0.06 |
| RV hypokinesis | 0.174 | 0.006 |
| TR severity | 0.277 | 0.001 |
| TR velocity | 0.181 | 0.007 |
Multivariable predictors of mortality at 1 year among the 134 subjects with echocardiographic data
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| NT-proBNP >2430 pg/mL | 8.65 | 2.7–27.8 | <0.001 |
| LVEF (per %) | 0.95 | 0.91–0.99 | 0.009 |
| RV dilation present | 2.98 | 1.05–12.8 | 0.04 |
| Systolic BP (per mmHg) | 0.97 | 0.96–0.99 | 0.01 |
| Ea (per cm/s) | 0.99 | 0.79–1.2 | 0.94 |
| Aa (per cm/s) | 1.1 | 0.91–1.4 | 0.27 |
| TR severity | 0.82 | 0.48–1.4 | 0.46 |
| Age (by decade) | 1.3 | 0.85–1.96 | 0.23 |
| Creatinine clearance decile | 1.1 | 0.91–1.45 | 0.24 |
| NYHA class | 1.2 | 0.85–1.60 | 0.34 |
| Heart rate decile | 1.05 | 0.85–1.28 | 0.67 |
| BUN decile | 1.34 | 0.89–2.0 | 0.17 |
| Troponin-T | 1.7 | 0.97–2.63 | 0.15 |
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| NT-proBNP >2430 pg/mL | 8.65 | 2.7–27.8 | <0.001 |
| LVEF (per %) | 0.95 | 0.91–0.99 | 0.009 |
| RV dilation present | 2.98 | 1.05–12.8 | 0.04 |
| Systolic BP (per mmHg) | 0.97 | 0.96–0.99 | 0.01 |
| Ea (per cm/s) | 0.99 | 0.79–1.2 | 0.94 |
| Aa (per cm/s) | 1.1 | 0.91–1.4 | 0.27 |
| TR severity | 0.82 | 0.48–1.4 | 0.46 |
| Age (by decade) | 1.3 | 0.85–1.96 | 0.23 |
| Creatinine clearance decile | 1.1 | 0.91–1.45 | 0.24 |
| NYHA class | 1.2 | 0.85–1.60 | 0.34 |
| Heart rate decile | 1.05 | 0.85–1.28 | 0.67 |
| BUN decile | 1.34 | 0.89–2.0 | 0.17 |
| Troponin-T | 1.7 | 0.97–2.63 | 0.15 |
Multivariable predictors of mortality at 1 year among the 134 subjects with echocardiographic data
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| NT-proBNP >2430 pg/mL | 8.65 | 2.7–27.8 | <0.001 |
| LVEF (per %) | 0.95 | 0.91–0.99 | 0.009 |
| RV dilation present | 2.98 | 1.05–12.8 | 0.04 |
| Systolic BP (per mmHg) | 0.97 | 0.96–0.99 | 0.01 |
| Ea (per cm/s) | 0.99 | 0.79–1.2 | 0.94 |
| Aa (per cm/s) | 1.1 | 0.91–1.4 | 0.27 |
| TR severity | 0.82 | 0.48–1.4 | 0.46 |
| Age (by decade) | 1.3 | 0.85–1.96 | 0.23 |
| Creatinine clearance decile | 1.1 | 0.91–1.45 | 0.24 |
| NYHA class | 1.2 | 0.85–1.60 | 0.34 |
| Heart rate decile | 1.05 | 0.85–1.28 | 0.67 |
| BUN decile | 1.34 | 0.89–2.0 | 0.17 |
| Troponin-T | 1.7 | 0.97–2.63 | 0.15 |
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| NT-proBNP >2430 pg/mL | 8.65 | 2.7–27.8 | <0.001 |
| LVEF (per %) | 0.95 | 0.91–0.99 | 0.009 |
| RV dilation present | 2.98 | 1.05–12.8 | 0.04 |
| Systolic BP (per mmHg) | 0.97 | 0.96–0.99 | 0.01 |
| Ea (per cm/s) | 0.99 | 0.79–1.2 | 0.94 |
| Aa (per cm/s) | 1.1 | 0.91–1.4 | 0.27 |
| TR severity | 0.82 | 0.48–1.4 | 0.46 |
| Age (by decade) | 1.3 | 0.85–1.96 | 0.23 |
| Creatinine clearance decile | 1.1 | 0.91–1.45 | 0.24 |
| NYHA class | 1.2 | 0.85–1.60 | 0.34 |
| Heart rate decile | 1.05 | 0.85–1.28 | 0.67 |
| BUN decile | 1.34 | 0.89–2.0 | 0.17 |
| Troponin-T | 1.7 | 0.97–2.63 | 0.15 |
References
Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort.
Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function.
Januzzi JL Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study.
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure.
Davis M, Espiner E, Richards G, Billings J, Town I, Neill A, Drennan C, Richard M, Turner J, Yandle T. Plasma brain natriuretic peptide in assessment of acute dyspnoea.
Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, Gardetto N, Wanner E, Maisel AS. Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings.
Troughton RW, Prior DL, Pereira JJ, Martin M, Fogarty A, Morehead A, Yandle TF, Richards AM, Starling RC, Young JB, Thomas JD, Klein AL. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function.
Vinch CS, Aurigemma GP, Hill JC, Gaasch WH, Volturo G, Tighe DA, Meyer TE. Usefulness of clinical variables, echocardiography, and levels of brain natriuretic peptide and norepinephrine to distinguish systolic and diastolic causes of acute heart failure.
Mak GS, DeMaria A, Clopton P, Maisel AS. Utility of B-natriuretic peptide in the evaluation of left ventricular diastolic function: comparison with tissue Doppler imaging recordings.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings.
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography.
Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL Jr. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study.
Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, Trawinski J, Boesgaard S, Aldershvile J. NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function.
McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, Tunstall-Pedoe H, McMurray JJ, Dargie HJ. Biochemical detection of left-ventricular systolic dysfunction.
O'Donoghue M, Chen A, Baggish AL, Anwaruddin S, Krauser DG, Tung R, Januzzi JL. The effects of ejection fraction on N-terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study.
Catuzzo B, Ciancamerla F, Bobbio M, Longo M, Trevi GP. In patients with severe systolic dysfunction, only brain natriuretic peptide is related to diastolic restrictive pattern.
Weber M, Arnold R, Rau M, Brandt R, Berkovitsch A, Mitrovic V, Hamm C. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis.
Cohn JN, Johnson G. Heart failure with normal ejection fraction. The V-HeFT Study. Veterans Administration Cooperative Study Group.
Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991.
Tsutsui H, Tsuchihashi M, Takeshita A. Mortality and readmission of hospitalized patients with congestive heart failure and preserved versus depressed systolic function.
McDermott MM, Feinglass J, Lee PI, Mehta S, Schmitt B, Lefevre F, Gheorghiade M. Systolic function, readmission rates, and survival among consecutively hospitalized patients with congestive heart failure.
Grayburn PA, Appleton CP, DeMaria AN, Greenberg B, Lowes B, Oh J, Plehn JF, Rahko P, St John Sutton M, Eichhorn EJ. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST).
Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography.
Xie GY, Berk MR, Smith MD, Gurley JC, DeMaria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure.
Giannuzzi P, Temporelli PL, Bosimini E, Silva P, Imparato A, Corra U, Galli M, Giordano A. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction.
Wang M, Yip G, Yu CM, Zhang Q, Zhang Y, Tse D, Kong SL, Sanderson JE. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function.
Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults.
Sun JP, James KB, Yang XS, Solankhi N, Shah MS, Arheart KL, Thomas JD, Stewart WJ. Comparison of mortality rates and progression of left ventricular dysfunction in patients with idiopathic dilated cardiomyopathy and dilated versus nondilated right ventricular cavities.
Hung J, Koelling T, Semigran MJ, Dec GW, Levine RA, Di Salvo TG. Usefulness of echocardiographic determined tricuspid regurgitation in predicting event-free survival in severe heart failure secondary to idiopathic-dilated cardiomyopathy or to ischemic cardiomyopathy.
Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure.
Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, Ohnishi M, Sugimoto Y, Kinoshita M. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction.
Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients.
Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure.
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes.
Olsen MH, Wachtell K, Tuxen C, Fossum E, Bang LE, Hall C, Ibsen H, Rokkedal J, Devereux RB, Hildebrandt P. N-terminal pro-brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study.
Groenning BA, Raymond I, Hildebrandt PR, Nilsson JC, Baumann M, Pedersen F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population.
Kirk V, Bay M, Parner J, Krogsgaard K, Herzog TM, Boesgaard S, Hassager C, Nielsen OW, Aldershvile J, Nielsen H. N-terminal proBNP and mortality in hospitalised patients with heart failure and preserved vs. reduced systolic function: data from the prospective Copenhagen Hospital Heart Failure Study (CHHF).
Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction.
Dokainish H, Zoghbi WA, Lakkis NM, Ambriz E, Patel R, Quinones MA, Nagueh SF. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure.
Gackowski A, Isnard R, Golmard JL, Pousset F, Carayon A, Montalescot G, Hulot JS, Thomas D, Piwowarska W, Komajda M. Comparison of echocardiography and plasma B-type natriuretic peptide for monitoring the response to treatment in acute heart failure.
Nielsen OW, McDonagh TA, Robb SD, Dargie HJ. Retrospective analysis of the cost-effectiveness of using plasma brain natriuretic peptide in screening for left ventricular systolic dysfunction in the general population.