-
PDF
- Split View
-
Views
-
Cite
Cite
Felix Mahfoud, Barbara Gärtner, Michael Kindermann, Christian Ukena, Katharina Gadomski, Karin Klingel, Reinhard Kandolf, Michael Böhm, Ingrid Kindermann, Virus serology in patients with suspected myocarditis: utility or futility?, European Heart Journal, Volume 32, Issue 7, April 2011, Pages 897–903, https://doi.org/10.1093/eurheartj/ehq493
Close - Share Icon Share
Abstract
Serological analyses of viral infection in suspected myocarditis are still widely used, although convincing evidence for their value is lacking. We determined prospectively the diagnostic value of virus serology in comparison with endomyocardial biopsy (EMB) including viral genome detection and immunohistochemistry in patients with clinically suspected myocarditis.
Virus serology and state-of-the-art evaluation of EMB were performed in 124 patients (age 40 ± 15 years) with suspected myocarditis. Endomyocardial biopsy was studied for inflammation with histological and immunohistological criteria. The viral genome was detected in the myocardium by polymerase chain reaction. Acute viral infection with enterovirus, adenovirus, parvovirus B19, cytomegalovirus, human herpesvirus, and Epstein-Barr virus was diagnosed by IgM or IgA in the initial sample or IgG seroconversion in the follow-up sample. Immunohistological signs of inflammation were present in 54 patients. The viral genome was detected in the myocardium of 58 patients (47%). In 20 patients (16%), acute viral infection was diagnosed by serology. Only in 5 out of 124 patients (4%), there was serological evidence of an infection with the same virus that was detected by EMB. Sensitivity and specificity of virus serology were 9 and 77%, respectively. The positive predictive value was 25% and the negative predictive value was 49%. The lack of correlation between serology and EMB remained also for patients with biopsy-proven myocarditis and patients with time from initial symptoms to EMB procedure of ≤1 month.
For patients with suspected myocarditis, virus serology has no relevance for the diagnosis of myocardial infection. Endomyocardial biopsy remains the gold standard in the diagnostic of viral myocarditis.
Introduction
Myocarditis is an inflammatory heart disease classified by clinical, immunohistological, and clinicopathological criteria and is usually caused by infectious agents.1,2 In Europe and North America, viral infections are the most common causes of myocarditis.3 The clinical picture of patients with myocarditis is highly variable, ranging from asymptomatic courses to severe illness with the necessity of intensive care therapy.4–6 The diagnosis of myocarditis is based on histopathological and immunohistological assessment of endomyocardial biopsies (EMBs).7 Molecular pathological analyses, such as PCR or in situ hybridization, allow the detection and quantification of the viral genome in the heart.8–10 Immunohistological signs of inflammation in the myocardium have recently been reported to predict poor outcome.11 The application of EMB might be underused due to lack of accessibility, clinical experience, and skills. Nevertheless, immediate diagnosis and treatment may be needed to prevent the development of inflammatory dilated cardiomyopathy (DCMi), an important cause of heart failure, which seems to be triggered by viral myocarditis in ∼35–50%.12,13 Serological analyses for the diagnosis of viral infection in patients with suspected myocarditis are still frequently used in clinical practice, although the assays are costly and data in comparison with state-of-the-art evaluation of EMB findings are lacking. The aim of this prospective study was to determine the diagnostic value of virus serology in comparison with analyses of EMB including viral genome detection in patients with clinically suspected myocarditis.
Methods
Study subjects
The study enrolled 124 patients with clinically suspected myocarditis, who underwent EMB at our institution between 1997 and 2009. Inclusion criteria were selected on the basis of previous studies and were in accordance with the recent guidelines of the American College of Cardiology, American Heart Association, and European Society of Cardiology. Patients were included if they had experienced an episode of a febrile infection of the bronchial tree, the gut, or the urinary tract within the last 6 months and if they had at least one of the following features not related to myocardial ischaemia: impaired global or regional left ventricular systolic function, a presentation with increased serum concentrations of myocardial necrosis markers, pericardial effusion of unknown reason, or sustained or non-sustained ventricular tachycardia or ventricular fibrillation of unknown origin. Before EMB, all patients underwent a complete history and physical examination, as well as selected laboratory studies such as C-reactive protein and leucocytes, 12-lead electrocardiogram (ECG), and echocardiography for measurement of left ventricular end-diastolic and end-systolic diameters with two-dimensionally guided M-mode. Patients with familial causes of cardiomyopathy were excluded from the study. The study was approved by the local ethics committee and all patients gave written informed consent to include their data in the study.
Virus serology
One serum sample for virus serology was collected before EMBs were taken. In 30 patients, follow-up serum samples were collected between 7 and 28 days after the initial serum sample. Acute viral infection was diagnosed by serological detection of IgM or IgA in the initial sample or IgG seroconversion between the initial and the follow-up sample in enzyme immuno- or immunofluorescence assays. In case of detection of IgG in the initial samples, no further analysis was done in follow-up samples, since no seroconversion could appear. In quantitative assays such as the neutralization assay or the complement fixation reaction, a four-fold increase in titre between the initial and the follow-up samples or a significantly high titre (>1:256 in the neutralization assay or >1:80 in the complement fixation reaction) defined an acute infection. Neutralization assays on GMK (green monkey kidney) cells for the subtypes B1-B5 were performed for detection of Coxsackievirus as well as the complement fixation reaction was for tested ECHO virus and adenoviruses (ADV) (Virion GmbH, München). An enterovirus (EV) IgA and IgM enzyme-immunoassay (EIA) was used for detection of coxsackie B5 and ECHO30 (Genzyme-Virotech, Germany). Moreover, human cytomegalovirus (CMV) (Abbott, Germany) and parvovirus B19 (PVB-19), IgG and IgM (Biotrin, Sinsheim and Medac, Germany) were tested by EIA. Immunofluorescence tests were used to detect IgM and IgG against human herpesvirus 6 (HHV-6) (Euroimmun, Germany), and Epstein-Barr virus (EBV)-capsid antigen (VCA), -IgG, -IgM and EBNA-1 IgG as previously described.14 All assays were applied according to the manufacturer's instructions.
Endomyocardial biopsy
Before EMB each patient underwent left heart catheterization with coronary angiography to exclude relevant coronary artery disease. The biopsy sample sites (left, right, or biventricular) were chosen according to pre-biopsy imaging studies using MRI (Siemens 1.5-T-Magnetom Sonata) and echocardiography to reduce sampling error and to maximize the specificity and sensitivity. Biopsies were taken by using a bioptome (H1518.02-A, Endoflex, Germany) from the left ventricle in 112 patients (90%), from the right ventricle in 10 patients (8%), and from both ventricles in 2 patients (2%). At least four biopsy specimens (median, n = 5) with a diameter between 1 and 3 mm were harvested immediately: two biopsy specimens were fixed in 4% buffered formaldehyde for haematoxylin and eosin, Masson-trichrome and Giemsa staining, and performance of immunohistology; two to three cardiac tissue samples were quick-frozen in liquid nitrogen or fixed in the RNAlater for molecular pathological examination including nested (RT-)PCR detection of viral genomes without a loss of sensitivity.15,16 Within 24 h biopsy specimens were investigated.
Histopathological analysis
Paraffin-embedded 5 µm thick tissue sections were stained with haematoxylin and eosin, Masson-trichrome, and Giemsa, and were examined by light microscopy. Histological analysis followed the Dallas criteria,8,9 which have previously been considered the gold standard for the bioptic evaluation in suspected myocarditis. According to these criteria, acute myocarditis is defined by lymphocytic infiltrates in association with myocyte necrosis while borderline myocarditis is characterized by the presence of inflammatory infiltrates without microscopic signs of myocyte injury. In the present study, the categories of acute and borderline myocarditis were combined and judged as a positive biopsy according to the Dallas criteria.
Immunohistochemistry
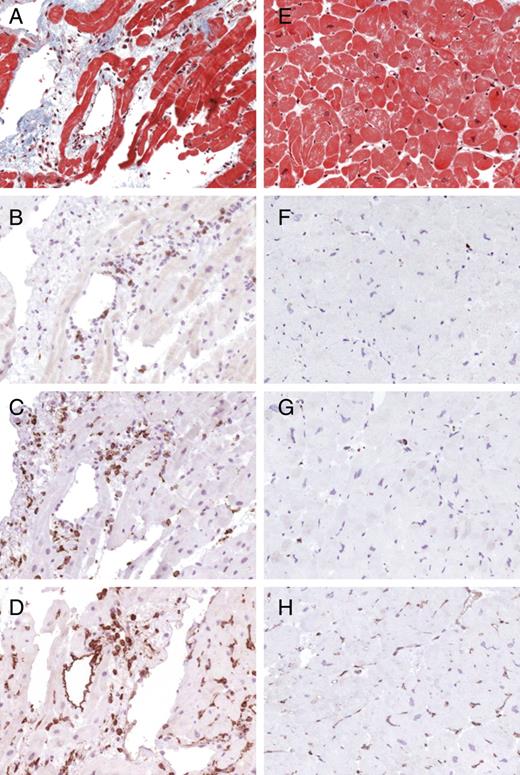
For immunohistological staining, paraffin-embedded tissue sections were treated with an avidin–biotin–immunoperoxidase method according to the protocol of the manufacturer (Vectastain Elite ABC Kit, Burlingame, CA, USA). The following antibodies were used for identification, localization, and characterization of the mononuclear cell infiltrates: CD3 for T cells (Novocastra Laboratories, GB), PGM1 for macrophages (CD68) (DAKO, Denmark), as well as HLA-DR-α (DAKO, Germany) to assess HLA class II expression in professional antigen-presenting immune cells. According to the World Health Organization and the International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies, EMBs were considered to be inflamed in the case of immunohistochemical detection of focal or diffuse mononuclear infiltrates [>14 leucocytes/mm² (CD3+ T lymphocytes and/or CD68+ macrophages)] in the myocardium, additionally to enhanced expression of HLA class II molecules.3,9,17Figure 1 illustrates typical examples of immunohistochemical staining showing T lymphocytes, macrophages, and expression of HLA class II molecules in the myocardium of a patient with myocarditis compared with uninflamed myocardium.
Typical histopathological findings in borderline myocarditis (A–D) and uninflamed hearts (E–H). Masson's trichrome staining is shown in (A) and (E). Examples of immunohistochemical staining showing CD3+ T lymphocytes (B and F), CD 68+ macrophages (C and G), and expression of HLA class II molecules (D and H).
Molecular detection of the viral genome
For detection of viral genomes, nested (RT-)PCR was performed with deep-frozen or RNAlater fixed EMB. Enterovirus species comprising Coxsackievirus group B and several types of Coxsackievirus group A as well as ECHO virus, parvovirus B19 (PVB-19), ADV, influenza virus A and B, human CMV, EBV and HHV-6 were evaluated by nested (RT-)PCR from biopsy specimens. For RT-PCR analyses, RNA was transcribed into cDNA by reverse-transcriptase according to the protocol of the manufacturer (AGS, Germany).15 The enzymatic amplification of cDNA was performed as nested PCR on a Perkin-Elmer GeneAmp PCR System 9600 (Applied Biosystems, Germany) in two 30-cycle-programmes.18 As an internal control for successful isolation of nucleic acids, the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was detected by PCR. A biopsy was considered to be positive for viral infection if the viral genome was detected by PCR and specificity was confirmed by automatic DNA sequencing of viral amplification products. In addition, viral nucleic acids were amplified from leucocytes of the peripheral blood with the same method and primers as in the cardiac tissue to identify systemic infection. Patients were prospectively analysed for all viruses, except a subset of 10 patients (8%) with retrospective viral genome detection of PVB-19 who were enrolled before April 1997.
Statistical analysis
All variables were tested for normal distribution by the Kolmogorov–Smirnov test. Continuous variables with normal distribution are expressed as mean ± standard deviation (SD). Continuous variables with non-normal distribution are summarized as the median and the inter-quartile range (IQR). For statistical analysis of two by two tables, Fisher's exact test was used. A value of P ≤ 0.05 was considered statistically significant. Using the results of the viral genome detection of EMB as the reference standard, sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and negative (LR−) as well as positive likelihood ratios (LR+) of virus serology were determined. All statistical analyses were performed with SPSS statistical software (version 17.0, SPSS, Inc., Chicago, IL, USA).
Results
Patient population
Baseline features at presentation are detailed in Table 1. Patients were relatively young (median 40, IQR 30–51 years) and mostly male (n = 82, 66%). Left ventricular end-diastolic diameters were dilated (median 59.1, IQR 52.0–66.2 mm) with impaired left ventricular ejection fraction (37.0 ± 19.2%). At admission, mean value of C-reactive protein was elevated (17.1 ± 24.6 mg/dL, n = 112) while mean leucocytes were within normal ranges (8033 ± 3070/µL, n = 112). Time between onset of symptoms and EMB was 17.6 weeks.
Baseline characteristics
| Characteristic . | Value, n = 124 . | Viral genome in EMB, n = 58 . | No viral genome in EMB, n = 66 . |
|---|---|---|---|
| Age, years | 40 (IQR 30–51) | 39 (IQR 23–49) | 41 (IQR 32–54) |
| Men (%) | 82 (66) | 42 (72) | 40 (61) |
| LV ejection fraction, % | 37.0 ± 19.2 | 42.9 ± 21.9 | 37.2 ± 18.9 |
| LV end diastolic diameter, mm | 59.1 (IQR 52.0–66.2) | 59.5 (IQR 52.0–65.7) | 58.0 (IQR 51.7–67.3) |
| C-reactive protein, mg/dL, n = 112 | 17.1 ± 24.6 | 13.1 ± 17.2 | 19.9 ± 28.6 |
| Leucocytes, µL−1, n = 112 | 8033 ± 3070 | 7981 ± 3829 | 8072 ± 2411 |
| Endomyocardial biopsy results (%) | |||
| Positive immunohistology | 54 (44) | 32 (55) | 22 (33) |
| Acute myocarditisb | 4 (3) | 2 (3) | 2 (3) |
| Borderline myocarditisb | 40 (32) | 24 (41) | 16 (24) |
| No myocarditisb | 70 (56) | 23 (40) | 47 (71) |
| Detection of viral genome | 58 (47) | 58 (100) | — |
| Enterovirus | 5 (4) | 5 (9) | — |
| Adenovirus | 3 (2) | 3 (5) | — |
| Parvovirus B19 | 33 (27) | 33 (57) | — |
| Cytomegalovirus | 0 | 0 | — |
| Human herpes 6 virus | 10 (8) | 10 (17) | — |
| Epstein-Barr virus | 7 (6) | 7 (12) | — |
| Infection with ≥2 viruses | 13 (10) | 13 (22) | — |
| Virus serology (%) | |||
| Positive serology | 20 (16) | 8 (14) | 12 (18) |
| Enterovirus | 10 (8) | 3 (5) | 7 (11) |
| Adenovirus | 1 (1) | 0 | 1 (2) |
| Parvovirus B19 | 2 (2) | 1 (2) | 1 (2) |
| Cytomegalovirus | 3 (2) | 1 (2) | 2 (3) |
| Human herpes 6 virus | 3 (2) | 2 (3) | 1 (2) |
| Epstein-Barr virus | 1 (1) | 1 (2) | 0 |
| Characteristic . | Value, n = 124 . | Viral genome in EMB, n = 58 . | No viral genome in EMB, n = 66 . |
|---|---|---|---|
| Age, years | 40 (IQR 30–51) | 39 (IQR 23–49) | 41 (IQR 32–54) |
| Men (%) | 82 (66) | 42 (72) | 40 (61) |
| LV ejection fraction, % | 37.0 ± 19.2 | 42.9 ± 21.9 | 37.2 ± 18.9 |
| LV end diastolic diameter, mm | 59.1 (IQR 52.0–66.2) | 59.5 (IQR 52.0–65.7) | 58.0 (IQR 51.7–67.3) |
| C-reactive protein, mg/dL, n = 112 | 17.1 ± 24.6 | 13.1 ± 17.2 | 19.9 ± 28.6 |
| Leucocytes, µL−1, n = 112 | 8033 ± 3070 | 7981 ± 3829 | 8072 ± 2411 |
| Endomyocardial biopsy results (%) | |||
| Positive immunohistology | 54 (44) | 32 (55) | 22 (33) |
| Acute myocarditisb | 4 (3) | 2 (3) | 2 (3) |
| Borderline myocarditisb | 40 (32) | 24 (41) | 16 (24) |
| No myocarditisb | 70 (56) | 23 (40) | 47 (71) |
| Detection of viral genome | 58 (47) | 58 (100) | — |
| Enterovirus | 5 (4) | 5 (9) | — |
| Adenovirus | 3 (2) | 3 (5) | — |
| Parvovirus B19 | 33 (27) | 33 (57) | — |
| Cytomegalovirus | 0 | 0 | — |
| Human herpes 6 virus | 10 (8) | 10 (17) | — |
| Epstein-Barr virus | 7 (6) | 7 (12) | — |
| Infection with ≥2 viruses | 13 (10) | 13 (22) | — |
| Virus serology (%) | |||
| Positive serology | 20 (16) | 8 (14) | 12 (18) |
| Enterovirus | 10 (8) | 3 (5) | 7 (11) |
| Adenovirus | 1 (1) | 0 | 1 (2) |
| Parvovirus B19 | 2 (2) | 1 (2) | 1 (2) |
| Cytomegalovirus | 3 (2) | 1 (2) | 2 (3) |
| Human herpes 6 virus | 3 (2) | 2 (3) | 1 (2) |
| Epstein-Barr virus | 1 (1) | 1 (2) | 0 |
Values are n (%), mean ± SD or median with IQR when appropriate. LV, left ventricular.
n, Number of patients for whom corresponding variable has been identified.
bHistopathology according to Dallas criteria.
Baseline characteristics
| Characteristic . | Value, n = 124 . | Viral genome in EMB, n = 58 . | No viral genome in EMB, n = 66 . |
|---|---|---|---|
| Age, years | 40 (IQR 30–51) | 39 (IQR 23–49) | 41 (IQR 32–54) |
| Men (%) | 82 (66) | 42 (72) | 40 (61) |
| LV ejection fraction, % | 37.0 ± 19.2 | 42.9 ± 21.9 | 37.2 ± 18.9 |
| LV end diastolic diameter, mm | 59.1 (IQR 52.0–66.2) | 59.5 (IQR 52.0–65.7) | 58.0 (IQR 51.7–67.3) |
| C-reactive protein, mg/dL, n = 112 | 17.1 ± 24.6 | 13.1 ± 17.2 | 19.9 ± 28.6 |
| Leucocytes, µL−1, n = 112 | 8033 ± 3070 | 7981 ± 3829 | 8072 ± 2411 |
| Endomyocardial biopsy results (%) | |||
| Positive immunohistology | 54 (44) | 32 (55) | 22 (33) |
| Acute myocarditisb | 4 (3) | 2 (3) | 2 (3) |
| Borderline myocarditisb | 40 (32) | 24 (41) | 16 (24) |
| No myocarditisb | 70 (56) | 23 (40) | 47 (71) |
| Detection of viral genome | 58 (47) | 58 (100) | — |
| Enterovirus | 5 (4) | 5 (9) | — |
| Adenovirus | 3 (2) | 3 (5) | — |
| Parvovirus B19 | 33 (27) | 33 (57) | — |
| Cytomegalovirus | 0 | 0 | — |
| Human herpes 6 virus | 10 (8) | 10 (17) | — |
| Epstein-Barr virus | 7 (6) | 7 (12) | — |
| Infection with ≥2 viruses | 13 (10) | 13 (22) | — |
| Virus serology (%) | |||
| Positive serology | 20 (16) | 8 (14) | 12 (18) |
| Enterovirus | 10 (8) | 3 (5) | 7 (11) |
| Adenovirus | 1 (1) | 0 | 1 (2) |
| Parvovirus B19 | 2 (2) | 1 (2) | 1 (2) |
| Cytomegalovirus | 3 (2) | 1 (2) | 2 (3) |
| Human herpes 6 virus | 3 (2) | 2 (3) | 1 (2) |
| Epstein-Barr virus | 1 (1) | 1 (2) | 0 |
| Characteristic . | Value, n = 124 . | Viral genome in EMB, n = 58 . | No viral genome in EMB, n = 66 . |
|---|---|---|---|
| Age, years | 40 (IQR 30–51) | 39 (IQR 23–49) | 41 (IQR 32–54) |
| Men (%) | 82 (66) | 42 (72) | 40 (61) |
| LV ejection fraction, % | 37.0 ± 19.2 | 42.9 ± 21.9 | 37.2 ± 18.9 |
| LV end diastolic diameter, mm | 59.1 (IQR 52.0–66.2) | 59.5 (IQR 52.0–65.7) | 58.0 (IQR 51.7–67.3) |
| C-reactive protein, mg/dL, n = 112 | 17.1 ± 24.6 | 13.1 ± 17.2 | 19.9 ± 28.6 |
| Leucocytes, µL−1, n = 112 | 8033 ± 3070 | 7981 ± 3829 | 8072 ± 2411 |
| Endomyocardial biopsy results (%) | |||
| Positive immunohistology | 54 (44) | 32 (55) | 22 (33) |
| Acute myocarditisb | 4 (3) | 2 (3) | 2 (3) |
| Borderline myocarditisb | 40 (32) | 24 (41) | 16 (24) |
| No myocarditisb | 70 (56) | 23 (40) | 47 (71) |
| Detection of viral genome | 58 (47) | 58 (100) | — |
| Enterovirus | 5 (4) | 5 (9) | — |
| Adenovirus | 3 (2) | 3 (5) | — |
| Parvovirus B19 | 33 (27) | 33 (57) | — |
| Cytomegalovirus | 0 | 0 | — |
| Human herpes 6 virus | 10 (8) | 10 (17) | — |
| Epstein-Barr virus | 7 (6) | 7 (12) | — |
| Infection with ≥2 viruses | 13 (10) | 13 (22) | — |
| Virus serology (%) | |||
| Positive serology | 20 (16) | 8 (14) | 12 (18) |
| Enterovirus | 10 (8) | 3 (5) | 7 (11) |
| Adenovirus | 1 (1) | 0 | 1 (2) |
| Parvovirus B19 | 2 (2) | 1 (2) | 1 (2) |
| Cytomegalovirus | 3 (2) | 1 (2) | 2 (3) |
| Human herpes 6 virus | 3 (2) | 2 (3) | 1 (2) |
| Epstein-Barr virus | 1 (1) | 1 (2) | 0 |
Values are n (%), mean ± SD or median with IQR when appropriate. LV, left ventricular.
n, Number of patients for whom corresponding variable has been identified.
bHistopathology according to Dallas criteria.
Endomyocardial biopsy and serological determinations
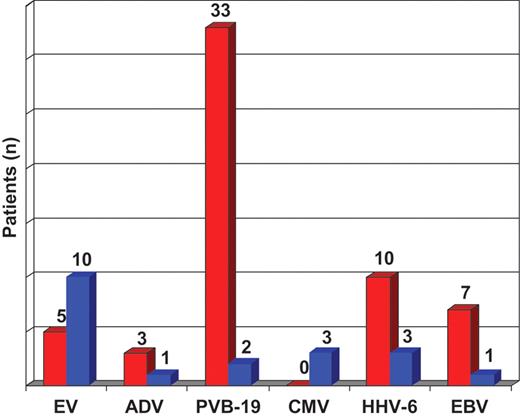
Histopathological diagnosis of myocarditis according to the Dallas criteria was made in 44 patients (35%), including 40 patients (32%) with borderline myocarditis (presence of inflammatory infiltrates without microscopic signs of myocyte injury) and 4 patients (3%) with acute myocarditis (presence of myocyte necrosis in association with lymphocytic infiltrates). Positive findings were more frequent after immunohistochemical staining, which revealed significant inflammatory cellular infiltrates in the specimens of 54 subjects (44%). In patients without signs of myocardial inflammation (n = 70, 56%), the phenotype of DCMi was observed in 47 patients (38%). Using nested (RT-)PCR, the viral genome was detected in the myocardium of 58 patients (47%), of whom 32 patients showed signs of inflammation (acute myocarditis, n = 2; positive immunohistology without myocyte necrosis, n = 30). The prevailing virus was PVB-19 (n = 33, 27%). Human herpesvirus 6 was identified in 10 (8%) and EBV in seven patients (6%). The so-called cardiotropic viruses were rarely detected: EV genome was amplified in five patients (4%) and ADV genomes in three patients (2%) (Figure 2). Thirteen patients (10%) showed infection with ≥2 viruses in EMB [co-detection of PVB-19 prevailed (11 of 13)]. The most frequent combination of myocardial co-infection was PVB-19 and HHV-6 (n = 7). In 54 patients with positive immunohistology, viral genome was detected in the myocardium of 32 subjects (59%). In 70 patients without immunohistochemical signs of inflammation, viral genome detection in the EMB was positive in 23 subjects (33%). In four patients, a possible blood contamination of EMB could not be excluded because PCR revealed genomes of the same virus species in the myocardium as well as in blood leucocytes.
Prevalence of RNA- and DNA viral infection in cardiac tissue (red) and serology (blue) in patients with suspected myocarditis. EV, enterovirus (RNA); ADV, adenovirus (DNA); PVB-19, parvovirus B19 (DNA); CMV, cytomegalovirus (DNA); HHV-6, human herpesvirus (DNA) 6; EBV, Epstein-Barr virus (DNA).
Evidence of an acute viral infection by serological determinations was found in the serum of 20 patients (16%), of whom only 5 patients had virus genome and only 6 patients showed evidence of inflammation in the myocardium. Enterovirus serology was positive in 10 patients (8%), HHV-6 in 3 patients (2%), CMV in 3 patients (2%), PVB-19 in 2 patients (2%), and ADV (1%) and EBV in 1 patient each (1%) (Figure 2). Three patients were infected with ≥2 viruses as diagnosed by serology [EV and HHV-6 (n = 2), EV and CMV (n = 1)].
Correlation of virus serology and viral detection in the myocardium
Only in 5 out of 124 patients (4%), there was serological evidence of an infection with the identical virus that was detected by EMB (Table 2). Interestingly, a positive virus serology was slightly more common in patients with negative biopsy (15 out of 66, 23%) than in patients with myocardial viral genome detection (5 out of 58, 9%). This association was of marginal significance (P = 0.04) and probably an incidental finding, since it lacks a pathophysiological rationale. The likelihood ratios of 0.38 (LR+) and 1.18 (LR−) would indicate that a positive serological finding would decrease, while a negative serological finding would increase the likelihood of myocardial virus genome detection. Taking all patients together (n = 124), the sensitivity for serological detection of a virus that was found in EMB was 9% and the specificity was 77%. The PPV and NPV were 25 and 49%, respectively. This held also true for patients with immunohistopathological proven myocarditis (n = 54) and patients with time from initial symptoms to the EMB procedure of ≤1 months (n = 76) (Table 2): the sensitivity was 6/11% and the specificity was 82/78%, the PPV was 33/31% and the NPV 38/49%.
Two by two table of virus serology and direct viral detection in EMB
| . | Biopsy negative . | Biopsy positive . | P . |
|---|---|---|---|
| All patients, n = 124 | |||
| Serology negative | 51 | 53 | |
| Serology positive | 15 | 5 | 0.04 |
| IHC positive, n = 54 | |||
| Serology negative | 18 | 30 | |
| Serology positive | 4 | 2 | 0.17 |
| Time from initial symptoms to EMB ≤1 month, n = 76 | |||
| Serology negative | 31 | 32 | |
| Serology positive | 9 | 4 | 0.19 |
| . | Biopsy negative . | Biopsy positive . | P . |
|---|---|---|---|
| All patients, n = 124 | |||
| Serology negative | 51 | 53 | |
| Serology positive | 15 | 5 | 0.04 |
| IHC positive, n = 54 | |||
| Serology negative | 18 | 30 | |
| Serology positive | 4 | 2 | 0.17 |
| Time from initial symptoms to EMB ≤1 month, n = 76 | |||
| Serology negative | 31 | 32 | |
| Serology positive | 9 | 4 | 0.19 |
EMB, endomyocardial biopsy; IHC, immunohistology; P, Fisher's exact test.
Two by two table of virus serology and direct viral detection in EMB
| . | Biopsy negative . | Biopsy positive . | P . |
|---|---|---|---|
| All patients, n = 124 | |||
| Serology negative | 51 | 53 | |
| Serology positive | 15 | 5 | 0.04 |
| IHC positive, n = 54 | |||
| Serology negative | 18 | 30 | |
| Serology positive | 4 | 2 | 0.17 |
| Time from initial symptoms to EMB ≤1 month, n = 76 | |||
| Serology negative | 31 | 32 | |
| Serology positive | 9 | 4 | 0.19 |
| . | Biopsy negative . | Biopsy positive . | P . |
|---|---|---|---|
| All patients, n = 124 | |||
| Serology negative | 51 | 53 | |
| Serology positive | 15 | 5 | 0.04 |
| IHC positive, n = 54 | |||
| Serology negative | 18 | 30 | |
| Serology positive | 4 | 2 | 0.17 |
| Time from initial symptoms to EMB ≤1 month, n = 76 | |||
| Serology negative | 31 | 32 | |
| Serology positive | 9 | 4 | 0.19 |
EMB, endomyocardial biopsy; IHC, immunohistology; P, Fisher's exact test.
Discussion
This study showed for the first time that the detection of acute viral infections by serological antibody assays does not correlate with the detection of viral genome in EMB in patients with suspected myocarditis.
Although not recommended in international guidelines,16 virus serology is still frequently used in clinical practice for the diagnosis of myocarditis.19 The determination of virus titres, in particular when follow-up examinations are correctly performed, is expensive and time-consuming. Seroconversion or a four-fold increase in the titre in paired serum samples (7–28 days after the initial serum) has been suggested to provide evidence for an acute viral infection.20 The initial serum sample has to be taken very early in the course of infection. However, patients are referred for diagnostics and medical treatment with a significant delay from the onset of the initial infection, potentially ranging from some weeks to a few months (in our study 17.6 weeks), when the acute viral infection and the acute phase of myocarditis have already resolved.4,6,21 Moreover, antibody results are influenced by the fact that various assays cover different periods after the infection. In EV infections, neutralizing antibodies appear relatively late in the course of infection and may persist for several months up to years.22 In contrast, parvovirus B19 (PVB-19) IgM is detectable only for a short period of ∼4–6 weeks and might fade in most patients before they develop any signs or symptoms of myocarditis.23 With the exception of EV detection by the neutralization assay, the determination of a viral infection by serology is only feasible at the time of symptom onset. The diagnostic value of serology is also limited by the fact that most viruses involved in the pathogenesis of myocarditis are highly prevalent in the population. Over 70% of the German population have been tested seropositive for PVB-19 IgG antibodies,24 although most of the infections remain asymptomatic. Similar observations have been reported for herpes infection.25 Therefore, primary infections might be difficult to detect since a production of IgM or IgA is not mandatory in patients with reactivation or reinfection. Particularly, in persistent infections, reactivation is common in healthy individuals (e.g. herpesvirus infections).26 The interpretation of serologic findings is further limited by the finding that Coxsackie B virus IgM is similar in patients with DCM and control subjects who shared the same environment, indicating local cross infection.27 Finally, infections with EBV or EV frequently cause a polyclonal stimulation of B cells resulting in a production of antibodies with different specificity and reactivity. This cross reaction further limits the interpretation of serological results. Taken together, the interpretation of antibody assays is complex and complicated by many confounders. The role of EMB in diagnosing myocarditis has been challenged by a lack of specificity, risk of complication, and sampling errors.16,21 By taking two to three EMB specimens from the affected region with the use of cardiac imaging (echocardiography and MRI), the diagnostic gain for detection of myocarditis can be enhanced to 50%.11 The complication rate of the procedure is related to the expertise of the cardiologist and varies between 0.3 and 5.5%.28–31 However, EMB provides important prognostic information since immunohistological signs of inflammation in the myocardium are related to poor outcome.11 Furthermore, there is evidence that in the case of PVB-19 the viral load in EMB specimens can be used to distinguish latent from acute viral infection: A viral load of ≥500 genome equivalents per microgram in EMB specimens has been shown to be a clinically relevant threshold for the maintenance of myocardial inflammation.10 Our data support the recommendation that histopathological and molecular analysis for viral infections in EMB remain the gold standard for the diagnoses of myocarditis.
This study has some potential limitations. One might be the sampling error by taking the specimens not directly from the area of infection and inflammation. Therefore, we chose the biopsy sample sites according to the findings of magnetic resonance imaging or echocardiography. Moreover, we analysed cardiotropic viruses which were described to trigger inflammatory cardiomyopathy. However, there is a little chance that unknown viruses could be involved in the development of myocarditis. Interestingly, detection of DNA viruses (CMV, EBV, PVB-19, ADV) in the myocardium was more common than detection of RNA viruses while serologically the evidence for acute viral infection was more often shown for RNA viruses (EV) (Table 1, Figure 2). This finding is supported by an earlier published study:32 In 624 patients with myocarditis, the most frequently amplified virus in the myocardium (using PCR) was ADV (n = 142, 23%), whereas EV (n = 46, 7%) was the most commonly detected virus in serology.32 This might be a result of the neutralization assays used to detect EV because the antibody titre remains stable in these assays over long periods. On the other hand, due to the activity of tissue ribonuclease in the myocardium,33 viral RNA is more unstable than viral DNA which may favour the detection of DNA viruses in EMB. The most frequently amplified virus in EMB was PVB-19, which is in line with previous observations.34 Quantitative assessments of the viral load in EMB specimens were not performed, since the methodology was not established when the study was initiated.10 Patients with an episode of suspected viral illness within 6 months prior to admission were included. That time period possibly lowered the frequency of positive results in serology and EMB, based on viral clearance from the myocardium. On an average our patients were probably less ill than, for example, those patients included in the multicentre Myocarditis Treatment Trial35 due to our broader inclusion criteria (in particular before the publication of the scientific statement from the AHA/ACC/ESC16 regarding the role of EMB in the management of cardiovascular disease in 2007). However, detection of inflammation by histological as well as immunohistological staining and detection of viral genome by PCR in the myocardium are comparable or even higher with previous published studies.3,35,36 Owing to the low prevalence of acute myocarditis (herein n = 4, 3%), the value of serological analyses in that small subgroup of patients remains unclear.
Conclusion
Based on our study, virus serology has no relevance for the diagnosis of myocardial infection in patients presenting with suspected myocarditis. Even in the subgroup of patients with histopathological signs of inflammation in the myocardium, a lack of correlation remains. While serological diagnosis is mandatory in different disease entities, e.g. in viral hepatitis or HIV, this is obviously not the case in suspected myocarditis. Serological examinations are costly and unreliable in clinical practice and should no longer be used as a standard tool in the work-up of patients with suspected myocarditis. Endomyocardial biopsy offers the possibility for an exact diagnosis and appears to be underused.
Funding
This work was supported by HOMFOR (Homburger Forschungsförderungsprogramm) and by the Ministry of Economy and Science of the Saarland. CU and MB are supported by the Deutsche Forschungsgemeinschaft (Klinische Forschergruppe KFO 196).
Conflict of interest: none declared
References
- myocarditis
- myocardium
- polymerase chain reaction
- endomyocardial biopsy
- enterovirus
- herpesvirus 4, human
- immunohistochemistry
- inflammation
- adenoviruses
- biopsy
- erythema infectiosum
- viral genome
- human parvovirus b19 virus
- serologic tests
- virus diseases
- infections
- cytomegalovirus
- diagnosis
- study of serum
- viruses




Comments
Dear Dr. Mavrogeni,
We appreciate your interest for the results of our study (1). You mentioned that also in your institution only 5% of patients with proven myocarditis showed serological evidence of an infection with the same virus detected by EMB. You also emphasize that viral genomes were even prevalent in patients undergoing bypass surgery without inflammatory cardiomyopathy, probably indicating innocent by-standers in the myocardium. However, it would be interesting to know which viruses were detected in your patient cohort. There is evidence indicating that viral genomes can also be found in patients with coronary artery disease and even in healthy individuals. In the recently published letter by Kandolf et al. (2) about human Parvovirus B19 (PVB-19) associated myocarditis, PVB -19 genomes were detected in the myocardium of 35% of the patients with dilated cardiomyopathy and in 8% of uninflamed hearts. However, viral load was significantly higher in patients with proven myocarditis and dilated cardiomyopathy than in those without myocardial inflammation. Based on these data the authors suggest that a viral load of >500 genome equivalents per microgram in EMB specimens was a clinically relevant threshold for the maintenance of myocardial inflammation. Hopefully further studies will provide more in-depth understanding of the immune- independent viral pathogenesis in inflamed and non-inflamed hearts and reactivation of latent infections.
1. Mahfoud F, G?rtner B, Kindermann M, Ukena C, Gadomski K, Klingel K, Kandolf R, B?hm M, Kindermann I. Virus serology in patients with suspected myocarditis: utility or futility? Eur Heart J. 2011;32(7):897- 903. 2. Bock CT, Klingel K, Kandolf R. Human parvovirus B19-associated myocarditis. N Engl J Med. 2010;362(13):1248-9.
Conflict of Interest:
None declared
Dear Editor,
I read with interest your recent publication supporting that virus serology has no relevance for diagnosis of myocarditis and myocardial biopsy remains the gold standard. Our results were in agreement with yours. Indeed, also in our institution, only in 5% of patients with myocarditis, there was serolological evidence of the same virus detected by EMB and in all of them a positive for myocarditis magnetic resonance (CMR) study using two out of three criteria according to JACC White paper (1) was found. Interestingly, viral genomes were also detected after intra-operative EMB in patients from our institution during by-pass operation with evidence of myocardial infarction but not of myocarditis in the pre-operative clinical evaluation and CMR study. In these patients there was no relationship between EMB and virus serology. This is an exciting finding, because available EMB data from patients without myocarditis are rather few (2, 3) and were not correlated with CMR evaluation. The presence of viral genomes in the myocardium of patients with documented coronary artery disease showed that the identification of positive virology in EMB without simultaneously clinical presentation and positive CMR or immunohistology, can not be used by itself to document the diagnosis of myocarditis. Additionally, in other studies, the enteroviral genome was detected in 12% of patients with dilated cardiomyopathy and 17% of the controls. Within the control group, the enteroviral genome was detected in 20% of patients with ischaemic heart disease, 10.5% with valvular heart disease and 20% with specific heart muscle disease (4). Different viral or microbial genomes in EMB can be innocent by-standers in myocardium and only under special conditions such as hypersensitivity, immune defects, special immunologic background etc can contribute to clinical overt myocarditis (4). Additionally, using the pooled data presented in JACC White paper (1), CMR showed that, if all sequences can be performed and two or more of the three tissue-based criteria are positive, myocardial inflammation can be predicted or ruled out with a diagnostic accuracy of 78%. At this point, CMR can be used as a filter for further patients' selection for EMB evaluation.
References
1. Friedrich M, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper L, White J, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009; 53:1475-87.)
2. Lotze U, Egerer R, Tresselt C, Gl?ck B, Dannberg G, Stelzner A, Figulla HR. Frequent detection of parvovirus B19 genome in the myocardium of adult patients with idiopathic dilated cardiomyopathy. Med Microbiol Immunol. 2004 May;193(2-3):75-82
3. Zhang H, Li Y, McClean DR, Richardson PJ, Florio R, Sheppard M, Morrison K, Latif N, Dunn MJ, Archard LC. Detection of enterovirus capsid protein VP1 in myocardium from cases of myocarditis or dilated cardiomyopathy by immunohistochemistry: further evidence of enterovirus persistence in myocytes. Med Microbiol Immunol. 2004 May; 193(2-3):109-14.
4. Keeling PJ, Jeffery S, Caforio AL, Taylor R, Bottazzo GF, Davies MJ, McKenna WJ. Similar prevalence of enteroviral genome within the myocardium from patients with idiopathic dilated cardiomyopathy and controls by the polymerase chain reaction. Br Heart J. 1992; 68(6):554-9.
5. Dennert R, Crijns HJ, Heymams S. Acute viral myocarditis. Eur Heart J 2008; 29:2073-82
Conflict of Interest:
None declared