-
PDF
- Split View
-
Views
-
Cite
Cite
Jeroen J. Bax, Victoria Delgado, Further insight into transcatheter and surgical aortic bioprosthetic valve thrombosis, European Heart Journal, Volume 38, Issue 28, 21 July 2017, Pages 2208–2210, https://doi.org/10.1093/eurheartj/ehx368
Close - Share Icon Share
This editorial refers to ‘Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves’†, by L. Sondergaard et al., on page 2201.
In the current issue of the journal, Sondergaard and colleagues report further on the topic of transcatheter and surgical bioprosthetic aortic valve thrombosis1 as detected with 4D computed tomography (CT). Specifically, the novelty of this article is the temporal development of bioprosthetic aortic valve thrombosis as detected on 4D CT: is there progression, regression, or are there no changes at all? This information would have major implications for the type and duration of oral anticoagulation or antiplatelet therapy.
For this purpose, the authors prospectively evaluated 84 patients, who underwent (per protocol) two 4D CT scans evaluating bioprosthetic aortic valves: 61 patients underwent transcatheter aortic valve replacement (TAVR) and 23 patients received a surgical bioprosthetic valve. A large variety of transcatheter heart valves were implanted, including CoreValve and EvolutR (Medtronic, MN, USA), Portico (St Jude Medical, MN, USA), and Sapien 3 (Edwards Lifesciences, CA, USA); the surgical bioprosthetic valves included Perimount (Edwards Lifesciences, CA, USA) and Trifecta (St Jude Medical, MN, USA).
The first 4D CT scan was performed between 1 and 6 months after valve replacement, whereas the second scan was performed 3–6 months after the first scan. From the 4D CT scan, different anatomical parameters were derived, including hypoattenuating leaflet thickening (HALT) and hypoattenuation affecting motion (HAM). Importantly, the CT parameters were assessed in a core laboratory. However, precise quantification of these parameters remains difficult and still hampers standardized assessment. Reality also learns that not all 4D CT scans are interpretable; in this study, 11 (13%) scans were not (fully) interpretable.1 Given the high clinical relevance of the findings, further optimization and standardization of the imaging protocol is needed.
What happened to the abnormalities on 4D CT over longer term follow-up?
The first 4D CT was performed at 140 ± 152 days after valve implantation, and HALT was noted in 32 patients (38.1% of the population), while HAM was noted in 17 patients (20.2%); there was no difference in the patients undergoing surgical or transcatheter valve replacement. Importantly, there was a significant range in time to first detection of valve thrombosis: ranging from within a month to more than a year after valve implantation—this could imply that HALT and HAM may develop early and later after valve implantation, although serial CT scans would be needed to prove this hypothesis; if, however, 4D CT would be the technique of choice for thrombosis detection, this observation then poses the question of when should the initial 4D CT be performed to detect thrombosis.
It is also interesting to note that the incidence of HALT in the current study was higher than in most previous studies, which reported incidences between 10 and 15%.2–5 At the same time, none of the patients in the current study showed significant stenosis on echocardiography (0%), which is lower than in previous studies, where the incidence of stenosis was ∼5%.2–5
The second CT scan was performed at 298 ± 141 days after valve implantation. Progression of abnormalities was noted in 15.5% of patients, whereas regression was observed in 10.7%, and no change occurred in 73.8% of patients. Progression includes from no thrombosis to HALT or from HALT to HAM. Importantly, 61.9% of the total cohort had normal CT scans at both time points.
How do the 4D CT findings relate to functional measurements derived from echocardiography?
Various previous studies demonstrated that HALT and HAM on 4D CT shortly after TAVR or surgical bioprosthetic aortic valve replacement were not often associated with haemodynamic (functional) abnormalities on echocardiography.2–5 The current study adds important information to our knowledge, namely that even when HALT and HAM persisted on 4D CT at 298 ± 141 days after valve implantation, the transvalvular mean and peak gradients did not change significantly. Throughout the entire study period, the mean echocardiographic gradient for the entire population never exceeded 10 mmHg, and none of the patients developed significant valvular stenosis or regurgitation, indicating the absence of haemodynamic consequences of the anatomical abnormalities. Furthermore, all patients were free of symptoms during the entire study period. Accordingly, there appears to be a ‘mismatch’ between anatomical and haemodynamic (functional) abnormalities of transcatheter or surgical bioprosthetic aortic valves (an example is presented in Figure 1).
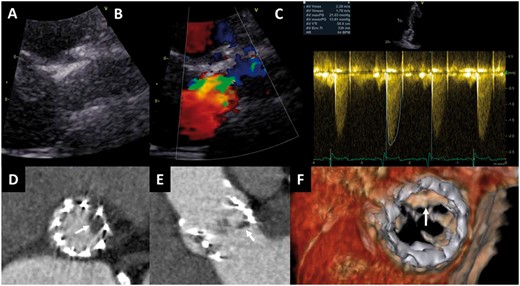
Mismatch between haemodynamics of transcatheter aortic valve on transthoracic echocardiography and anatomic findings on multidetector row computed tomography. An 85-year-old female treated with a 23 mm SAPIEN valve who underwent transthoracic echocardiography and multidetector row computed tomography at 2 months follow-up. The patient was asymptomatic. Echocardiography (parasternal long-axis view) showed a normal aspect of the valve leaflets (A), and mild turbulent flow (colour Doppler, B), with mean and peak gradients of 13 and 21 mmHg, and calculated aortic valve area of 1.3 cm2 (C). On multidetector row computed tomography, the reconstructed short-axis view (D) and the sagittal view (E) show hypoattenuated leaflet thickening of the left coronary cusp; four-dimensional volume rendering of the valve shows the thickening of the leaflet with restrictive motion in systole (F).
What are the implications for anticoagulation therapy?
Among the 24 patients using oral anticoagulation, none showed progression, whereas 21 were stable and 3 showed regression. Among the 60 patients using antiplatelet or no therapy, progression was observed in 13 patients, whereas 41 were stable and 6 showed regression. Of note, ‘stability’ includes stable abnormalities (without progression/regression), but also includes completely normal prostheses, and therefore limits precise interpretation of the data.
When the results of 4D CT were related to the use of oral anticoagulation or antiplatelet therapy (or no therapy), the following observations were of interest.
The first important observation is that progression was absent in all patients using anticoagulation, and that stability was noted in 21 patients, underscoring that anticoagulation stabilizes the thrombotic process. Importantly, the timing of the second scan was 298 ± 141 days after valve implantation, which means that with oral anticoagulation there was stability, but at the same time no regression, and one can argue that oral anticoagulation may thus be needed for long periods. How long cannot currently be defined, since more follow-up CT scans would have been needed to obtain a better understanding of the time-course of the thrombotic process.
The second important observation is that from the current data,1 it is unclear how many stable patients had normal 4D CT scans, and how many with HALT or HAM on the CT scan were stable. This hampers precise interpretation of data, since stable with HALT or HAM is a different situation from stable without any abnormalities on CT. Moreover, 61.9% of patients had normal valves at both 4D CT scans, of whom a significant percentage did not develop HALT or HAM despite the absence of oral anticoagulation, implying that treatment with anticoagulation may not be warranted in all patients, but should be personalized to patients who need it. Moreover, many patients prefer a surgical bioprosthetic aortic valve over a mechanical valve to avoid life-long anticoagulation, and many patients are older and frail with multiple co-morbidities that increase bleeding risk. For these reasons, it would be preferred to have an imaging technique to guide individual patient management with regards to choice of anticoagulation or antiplatelet treatment. Since echocardiography may not be sensitive enough to detect these abnormalities (the ‘mismatch’ between 4D CT and echocardiography described above), 4D CT is probably the best modality to guide therapy (initiation and continuation). However, radiation and contrast limit the use of 4D CT. What is more important, the current data show that thrombosis can occur early and late after valve implantation—and thus early and late 4D CT are needed for optimal thrombosis detection.
The third important observation is that stability was also noted in 41 patients using antiplatelet therapy (or no therapy), indicating that oral anticoagulation is not needed in every patient to prevent or stabilize thrombosis.
What further information is needed in the future?
Since it appears that valve thrombosis can occur early and late after surgical or transcatheter valve implantation,1 more serial 4D CT studies are needed to determine when thrombosis mostly occurs. These serial follow-up studies will also provide more information on the development of thrombosis (progression, stability, or regression). Ideally, another imaging technique without radiation is needed for serial imaging. Finally, better understanding of the clinical relevance of the CT findings in relation to the absence of echocardiographic abnormalities (‘mismatch’) is needed. All this information will help to determine optimal therapy. Whether patient-tailored treatment will ever be feasible or whether every patient will receive life-long oral anticoagulation (with relatively high bleeding risk) remains to be determined.
Conflict of interest: none declared.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.