-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-Pierre Després, Abdominal obesity: the most prevalent cause of the metabolic syndrome and related cardiometabolic risk, European Heart Journal Supplements, Volume 8, Issue suppl_B, May 2006, Pages B4–B12, https://doi.org/10.1093/eurheartj/sul002
Close - Share Icon Share
Abstract
Abdominal obesity, due to intra-abdominal adiposity, drives the progression of multiple cardiometabolic risk factors independently of body mass index. This occurs both through altered secretion of adipocyte-derived biologically active substances (adipokines), including free fatty acids, adiponectin, interleukin-6, tumour necrosis factor alpha, and plasminogen activator inhibitor-1, and through exacerbation of insulin resistance and associated cardiometabolic risk factors. The prevalence of abdominal obesity is increasing in western populations, due to a combination of low physical activity and high-energy diets, and also in developing countries, where it is associated with the urbanization of populations. The measurement of waist circumference, together with an additional comorbidity, readily identifies the presence of increased cardiometabolic risk associated with abdominal obesity. For example, >80% men with waist circumference ≥90 cm and triglycerides (TG) ≥2 mmol/L were found to have an atherogenic triad of elevated apolipoprotein B, fasting hyperinsulinaemia, and small, dense LDL, which had been strongly associated with adverse cardiovascular outcomes in a previous observational study. Accordingly, measurement of waist circumference should become a standard component of cardiovascular risk evaluation in routine clinical practice. Lifestyle modification remains the initial intervention of choice for this population, with pharmacological modulation of risk factors where this is insufficiently effective. Looking ahead, the initial results of randomized trials with rimonabant, the first CB1 receptor blocker, indicate the potential of correcting overactivation of the endogenous endocannabinoid system for simultaneous improvement of multiple cardiometabolic risk factors.
Introduction
Abdominal obesity is emerging as an important driving force behind the deterioration of cardiometabolic risk in the general population. Patients with evidence of cardiovascular disease often display abdominal obesity,1,2 and observational studies have identified abdominal obesity as a predictor of adverse metabolic or cardiovascular outcomes independently of body mass index (BMI).3–13 Moreover, we have only recently begun to understand the important endocrine and paracrine functions of intra-abdominal adipocytes, and the complex interactions leading to a diabetogenic and atherogenic metabolic risk profile.14 The purposes of this review are to explore the pathophysiological links between abdominal obesity and elevated cardiometabolic risk, to evaluate strategies to identify patients most at risk through the presence of intra-abdominal adiposity, and to consider the implications for intervention to improve cardiovascular outcomes in these patients.
Development of abdominal obesity
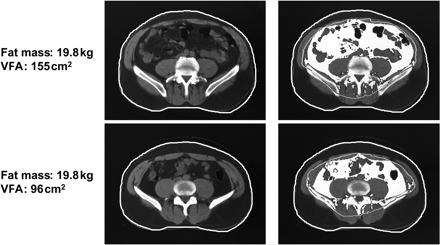
Typically, upper body obesity (android, ‘apple shape’ obesity) is more commonly found in men, whereas lower body obesity (gynoid, ‘pear shape’) is more commonly found in women. Upper body obesity receives contributions from adiposity in subcutaneous and intra-abdominal compartments. Intra-abdominal fat (visceral fat) has been defined as the fat located around the viscera and within the peritoneum, the dorsal border of the intestines and the ventral surface of the kidney.15 Intra-abdominal fat accumulation can occur in men or women, and BMI does not provide a reliable indication of the extent of intra-abdominal adiposity. For example, Figure 1 shows computed tomography (CT) scans of two men with a similar BMI and with the same amount of total body fat. Nonetheless, the visceral fat area (VFA) on the CT scan in the subject of the top panel is >50% higher than the other man, despite similar BMI values. It is therefore important to predict intra-abdominal adiposity carefully in clinical practice in order to better assess the related cardiometabolic risk.
Although there is solid evidence that body fat distribution (and therefore intra-abdominal obesity) has a very significant genetic basis, abdominal obesity will only develop in the presence of a positive energy balance. Unfortunately, because of the ‘toxic’ environment that we have designed for ourselves, an increasing proportion of our population is sedentary and exposed to an energy-dense refined diet favouring the development of obesity. As a result, this increasing tendency towards sedentary habits and an excessive intake of high-energy foods are efficient promoters of abdominal obesity. Recent evidence confirms a high prevalence of abdominal obesity among genetically susceptible individuals. In the USA National Health and Nutrition Examination Survey (NHANES) cohort examined between 1988 and 1994, 30% of men and 46% of women were abdominally obese,16 according to the arbitrary waist circumference cut-offs of >102 cm in men and of >88 cm in women proposed by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III).17 By 1999–2000, these figures had risen to 36 and 52%, respectively. In a European population comprising 9596 participants within the WHO Monica Study, the average waist circumference increased by 1 cm between 1989–90 and 1994–95, with the prevalence of abdominal obesity (defined as >80th percentile for men or women, equating to waist circumference >103 cm for men and >92 cm for women) increasing by >3% over this short time interval.18 Increasing urbanization of populations in the developing world, associated with decreased physical activity and increased energy intake, is driving an increased prevalence of diabetes and cardiovascular disease and there is no evidence that this phenomenon will plateau.19–22
Abdominal obesity and elevated cardiometabolic risk
Abdominal obesity, insulin resistance, and the metabolic syndrome
The prognostic importance of high waist circumference has been recognized within the diagnostic criteria to identify individuals with features of the metabolic syndrome. The NCEP-ATP III criteria, dating from 2001, include high waist circumference (>102 cm for men and >88 cm for women) along with criteria relating to elevated TG, low HDL-cholesterol, high blood pressure, and high fasting plasma glucose (FPG).17 Patients need to meet any three of the five criteria. The International Diabetes Federation (IDF) has gone further and made the presence of abdominal obesity a requirement for the diagnosis of metabolic syndrome, along with two of four other criteria similar to those used by the NCEP-ATP III.23 The IDF criteria use lower cut-off values for waist circumference than earlier criteria, and ethnic-specific values are given based on some epidemiological evidence. These criteria are (men/women) ≥94/≥80 cm for Europid subjects; ≥90/≥80 cm for South Asian subjects; ≥90/≥80 cm for Chinese subjects, and ≥85/≥90 cm for Japanese subjects, with criteria for Europids to be used for other ethnicities in the absence of specific recommendations. Although it is clear that lower waist cut-offs are required in some populations of the world such as Asia, the values proposed to define abdominal obesity have not been based on a rigorous analysis and comparison of standardized measures of visceral adiposity, except for Japan, and cardiometabolic risk factors/markers across populations. Therefore, the recent IDF criteria will have to be subjected to further validation against ‘hard’ clinical endpoints.
It is important to remember that the above clinical variables are diagnostic criteria for and not definitions of the metabolic syndrome.24 A number of other cardiometabolic risk factors are associated with the metabolic syndrome beyond these criteria, such as impaired fibrinolysis and a hypercoagulable state,25 chronic low-grade inflammation,26,27 and the appearance of atherogenic small, dense lipoproteins.28 Many of these cardiometabolic risk factors are driven by a combination of insulin resistance and abdominal obesity resulting from excess intra-abdominal adiposity. For example, insulin resistance promotes the atherogenic dyslipidaemia that is characterized by elevated TG, low HDL-cholesterol, and small, dense LDL.29 The prognostic importance of the high TG–low HDL-cholesterol dyslipidaemic state has been well described,17 whereas the small, dense LDL phenotype was shown to be significantly associated with adverse cardiovascular outcomes in the Québec Cardiovascular Study.13 A shift towards small, dense HDL particles has also been identified as part of the cluster of cardiometabolic risk factors accompanying abdominal obesity and insulin resistance.30
Measures of insulin resistance correlate significantly with the degree of intra-abdominal adiposity in humans.31,32 In a study where obese men matched for total adiposity but with low and high intra-abdominal adiposity were compared for glucose tolerance, it was found that plasma levels of glucose and insulin during an oral glucose tolerance test were similar in lean subjects and in obese subjects with low intra-abdominal adiposity.33 However, glucose tolerance was deteriorated only among subjects with elevated intra-abdominal adiposity, indicating the presence of insulin resistance and glucose intolerance. In another study conducted in older subjects (>60 years), it was also found that intra-abdominal adiposity was associated with multiple adverse changes to the lipid profile before and after adjustment for overall adiposity.34
Importantly, intra-abdominal adiposity appears to interact with other cardiometabolic risk factors to adversely influence overall cardiometabolic risk. An analysis from the Québec Health Survey stratified subjects for BMI and then for waist circumference and studied the relationships between indices of obesity, hyperinsulinaemia, and blood pressure in the resulting subgroups.12 Variations in waist circumference, rather than BMI, were found to explain the well-known relationships between obesity, insulin resistance, and hypertension. In another study, stratification of 569 men for FPG revealed no significant association between the presence of impaired fasting glucose (IFG) (FPG 6.1–6.9 mmol/L) and the presence of coronary artery disease (CAD) on coronary angiography in subjects without abdominal obesity.13 In contrast, subjects with IFG, abdominal obesity, and hypertriglyceridaemia were more than eight-fold more likely to have CAD than subjects without these risk factors. Thus, it appears important in clinical practice to consider additional risk factors, such as abdominal obesity and TG, when evaluating the importance of dysglycaemia as a cardiovascular risk factor.
It should be noted, however, that the relationship between abdominal obesity and insulin resistance is influenced by genetic factors. South Asians, for example, tend to display insulin resistance at all levels of abdominal obesity and these subjects will develop type 2 diabetes or coronary heart disease (CHD) at lower levels of obesity than other populations.35 In this regard, there is an urgent need for good descriptive imaging and metabolic data to verify whether such greater susceptibility to the comorbidities of abdominal obesity are explained by a greater accumulation of visceral adipose tissue or by a more substantial metabolic deterioration for a given level of intra-abdominal fat.
Direct modulation of cardiometabolic risk by intra-abdominal adiposity
Overview of adipokines
Excess intra-abdominal adiposity has the potential to influence metabolism and cardiometabolic risk directly, through alterations in the secretion of adipokines (Table 1). Abdominal obesity promotes increased secretion of a range of metabolites and of biologically active substances, including glycerol, free fatty acids (FFA), inflammatory mediators [e.g. tumour necrosis factor alpha (TNFα) and interleukin-6 (IL-6)], plasminogen activator inhibitor-1 (PAI-1), and C-reactive protein.14,36 The secretion of adiponectin, an apparently cardioprotective adipokine, has been shown to be reduced in abdominally obese patients.14,36
Acute exposure of skeletal muscle to elevated levels of FFA induces insulin resistance,37 whereas chronic exposure of the pancreas to elevated FFA impairs β-cell function.38 Observational evidence suggested a two-fold increase in the risk of ischaemic heart disease associated with elevated plasma FFA (top vs. lowest tertile) after correction for non-lipid risk factors, although further multivariate adjustment for lipid parameters and insulin weakened the association.39 The majority of circulating FFA originates from upper-body subcutaneous adipocytes, whereas intra-abdominal fat content has been positively correlated with splanchnic FFA levels which may contribute to the liver fat accumulation commonly found in abdominal obesity.40
Leptin is an adipokine involved in the regulation of satiety and energy intake.36 Levels of leptin in the plasma increase during the development of obesity and decline during weight loss. However, the plasma concentration of leptin is not determined primarily by the amount of visceral fat present and correlates more strongly with subcutaneous adiposity.41
Pro-inflammatory adipokines and atherogenesis
Atherosclerosis has been shown to have an inflammatory component,42 and pro-inflammatory adipokines may be important mediators of atherogenesis in abdominally obese subjects.43 Adipocytes, particularly visceral adipocytes, secrete numerous pro-inflammatory adipokines, such as TNFα and IL-6. TNFα is a paracrine mediator in adipocytes and appears to act locally to reduce the insulin sensitivity of adipocytes.36,44 This action would tend to exacerbate FFA release, inducing an atherogenic dyslipidaemia.29 TNFα also increases the secretion of other inflammatory mediators.36
IL-6 is a systemic adipokine, which not only impairs insulin sensitivity, but is also a major determinant of hepatic production of C-reactive protein,45 the most important source of this inflammatory marker. A study in 16 well-controlled type 2 diabetes patients showed that circulating levels of IL-6 correlate strongly with VFA, quantified by magnetic resonance imaging.45 In addition, the stiffness of the carotid artery, an index of atherosclerosis, correlated with both VFA and with levels of IL-6 and C-reactive protein in this study. However, the correlation with intra-abdominal adiposity was attenuated on multivariate analysis, when levels of the inflammatory markers were included, whereas the relationship between carotid stiffness and IL-6 remained strong. Thus, intra-abdominal adipocyte-derived IL-6 could be involved in the accelerated atherosclerosis of type 2 diabetic patients. The prognostic importance of IL-6 for cardiovascular outcomes was studied in a cohort of 1982 men, free of ischaemic heart disease at baseline and followed for 13 years, in the Québec Cardiovascular Study.46 An elevated IL-6 level (highest vs. lowest quartile) was associated with an increase of 70% in the risk of a first fatal ischaemic heart disease event or a non-fatal myocardial infarction (MI), after extensive multivariate adjustment for C-reactive protein and fibrinogen levels, together with standard cardiometabolic risk factors (age, BMI, systolic blood pressure, diabetes, smoking, medication, LDL-cholesterol, HDL-cholesterol, and TG).
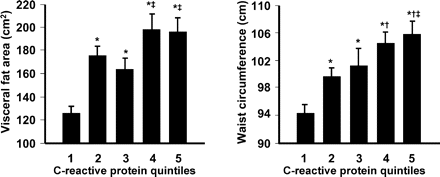
Circulating levels of C-reactive protein are elevated in subjects with abdominal obesity, and conversely, subjects with elevated C-reactive protein tend to have intra-abdominal adiposity (Figure 2).27 C-reactive protein was a significant predictor of adverse outcomes in the analysis from the Québec Cardiovascular Study, described above, although adjustment for other risk factors/markers markedly attenuated this relationship.46 Many other studies have also implicated elevated C-reactive protein concentrations as a marker of increased risk of CHD or stroke.47,48
PAI-1 is secreted from intra-abdominal adipocytes, although mainly from platelets and the vascular endothelium.36 This acute phase protein is elevated in inflammatory states49 and promotes a pro-coagulant state by inhibiting tissue plasminogen activator, thus increasing the risk of an intravascular thrombus.50 Plasma PAI-1 levels are increased in abdominally obese subjects51 and are predictive of adverse cardiovascular outcomes.50
Adiponectin: a cardioprotective adipokine
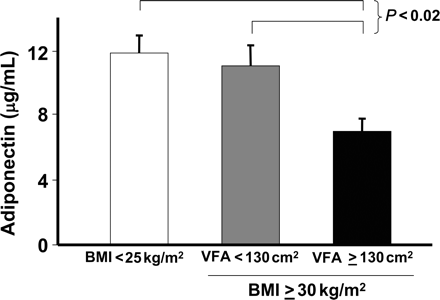
Plasma adiponectin levels have been shown to be inversely proportional to the severity of intra-abdominal adiposity.41,52 When a group of 196 men were stratified into lean and obese groups, and further stratified on the basis of their VFA assessed by CT, it was found that adiponectin levels were similar in the lean group and in the obese group with low VFA.52 However, obese subjects with high intra-abdominal adiposity were characterized by markedly reduced plasma adiponectin levels (Figure 3). These data confirm the dependence of adiponectin levels on intra-abdominal adiposity, rather than on obesity per se. Indeed, intra-abdominal adiposity was the only independent predictor of adiponectin levels in this study.
Adiponectin has been shown to have many favourable metabolic properties. For instance, it improves insulin sensitivity and glycaemic control,52,53 and levels of this adipokine correlate positively with levels of HDL-cholesterol and inversely with TG or PAI-1.52,54 The anti-atherogenic actions of adiponectin appears to be multifactorial, including inhibition of endothelial activation, reduced conversion of macrophages to foam cells, and inhibition of the smooth muscle proliferation and arterial remodelling that characterizes the development of the mature atherosclerotic plaque.55 Low adiponectin levels have been associated with adverse cardiovascular outcomes. For example, in a 6-year follow-up of subjects in the Physicians Health Study, the risk of MI increased as adiponectin levels decreased, with a doubling of adiponectin associated with a risk reduction of 20% after multivariate adjustment for lipids, HbA1C, C-reactive protein, age, smoking, month of blood draw, BMI, family history of MI, history of diabetes and hypertension, alcohol, and physical activity.56
Prognostic value of abdominal obesity beyond BMI
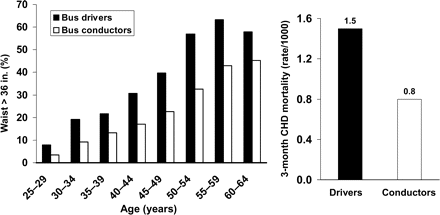
The strong relationships between abdominal obesity, insulin resistance, and cardiometabolic risk factors, described above, are suggestive of an important role for intra-abdominal adiposity in the pathogenesis of cardiovascular disease. A link between abdominal obesity and increased cardiometabolic risk was suggested almost six decades ago by Vague as well as in two elegant early epidemiological studies that investigated the links between occupational physical activity, adiposity, and outcomes. Specifically, bus drivers and bus conductors in London, UK, were studied. The drivers had an almost completely sedentary occupation, whereas conductors were more active, as they needed to walk around the upper and lower decks of buses to collect fares and issue tickets. A markedly higher incidence of early (3 months) mortality following a first CHD event had been observed among the sedentary drivers (Figure 4, right panel).57 A similar difference in this outcome was observed between sedentary telephone operators and more active postmen. A few years later, a study set out to investigate whether differences in the body shape between these groups, using available records of uniform sizes, might explain the difference in outcomes. This study demonstrated a clear difference in the waist circumference of drivers' uniform trousers, which was indicative of upper body obesity and suggestive of abdominal obesity (Figure 4, left panel).58
The five decades of clinical research undertaken since this pioneering study have confirmed the prognostic importance of abdominal obesity. An increased waist–hip ratio was found to account for ∼20% of the population-attributable risk of a first MI after adjustment for a range of other cardiometabolic risk factors in the INTERHEART study, a case–control study involving 29 972 subjects from 52 countries.3 Other studies have shown abdominal obesity to be a significant predictor of cardiovascular mortality,5,8 the development of CAD,9,13 type 2 diabetes,7,10,11 or the metabolic syndrome.6,9
Importantly, some of these studies have demonstrated the adverse prognosis associated with abdominal obesity, independently of BMI. BMI did not predict significantly the development of major coronary events in a retrospective cohort study in 756 patients undergoing coronary angiography after adjustment for standard cardiometabolic risk factors and abdominal obesity (Table 2).5 In contrast, high waist circumference significantly predicted the development of major coronary events after adjustment for the same standard cardiometabolic risk factors and BMI (Table 2). Similarly, an analysis from a large cohort of 44 702 women enrolled in the Nurses Health Study, aged 40–65 and free of CHD at baseline, stratified subjects into tertiles for BMI and waist circumference.7 The age-adjusted risk of CHD during 8 years of follow-up increased with increasing waist circumference for each tertile of BMI. The age-adjusted incidence of CHD was similar between subjects with the lowest BMI and highest waist circumference (83/100 000 person-years) and subjects with the highest BMI and lowest waist circumference (77/100 000 person-years). Subjects with both high BMI and high waist circumference had the highest cardiovascular risk, with an age-adjusted CHD incidence of 128/100 000 patient-years. There is no doubt that measurement of waist circumference adds clinically significant prognostic information to BMI measurement relating to the risk of developing cardiovascular disease.
Implications for therapy
Identifying high-risk patients for interventions
The evidence reviewed above shows that abdominal obesity is closely involved in the development of multiple cardiometabolic risk factors, including those associated with the metabolic syndrome. The large and growing abdominally obese population includes a substantial number of patients who are at increased risk of adverse cardiometabolic outcomes. In this regard, the NCEP-ATP III guidelines emphasized that the most prevalent form of the metabolic syndrome that physicians will encounter is associated with abdominal obesity.43 In relying on the classical risk factors that define the metabolic syndrome, however, we run the risk of missing important information captured by the presence of other potentially important cardiometabolic risk factors. For example, a triad of non-traditional cardiometabolic risk factors, elevated apolipoprotein B (ApoB), fasting hyperinsulinaemia, and small, dense LDL conferred a five-fold elevation in the risk of developing ischaemic heart disease, after adjustment for other lipid parameters, compared with subjects with not more than one of these risk factors, in a 5-year prospective case–control analysis of the Québec Cardiovascular Study.59
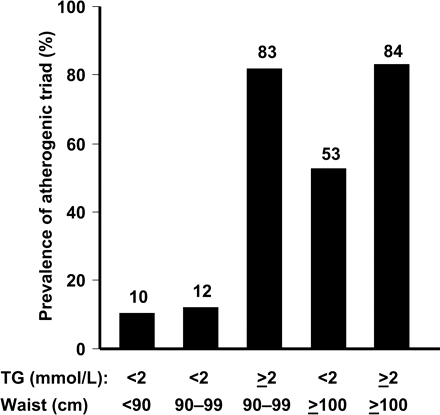
However, the above elements of the atherogenic triad are not measured in routine clinical practice, and a more practicable means of identifying this high-risk subgroup is required. Accordingly, we have identified the ‘hypertriglyceridaemic waist’, a combination of high waist circumference and hypertriglyceridaemia, a straightforward and useful means of identifying abdominally obese patients with excess visceral fat and with the atherogenic triad. The utility of hypertriglyceridaemic waist was determined in a study in 185 men without symptoms of cardiovascular disease stratified for different values of TG and waist circumference.60 More than 80% subjects with modest hypertriglyceridaemia (≥2 mmol/L) together with high waist circumference (≥90 or ≥100 cm) were found to have the atherogenic triad (Figure 5). In contrast, a waist girth below 90 cm combined with TG concentrations below 2.0 mmol/L was associated with a low probability (∼10%) of being characterized by visceral obesity and related metabolic abnormalities. Thus, measurement of waist circumference and TG, two simple clinical measurements suitable for routine clinical use, clearly identifies a high proportion of a subgroup of individuals at markedly elevated cardiometabolic risk.
Interventions to manage cardiometabolic risk in abdominal obesity
When managing the prevalent form of the metabolic syndrome, NCEP-ATP III recommend to treat abdominal obesity and its associated insulin resistance first, as these are root causes of the overall elevation of cardiometabolic risk. Current management guidelines support the use of lifestyle interventions (diet and exercise), as this strategy has the potential to improve all cardiometabolic risk factors.17 Where necessary, the individual complications of abdominal obesity and insulin resistance, such as atherogenic dyslipidaemia, hypertension, a pro-coagulant state, or inflammation, can then be managed. However, lifestyle modifications are often unsuccessful, due in part to insufficient patient compliance with these regimens to induce long-term weight loss and maintenance. Under such circumstances, pharmacotherapy can be justified to manage elevated cardiometabolic risk.
Recent research has identified overactivity of the endocannabinoid system, acting via the CB1 receptor, as an important factor in the pathogenesis of cardiometabolic risk.61 Rimonabant is the first selective CB1 blocker to undergo clinical evaluation and has been extensively evaluated in patients with obesity and associated risk factors within the Rimonabant In Obesity (RIO) trial programme. Table 3 shows the effects of rimonabant on key cardiometabolic risk factors in two of these trials, RIO-Europe62 and RIO-Lipids.63 RIO-Europe recruited a population of obese (BMI≥30 kg/m2) or overweight (BMI>27 kg/m2) patients with at least one cardiovascular comorbidity, whereas RIO-Lipids recruited obese (BMI≥30 kg/m2) or overweight (BMI>27 kg/m2) patients with untreated atherogenic dyslipidaemia, defined by hypertriglyceridaemia or an elevated total cholesterol:HDL-cholesterol ratio. These double blind, placebo controlled, randomized trials evaluated rimonabant 5 and 20 mg once daily in addition to a mild hypocaloric diet. Treatment with rimonabant 20 mg for 1 year resulted in marked and significant improvements relative to placebo in a number of cardiometabolic risk factors, including waist circumference, body weight, HDL-cholesterol, TG, and blood pressure. Importantly, statistical analysis showed that about half of the improvements in HDL-cholesterol and triglyceride levels were independent of weight loss, consistent with a direct action of rimonabant on cardiometabolic risk. In RIO-Europe, indices of glycaemic control and insulin sensitivity also improved during treatment with rimonabant, and there was a 46% increase in plasma adiponectin vs. placebo with rimonabant 20 mg in RIO-Lipids. Rimonabant 5 mg results were either similar to those of placebo or intermediate to those of placebo and rimonabant 20 mg. Rimonabant was generally well tolerated.
Conclusions
A growing database of clinical evidence implicates intra-abdominal adiposity as a powerful driving force for elevated cardiometabolic risk. This association appears to arise directly, via secretion of adipokines, and indirectly, through promotion of insulin resistance. Addressing intra-abdominal adiposity should play a central role in future strategies aimed at improving cardiovascular outcomes in patients with abdominal obesity and its associated cardiometabolic risk factors.
Conflict of interest: J.-P.D. has received consulting or lecture fees from Abbott Laboratories, AstraZeneca, Fournier Pharma, GlaxoSmithKline, Merck, Pfizer, Pharmacia, and sanofi-aventis and grant support from Fournier Pharma, GlaxoSmithKline, Merck, Pfizer, and sanofi-aventis. J.-P.D. is Scientific Director of the International Chair on Cardiometabolic Risk which is supported by an unrestricted grant awarded to Université Laval by sanofi-aventis.
CT scans from two subjects with comparable BMI illustrating adiposity phenotypes characterized mainly by intra-abdominal adiposity (top panels) and subcutaneous adiposity (bottom panels). Subcutaneous fat is shown in black under the skin, and visceral fat area (VFA) in white. Scans were made at the L4-L5 level. Reproduced with permission from Tchernof A, Després JP. Obesity and lipoprotein metabolism. In: Kopelman PG, ed. Clinical Obesity, UK: Blackwell Science Ltd; 1998. p176–204.
Overview of key adipokines
| Adipokine . | Key properties . | Secretion in abdominal obesity . |
|---|---|---|
. | ||
| Adiponectin | Anti-atherogenic, reduces risk of developing diabetes | ↓ |
| ↓Differentiation of macrophages into foam cells | ||
| ↓Atherogenic vascular remodelling | ||
| ↓Hepatic glucose output | ||
| ↑Insulin sensitivity | ||
| IL-6 | Promotes inflammation, pro-atherogenic, promotes diabetes | ↑ |
| ↑Vascular inflammation | ||
| ↑Hepatic C-reactive protein production | ||
| ↓Insulin signalling | ||
| TNFα | Pro-atherogenic/pro-diabetic | ↑ |
| Paracrine role in the adipocyte | ||
| ↓Insulin signalling | ||
| ↑Secretion of other pro-inflammatory mediators | ||
| C-reactive protein | Promotes inflammation, pro-atherogenic | ↑ |
| Marker of chronic low-grade inflammation | ||
| Predicts adverse cardiovascular outcomes | ||
| PAI-1 | Pro-atherogenic, pro-coagulant | ↑ |
| ↑Atherothrombotic risk | ||
| Resistin | Exacerbates insulin resistance | ↑ |
| ↓Insulin signalling | ||
| ↓Endothelial function | ||
| ↑Vascular smooth muscle proliferation | ||
| Adipokine . | Key properties . | Secretion in abdominal obesity . |
|---|---|---|
. | ||
| Adiponectin | Anti-atherogenic, reduces risk of developing diabetes | ↓ |
| ↓Differentiation of macrophages into foam cells | ||
| ↓Atherogenic vascular remodelling | ||
| ↓Hepatic glucose output | ||
| ↑Insulin sensitivity | ||
| IL-6 | Promotes inflammation, pro-atherogenic, promotes diabetes | ↑ |
| ↑Vascular inflammation | ||
| ↑Hepatic C-reactive protein production | ||
| ↓Insulin signalling | ||
| TNFα | Pro-atherogenic/pro-diabetic | ↑ |
| Paracrine role in the adipocyte | ||
| ↓Insulin signalling | ||
| ↑Secretion of other pro-inflammatory mediators | ||
| C-reactive protein | Promotes inflammation, pro-atherogenic | ↑ |
| Marker of chronic low-grade inflammation | ||
| Predicts adverse cardiovascular outcomes | ||
| PAI-1 | Pro-atherogenic, pro-coagulant | ↑ |
| ↑Atherothrombotic risk | ||
| Resistin | Exacerbates insulin resistance | ↑ |
| ↓Insulin signalling | ||
| ↓Endothelial function | ||
| ↑Vascular smooth muscle proliferation | ||
See text for explanation and references.
Overview of key adipokines
| Adipokine . | Key properties . | Secretion in abdominal obesity . |
|---|---|---|
. | ||
| Adiponectin | Anti-atherogenic, reduces risk of developing diabetes | ↓ |
| ↓Differentiation of macrophages into foam cells | ||
| ↓Atherogenic vascular remodelling | ||
| ↓Hepatic glucose output | ||
| ↑Insulin sensitivity | ||
| IL-6 | Promotes inflammation, pro-atherogenic, promotes diabetes | ↑ |
| ↑Vascular inflammation | ||
| ↑Hepatic C-reactive protein production | ||
| ↓Insulin signalling | ||
| TNFα | Pro-atherogenic/pro-diabetic | ↑ |
| Paracrine role in the adipocyte | ||
| ↓Insulin signalling | ||
| ↑Secretion of other pro-inflammatory mediators | ||
| C-reactive protein | Promotes inflammation, pro-atherogenic | ↑ |
| Marker of chronic low-grade inflammation | ||
| Predicts adverse cardiovascular outcomes | ||
| PAI-1 | Pro-atherogenic, pro-coagulant | ↑ |
| ↑Atherothrombotic risk | ||
| Resistin | Exacerbates insulin resistance | ↑ |
| ↓Insulin signalling | ||
| ↓Endothelial function | ||
| ↑Vascular smooth muscle proliferation | ||
| Adipokine . | Key properties . | Secretion in abdominal obesity . |
|---|---|---|
. | ||
| Adiponectin | Anti-atherogenic, reduces risk of developing diabetes | ↓ |
| ↓Differentiation of macrophages into foam cells | ||
| ↓Atherogenic vascular remodelling | ||
| ↓Hepatic glucose output | ||
| ↑Insulin sensitivity | ||
| IL-6 | Promotes inflammation, pro-atherogenic, promotes diabetes | ↑ |
| ↑Vascular inflammation | ||
| ↑Hepatic C-reactive protein production | ||
| ↓Insulin signalling | ||
| TNFα | Pro-atherogenic/pro-diabetic | ↑ |
| Paracrine role in the adipocyte | ||
| ↓Insulin signalling | ||
| ↑Secretion of other pro-inflammatory mediators | ||
| C-reactive protein | Promotes inflammation, pro-atherogenic | ↑ |
| Marker of chronic low-grade inflammation | ||
| Predicts adverse cardiovascular outcomes | ||
| PAI-1 | Pro-atherogenic, pro-coagulant | ↑ |
| ↑Atherothrombotic risk | ||
| Resistin | Exacerbates insulin resistance | ↑ |
| ↓Insulin signalling | ||
| ↓Endothelial function | ||
| ↑Vascular smooth muscle proliferation | ||
See text for explanation and references.
Association of intra-abdominal adiposity (VFA on CT scans) with elevated C-reactive protein. Significance of results: P<0.0001 vs. (asterisk) quintile 1; (dagger) quintile 2; (double dagger) quintile 3. Reproduced with permission from Lemieux et al.27
Plasma adiponectin levels in healthy non-obese controls and in obese men with either low or high levels of visceral fat area (VFA). Data are from a study of 39 non-obese men and two groups of 15 obese men stratified for VFA measured using CT scanning. Reproduced with permission from Cote et al.52 Copyright 2005, The Endocrine Society.
Associations between occupational physical activity, obesity, and mortality in the 3 months following a first CHD event in transport workers in London, UK. Proportions with waist >36 in. were adjusted for subjects' height; 36 in. is equivalent to 91.4 cm. Between 58 and 214 men were studied for each age group in either occupation. Mortality data are standardized mortality rates for individuals aged 35–64 for years 1949–52. Drawn from data presented by Morris et al.57 and Heady et al.58
Prognostic value of high waist circumference beyond BMI: data from an analysis of 756 patients undergoing coronary angiography.5
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | Odds ratio (95% CI) . | P-value . | Odds ratio (95% CI) . | P-value . |
. | ||||
| Vascular mortality | ||||
| BMI | 1.04 (0.62–1.73) | 0.886 | 0.35 (0.10–1.20) | 0.095 |
| Waist circumference | 2.31 (1.16–4.60) | 0.017 | 8.71 (1.78–42.68) | 0.008 |
| Major coronary events | ||||
| BMI | 0.99 (0.63–1.57) | 0.965 | 0.47 (0.18–1.24) | 0.128 |
| Waist circumference | 2.05 (1.06–3.94) | 0.032 | 4.55 (1.12–18.48) | 0.034 |
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | Odds ratio (95% CI) . | P-value . | Odds ratio (95% CI) . | P-value . |
. | ||||
| Vascular mortality | ||||
| BMI | 1.04 (0.62–1.73) | 0.886 | 0.35 (0.10–1.20) | 0.095 |
| Waist circumference | 2.31 (1.16–4.60) | 0.017 | 8.71 (1.78–42.68) | 0.008 |
| Major coronary events | ||||
| BMI | 0.99 (0.63–1.57) | 0.965 | 0.47 (0.18–1.24) | 0.128 |
| Waist circumference | 2.05 (1.06–3.94) | 0.032 | 4.55 (1.12–18.48) | 0.034 |
For BMI, data shown are standardized odds ratios adjusted for age, gender, smoking and total cholesterol. Similar adjustments were made for waist circumference including adjustment for BMI. Major coronary events were defined as fatal/non-fatal MI, sudden cardiac death, or mortality from congestive heart failure of ischaemic aetiology.
Prognostic value of high waist circumference beyond BMI: data from an analysis of 756 patients undergoing coronary angiography.5
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | Odds ratio (95% CI) . | P-value . | Odds ratio (95% CI) . | P-value . |
. | ||||
| Vascular mortality | ||||
| BMI | 1.04 (0.62–1.73) | 0.886 | 0.35 (0.10–1.20) | 0.095 |
| Waist circumference | 2.31 (1.16–4.60) | 0.017 | 8.71 (1.78–42.68) | 0.008 |
| Major coronary events | ||||
| BMI | 0.99 (0.63–1.57) | 0.965 | 0.47 (0.18–1.24) | 0.128 |
| Waist circumference | 2.05 (1.06–3.94) | 0.032 | 4.55 (1.12–18.48) | 0.034 |
| . | Men . | Women . | ||
|---|---|---|---|---|
| . | Odds ratio (95% CI) . | P-value . | Odds ratio (95% CI) . | P-value . |
. | ||||
| Vascular mortality | ||||
| BMI | 1.04 (0.62–1.73) | 0.886 | 0.35 (0.10–1.20) | 0.095 |
| Waist circumference | 2.31 (1.16–4.60) | 0.017 | 8.71 (1.78–42.68) | 0.008 |
| Major coronary events | ||||
| BMI | 0.99 (0.63–1.57) | 0.965 | 0.47 (0.18–1.24) | 0.128 |
| Waist circumference | 2.05 (1.06–3.94) | 0.032 | 4.55 (1.12–18.48) | 0.034 |
For BMI, data shown are standardized odds ratios adjusted for age, gender, smoking and total cholesterol. Similar adjustments were made for waist circumference including adjustment for BMI. Major coronary events were defined as fatal/non-fatal MI, sudden cardiac death, or mortality from congestive heart failure of ischaemic aetiology.
Prevalence of an atherogenic triad of elevated ApoB, fasting hyperinsulinaemia, and small, dense LDL according to the TG and waist circumference. Reproduced with permission from Lemieux et al.60
| . | RIO-Europe . | RIO-Lipids . |
|---|---|---|
. | ||
| Waist circumference (cm) | −4.2a | −4.7a |
| Body weight (kg) | −4.7a | −5.4a |
| HDL-cholesterol (%) | +8.9a | +8.1a |
| Triglycerides (%) | −15.1a | −12.4a |
| Systolic BP (mmHg) | −1.2 | −1.8b |
| Diastolic BP (mmHg) | −1.0 | −1.5b |
| . | RIO-Europe . | RIO-Lipids . |
|---|---|---|
. | ||
| Waist circumference (cm) | −4.2a | −4.7a |
| Body weight (kg) | −4.7a | −5.4a |
| HDL-cholesterol (%) | +8.9a | +8.1a |
| Triglycerides (%) | −15.1a | −12.4a |
| Systolic BP (mmHg) | −1.2 | −1.8b |
| Diastolic BP (mmHg) | −1.0 | −1.5b |
Data are least-squares mean treatment differences or differences between mean changes on placebo and rimonabant 20 mg from the intent-to-treat population of the trials. Patients were randomized to receive placebo, rimonabant 5 mg (data not shown) or rimonabant 20 mg for 1 year in addition to a mild hypocaloric diet (−600 kcal/day).
aSignificance vs. placebo, P<0.001.
bSignificance vs. placebo, P<0.05.
| . | RIO-Europe . | RIO-Lipids . |
|---|---|---|
. | ||
| Waist circumference (cm) | −4.2a | −4.7a |
| Body weight (kg) | −4.7a | −5.4a |
| HDL-cholesterol (%) | +8.9a | +8.1a |
| Triglycerides (%) | −15.1a | −12.4a |
| Systolic BP (mmHg) | −1.2 | −1.8b |
| Diastolic BP (mmHg) | −1.0 | −1.5b |
| . | RIO-Europe . | RIO-Lipids . |
|---|---|---|
. | ||
| Waist circumference (cm) | −4.2a | −4.7a |
| Body weight (kg) | −4.7a | −5.4a |
| HDL-cholesterol (%) | +8.9a | +8.1a |
| Triglycerides (%) | −15.1a | −12.4a |
| Systolic BP (mmHg) | −1.2 | −1.8b |
| Diastolic BP (mmHg) | −1.0 | −1.5b |
Data are least-squares mean treatment differences or differences between mean changes on placebo and rimonabant 20 mg from the intent-to-treat population of the trials. Patients were randomized to receive placebo, rimonabant 5 mg (data not shown) or rimonabant 20 mg for 1 year in addition to a mild hypocaloric diet (−600 kcal/day).
aSignificance vs. placebo, P<0.001.
bSignificance vs. placebo, P<0.05.
References
Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm.
Sonmez K, Akcakoyun M, Akcay A, Demir D, Duran NE, Gencbay M, Degertekin M, Turan F. Which method should be used to determine the obesity, in patients with coronary artery disease? (body mass index, waist circumference or waist-hip ratio).
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Razak F, Sharma AM, Anand SS; INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study.
Hoefle G, Saely CH, Aczel S, Benzer W, Marte T, Langer P, Drexel H. Impact of total and central obesity on vascular mortality in patients undergoing coronary angiography.
Han TS, Williams K, Sattar N, Hunt KJ, Lean MEJ, Haffner SM. Analysis of obesity and hyperinsulinemia in the development of metabolic syndrome: San Antonio Heart Study.
Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women.
Empana JP, Ducimetiere P, Charles MA, Jouven X. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men: the Paris Prospective Study I.
Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases.
Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men.
Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study.
Poirier P, Lemieux I, Mauriege P, Dewailly E, Blanchet C, Bergeron J, Despres JP. Impact of waist circumference on the relationship between blood pressure and insulin: the Quebec Health Survey.
St-Pierre J, Lemieux I, Vohl MC, Perron P, Tremblay G, Despres JP, Gaudet D. Contribution of abdominal obesity and hypertriglyceridemia to impaired fasting glucose and coronary artery disease.
Kobayashi K. Adipokines: therapeutic targets for metabolic syndrome.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome.
Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III).
Liese AD, Döring A, Hense HW, Keil U. Five year changes in waist circumference, body mass index and obesity in Augsburg, Germany
Sobngwi E, Mbanya JC, Unwin NC, Porcher R, Kengne AP, Fezeu L, Minkoulou EM, Tournoux C, Gautier JF, Aspray TJ, Alberti K. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon.
Gao M, Ikeda K, Hattori H, Miura A, Nara Y, Yamori Y. Cardiovascular risk factors emerging in Chinese populations undergoing urbanization.
Ramachandran A, Snehalatha C, Latha E, Manoharan M, Vijay V. Impacts of urbanisation on the lifestyle and on the prevalence of diabetes in native Asian Indian population.
Taufa T, Benjamin AL. Diabetes: the by-product of westernization in Papua New Guinea.
Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition.
Despres JP. Our passive lifestyle, our toxic diet, and the atherogenic/diabetogenic metabolic syndrome: can we afford to be sedentary and unfit?
Nieuwdorp M, Stroes ES, Meijers JC, Buller H. Hypercoagulability in the metabolic syndrome.
Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome.
Lemieux I, Pascot A, Prud'homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity.
Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes.
Pascot A, Lemieux I, Prud'homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Despres JP. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity.
Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men.
Faria AN, Ribeiro Filho FF, Gouveia Ferreira SR, Zanella MT. Impact of visceral fat on blood pressure and insulin sensitivity in hypertensive obese women.
Pouliot MC, Despres JP, Nadeau A et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels.
Weltman A, Despres JP, Clasey JL, Weltman JY, Wideman L, Kanaley J, Patrie J, Bergeron J, Thorner MO, Bouchard C, Hartman ML. Impact of abdominal visceral fat, growth hormone, fitness, and insulin on lipids and lipoproteins in older adults.
Bajaj M, Banerji MA. Type 2 diabetes in South Asians: a pathophysiologic focus on the Asian-Indian epidemic.
Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis.
Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects.
Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships.
Pirro M, Mauriege P, Tchernof A, Cantin B, Dagenais GR, Despres JP, Lamarche B. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Quebec Cardiovascular Study.
Miles JM, Jensen MD. Counterpoint: visceral adiposity is not causally related to insulin resistance.
Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution.
Despres JP. Inflammation and cardiovascular disease: is abdominal obesity the missing link?
Skolnik EY, Marcusohn J. Inhibition of insulin receptor signaling by TNF: potential role in obesity and non-insulin-dependent diabetes mellitus.
Diamant M, Lamb HJ, van de Ree MA, Endert EL, Groeneveld Y, Bots ML, Kostense PJ, Radder JK. The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes.
St-Pierre AC, Cantin B, Bergeron J, Pirro M, Dagenais GR, Despres JP, Lamarche B. Inflammatory markers and long-term risk of ischemic heart disease in men A 13-year follow-up of the Quebec Cardiovascular Study.
Torres JL, Ridker PM. High sensitivity C-reactive protein in clinical practice.
Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis.
Alessi MC, Juhan-Vague I. Contribution of PAI-1 in cardiovascular pathology.
Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease.
Cigolini M, Targher G, Bergamo Andreis IA, Tonoli M, Agostino G, De Sandre G. Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men.
Cote M, Mauriege P, Bergeron J, Almeras N, Tremblay A, Lemieux I, Despres JP. Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein-lipid levels in men.
Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function.
Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes.
Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome.
Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men.
Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work.
Heady JA, Morris JN, Raffle PA. Physique of London busmen; epidemiology of uniforms.
Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men: prospective results from the Québec Cardiovascular Study.
Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Despres JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men?
Pagotto U, Vicennati V, Pasquali R. The endocannabinoid system and the treatment of obesity.
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study.