-
PDF
- Split View
-
Views
-
Cite
Cite
Massimo Stefano Silvetti, Fabrizio Drago, Giorgia Grutter, Antonella De Santis, Vincenzo Di Ciommo, Lucilla Ravà, Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients, EP Europace, Volume 8, Issue 7, July 2006, Pages 530–536, https://doi.org/10.1093/europace/eul062
Close - Share Icon Share
Abstract
Aims The aim of this study was to evaluate long-term outcome of pacemakers (PMs) in paediatric patients.
Methods and results Patients' data were retrospectively reviewed. We recorded the techniques and systems used, any complication, and outcome. Endocardial leads were inserted by transcutaneous puncture of subclavian vein and fixed with a non-absorbable ligature, and epicardial leads by standard surgical technique. Lead survival was calculated and plotted with the product limit method of Kaplan–Meier. Between 1982 and 2002, 292 patients, aged 8±7 years (range 1 day–18 years), underwent PM implantation: the first PM had endocardial leads in 165 patients and epicardial in 127 patients. Structural heart disease (HD) was present in 239 patients. Follow-up was 5±4 (range 0.1–18) years. There were no pacing-related deaths. In total, 211 endocardial implantation procedures with 90 atrial and 165 ventricular leads and 145 epicardial procedures with 103 atrial and 123 ventricular leads were performed. Early (<3 months) complications: haemothorax occurred in 3.5% of endocardial leads and dislodgement was not significantly different for atrial and ventricular endocardial leads. Late complications: 63 leads failed (48 epicardial), with the worst outcome for conventional epicardial leads (31 vs. 9% endocardial, P<0.05; steroid eluting 8% epicardial vs. 5% endocardial, P=NS). Endocardial atrial leads failed (7%) in operated HD and ventricular leads failed (6%) after body growth, without difference in estimated mean survival time (11 years). Early and late PM infection/erosion was ∼2% in all patients.
Conclusion Pacing in children shows good results, but complications are frequent and related to leads. Endocardial pacing showed better long-term outcome.
Introduction
Permanent cardiac pacing in paediatric patients is performed in few Paediatric Cardiology and Heart Surgery centres, and with better results of surgical repair of congenital heart disease (CHD), the number of children who require a permanent pacemaker (PM) is increasing. Studies in children with PM usually have a small sample size. Large and recent studies are rare.1,2 Cardiac pacing in children is safe and reliable, but concerns have been raised about the long-term outcome. The aim of this study was to report our 20-year experience of permanent pacing in a large paediatric cohort.
Methods
Between October 1982 and March 2002, 292 patients underwent PM implantation and have been followed-up at the Cardiac Arrhythmia Service of Cardiology and Heart Surgery Department of Bambino Gesù Paediatric Hospital. We have retrospectively analysed all data of these patients. We recorded the first PM implantation, either with endocardial pacing or with epicardial pacing. We also defined PM revisions when new PM and lead(s) were implanted, such as upgrading of single-chamber to dual-chamber PM, positioning of a new lead in the case of lead removal or abandonment, and changing an epicardial to an endocardial pacing system (and vice versa). A replacement procedure was identified when only the PM generator was replaced, without inserting a lead. Complications of permanent pacing were divided into early (occurring in the first 3 months after the procedure) and late complications. The complications were defined according to a recent study.3
Implantation technique
Endocardial or epicardial pacing was chosen according to the characteristics of the patient: in the presence of intracardiac right-to-left shunt or single ventricle physiology, concomitant heart surgery, lack of venous access to the heart chambers, epicardial pacing was always used, and it was preferred in children of small size.1 The choice of endocardial or epicardial pacing was also related to the year in which the procedure was performed, according to the experience of the operators and to advances in generator and lead technology: epicardial pacing was generally performed in the first decade of our experience, then, since 1990, endocardial pacing was increasingly used, even in newborns and infants. With the availability of steroid-eluting leads, there was a revival in the use of epicardial pacing in children <10–15 kg.
The majority of procedures was performed under general anaesthesia. Antibiotic prophylaxis was routinely given perioperatively to all patients, according to the hospital protocols that changed over these years: kefamandol, kefamezine, keftazidime, and amoxicillin plus clavulanic acid (still in use) were consecutively used. Patients were kept on bed rest for 24–48 h. After discharge, patients were followed-up at 1 month, 3 months, and then every 6 months or as needed.
Endocardial pacing
The endocardial leads were inserted by transcutaneous puncture of the left or right (rarely) subclavian vein. Atrial leads were placed in the right atrial appendage or appendage remnant in patients with prior cardiac surgery and in the left atrial roof in patients with transposition of the great arteries (TGA) {S, D, D} after Mustard palliation. Since 2000, when neither the right atrial appendage nor the appendage remnant was adequate, an active fixation lead was screwed into the right atrial free wall. Ventricular leads were placed in the right ventricular apex, in the left ventricular apex in patients with congenitally corrected TGA {S, L, L}, and in patients with TGA {S, D, D} after Mustard palliation. Leads were fixed to the subcutaneous tissues with slow absorbing ligature.4 Passive (tined) and, since 2000, also active fixation (screw-in) leads were used. Unipolar leads were usually used in ventricular pacing, and bipolar leads in atrial pacing. Implantation of dual-chamber PM with endocardial leads was generally performed in adolescents or in children with a body weight >25–30 kg. After lead positioning, the PM pocket was created in pre-pectoral area.
Epicardial pacing
In epicardial pacing, the PM generator was placed in the abdominal wall in a subcutaneous or in a submuscular (generally in neonates and infants) pocket. The leads, usually unipolar, were inserted by the standard surgical techniques through sternotomy, thoracotomy, or a subxiphoid approach, based on anatomical or operative characteristics.1,5 The placement of right atrial and ventricular epicardial leads was the standard approach; leads were rarely positioned in left atrium.1,5,6
Statistical analysis
Statistical analysis was carried out using the Stata package, version 8.0 [StataCorp. (2003) Stata Statistical Software: Release 8.0. College Station, Stata Corporation, TX, USA]. Proportions or, when appropriate, means or medians together with standard deviation and range were computed. Pacing thresholds at implantation were compared between endocardial pacing and epicardial pacing by means of Student's t-test. A P-value less than 0.05 was considered significant.
The Kaplan–Meier method and log-rank test were used to study the longevity of the leads. Failures included lead fracture and lead malfunction because of pacing or sensing defects. Cases of lead removal because of extraneous causes (e.g. new cardiac surgery), infections, cardiac transplantation, and patient death unrelated to pacing were considered as ‘censored’ or lost to follow-up. A Cox multivariate proportional hazard model was used to explore factors associated with the longevity of the lead. Variables included in the model as predictors were lead type and positioning (coded as conventional endocardial, steroid-eluting endocardial, conventional epicardial, and steroid-eluting epicardial), site (atrial vs. ventricular), patient age and gender, number of procedures, and, as a continuous variable, year of implantation.
Results (Table 1)
There were 175 (60%) boys and 117 girls. Age and weight at PM implantation were 7.0±6.5 years (1 day–18 years), median 7 years and 16±19 kg (1.5–88 kg), median 9 kg. About one-third of patients was <2 years of age at first implantation and a quarter was between 2 and 6.
Patient characteristics
| . | Number (%) . |
|---|---|
| Sex | |
| Male | 175 (60%) |
| Female | 117 (40%) |
| Arrhythmias | |
| Congenital/acquired AVB | 126 (43%) |
| Post-operative AVB | 64 (22%) |
| Sinus node dysfunction | 89 (31%) |
| HOCM | 9 (3%) |
| DCM | 4 (1%) |
| Heart disease | |
| TGA {S, D, D} post-Mustard operation | 44 (15%) |
| Univentricular heart post-Fontan operation | 42 (14%) |
| Ventricular septal defect | 35 (12%) |
| Atrioventricular septal defect | 26 (9%) |
| Cardiomyopathy | 25 (9%) |
| Tetralogy of Fallot | 23 (8%) |
| Congenitally corrected TGA {S, L, L} | 14 (5%) |
| Other HD | 17 (6%) |
| No structural HD | 66 (22%) |
| . | Number (%) . |
|---|---|
| Sex | |
| Male | 175 (60%) |
| Female | 117 (40%) |
| Arrhythmias | |
| Congenital/acquired AVB | 126 (43%) |
| Post-operative AVB | 64 (22%) |
| Sinus node dysfunction | 89 (31%) |
| HOCM | 9 (3%) |
| DCM | 4 (1%) |
| Heart disease | |
| TGA {S, D, D} post-Mustard operation | 44 (15%) |
| Univentricular heart post-Fontan operation | 42 (14%) |
| Ventricular septal defect | 35 (12%) |
| Atrioventricular septal defect | 26 (9%) |
| Cardiomyopathy | 25 (9%) |
| Tetralogy of Fallot | 23 (8%) |
| Congenitally corrected TGA {S, L, L} | 14 (5%) |
| Other HD | 17 (6%) |
| No structural HD | 66 (22%) |
DCM, dilated cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; TGA, transposition of great arteries; HD, heart disease; AVB, atrioventricular block.
Patient characteristics
| . | Number (%) . |
|---|---|
| Sex | |
| Male | 175 (60%) |
| Female | 117 (40%) |
| Arrhythmias | |
| Congenital/acquired AVB | 126 (43%) |
| Post-operative AVB | 64 (22%) |
| Sinus node dysfunction | 89 (31%) |
| HOCM | 9 (3%) |
| DCM | 4 (1%) |
| Heart disease | |
| TGA {S, D, D} post-Mustard operation | 44 (15%) |
| Univentricular heart post-Fontan operation | 42 (14%) |
| Ventricular septal defect | 35 (12%) |
| Atrioventricular septal defect | 26 (9%) |
| Cardiomyopathy | 25 (9%) |
| Tetralogy of Fallot | 23 (8%) |
| Congenitally corrected TGA {S, L, L} | 14 (5%) |
| Other HD | 17 (6%) |
| No structural HD | 66 (22%) |
| . | Number (%) . |
|---|---|
| Sex | |
| Male | 175 (60%) |
| Female | 117 (40%) |
| Arrhythmias | |
| Congenital/acquired AVB | 126 (43%) |
| Post-operative AVB | 64 (22%) |
| Sinus node dysfunction | 89 (31%) |
| HOCM | 9 (3%) |
| DCM | 4 (1%) |
| Heart disease | |
| TGA {S, D, D} post-Mustard operation | 44 (15%) |
| Univentricular heart post-Fontan operation | 42 (14%) |
| Ventricular septal defect | 35 (12%) |
| Atrioventricular septal defect | 26 (9%) |
| Cardiomyopathy | 25 (9%) |
| Tetralogy of Fallot | 23 (8%) |
| Congenitally corrected TGA {S, L, L} | 14 (5%) |
| Other HD | 17 (6%) |
| No structural HD | 66 (22%) |
DCM, dilated cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; TGA, transposition of great arteries; HD, heart disease; AVB, atrioventricular block.
Arrhythmias (Table 1)
The indications for pacing followed international7,8 and national9 guidelines. In the group of congenital or acquired atrioventricular block (AVB), there were 16 patients with paroxysmal AVB; 34 of 89 patients with sinus node dysfunction (SND) had the bradycardia–tachycardia syndrome.
Heart disease (Table 1)
Congenital or acquired HD was present in 226 patients, whereas 66 patients (23%) had no structural HD.
Pacemaker implanted (Table 2)
The first pacing system was implanted with endocardial leads in 165 patients and with epicardial leads in 127 patients. A total of 515 PMs was implanted, 259 with endocardial pacing and 256 with epicardial pacing, the number of procedures per patient was 2±1 (range 1–6).
Implantable generators used (n=515 PMs)
| Manufacturer . | Model . | Number . |
|---|---|---|
| Medtronic | Kappa SR 701 | 103 |
| Kappa DR 701 | 60 | |
| Kappa VDD 701 | 4 | |
| Thera SR 8940/8960 | 77 | |
| Thera DR 7940/7960 | 50 | |
| Legend 8416/8417 | 22 | |
| Legend II 8424 | 13 | |
| Minuet 7108 | 13 | |
| Microminix 8360 | 4 | |
| Minix 8340 | 3 | |
| AT 501 | 6 | |
| Elite 7077 | 1 | |
| Elite II 7086 | 2 | |
| Intermedics | Dash | 22 |
| Cosmos II | 8 | |
| Telectronics | Reflex 8220 | 17 |
| Reflex 8222 | 13 | |
| Quadra 9221 | 36 | |
| Optima 5281 | 3 | |
| Vitatron | Ultrafinesse 203-1 | 9 |
| Saphir VDD | 2 | |
| Biotronik | Inos 2 CLS | 6 |
| Cordis | 233 | 8 |
| 337 | 5 | |
| 402 | 28 | |
| Manufacturer . | Model . | Number . |
|---|---|---|
| Medtronic | Kappa SR 701 | 103 |
| Kappa DR 701 | 60 | |
| Kappa VDD 701 | 4 | |
| Thera SR 8940/8960 | 77 | |
| Thera DR 7940/7960 | 50 | |
| Legend 8416/8417 | 22 | |
| Legend II 8424 | 13 | |
| Minuet 7108 | 13 | |
| Microminix 8360 | 4 | |
| Minix 8340 | 3 | |
| AT 501 | 6 | |
| Elite 7077 | 1 | |
| Elite II 7086 | 2 | |
| Intermedics | Dash | 22 |
| Cosmos II | 8 | |
| Telectronics | Reflex 8220 | 17 |
| Reflex 8222 | 13 | |
| Quadra 9221 | 36 | |
| Optima 5281 | 3 | |
| Vitatron | Ultrafinesse 203-1 | 9 |
| Saphir VDD | 2 | |
| Biotronik | Inos 2 CLS | 6 |
| Cordis | 233 | 8 |
| 337 | 5 | |
| 402 | 28 | |
Implantable generators used (n=515 PMs)
| Manufacturer . | Model . | Number . |
|---|---|---|
| Medtronic | Kappa SR 701 | 103 |
| Kappa DR 701 | 60 | |
| Kappa VDD 701 | 4 | |
| Thera SR 8940/8960 | 77 | |
| Thera DR 7940/7960 | 50 | |
| Legend 8416/8417 | 22 | |
| Legend II 8424 | 13 | |
| Minuet 7108 | 13 | |
| Microminix 8360 | 4 | |
| Minix 8340 | 3 | |
| AT 501 | 6 | |
| Elite 7077 | 1 | |
| Elite II 7086 | 2 | |
| Intermedics | Dash | 22 |
| Cosmos II | 8 | |
| Telectronics | Reflex 8220 | 17 |
| Reflex 8222 | 13 | |
| Quadra 9221 | 36 | |
| Optima 5281 | 3 | |
| Vitatron | Ultrafinesse 203-1 | 9 |
| Saphir VDD | 2 | |
| Biotronik | Inos 2 CLS | 6 |
| Cordis | 233 | 8 |
| 337 | 5 | |
| 402 | 28 | |
| Manufacturer . | Model . | Number . |
|---|---|---|
| Medtronic | Kappa SR 701 | 103 |
| Kappa DR 701 | 60 | |
| Kappa VDD 701 | 4 | |
| Thera SR 8940/8960 | 77 | |
| Thera DR 7940/7960 | 50 | |
| Legend 8416/8417 | 22 | |
| Legend II 8424 | 13 | |
| Minuet 7108 | 13 | |
| Microminix 8360 | 4 | |
| Minix 8340 | 3 | |
| AT 501 | 6 | |
| Elite 7077 | 1 | |
| Elite II 7086 | 2 | |
| Intermedics | Dash | 22 |
| Cosmos II | 8 | |
| Telectronics | Reflex 8220 | 17 |
| Reflex 8222 | 13 | |
| Quadra 9221 | 36 | |
| Optima 5281 | 3 | |
| Vitatron | Ultrafinesse 203-1 | 9 |
| Saphir VDD | 2 | |
| Biotronik | Inos 2 CLS | 6 |
| Cordis | 233 | 8 |
| 337 | 5 | |
| 402 | 28 | |
Endocardial pacing
Considering all the pacing system implantations (first PM implantations and PM revisions), a total of 211 PMs and lead implantation procedures with 90 endocardial atrial leads and 165 endocardial ventricular leads (Table 3) was performed at the age of 9±7 years (range 1 day–18 years, median 8 years).
Leads (n=480)
| . | Endocardial leads (n=254) . | Epicardial leads (n=226) . |
|---|---|---|
| Atrial | 90 | 103 |
| Ventricular | 164 | 123 |
| Conventional leads | 46 (18%) | 127 (56%) |
| Steroid-eluting leads | 208 (82%) | 99 (44%) |
| Failures | 15 (6%)* | 48 (21%)* |
| Failures among atrial leads | 6 (7%) | 16 (16%) |
| Failures among ventricular leads | 9 (6%) | 32 (26%) |
| Failures among conventional leads | 4 (9%)* | 40 (31%)* |
| Failures among steroid-eluting leads | 11 (5%)** | 8 (8%)** |
| . | Endocardial leads (n=254) . | Epicardial leads (n=226) . |
|---|---|---|
| Atrial | 90 | 103 |
| Ventricular | 164 | 123 |
| Conventional leads | 46 (18%) | 127 (56%) |
| Steroid-eluting leads | 208 (82%) | 99 (44%) |
| Failures | 15 (6%)* | 48 (21%)* |
| Failures among atrial leads | 6 (7%) | 16 (16%) |
| Failures among ventricular leads | 9 (6%) | 32 (26%) |
| Failures among conventional leads | 4 (9%)* | 40 (31%)* |
| Failures among steroid-eluting leads | 11 (5%)** | 8 (8%)** |
*P<0.05 (χ2 test).
**P=not significant.
Leads (n=480)
| . | Endocardial leads (n=254) . | Epicardial leads (n=226) . |
|---|---|---|
| Atrial | 90 | 103 |
| Ventricular | 164 | 123 |
| Conventional leads | 46 (18%) | 127 (56%) |
| Steroid-eluting leads | 208 (82%) | 99 (44%) |
| Failures | 15 (6%)* | 48 (21%)* |
| Failures among atrial leads | 6 (7%) | 16 (16%) |
| Failures among ventricular leads | 9 (6%) | 32 (26%) |
| Failures among conventional leads | 4 (9%)* | 40 (31%)* |
| Failures among steroid-eluting leads | 11 (5%)** | 8 (8%)** |
| . | Endocardial leads (n=254) . | Epicardial leads (n=226) . |
|---|---|---|
| Atrial | 90 | 103 |
| Ventricular | 164 | 123 |
| Conventional leads | 46 (18%) | 127 (56%) |
| Steroid-eluting leads | 208 (82%) | 99 (44%) |
| Failures | 15 (6%)* | 48 (21%)* |
| Failures among atrial leads | 6 (7%) | 16 (16%) |
| Failures among ventricular leads | 9 (6%) | 32 (26%) |
| Failures among conventional leads | 4 (9%)* | 40 (31%)* |
| Failures among steroid-eluting leads | 11 (5%)** | 8 (8%)** |
*P<0.05 (χ2 test).
**P=not significant.
Epicardial pacing
A total of 145 PMs and lead implantation procedures has been performed with 103 atrial and 123 ventricular leads (Table 3) at the age of 6±7 years (range 1 day–18 years, median 4 years).
Leads, pacing modes
Leads used are reported in Table 3. Out of 480 implanted leads, 254 were endocardial and 226 were epicardial; the proportion of the steroid-eluting type was 74% (n=192) among the former and 42% (n=95) among the latter. All endocardial leads were passive fixation (tined), except Medtronic 5076 (screw-in), six atrial leads (7%) and six ventricular leads (4%). All epicardial leads were unipolar, except Medtronic 4968: only two of these leads were implanted in the atrium (2%) and two in the ventricle (1.5%). Pacing modes are reported in Table 4.
Pacing modes
| . | AAI/R endo . | AAI/R epi . | VVI/R endo . | VVI/R epi . | DDD/R endo . | DDD/R epi . |
|---|---|---|---|---|---|---|
| Patients | 36 | 18 | 93 (two VDD) | 33 | 41 | 69 |
| Age impl (years) | 11±7 (median 11) | 6±4 (median 6) | 6±5 (median 4) | 4±5 (median 2) | 12±8 (median 12) | 7±8 (median 5) |
| LR (bpm) | 77±11 | 88±9 | 72±14 | 83±19 | 66±11 | 83±15 |
| UR/mtr (bpm) | 159±15 | 148±16 | 164±16 | 165±14 | 156±19 | 157±22 |
| Upgrading | 1 | 3 | 13 (4 VDD) | 2 | ||
| Downgrading | 5 VVI, 2 VDD | 11 AAI, 9 VVI, 1 VDD | ||||
| . | AAI/R endo . | AAI/R epi . | VVI/R endo . | VVI/R epi . | DDD/R endo . | DDD/R epi . |
|---|---|---|---|---|---|---|
| Patients | 36 | 18 | 93 (two VDD) | 33 | 41 | 69 |
| Age impl (years) | 11±7 (median 11) | 6±4 (median 6) | 6±5 (median 4) | 4±5 (median 2) | 12±8 (median 12) | 7±8 (median 5) |
| LR (bpm) | 77±11 | 88±9 | 72±14 | 83±19 | 66±11 | 83±15 |
| UR/mtr (bpm) | 159±15 | 148±16 | 164±16 | 165±14 | 156±19 | 157±22 |
| Upgrading | 1 | 3 | 13 (4 VDD) | 2 | ||
| Downgrading | 5 VVI, 2 VDD | 11 AAI, 9 VVI, 1 VDD | ||||
Data are in absolute numbers and are expressed as mean±standard deviation (the median is reported when different from the mean). Endo, endocardial leads; Epi, epicardial leads; impl, implantation; LR, lower pacing rate; mtr, maximum tracking rate; UR, upper pacing rate. Note the different LR, higher in AAI(R) to provide overdrive atrial pacing,20 lower in DDD pacing to obtain maximum atrial sensed-ventricular paced rhythm. In epicardial pacing, as the age at implantation is lower than in endocardial pacing, the LR is higher.
Pacing modes
| . | AAI/R endo . | AAI/R epi . | VVI/R endo . | VVI/R epi . | DDD/R endo . | DDD/R epi . |
|---|---|---|---|---|---|---|
| Patients | 36 | 18 | 93 (two VDD) | 33 | 41 | 69 |
| Age impl (years) | 11±7 (median 11) | 6±4 (median 6) | 6±5 (median 4) | 4±5 (median 2) | 12±8 (median 12) | 7±8 (median 5) |
| LR (bpm) | 77±11 | 88±9 | 72±14 | 83±19 | 66±11 | 83±15 |
| UR/mtr (bpm) | 159±15 | 148±16 | 164±16 | 165±14 | 156±19 | 157±22 |
| Upgrading | 1 | 3 | 13 (4 VDD) | 2 | ||
| Downgrading | 5 VVI, 2 VDD | 11 AAI, 9 VVI, 1 VDD | ||||
| . | AAI/R endo . | AAI/R epi . | VVI/R endo . | VVI/R epi . | DDD/R endo . | DDD/R epi . |
|---|---|---|---|---|---|---|
| Patients | 36 | 18 | 93 (two VDD) | 33 | 41 | 69 |
| Age impl (years) | 11±7 (median 11) | 6±4 (median 6) | 6±5 (median 4) | 4±5 (median 2) | 12±8 (median 12) | 7±8 (median 5) |
| LR (bpm) | 77±11 | 88±9 | 72±14 | 83±19 | 66±11 | 83±15 |
| UR/mtr (bpm) | 159±15 | 148±16 | 164±16 | 165±14 | 156±19 | 157±22 |
| Upgrading | 1 | 3 | 13 (4 VDD) | 2 | ||
| Downgrading | 5 VVI, 2 VDD | 11 AAI, 9 VVI, 1 VDD | ||||
Data are in absolute numbers and are expressed as mean±standard deviation (the median is reported when different from the mean). Endo, endocardial leads; Epi, epicardial leads; impl, implantation; LR, lower pacing rate; mtr, maximum tracking rate; UR, upper pacing rate. Note the different LR, higher in AAI(R) to provide overdrive atrial pacing,20 lower in DDD pacing to obtain maximum atrial sensed-ventricular paced rhythm. In epicardial pacing, as the age at implantation is lower than in endocardial pacing, the LR is higher.
Pacing thresholds
Thresholds at implantation at 0.50 ms of pulse duration were 0.5±0.3 V for endocardial leads and significantly higher (1.2±0.5 V) for epicardial leads (P<0.05).
Follow-up
The duration of follow-up was 5±4 years (1 month–18 years).
None of the 15 deaths documented was attributed to pacing failure. Pacing systems have been explanted and not replaced after heart transplantation (n=6), systemic infection and endocarditis (n=1), and resolution of post-operative brady–tachy syndrome (n=1). Forty-nine patients were lost to follow-up as they are seen at other institutions.
Pacemaker revisions
Pacemaker revisions account for 56 procedures.
Switch of pacing system (from epicardial to endocardial pacing and vice versa)
Epicardial pacing was replaced with endocardial pacing for lead malfunction (n=24) and pocket infection/erosion (n=7). An epicardial pacing system replaced an endocardial system because of concomitant heart surgery (n=3) and infection/erosion (n=3).
System up-/downgrading (Table 4)
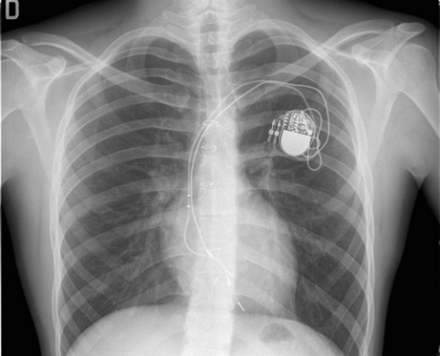
System upgrading was performed in 19 patients. The occurrence of symptomatic (fatigue and exercise intolerance) second degree AVB (n=4, 7% of all atrial-paced patients) was the indication to upgrade AAI to DDD PM; VVI PM was upgraded to DDD PM for pre-syncope (n=1) and systemic ventricular dysfunction (left ventricular ejection fraction <40% with single-plane disk method with apical four-chamber view on echocardiography) (n=10). In four patients, a VVI PM with a malfunctioning lead was substituted by a VDD system (Figure 1).
Postero-anterior view of chest X-ray of patient, 15 years old (height 192 cm) implanted at the age of 10 years (height 146 cm) with a VVIR PM for post-operative AV block. After 5 years, the ventricular lead is stretched and the tip seems displaced beneath the tricuspid valve; abnormal threshold increase with loss of capture was documented. The system was upgraded with a VDD PM and lead using the same left subclavian vein. The old lead was not removed.
System downgrading was performed in the case of lead malfunction or when a DDD PM was implanted for SND and AV conduction was not impaired during follow-up.
Early complications, endocardial/epicardial pacing
Infection/erosion
There were no differences in the infection/erosion rate between endocardial (n=3, 1%) and epicardial (n=5, 2%) systems and between single-chamber and dual-chamber PM.
Haemothorax/vascular haemorrhage
Haemothorax or vascular haemorrhage requiring either chest drainage or blood transfusion occurred in seven patients, 3.5% of transvenous procedures, and the frequency was similar for atrial, ventricular, and dual-chamber PM.
Lead dislodgement
The early dislodgement rate was not significantly higher for atrial (n=6, 7%) than for ventricular (n=6, 4%) endocardial leads. The early dislodgement rate for all transvenous leads was nearly 5%.
Inappropriate muscle stimulation
Early inappropriate diaphragmatic stimulation via the phrenic nerve occurred in four atrial and one ventricular endocardial leads. This symptom disappeared with the use of bipolar stimulation at low pacing output, except in one DDD PM that was downgraded to VDD pacing. Inappropriate abdominal wall muscle stimulation occurred in two epicardial leads (one ventricular and one atrial) and was treated with pacing output reduction or DDD downgrading to VDD.
Late complications, endocardial/epicardial pacing
Infection/erosion
As for early complications, there were no differences in the infection/erosion rate between endocardial (n=5, 2%) and epicardial (n=5, 2%) systems and between single-chamber and dual-chamber PM.
Lead failure
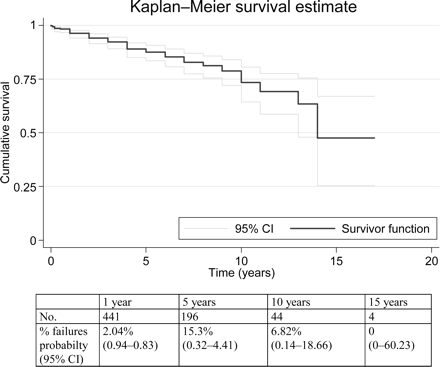
During the study period, there were 63 lead failures because of pacing and/or sensing defects: 15 in the endocardial and 48 in the epicardial group (Table 3). The overall longevity of the leads, independently from site of pacing and type, is presented in Figure 2. At 10 years, ∼75% of the leads were still functioning, and the proportion decreases to 50% at 15 years. Significant differences exist between endocardial and epicardial leads (Figure 3) and between conventional and steroid-eluting leads (Figure 4). As expected, the proportion of failures was higher among the epicardial leads of conventional type. There is no significant difference between conventional and steroid-eluting endocardial lead outcomes and between steroid-eluting endocardial and epicardial lead outcomes (Table 3).
Lead survival curve with 95% confidence interval (CI) in overall population.
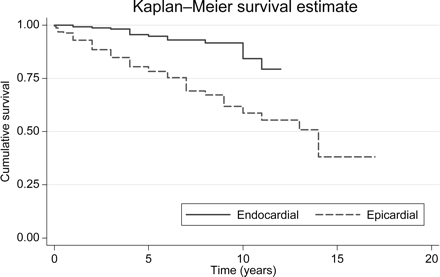
Lead survival curve according to lead type (log-rank test χ2(1)=29.34, P=0.0001).
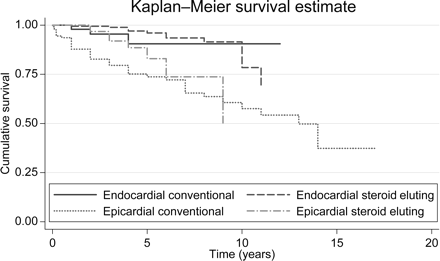
Cumulative survival of pacemaker leads according to lead type (log-rank test χ2(3)=34.63, P=0.0001).
Figure 4 shows the estimated longevity of leads by lead type, using the Kaplan–Meier method. According to the estimated curves, endocardial leads have the best performance, whereas conventional epicardial leads fare worst.
Table 5 reports the risk of lead failure by Cox's proportional hazard model. When all the variables are in the model, conventional epicardial leads show the worst performance, with an approximately five-fold greater risk of failure compared with the conventional endocardial leads (P=0.004). The number of previous procedures had no negative impact on lead longevity and subsequent procedures. None of the other factors have a statistically significant influence on lead lifetime.
Risk of failure of different lead types adjusted for lead type, patient's gender, age at implantation, year of implantation, and number of procedures (multivariate Cox proportional hazard model)
| . | Hazard ratio . | P>|z| . | 95% CI . | |
|---|---|---|---|---|
| Lead type | ||||
| Conventional endocardiala | 1 | — | — | |
| Steroid-eluting endocardial | 1.31 | 0.839 | 0.34 | 3.73 |
| Conventional epicardial | 4.83 | 0.004 | 1.66 | 14.05 |
| Steroid-eluting epicardial | 2.75 | 0.115 | 0.78 | 9.65 |
| Lead site | ||||
| Atriala | 1 | — | — | |
| Ventricular | 1.27 | 0.385 | 0.74 | 2.16 |
| Age (years) | ||||
| <2a | 1 | — | — | |
| 2–6 | 0.64 | 0.195 | 0.33 | 1.25 |
| 6–10 | 0.88 | 0.718 | 0.44 | 1.77 |
| >10 | 0.64 | 0.292 | 0.28 | 1.47 |
| Gender | ||||
| Malea | 1 | — | — | |
| Female | 0.72 | 0.219 | 0.42 | 1.22 |
| Year of implantation | 0.71 | 0.351 | 0.35 | 1.45 |
| Number of procedures | 0.67 | 0.017 | 0.48 | 0.93 |
| . | Hazard ratio . | P>|z| . | 95% CI . | |
|---|---|---|---|---|
| Lead type | ||||
| Conventional endocardiala | 1 | — | — | |
| Steroid-eluting endocardial | 1.31 | 0.839 | 0.34 | 3.73 |
| Conventional epicardial | 4.83 | 0.004 | 1.66 | 14.05 |
| Steroid-eluting epicardial | 2.75 | 0.115 | 0.78 | 9.65 |
| Lead site | ||||
| Atriala | 1 | — | — | |
| Ventricular | 1.27 | 0.385 | 0.74 | 2.16 |
| Age (years) | ||||
| <2a | 1 | — | — | |
| 2–6 | 0.64 | 0.195 | 0.33 | 1.25 |
| 6–10 | 0.88 | 0.718 | 0.44 | 1.77 |
| >10 | 0.64 | 0.292 | 0.28 | 1.47 |
| Gender | ||||
| Malea | 1 | — | — | |
| Female | 0.72 | 0.219 | 0.42 | 1.22 |
| Year of implantation | 0.71 | 0.351 | 0.35 | 1.45 |
| Number of procedures | 0.67 | 0.017 | 0.48 | 0.93 |
aReference category.
Risk of failure of different lead types adjusted for lead type, patient's gender, age at implantation, year of implantation, and number of procedures (multivariate Cox proportional hazard model)
| . | Hazard ratio . | P>|z| . | 95% CI . | |
|---|---|---|---|---|
| Lead type | ||||
| Conventional endocardiala | 1 | — | — | |
| Steroid-eluting endocardial | 1.31 | 0.839 | 0.34 | 3.73 |
| Conventional epicardial | 4.83 | 0.004 | 1.66 | 14.05 |
| Steroid-eluting epicardial | 2.75 | 0.115 | 0.78 | 9.65 |
| Lead site | ||||
| Atriala | 1 | — | — | |
| Ventricular | 1.27 | 0.385 | 0.74 | 2.16 |
| Age (years) | ||||
| <2a | 1 | — | — | |
| 2–6 | 0.64 | 0.195 | 0.33 | 1.25 |
| 6–10 | 0.88 | 0.718 | 0.44 | 1.77 |
| >10 | 0.64 | 0.292 | 0.28 | 1.47 |
| Gender | ||||
| Malea | 1 | — | — | |
| Female | 0.72 | 0.219 | 0.42 | 1.22 |
| Year of implantation | 0.71 | 0.351 | 0.35 | 1.45 |
| Number of procedures | 0.67 | 0.017 | 0.48 | 0.93 |
| . | Hazard ratio . | P>|z| . | 95% CI . | |
|---|---|---|---|---|
| Lead type | ||||
| Conventional endocardiala | 1 | — | — | |
| Steroid-eluting endocardial | 1.31 | 0.839 | 0.34 | 3.73 |
| Conventional epicardial | 4.83 | 0.004 | 1.66 | 14.05 |
| Steroid-eluting epicardial | 2.75 | 0.115 | 0.78 | 9.65 |
| Lead site | ||||
| Atriala | 1 | — | — | |
| Ventricular | 1.27 | 0.385 | 0.74 | 2.16 |
| Age (years) | ||||
| <2a | 1 | — | — | |
| 2–6 | 0.64 | 0.195 | 0.33 | 1.25 |
| 6–10 | 0.88 | 0.718 | 0.44 | 1.77 |
| >10 | 0.64 | 0.292 | 0.28 | 1.47 |
| Gender | ||||
| Malea | 1 | — | — | |
| Female | 0.72 | 0.219 | 0.42 | 1.22 |
| Year of implantation | 0.71 | 0.351 | 0.35 | 1.45 |
| Number of procedures | 0.67 | 0.017 | 0.48 | 0.93 |
aReference category.
Of 165 endocardial ventricular leads, 9 (6%) had to be substituted, and among 90 endocardial atrial leads, 6 (7%) also had to be substituted (or abandoned), with no difference in the estimated mean survival time (11 years). There was no difference between survival of the different endocardial leads used.
Nine endocardial ventricular leads, all connected to VVI/R PM, were removed (two patients) or abandoned and replaced for failure to capture or excessive threshold increase. Chest X-rays showed loss of lead slack because of patient's growth (Figure 1). These patients underwent PM implantation for congenital or post-operative complete AVB at the age of 6±3 (2–10) years. The thresholds at implantation were comparable with those of other patients. In general, threshold increase was sudden, and the time to lead failure was 6±3 (3–11) years.
Six endocardial atrial leads (two AAI and four DDD PMs) implanted in patients aged 7±3 (4–12) have been abandoned, for capture or sensing failure, after 3±2 years. The leads were passive fixation. All but one patient previously underwent a Mustard procedure.
Epicardial lead failure (Table 4) occurred in 21% (n=48) of all leads. The time to lead failure was not significantly different for atrial (4±3, range 0.3–11 years) and for ventricular leads (4±3, range 0.2–14 years). Epicardial lead fracture occurred in 7% of all leads, with no differences between atrial and ventricular leads. An analysis of epicardial lead failure according to surgical approach was not performed.
Inappropriate muscle stimulation
Late inappropriate pectoral stimulation occurred in two patients with unipolar endocardial ventricular leads (DDD PM) and was treated with pacing output reduction, and in two DDD-paced patients with epicardial unipolar ventricular leads and was treated with pacing output reduction or pacing system replacement.
Discussion
The implantation of a permanent PM in children and adolescents is a procedure with a generally favourable outcome, but children might be more prone to complications because of their more active lifestyle, their higher frequency of traumatic events, and localized or systemic infections that may affect the pacing system. Despite recent technical progress, pacing leads remain the ‘weakest link’ of the permanent pacing system,10 particularly in a growing patient. Moreover, in the era of modern PM technology, a rate of 14% of complications is still reported in adults.3 Concerns have been raised about the long-term efficacy and safety of endocardial leads in children in terms of high rate of lead abandonment,11 valvular integrity, and venous obstruction.1,12,13
In our patients, PM-related deaths were not documented, but serious complications that needed re-operations or a revision of the PM system have been necessary. Early complications are related to infections (∼2%) and, in endocardial pacing, to haemothorax or vascular haemorrhage (3.5%) or to lead dislodgement (5%), the latter due to the frequent impossibility to maintain the child quiet and calm for the first 24 h. In addition, there are 2% late infective complications.
In general, long-term complications are lead-related. As a rule, the less leads implanted, the complications in the future. Among endocardial leads, there were no significant differences between steroid-eluting and conventional leads. Actually, lead failure occurred in 6% of endocardial leads and in 21% of epicardial leads (Table 3). Conventional epicardial leads showed a significantly poorer outcome than other leads. Other authors have described lead failure in 15–20% of epicardially paced patients or complications requiring re-operation.1,5 The performance and longevity of steroid-eluting epicardial leads have been described to be comparable with those of endocardial leads,1,2,5,14 without differences in survival between atrial and ventricular leads,1 although exit block was described in up to 19% of leads.15 In our experience, steroid-eluting epicardial leads implanted in children showed no significant increase in failures when compared with steroid-eluting endocardial leads (Table 3).
The high failure rate of epicardial leads is related not only to threshold increase and exit block, but also to fracture, due to somatic growth and to frequent traumatic events occurring in children. Lead fracture rate was similar in all epicardial lead models. No endocardial lead fracture was detected in our experience, whereas fractures have been documented in adults.3,10 Ventricular leads failed more frequently than atrial leads, but steroid-eluting leads were implanted more frequently in atrial than in ventricular pacing.
Complex post-operative atrial anatomy was the major cause of tined endocardial atrial lead failure in our experience; an increased incidence of atrial lead failure has been already reported.11 This may be caused by the difficult in positioning and fixing atrial leads after extensive atrial surgery. For this reason, we now prefer screw-in atrial leads in patients with prior cardiac surgery.
Slow absorbing ligatures4 were used as a solution to the problem of growth (Figure 5), but this does not seem to be the ideal solution: the frequency of late endocardial ventricular lead failure is 6% ∼6 years after the implant in childhood and occurred principally after the growth of patient with loss of lead slack (Figure 1). For this reason, since 2000, we added an atrial loop to ventricular leads in the hope that this combination of methods results in better long-term outcome. The efficacy of this combined technique has to be verified in the future. The survival rate of endocardial leads in our study is higher than that reported in 199311 probably due to the improvement in lead technology. Thin, unipolar, tined ventricular leads were preferred in children because larger leads potentially increase the risk for venous thrombosis.12,13 In our patients, chronic venous occlusion (often asymptomatic) has not been systematically assessed and this is a limitation of this study. Doppler echography of the subclavian vein was performed only before transvenous upgrading procedures, and in this small group of patients, until March 2002, we have not found any venous occlusion or significant obstruction.
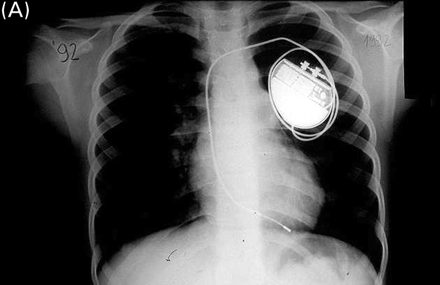
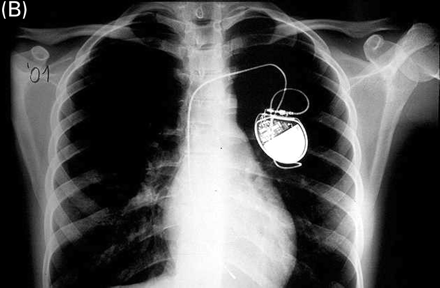
Chest X-ray of a girl at the age of 9 years in 1992 (A) and in 2001 (B). The ventricular lead, implanted in 1990, was fixed to the subcutaneous tissue with a slowly absorbable ligature. After patient's growth, the lead maintains an adequate position with normal function.
It is clear that great experience in the field of paediatric electrophysiology and cardiac pacing may yield an advantage in terms of outcomes or complications. Therefore, the large series and the long follow-up described here may be helpful in the understanding of how to pace this population. We think that careful and complete follow-up evaluation is at least as useful as the large number of cases, because the experience acquired and the knowledge of the complications that have occurred may help in new decision-making processes. This is true in any field of medicine, but, particularly in paediatric pacing, continuing technical innovations can make accumulated experience in a particular procedure quickly redundant and demand new experience perhaps on a better basis. This makes paediatric pacing more difficult, but also more fascinating and challenging than any other paediatric field.
Therefore, on the basis of our long experience, our policy is now as follows.
In patients with AVB and normal ventricular function, the pacing mode is VVIR in childhood changing to VDD/DDD during adolescence.16–18
DDD pacing with transvenous leads is technically feasible in small children, but may not be needed.
When patients are too young to receive a pacing system with transvenous leads, epicardial pacing is indicated. It still seems prudent to defer endocardial pacing until the child has reached a weight of 15–20 kg to minimize the risk of vascular injury and thrombosis or late lead failure due to inappropriate lead stretching with somatic growth.
The addition of an atrial loop18 to the slow absorbing ligature might produce a better outcome for ventricular endocardial leads implanted before the adolescence.
At younger age, the atrium may be too small for the J-shaped atrial lead, and the epicardial approach should be preferred if atrial pacing is necessary.
Inappropriate muscle stimulation occurs both in transvenous and in epicardial pacing. This is mainly related to the use of unipolar leads and to the general anaesthesia with muscular paralysis during the implant procedure. To reduce this occurrence, leads must be tested with high pacing output (10 V and 1.5 ms) when anaesthesia is light.
As SND is a long-term post-operative complication, atrial PMs are implanted in patients with operated congenital HDs19 at an age similar to that of DDD-paced patients. Higher rates can be selected in atrial pacing than in VVIR and DDD pacing for the prevention of atrial tachyarrhythmias.20
When AVB occurs in AAI/R-paced patients and causes symptoms, patients can be successfully upgraded to dual-chamber stimulation (with either endocardial or epicardial pacing). Thus, with transvenous pacing, it seems prudent to perform an initial implantation of a single-chamber atrial PM, and then to upgrade the system if AVB occurs, whereas in epicardial pacing, we suggest implantation of a dual-chamber system in the case of SND to avoid a future surgical procedure if AV conduction becomes impaired.
Conclusions
In conclusion, permanent pacing in paediatric patients is generally safe and has a favourable long-term outcome, but there remains a high rate of complications, mainly related to leads. This is of particular concern in children who need a lifetime of pacing. Therefore, we suggest minimizing the number of leads implanted. With modern technology,21 transvenous and epicardial pacing are initially comparable. However, epicardial pacing shows more long-term complications. In the older child or in the adolescent, endocardial pacing should be considered to be the first choice.
Acknowledgements
In memory of our master in paediatric pacing, P. Ragonese and of C. Squitieri. Other doctors who performed the implantation of PM are S. Albanese, A. Amodeo, A. Carotti, D. Di Carlo, E. Mazzera, R.M. Di Donato (Paediatric Heart Surgery), and P. Guccione (Paediatric Cardiology). We thank M. Cuttini (Epidemiologic Unit) for her help in statistical analysis.