-
PDF
- Split View
-
Views
-
Cite
Cite
Jacqueline Barrett, Moyez Jiwa, Peter Rose, William Hamilton, Pathways to the diagnosis of colorectal cancer: an observational study in three UK cities, Family Practice, Volume 23, Issue 1, February 2006, Pages 15–19, https://doi.org/10.1093/fampra/cmi093
Close - Share Icon Share
Abstract
Background. Colorectal cancer can present in a variety of ways, and with any of several symptoms. Different referral routes from primary to secondary care cater for these different presentations. The route that has received most investment in the UK National Health Service is the 2-week clinic, but the proportions of patients taking this and other routes to diagnosis are largely unknown.
Methods. We designed an observational audit in Exeter, Oxford and Sheffield, UK. Colorectal cancers diagnosed in 2002 from participating practices were identified and the presence and timing of seven important clinical features noted: diarrhoea, constipation, rectal bleeding, abdominal pain, the finding of an abdominal or rectal mass on examination, anaemia and positive faecal occult blood tests. The referral pathways to secondary care were identified.
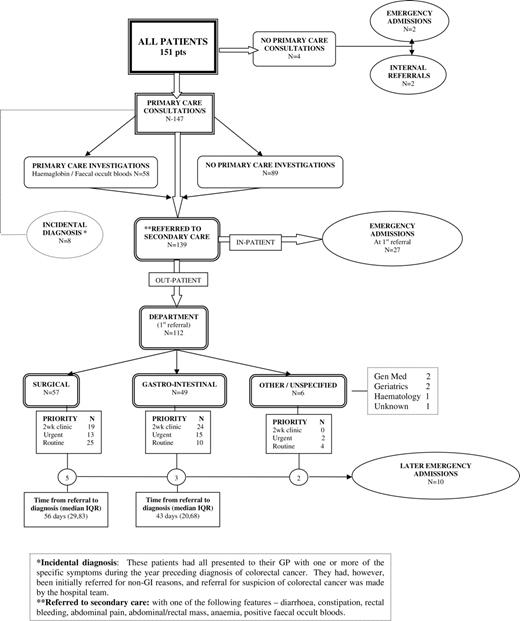
Results. Of the 151 patients studied, 112 (74%) were referred with at least one clinical feature of colorectal cancer to a specialist. Only 43 of these (28% of the total) were referred to a 2-week clinic; 39 patients (26% of the total) had an emergency admission, of whom 10 (7%) had their emergency admission after referral to a specialist for investigation but before a diagnosis had been established. The time intervals between the first consultation with a symptom of cancer and referral were mostly short.
Conclusion. Patients with colorectal cancer travel several different pathways to diagnosis. The pathway with the most resources—the 2-week clinic—is used by a minority of patients.
Barrett J, Jiwa M, Rose P and Hamilton W. Pathways to the diagnosis of colorectal cancer: an observational study in three UK cities. Family Practice 2006; 23: 15–19.
Introduction
The diagnosis of colorectal cancer is not straightforward. First, the cancer can be discovered at any stage during progression from asymptomatic cancer identified by screening, through consultation with symptoms with a GP, to presentation as a surgical emergency. Each of these three main modes of presentation has a different clinical path from identification of the initial problem to eventual diagnosis.
In order of frequency, the first mode of presentation is by screening, though the proportion of cancers diagnosed this way in the UK has been low until recently. A national programme of screening by home faecal occult blood testing of those aged 60–69 years is planned to begin in 2006,1 based in part upon pilot studies which examined patients aged 50–70 years.2 The second mode of presentation is as an emergency: around a quarter of colorectal cancers present in this way, usually with bowel obstruction or perforation.3–9 Although the majority of patients with bowel obstruction have had symptoms for a very short time, some have had persistent symptoms before the emergency presentation.8,10 The third, and commonest, mode of presentation is to primary care with non-urgent symptoms. Many symptoms have been described, and they can occur singly or in combination. Some of these are localised, such as abdominal pain or rectal bleeding, whereas others are systemic, such as loss of weight or anaemia. UK GPs manage patients with abdominal symptoms on a daily basis.11 On average each refers between 6 and 12 patients with lower bowel symptoms each year to colorectal surgeons or gastroenterologists for investigation of possible colorectal cancer, with the choice between the two depending largely on local provision of services.12 As only 1 patient in 1800 (or roughly 1 patient per whole-time GP) develops colorectal cancer each year,13 most of the patients referred for investigation are found to have benign conditions.14 Patients with systemic features may be referred to other specialities, such as haematology or geriatric medicine.
These different pathways are important, as the considerable increase in investment in cancer diagnostic services in the UK National Health Service (NHS)15 has presumed that the large majority of patients follow a single pathway—namely, referral of patients presenting to primary care with a suspicious symptom to a 2-week clinic. Referrals to these clinics are guided by the Referral Guidelines for Suspected Cancer.16,17 These guidelines were purported to identify 90% of cases of bowel cancer.17 However, secondary care audits have suggested that the introduction of 2-week clinics in 2000 has not increased the proportion of cases referred via the appropriate pathway, with the majority of cases bypassing such clinics.9,18,19 These patients are seeing no benefit from this increased investment. Worse still, as resources are redirected to 2-week clinics, the waits in standard clinics have risen. Perversely, patients referred outside the 2-week system could have been disadvantaged.20
No primary care study has identified—and quantified—the particular pathways patients take to their diagnosis of colorectal cancer. We set out to do this.
Methods
This was an observational audit from three UK cities—Exeter (78 cases), Oxford (32 cases) and Sheffield (41 cases). The Exeter cases consisted of all patients diagnosed in 2002 who had previously been part of the CAPER study.21 Practices in the local research networks in Oxford and Sheffield were invited to identify the first five colorectal cancer cases diagnosed after 1 January 2002. The upper limit of five was chosen because the study was unfunded, relying upon the goodwill of GPs in completing the proforma. None of the cities was participating in the colorectal cancer pilot study.
A proforma for the data collection was designed and piloted using all Exeter cases diagnosed in 1998. The Exeter data were collected by a research assistant (JB) and the Oxford and Sheffield data by the patients' own GPs. If the patient had died and the notes were not held at the GP's surgery, then they were retrieved from the relevant Health Authority. The GP notes for 1 year pre-diagnosis were examined for seven features deemed to be particularly relevant for colorectal cancer—any consultation with diarrhoea, constipation, rectal bleeding, abdominal pain, the finding of an abdominal or rectal mass on examination, anaemia (defined as a haemoglobin below the local laboratory's normal threshold) and positive faecal occult blood tests.21,22 The first mention of these features in the notes was identified and used for analysis, as all of these symptoms may be present for up to a year before diagnosis.21 This was the case even if the symptom had been given an alternative diagnostic label, as it was not possible to know whether this was a misdiagnosis. Furthermore, this definition of the first symptom encouraged GPs who were completing the proformas in Oxford or Sheffield to report what—in retrospect—may have been a diagnostic delay. Referral data were collected, including the hospital department and referral priority—the 2-week clinic, urgent, routine or emergency. Where available, the Duke's staging and site of the tumour, categorised as either right-sided (from the caecum to the transverse colon) or left-sided (from the splenic flexure to the rectum), were also recorded.
Analysis
The data are presented using simple descriptive statistics. As the time intervals were not normally distributed, medians and interquartile ranges (IQRs) are shown. The primary aim was to identify the pathways leading up to diagnosis; therefore comparative tests across the three centres were not applied. We used Stata version 8.23
Results
We studied 151 patents with a colorectal cancer diagnosed in 2002. Details of the patients and their tumours are shown in Table 1.
Patient demographics and tumour characteristics
| Median (IQR) age in years . | Sex . | . | Duke's stage . | . | . | . | Tumour site . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Male . | Female . | A . | B . | C . | D . | Left-sided . | Right-sided . | |||||
| 73 (66, 80) | 86 | 65 | 16 (14%) | 52 (45%) | 34 (30%) | 12 (11%) | 106 (73%) | 39 (27%) | |||||
| Median (IQR) age in years . | Sex . | . | Duke's stage . | . | . | . | Tumour site . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Male . | Female . | A . | B . | C . | D . | Left-sided . | Right-sided . | |||||
| 73 (66, 80) | 86 | 65 | 16 (14%) | 52 (45%) | 34 (30%) | 12 (11%) | 106 (73%) | 39 (27%) | |||||
IQR = interquartile range.
One subject had missing data for age; 37 for Duke's staging; 6 for tumour site.
Patient demographics and tumour characteristics
| Median (IQR) age in years . | Sex . | . | Duke's stage . | . | . | . | Tumour site . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Male . | Female . | A . | B . | C . | D . | Left-sided . | Right-sided . | |||||
| 73 (66, 80) | 86 | 65 | 16 (14%) | 52 (45%) | 34 (30%) | 12 (11%) | 106 (73%) | 39 (27%) | |||||
| Median (IQR) age in years . | Sex . | . | Duke's stage . | . | . | . | Tumour site . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Male . | Female . | A . | B . | C . | D . | Left-sided . | Right-sided . | |||||
| 73 (66, 80) | 86 | 65 | 16 (14%) | 52 (45%) | 34 (30%) | 12 (11%) | 106 (73%) | 39 (27%) | |||||
IQR = interquartile range.
One subject had missing data for age; 37 for Duke's staging; 6 for tumour site.
The age range was 40–97 years with a median age of 73 years (IQR 66, 80). Left-sided tumours were present in 106 patients (73%), 44 of these (33% of the total) being rectal and 37 (26%) being sigmoid tumours. There were 39 right-sided tumours (27%), with the commonest site being the caecum, accounting for 17 of these (12%).
Time intervals
Time intervals were calculated in days. The median (IQR) time interval from the first consultation to the first referral (n = 140) was 6 days (0, 56.25); from the first referral to diagnosis (n = 143), 42 days (21, 76); from the first consultation through to diagnosis (n = 147), 69 days (35, 188).
The time intervals from the first presentation of a specific feature (which may not have been at the first consultation) to referral are shown in Table 2. Delay was longest in patients with positive faecal occult blood tests (14 days), although only 11 patients had this feature.
Time from first consultation with a specific feature to referral
| Feature . | n . | Median (IQR) interval in days . |
|---|---|---|
| Abdominal mass | 19 | 0 (0, 1) |
| Rectal bleeding | 47 | 2 (0, 7) |
| Diarrhoea | 33 | 4 (0, 44) |
| Abdominal pain | 45 | 5 (0, 69) |
| Constipation | 27 | 7 (1, 182) |
| Anaemia | 38 | 11 (0, 54) |
| Positive faecal occult blood test | 11 | 14 (8, 23) |
| Feature . | n . | Median (IQR) interval in days . |
|---|---|---|
| Abdominal mass | 19 | 0 (0, 1) |
| Rectal bleeding | 47 | 2 (0, 7) |
| Diarrhoea | 33 | 4 (0, 44) |
| Abdominal pain | 45 | 5 (0, 69) |
| Constipation | 27 | 7 (1, 182) |
| Anaemia | 38 | 11 (0, 54) |
| Positive faecal occult blood test | 11 | 14 (8, 23) |
IQR = interquartile range.
Time from first consultation with a specific feature to referral
| Feature . | n . | Median (IQR) interval in days . |
|---|---|---|
| Abdominal mass | 19 | 0 (0, 1) |
| Rectal bleeding | 47 | 2 (0, 7) |
| Diarrhoea | 33 | 4 (0, 44) |
| Abdominal pain | 45 | 5 (0, 69) |
| Constipation | 27 | 7 (1, 182) |
| Anaemia | 38 | 11 (0, 54) |
| Positive faecal occult blood test | 11 | 14 (8, 23) |
| Feature . | n . | Median (IQR) interval in days . |
|---|---|---|
| Abdominal mass | 19 | 0 (0, 1) |
| Rectal bleeding | 47 | 2 (0, 7) |
| Diarrhoea | 33 | 4 (0, 44) |
| Abdominal pain | 45 | 5 (0, 69) |
| Constipation | 27 | 7 (1, 182) |
| Anaemia | 38 | 11 (0, 54) |
| Positive faecal occult blood test | 11 | 14 (8, 23) |
IQR = interquartile range.
Pathways
The pathways from the first presentation in primary care with a high-risk symptom to referral and diagnosis in secondary care are shown in Figure 1. Only 43 patients, or 28% of the total, were diagnosed after referral to a 2-week clinic. Two referrals were private: one surgical and one gastrointestinal. They have been classified as urgent, though both were seen by the specialist within a week of referral.
Discussion
This article describes the pathways to diagnosis of 151 colorectal cancer patients from three cities in the UK, as determined by an audit of their GP notes for 1 year pre-diagnosis. Unlike other studies, we use primary rather than secondary care data, which explains why the tumour site and staging data are partial. The stage and site of the tumours, and the proportion presenting as an emergency, are similar to previous reports, so our cases are likely to be generalisable to other UK areas. The weakness of the study is that the audit data are retrospective and reliant upon the quality of the GPs' records. It is also possible that GPs in research networks—and thus participating in this study—are better at selecting patients for investigation of possible cancer: this possibility has never been studied. We do not know whether GPs in Oxford or Sheffield complied wholly with the instructions to collect their first five cancers or whether they omitted to report (or partially reported) cases that they felt had not been correctly managed.
The main finding is confirmation of the multiple pathways to diagnosis. Only 102 out of 151 patients (68%) were diagnosed as outpatients, mainly as the result of the intervention of an emergency situation before or during the referral process. Twenty-eight per cent of cases were referred to 2-week clinics. Most referrals were to surgery or gastroenterology, with only six referrals to other specialities; although a further eight had reported symptoms possibly indicative of colorectal cancer to their GPs, the suspicion of cancer—and referral—came from a hospital team.
Delays were relatively minor within primary care, with the median time from the first primary care consultation with a suspicious symptom to referral being short, although the upper end of the interquartile range was long for patients with abdominal pain, constipation and anaemia. Change in bowel habit is the feature of colorectal cancer reported to be most associated with delay in diagnosis, particularly diarrhoea in terms of accounting for patient delay24 and constipation for doctor delay. Our study did not find this: it is possible doctors have become more alert to the possibility of colorectal cancer with these symptoms; alternatively, the trigger for referral may have been multiple symptoms, one of which was diarrhoea or constipation. Although the interval between referral and diagnosis for patients referred was relatively short, at 56 and 43 days for surgical and gastrointestinal clinics, respectively, this was not short enough for the 10 patients who developed a surgical emergency while already in the secondary care system. We cannot know how many of those patients could have been identified and their emergency pre-empted by earlier diagnosis, but this is clearly an area with potential for improvement.
Only one-third of patients had a haemoglobin estimation in primary care, despite the importance of identifying anaemia when colorectal cancer is a possibility. Fewer had faecal occult blood testing. These tests may not be appropriate in all circumstances, for example if referral is already indicated because of a rectal or abdominal mass. However, it is possible that testing haemoglobin and faecal occult bloods in patients with symptoms of possible colorectal cancer in a more systematic fashion would allow earlier referral. Patients with positive faecal occult blood tests and those with anaemia also suffered longer delays in referral.
It is unlikely that all patients will ever be fitted into simple referral pathways. However, our study shows that a minority of patients are travelling along the route that has been given most resources. This may be improved by increased testing in primary care, further refinement of referral guidelines and research to identify patients at risk of a surgical emergency.
Declaration
Ethical approval: not required as this was an audit.
Funding: no project funding. JB is funded through an NHS research practice (Barnfield Hill, Exeter) and WH holds an NHS Researcher Development Award.
Conflicts of interest: none.
We wish to thank the GPs from all three cities for their enthusiasm and help.
References
UK Colorectal Cancer Screening Pilot Group. Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom.
Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location.
Kemppainen M, Raiha I, Rajala T, Sourander L. Delay in diagnosis of colorectal cancer in elderly patients.
Kyle SM, Isbister WH, Yeong ML. Presentation, duration of symptoms and staging of colorectal carcinoma.
Speights VO, Johnson MW, Stoltenberg PH, Rappaport ES, Helbert B, Riggs M. Colorectal cancer: current trends in initial clinical manifestations.
Umpleby HC, Bristol JB, Rainey JB, Williamson RC. Survival of 727 patients with single carcinomas of the large bowel.
Mulcahy HE, O'Donoghue DP. Duration of colorectal cancer symptoms and survival: the effect of confounding clinical and pathological variables.
Trickett JP, Donaldson DR, Bearn PE, Scott HJ, Hassall AC. A study on the routes of referral for patients with colorectal cancer and its effect on the time to surgery and pathological stage.
Hargarten SW, Richards MJ, Anderson AJ, Roberts MJ. Cancer presentation in the emergency department: a failure of primary care.
Chaplin A, Curless R, Thomson R, Barton R. Prevalence of lower gastrointestinal symptoms and associated consultation behaviour in a British elderly population determined by face-to-face interview.
Jiwa M, Walters S, Mathers N. Referral letters to colorectal surgeons: the impact of peer-mediated feedback.
Metcalf JV, Smith J, Jones R, Record CO. Incidence and causes of rectal bleeding in general practice as detected by colonoscopy.
Department of Health. The NHS Cancer Plan: A Plan for Investment. A Plan for Reform. London: HMSO;
Department of Health. Referral Guidelines for Suspected Cancer. London: Department of Health;
Scottish Executive. Scottish Referral Guidelines for Suspected Cancer. Edinburgh: Scottish Executive;
Boulton-Jones J, Gamble S, Robinson MH, Goddard W, Long R, Teahon K. The impact of the two-week wait scheme for suspected gastrointestinal cancers.
Flashman K, O'Leary DP, Senapati A, Thompson MR. The Department of Health's ‘two week standard’ for bowel cancer: is it working?
Jones R, Rubin G, Hungin P. Is the two week rule for cancer referrals working?
Hamilton W, Round A, Sharp D, Peters T. Clinical features of colorectal cancer before diagnosis: a population-based case-control study.
Hamilton W, Sharp D. Diagnosis of colorectal cancer in primary care: the evidence base for guidelines.
Author notes
aBarnfield Hill Research Practice, Caper Unit, Halford Wing, Dean Clarke House, Exeter EX1 1PQ, UK, bUniversity of Sheffield, Institute of Primary Care and General Practice, Community Science Centre, Northern General Hospital, Sheffield S5 7AU, UK, cDepartment of Primary Health Care, University of Oxford, Old Road Campus, Headington, Oxford OX3 7LF, UK, dAcademic Unit of Primary Health Care, Department of Community Based Medicine, University of Bristol, The Grange, 1 Woodland Road, Bristol BS8 1AU, UK.