-
PDF
- Split View
-
Views
-
Cite
Cite
Kaisu H. Pitkälä, Jouko V. Laurila, Timo E. Strandberg, Reijo S. Tilvis, Multicomponent Geriatric Intervention for Elderly Inpatients With Delirium: A Randomized, Controlled Trial, The Journals of Gerontology: Series A, Volume 61, Issue 2, February 2006, Pages 176–181, https://doi.org/10.1093/gerona/61.2.176
Close - Share Icon Share
Abstract
Background. Delirium is a common syndrome with poor prognosis affecting elderly inpatients. Treatment is mainly based on common sense with wide variations in practice. We investigated whether intensified, multicomponent geriatric treatment could improve the prognosis of delirious patients.
Methods. We performed a randomized, controlled trial of 174 patients with delirium in six general medicine units from an acute hospital in Helsinki, Finland. The intervention group received individually tailored geriatric treatment. The primary endpoint was the sum of those deceased individuals and the patients permanently institutionalized. Secondary endpoints included the number of days in hospitals and other institutions, delirium intensity, and cognition.
Results. The mean age of patients was 83 years, and 31% had previous dementia. The intervention group (N = 87) received significantly more acetylcholinesterase inhibitors (58.6% vs 9.2%), atypical antipsychotics (69.8% vs 30.2%), specialist consultations (49.4% vs 28.7%), hip protectors (88.5% vs 3.4%), physiotherapy (87.4% vs 47.1%), and fewer conventional neuroleptics (8.0% vs 23.0%) than did the control group (N = 87). During the 1-year follow-up, 60.9% of the intervention group and 64.4% of controls were either deceased or permanently institutionalized (p =.638). The intervention group spent a mean of 126 days in institutions, and the control group 140 days (p =.688). Delirium was, however, alleviated more rapidly during hospitalization, and cognition improved significantly at 6 months in the intervention group.
Conclusions. Faster alleviation of delirium and improved cognition justify good, comprehensive geriatric care for these patients although treatment produced no significant improvements in hard endpoints of prognosis.
Delirium is a serious neuropsychiatric syndrome complicating somatic illnesses. It affects approximately 25% of older medical inpatients (1). Prospective studies have related delirium to increased mortality (2–4), need for permanent institutional care (4–7), longer hospital stays (4–7), poor functional outcome (7,8), and dementia (2,8). Especially, delirium has been shown to have an independent effect on mortality (2–4) and admissions to permanent institutional care (4). Increased need for hospital and institutional days produces substantial costs for health and social care (9).
Prevention of delirium by comprehensive geriatric intervention has been effective (9,10). Prevention has, however, left 10%–30% of elderly inpatients suffering from delirium (9,10) with little known about their best possible treatment. Only a few randomized studies have focused on treatment of full-blown delirium. Cole and colleagues showed that geriatric consultation for delirious inpatients may be effective in more rapidly improving their cognition (11), but showed no effect on mortality, institutionalization, or length of hospital stay (11,12). Treatment of delirium is thus mainly based on common sense, with large variations in practice (13).
A fairly large body of evidence indicates that comprehensive geriatric assessment and intervention are effective (14). Since the studies by Cole and colleagues, more evidence has accumulated on pathophysiologic mechanisms of delirium. Central cholinergic deficiency with simultaneous relative dopamine excess may play a central role in the development of delirium (15,16). Thus, acetylcholinesterase inhibitors (ChEIs) and small-dose dopamine antagonists might be effective in treating symptoms of delirium.
The aim of this randomized controlled study was to investigate whether a comprehensive geriatric assessment and individually tailored treatment are effective in reducing mortality and permanent institutional care among patients with delirium. We also wanted to determine whether this treatment is beneficial in reducing the number of days spent in institutions, alleviating delirium, or improving cognition or physical functioning of these patients.
Methods
Setting and Participants
Potential participants were consecutive patients (>69 years) admitted to the general medicine service at one Helsinki City hospital from September 20, 2001 through November 24, 2002. This hospital, with 156 acute beds and six units for general medicine, serves a population (>100,000 inhabitants) of the western area of Helsinki. Systematic methods on screening or preventing delirium are not used in this hospital.
Exclusion criteria included life expectancy of less than 6 months (e.g., metastatic cancer, severe stroke), inability to obtain informed consent within 2 working days, admission from permanent institutional care to the hospital, or refusal. Because all participants were delirious, informed consent was obtained from each patient's closest proxy (100%). The study was approved by Helsinki University Hospital and the Helsinki City ethics committees.
Screening and Confirming Delirium
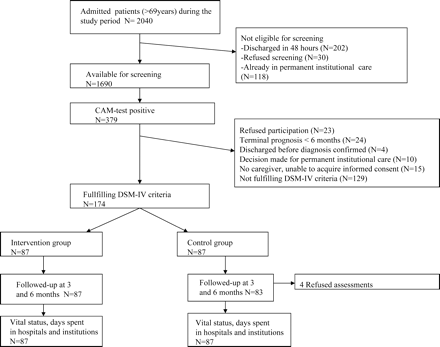
Patients were screened within 2 working days of their admission by two study nurses who had undergone detailed training and followed standard procedures. The initial screening included the Confusion Assessment Method (17), Mini-Mental State Examination (MMSE) (18), Digit span (19), a proxy interview, and a review of medical records (Figure 1). Those patients with a positive result on the Confusion Assessment Method test were assessed by the study physician, who confirmed the inclusion criteria and the diagnosis of delirium by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (20).
Design and Randomization
Patients were randomly allocated by means of computer-generated random numbers to undergo the intervention or to receive the usual care. When delirium was confirmed, the study assistant called by telephone to a randomization staff member who had not seen the patients or their clinical records. She assigned the next number from the computer and the group assignment to the patient.
Assessments
All participants underwent interviews and assessments at baseline, at 3 months, and at 6 months. These included assessment of cognitive functioning by the MMSE (18), of activities of daily living by the Barthel index (21) and the instrumental activities of daily living scale (22), of depression by the Geriatric Depression Scale (23), and of nutrition by the Mini-Nutritional Assessment (24). Severity of delirium was measured daily by the Memorial Delirium Assessment Scale (MDAS) (scored 0–30, 30 being worst; 25) during the first week of hospitalization and every second day thereafter.
Each patient's proxy was interviewed at baseline, at 3 months, and at 6 months concerning the patient's prior cognition, functioning, and symptoms. This information helped us to complete the Barthel index and the instrumental activities of daily living and Mini-Nutritional Assessment scales. The patients' medical records were carefully reviewed to obtain data on medical comorbidities and a list of medications. Comorbidity was assessed by the Charlson comorbidity index, a weighted index taking into account the number and severity of comorbid conditions (26).
Each patient's premorbid dementia status was based on information from interviews of proxies, the Clinical Dementia Rating Scale (27), DSM-IV criteria of dementia (20), and reviews of medical records confirming whether patients had undergone full assessment for diagnosis of dementia. If the patient fulfilled the dementia criteria of DSM-IV, scored 2 or 3 on the Clinical Dementia Rating Scale, or had a dementia diagnosis made by a specialist who used all standard diagnostic tests, then the patient was considered to have premorbid dementia.
The patients were followed-up for 1 year. Four patients from the control group refused follow-up assessments but allowed us to retrieve from central registries the status of their primary endpoint measures and their use of health and social services. Thus, the data on vital status and the number of days spent in hospitals and institutions were 100% complete.
Intervention
Table 1 describes all details of the intervention. Comprehensive geriatric assessment was performed at baseline. Particular attention was paid to careful diagnostics of the underlying etiological conditions. Only severely agitated patients received intramuscular haloperidol. A few effective general treatments were implemented for all patients (28–30). After the acute phase of delirium, all patients not recovering from impaired cognition (MMSE < 24) in the intervention group underwent detailed diagnostics of dementia with brain magnetic resonance imaging (MRI) or computed tomography (CT) scans and thereafter received ChEIs.
Outcome Measures
The primary endpoint was a combined endpoint (the number of patients discharged to permanent institutional care or deceased). In Helsinki, a decision for permanent institutional care leads to economic changes in the patient's status, so the decision is officially recorded in electronic medical records. In addition, we counted the days spent in an acute hospital during the delirium episode, total number of days in acute hospitals during the 1-year follow-up, total number of days spent in any institution (hospital or permanent institutional care) and in permanent institutional care during the follow-up year, and the number of days spent in community care before permanent institutional care. Mortality data were determined from medical records and central registries, and all days spent in institutions during the 1-year follow-up were retrieved from patients' medical records in all their area hospitals and from social care registries. Changes in the intensity of delirium were determined with the MDAS (25), in cognition with the MMSE (18), and in physical functioning with the Barthel index (21).
Statistical Methods
Sample size was calculated based on the data from several prognostic studies with similar populations (4,6,8). The combined endpoint range has varied between 60% and 75%. With a type I error of 5% and with 80% power, we would need 58–91 patients in each group to show a 20% difference between groups.
Data were analyzed by the NCSS for Windows statistical program (www.ncss.com). Patients in the intervention group were compared with those in the control group according to baseline variables and various types of treatments (with χ2 test or Fisher's exact test for categorical variables, and the Mann–Whitney U test for continuous variables). Endpoints were compared between the groups on an “intention-to-treat” basis. Proportions of those patients who were deceased or institutionalized during the follow-up year in the intervention group were compared (using the χ2 test) with the proportion of those patients from the control group. Days spent in acute hospitals, total number of days in any institutions, and number of days in permanent institutional care or in community care were compared between the groups with the Mann–Whitney U test. Confidence intervals were calculated (31). Analysis of variance for repeated measures was used to detect differences in changes in MMSE scores, and of the Barthel index between the groups. Baseline scores were used as covariates in these analyses. Those values not normally distributed were logarithmically transformed for these operations. A sustained improvement of four points or more in the MDAS score was used to detect significant improvement in symptoms of delirium. Product limit estimate was used to illustrate information on the cumulative proportions improving in symptoms of delirium. The proportional hazards model was used to identify adjusted differences between groups. In post hoc analyses we used the Cox proportional hazards model to illustrate the significance of various variables and treatments on the prognosis.
Results
Patients were old and frail with a mean age of 83 years; and 31% of patients had prior dementia (Table 2). The groups were balanced at baseline.
We detected a high number of probable etiological causes for delirium (N = 193) in the intervention group. Infections, metabolic conditions, and medications were the most common. Each patient had often 2–3 simultaneous etiological conditions causing or worsening delirium. During the patients' index hospitalization, delirium contributed to a high number of complications, e.g., 16.1% of patients in the intervention group developed new fractures during their hospitalization (19.1% in the control group).
The contents of treatment differed significantly between the groups (Table 3). The intervention group received significantly more atypical antipsychotics, fewer conventional neuroleptics, and more ChEIs than did the control group. They also received more calcium–vitamin D supplements and protein supplements, and used more hip protectors. The intervention group also received more physical therapy during their hospitalization, and received more specialist consultations.
The prognosis of all patients was very poor, with no significant differences between groups in primary endpoints (Table 4). During the follow-up year, 60.0% of the patients in the intervention group versus 64.4% in the control group (p =.638) had either died or were admitted to permanent institutional care; 42.5% in the intervention group and 51.7% in the control group had been admitted to permanent institutional care (p =.224). The results were essentially the same when early deaths (<11 days) were omitted from the analyses (p =.210; log rank test). Before death or admission to permanent institutional care, patients in the intervention group and control group were in community care (at home or acute care) for a mean of 237 and 202 days, respectively (p =.060). However, this difference was compensated for by the higher number of days spent in acute hospitals—52 in the intervention group and 42 in the control group (p =.032). The total number of institutional days did not differ between groups.
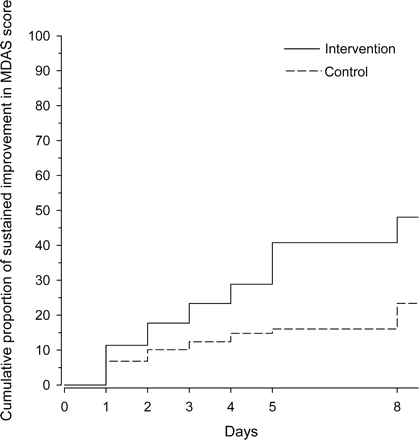
The intensity and symptoms of delirium were alleviated significantly faster in the intervention group (Figure 2). Cognition, according to MMSE score, had significantly improved at 6 months after the baseline in the intervention group than in the control group (18.4 vs 15.8, p =.047). There were no differences between groups as to change in physical functioning according to the Barthel index (Table 5).
Discussion
In this very frail patient group, intensified multicomponent geriatric treatment did alleviate symptoms of delirium earlier than in the control group, and improved cognition of survivors in the intervention group in 6 months of follow-up. We were, however, unable to influence the major endpoints, mortality or the proportion of patients admitted to permanent institutional care. A trend did appear toward a lower number of days spent in permanent institutional care for the intervention group but was compensated for by their higher number of days in acute hospitals.
Our trial includes novel aspects compared with the two previously published trials (11,12). In addition to our geriatric consultation and tailored treatment of delirium, our intervention included theoretically interesting ChEIs and atypical antipsychotics. We hypothesized that these drugs might prevent complications related to hyperactive delirium and development of dementia. Along with the comprehensive geriatric care, they may have had a role in reducing the length of delirium during the hospitalization and improving cognition at 6 months. There are theories of pathophysiology (15,16) and also intervention studies (32,33) supporting this hypothesis, but further testing of their effectiveness is needed. Delirium causes great suffering for patients (34). Therefore, the reduced duration of delirium probably has a significant impact on these patients' quality of life. The same applies for the improved cognition. Unfortunately, our intervention did not improve patients' general prognosis. Our results support those produced by a Canadian research group (11,12), who showed in their earlier study that the cognition of delirious patients may be improved faster by geriatric multicomponent treatment. However, neither could they show any effect on mortality, institutionalization, or length of hospital stay.
The intervention group received treatments different from those of the control group (Table 3). Hip protectors (28), protein supplements (29), and calcium–vitamin D supplements (30) were a part of the study design because they have been shown to be effective in frail patients. We succeeded in achieving a high adherence to these treatments.
Only 28% of the control patients had a diagnosis of delirium or confusion in their medical records; this figure was similar to those in prior studies showing underdetection of delirium (35). We did not particularly point out the recognized control cases of delirium for the attending physicians although they might have noticed them from the study nurses assessing them daily. The under-recognition of delirium may explain the vast differences in the treatments and the somewhat more rapid discharge of control patients.
In the case of full-blown delirium, our type of intervention may be called “too little too late” to produce a significant difference in prognosis. In view of this, even more effort should be focused on prevention of delirium among such patients (9,10). It may be questioned whether some elements in our intervention have worked unfavorably. Therefore, we performed post hoc analyses to clarify which patient characteristics and which factors in our intervention impacted prognosis. In the Cox proportional hazards model, age, gender, Charlson comorbidity score, poor cognition, impaired physical functioning, and the elements of our intervention were added as shown in Table 3; only a low Barthel index score showed a significant impact on mortality (hazard ratio, 2.1; 95% confidence interval, 1.1–4.0). Nutritional supplements seemed to protect against death (hazard ratio, 0.3; 95% confidence interval, 0.1–0.8). Antipsychotics or ChEIs did not affect mortality.
Some other elements in the design of our study design may, however, have diluted our results. First, the participants with few exclusion criteria included 5% very frail patients dying within a week from recruitment, thus diluting the results. Second, the control group also had normal good care in the same hospital. Our pragmatic trial shows the effect of geriatric consultation in a “real life” situation. Because our intervening geriatrician had to serve as one member of six teams in six units, this sometimes led to compromises that could have been avoided with a single, well-trained geriatric team. A well-trained geriatric team or home-based intervention might have led to different results.
Our study is the third randomized trial showing no effect of geriatric intervention on the prognosis for delirium. Good, comprehensive geriatric treatment is justified in this patient group because of more effective alleviation of delirium and improved cognition. However, individual cases deserve careful tailoring of treatment and evaluation whether they benefit from active, curative treatment or good palliative care.
Decision Editor: Luigi Ferrucci, MD, PhD
Design of the study. CAM = Confusion Assessment Method; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders
Cumulative proportion of patients with sustained improvement in symptoms of delirium. A permanent four point or more improvement in Memorial Delirium Assessment Scale (MDAS) score was considered the cut point for improvement. Baseline adjusted p =.002 between the groups
Contents of Intervention.
| 1. Accurate recognition of delirium with detailed diagnostics of the underlying conditions. |
| 2. Comprehensive geriatric assessment and treatments: |
| – History, interview of the caregiver |
| – Physical examination |
| – Cognition |
| – Physical functioning |
| – Screening of depression |
| – Nutrition |
| – Reviewing medications |
| 3. Avoiding conventional neuroleptics and administering atypical antipsychotics for hyperactive/psychotic symptoms |
| 4. Orientation with calendars, clocks, and photos |
| 5. Physiotherapy |
| 6. General geriatric interventions: |
| – Nutritional supplements for those at risk of malnutrition or malnourished |
| – Calcium + vitamin D supplements |
| – Hip protectors |
| 7. Cholinesterase inhibitors if the patient's cognition did not improve to MMSE >23; treatable causes for dementia or contraindications were screened with CT/MRI scans and laboratory tests |
| 8. Comprehensive discharge planning |
| – Consultation of social worker |
| – Occupational therapist's home visit |
| – Discharge planning with the caregivers |
| 1. Accurate recognition of delirium with detailed diagnostics of the underlying conditions. |
| 2. Comprehensive geriatric assessment and treatments: |
| – History, interview of the caregiver |
| – Physical examination |
| – Cognition |
| – Physical functioning |
| – Screening of depression |
| – Nutrition |
| – Reviewing medications |
| 3. Avoiding conventional neuroleptics and administering atypical antipsychotics for hyperactive/psychotic symptoms |
| 4. Orientation with calendars, clocks, and photos |
| 5. Physiotherapy |
| 6. General geriatric interventions: |
| – Nutritional supplements for those at risk of malnutrition or malnourished |
| – Calcium + vitamin D supplements |
| – Hip protectors |
| 7. Cholinesterase inhibitors if the patient's cognition did not improve to MMSE >23; treatable causes for dementia or contraindications were screened with CT/MRI scans and laboratory tests |
| 8. Comprehensive discharge planning |
| – Consultation of social worker |
| – Occupational therapist's home visit |
| – Discharge planning with the caregivers |
Note: MMSE = Mini-Mental State Examination; CT/MRI = computed tomography/magnetic resonance imaging.
Contents of Intervention.
| 1. Accurate recognition of delirium with detailed diagnostics of the underlying conditions. |
| 2. Comprehensive geriatric assessment and treatments: |
| – History, interview of the caregiver |
| – Physical examination |
| – Cognition |
| – Physical functioning |
| – Screening of depression |
| – Nutrition |
| – Reviewing medications |
| 3. Avoiding conventional neuroleptics and administering atypical antipsychotics for hyperactive/psychotic symptoms |
| 4. Orientation with calendars, clocks, and photos |
| 5. Physiotherapy |
| 6. General geriatric interventions: |
| – Nutritional supplements for those at risk of malnutrition or malnourished |
| – Calcium + vitamin D supplements |
| – Hip protectors |
| 7. Cholinesterase inhibitors if the patient's cognition did not improve to MMSE >23; treatable causes for dementia or contraindications were screened with CT/MRI scans and laboratory tests |
| 8. Comprehensive discharge planning |
| – Consultation of social worker |
| – Occupational therapist's home visit |
| – Discharge planning with the caregivers |
| 1. Accurate recognition of delirium with detailed diagnostics of the underlying conditions. |
| 2. Comprehensive geriatric assessment and treatments: |
| – History, interview of the caregiver |
| – Physical examination |
| – Cognition |
| – Physical functioning |
| – Screening of depression |
| – Nutrition |
| – Reviewing medications |
| 3. Avoiding conventional neuroleptics and administering atypical antipsychotics for hyperactive/psychotic symptoms |
| 4. Orientation with calendars, clocks, and photos |
| 5. Physiotherapy |
| 6. General geriatric interventions: |
| – Nutritional supplements for those at risk of malnutrition or malnourished |
| – Calcium + vitamin D supplements |
| – Hip protectors |
| 7. Cholinesterase inhibitors if the patient's cognition did not improve to MMSE >23; treatable causes for dementia or contraindications were screened with CT/MRI scans and laboratory tests |
| 8. Comprehensive discharge planning |
| – Consultation of social worker |
| – Occupational therapist's home visit |
| – Discharge planning with the caregivers |
Note: MMSE = Mini-Mental State Examination; CT/MRI = computed tomography/magnetic resonance imaging.
Baseline Characteristics.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 83.8 (5.6) | 83.3 (6.2) | .530* | |||
| Females, % | 75.9 | 71.3 | .492† | |||
| Widowed, % | 49.4 | 50.6 | .935† | |||
| Education, ≤ primary school, % | 46.5 | 54.0 | .255† | |||
| Use of alcohol > once a week, % | 9.2 | 8.0 | .787† | |||
| Prior dementia, % | 26.4 | 34.5 | .249† | |||
| Charlson comorbidity index, mean (SD) | 2.6 (2.0) | 2.3 (1.9) | .272* | |||
| Admission diagnoses, % | ||||||
| Infections | 33.3 | 25.3 | .244† | |||
| Trauma/fracture | 23.0 | 24.1 | .858† | |||
| Postoperative/pain | 8.0 | 9.2 | .787† | |||
| Cardiovascular disorders | 18.4 | 19.5 | .847† | |||
| Metabolic disturbances | 5.7 | 5.7 | 1.000† | |||
| Neurological disorders | 4.6 | 6.9 | .515† | |||
| Other | 5.7 | 9.2 | .387† | |||
| Drugs at admission, % | ||||||
| Conventional neuroleptic | 21.8 | 23.7 | .719† | |||
| Atypical antipsychotic | 14.9 | 13.8 | .829† | |||
| Cholinesterase inhibitor | 4.6 | 6.9 | .515† | |||
| Systolic BP, mean (SD) | 131.3 (23.4) | 133.1 (22.1) | .496* | |||
| Diastolic BP, mean (SD) | 71.2 (11.3) | 71.7 (11.7) | .785* | |||
| Pulse, mean (SD) | 73.5 (11.4) | 74.9 (13.1) | .353* | |||
| MNA >23.5 (well-nourished), % | 9.2 | 13.8 | .245† | |||
| BMI <19 kg/m2, % | 27.5 | 31.0 | .617† | |||
| Depression: GDS, mean (SD) | 6.0 (2.9) | 5.3 (3.4) | .08* | |||
| Physical functioning: Barthel index, mean (SD) | 79.0 (20.9) | 79.1 (18.4) | .63* | |||
| Instrumental activities of daily living, mean (SD) | 3.5 (6.5) | 2.8 (2.3) | .608* | |||
| Clinical Dementia Rating Scale >1, % | 10.3 | 12.6 | .635† | |||
| Cognition: MMSE, mean (SD) | 15.2 (5.1) | 13.5 (5.4) | .052* | |||
| Delirium intensity: MDAS, mean (SD) | 12.3 (4.8) | 12.8 (5.3) | .522* | |||
| Mean number of medications (SD) | 7.8 (4.0) | 6.8 (3.5) | .118* | |||
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 83.8 (5.6) | 83.3 (6.2) | .530* | |||
| Females, % | 75.9 | 71.3 | .492† | |||
| Widowed, % | 49.4 | 50.6 | .935† | |||
| Education, ≤ primary school, % | 46.5 | 54.0 | .255† | |||
| Use of alcohol > once a week, % | 9.2 | 8.0 | .787† | |||
| Prior dementia, % | 26.4 | 34.5 | .249† | |||
| Charlson comorbidity index, mean (SD) | 2.6 (2.0) | 2.3 (1.9) | .272* | |||
| Admission diagnoses, % | ||||||
| Infections | 33.3 | 25.3 | .244† | |||
| Trauma/fracture | 23.0 | 24.1 | .858† | |||
| Postoperative/pain | 8.0 | 9.2 | .787† | |||
| Cardiovascular disorders | 18.4 | 19.5 | .847† | |||
| Metabolic disturbances | 5.7 | 5.7 | 1.000† | |||
| Neurological disorders | 4.6 | 6.9 | .515† | |||
| Other | 5.7 | 9.2 | .387† | |||
| Drugs at admission, % | ||||||
| Conventional neuroleptic | 21.8 | 23.7 | .719† | |||
| Atypical antipsychotic | 14.9 | 13.8 | .829† | |||
| Cholinesterase inhibitor | 4.6 | 6.9 | .515† | |||
| Systolic BP, mean (SD) | 131.3 (23.4) | 133.1 (22.1) | .496* | |||
| Diastolic BP, mean (SD) | 71.2 (11.3) | 71.7 (11.7) | .785* | |||
| Pulse, mean (SD) | 73.5 (11.4) | 74.9 (13.1) | .353* | |||
| MNA >23.5 (well-nourished), % | 9.2 | 13.8 | .245† | |||
| BMI <19 kg/m2, % | 27.5 | 31.0 | .617† | |||
| Depression: GDS, mean (SD) | 6.0 (2.9) | 5.3 (3.4) | .08* | |||
| Physical functioning: Barthel index, mean (SD) | 79.0 (20.9) | 79.1 (18.4) | .63* | |||
| Instrumental activities of daily living, mean (SD) | 3.5 (6.5) | 2.8 (2.3) | .608* | |||
| Clinical Dementia Rating Scale >1, % | 10.3 | 12.6 | .635† | |||
| Cognition: MMSE, mean (SD) | 15.2 (5.1) | 13.5 (5.4) | .052* | |||
| Delirium intensity: MDAS, mean (SD) | 12.3 (4.8) | 12.8 (5.3) | .522* | |||
| Mean number of medications (SD) | 7.8 (4.0) | 6.8 (3.5) | .118* | |||
Note: *Mann–Whitney U test.
†Chi-square test.
SD = standard deviation; BP = blood pressure; MNA = Mini-Nutritional Assessment; BMI = body mass index; GDS = Geriatric Depression Scale; MMSE = Mini-Mental State Examination; MDAS = Memorial Delirium Assessment Scale.
Baseline Characteristics.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 83.8 (5.6) | 83.3 (6.2) | .530* | |||
| Females, % | 75.9 | 71.3 | .492† | |||
| Widowed, % | 49.4 | 50.6 | .935† | |||
| Education, ≤ primary school, % | 46.5 | 54.0 | .255† | |||
| Use of alcohol > once a week, % | 9.2 | 8.0 | .787† | |||
| Prior dementia, % | 26.4 | 34.5 | .249† | |||
| Charlson comorbidity index, mean (SD) | 2.6 (2.0) | 2.3 (1.9) | .272* | |||
| Admission diagnoses, % | ||||||
| Infections | 33.3 | 25.3 | .244† | |||
| Trauma/fracture | 23.0 | 24.1 | .858† | |||
| Postoperative/pain | 8.0 | 9.2 | .787† | |||
| Cardiovascular disorders | 18.4 | 19.5 | .847† | |||
| Metabolic disturbances | 5.7 | 5.7 | 1.000† | |||
| Neurological disorders | 4.6 | 6.9 | .515† | |||
| Other | 5.7 | 9.2 | .387† | |||
| Drugs at admission, % | ||||||
| Conventional neuroleptic | 21.8 | 23.7 | .719† | |||
| Atypical antipsychotic | 14.9 | 13.8 | .829† | |||
| Cholinesterase inhibitor | 4.6 | 6.9 | .515† | |||
| Systolic BP, mean (SD) | 131.3 (23.4) | 133.1 (22.1) | .496* | |||
| Diastolic BP, mean (SD) | 71.2 (11.3) | 71.7 (11.7) | .785* | |||
| Pulse, mean (SD) | 73.5 (11.4) | 74.9 (13.1) | .353* | |||
| MNA >23.5 (well-nourished), % | 9.2 | 13.8 | .245† | |||
| BMI <19 kg/m2, % | 27.5 | 31.0 | .617† | |||
| Depression: GDS, mean (SD) | 6.0 (2.9) | 5.3 (3.4) | .08* | |||
| Physical functioning: Barthel index, mean (SD) | 79.0 (20.9) | 79.1 (18.4) | .63* | |||
| Instrumental activities of daily living, mean (SD) | 3.5 (6.5) | 2.8 (2.3) | .608* | |||
| Clinical Dementia Rating Scale >1, % | 10.3 | 12.6 | .635† | |||
| Cognition: MMSE, mean (SD) | 15.2 (5.1) | 13.5 (5.4) | .052* | |||
| Delirium intensity: MDAS, mean (SD) | 12.3 (4.8) | 12.8 (5.3) | .522* | |||
| Mean number of medications (SD) | 7.8 (4.0) | 6.8 (3.5) | .118* | |||
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | |||
|---|---|---|---|---|---|---|
| Mean age (SD) | 83.8 (5.6) | 83.3 (6.2) | .530* | |||
| Females, % | 75.9 | 71.3 | .492† | |||
| Widowed, % | 49.4 | 50.6 | .935† | |||
| Education, ≤ primary school, % | 46.5 | 54.0 | .255† | |||
| Use of alcohol > once a week, % | 9.2 | 8.0 | .787† | |||
| Prior dementia, % | 26.4 | 34.5 | .249† | |||
| Charlson comorbidity index, mean (SD) | 2.6 (2.0) | 2.3 (1.9) | .272* | |||
| Admission diagnoses, % | ||||||
| Infections | 33.3 | 25.3 | .244† | |||
| Trauma/fracture | 23.0 | 24.1 | .858† | |||
| Postoperative/pain | 8.0 | 9.2 | .787† | |||
| Cardiovascular disorders | 18.4 | 19.5 | .847† | |||
| Metabolic disturbances | 5.7 | 5.7 | 1.000† | |||
| Neurological disorders | 4.6 | 6.9 | .515† | |||
| Other | 5.7 | 9.2 | .387† | |||
| Drugs at admission, % | ||||||
| Conventional neuroleptic | 21.8 | 23.7 | .719† | |||
| Atypical antipsychotic | 14.9 | 13.8 | .829† | |||
| Cholinesterase inhibitor | 4.6 | 6.9 | .515† | |||
| Systolic BP, mean (SD) | 131.3 (23.4) | 133.1 (22.1) | .496* | |||
| Diastolic BP, mean (SD) | 71.2 (11.3) | 71.7 (11.7) | .785* | |||
| Pulse, mean (SD) | 73.5 (11.4) | 74.9 (13.1) | .353* | |||
| MNA >23.5 (well-nourished), % | 9.2 | 13.8 | .245† | |||
| BMI <19 kg/m2, % | 27.5 | 31.0 | .617† | |||
| Depression: GDS, mean (SD) | 6.0 (2.9) | 5.3 (3.4) | .08* | |||
| Physical functioning: Barthel index, mean (SD) | 79.0 (20.9) | 79.1 (18.4) | .63* | |||
| Instrumental activities of daily living, mean (SD) | 3.5 (6.5) | 2.8 (2.3) | .608* | |||
| Clinical Dementia Rating Scale >1, % | 10.3 | 12.6 | .635† | |||
| Cognition: MMSE, mean (SD) | 15.2 (5.1) | 13.5 (5.4) | .052* | |||
| Delirium intensity: MDAS, mean (SD) | 12.3 (4.8) | 12.8 (5.3) | .522* | |||
| Mean number of medications (SD) | 7.8 (4.0) | 6.8 (3.5) | .118* | |||
Note: *Mann–Whitney U test.
†Chi-square test.
SD = standard deviation; BP = blood pressure; MNA = Mini-Nutritional Assessment; BMI = body mass index; GDS = Geriatric Depression Scale; MMSE = Mini-Mental State Examination; MDAS = Memorial Delirium Assessment Scale.
Percentage of Patients Receiving Various Types of Treatments, Specialist Consultations, or Brain Scans During Their Initial Hospital Stay.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Atypical antipsychotics | 69.0 | 29.9 | <.001 |
| Conventional neuroleptics | 8.0 | 23.0 | .006 |
| Acetylcholinesterase inhibitors | 58.6 | 9.2 | <.001 |
| Vitamin D–calcium supplements | 77.0 | 9.2 | <.001 |
| Nutritional supplements | 92.0 | 0.0 | <.001 |
| Hip protectors | 90.8 | 1.1 | <.001 |
| Physical therapy | 89.7 | 44.8 | <.001 |
| Specialist consultations | 49.4 | 28.7 | .005 |
| CT or MRI scans | 51.7 | 8.0 | <.001 |
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Atypical antipsychotics | 69.0 | 29.9 | <.001 |
| Conventional neuroleptics | 8.0 | 23.0 | .006 |
| Acetylcholinesterase inhibitors | 58.6 | 9.2 | <.001 |
| Vitamin D–calcium supplements | 77.0 | 9.2 | <.001 |
| Nutritional supplements | 92.0 | 0.0 | <.001 |
| Hip protectors | 90.8 | 1.1 | <.001 |
| Physical therapy | 89.7 | 44.8 | <.001 |
| Specialist consultations | 49.4 | 28.7 | .005 |
| CT or MRI scans | 51.7 | 8.0 | <.001 |
Note: *Chi-square test.
CT = computed tomography; MRI = magnetic resonance imaging.
Percentage of Patients Receiving Various Types of Treatments, Specialist Consultations, or Brain Scans During Their Initial Hospital Stay.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Atypical antipsychotics | 69.0 | 29.9 | <.001 |
| Conventional neuroleptics | 8.0 | 23.0 | .006 |
| Acetylcholinesterase inhibitors | 58.6 | 9.2 | <.001 |
| Vitamin D–calcium supplements | 77.0 | 9.2 | <.001 |
| Nutritional supplements | 92.0 | 0.0 | <.001 |
| Hip protectors | 90.8 | 1.1 | <.001 |
| Physical therapy | 89.7 | 44.8 | <.001 |
| Specialist consultations | 49.4 | 28.7 | .005 |
| CT or MRI scans | 51.7 | 8.0 | <.001 |
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Atypical antipsychotics | 69.0 | 29.9 | <.001 |
| Conventional neuroleptics | 8.0 | 23.0 | .006 |
| Acetylcholinesterase inhibitors | 58.6 | 9.2 | <.001 |
| Vitamin D–calcium supplements | 77.0 | 9.2 | <.001 |
| Nutritional supplements | 92.0 | 0.0 | <.001 |
| Hip protectors | 90.8 | 1.1 | <.001 |
| Physical therapy | 89.7 | 44.8 | <.001 |
| Specialist consultations | 49.4 | 28.7 | .005 |
| CT or MRI scans | 51.7 | 8.0 | <.001 |
Note: *Chi-square test.
CT = computed tomography; MRI = magnetic resonance imaging.
Primary Endpoints and Use of Institutional Care.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | 95% Confidence Intervals for Differences Between Proportions or Medians . | ||||
|---|---|---|---|---|---|---|---|---|
| Admitted to permanent institutional care, % | 42.5 | 51.7 | .224* | −24.0 to 5.6 | ||||
| Deceased, % | 34.5 | 29.9 | .516* | −9.3 to 18.5 | ||||
| Institutionalized or deceased, % | 60.9 | 64.4 | .638* | −17.8 to 10.9 | ||||
| No. of days in acute hospital during the delirium | ||||||||
| Mean (SD) | 29.3 (25.6) | 22.4 (18.4) | ||||||
| Median (Min-Max) | 21 (2–110) | 16 (1–90) | .171† | −9 to 1 | ||||
| No. of days before permanent institutional care | ||||||||
| Mean (SD) | 236.5 (140.1) | 202.1 (153.5) | ||||||
| Median (Min-Max) | 354 (21–365) | 216.5 (9–365) | .060† | −39 to 0 | ||||
| No. of days before permanent institutional care or death | ||||||||
| Mean (SD) | 202.9 (147.2) | 182.2 (153.3) | ||||||
| Median (Min-Max) | 181 (3–365) | 99 (5–365) | .189† | −29 to 0 | ||||
| No. of acute hospital days during the follow-up year | ||||||||
| Mean (SD) | 52.5 (37.2) | 42.3 (35.5) | ||||||
| Median (Min-Max) | 47 (2–202) | 30 (2–176) | .032† | −19 to −1 | ||||
| No. of days in long-term care during the follow-up year | ||||||||
| Mean (SD) | 73.0 (112.6) | 97.6 (129.8) | ||||||
| Median (Min-Max) | 0 (0–346) | 0 (0–352) | .179† | 0 to 0 | ||||
| No. of days in any institution during the follow-up year | ||||||||
| Mean (SD) | 125.7 (128.6) | 139.9 (138.2) | ||||||
| Median (Min-Max) | 61 (2–365) | 77 (2–365) | .688† | −13 to 23 | ||||
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | 95% Confidence Intervals for Differences Between Proportions or Medians . | ||||
|---|---|---|---|---|---|---|---|---|
| Admitted to permanent institutional care, % | 42.5 | 51.7 | .224* | −24.0 to 5.6 | ||||
| Deceased, % | 34.5 | 29.9 | .516* | −9.3 to 18.5 | ||||
| Institutionalized or deceased, % | 60.9 | 64.4 | .638* | −17.8 to 10.9 | ||||
| No. of days in acute hospital during the delirium | ||||||||
| Mean (SD) | 29.3 (25.6) | 22.4 (18.4) | ||||||
| Median (Min-Max) | 21 (2–110) | 16 (1–90) | .171† | −9 to 1 | ||||
| No. of days before permanent institutional care | ||||||||
| Mean (SD) | 236.5 (140.1) | 202.1 (153.5) | ||||||
| Median (Min-Max) | 354 (21–365) | 216.5 (9–365) | .060† | −39 to 0 | ||||
| No. of days before permanent institutional care or death | ||||||||
| Mean (SD) | 202.9 (147.2) | 182.2 (153.3) | ||||||
| Median (Min-Max) | 181 (3–365) | 99 (5–365) | .189† | −29 to 0 | ||||
| No. of acute hospital days during the follow-up year | ||||||||
| Mean (SD) | 52.5 (37.2) | 42.3 (35.5) | ||||||
| Median (Min-Max) | 47 (2–202) | 30 (2–176) | .032† | −19 to −1 | ||||
| No. of days in long-term care during the follow-up year | ||||||||
| Mean (SD) | 73.0 (112.6) | 97.6 (129.8) | ||||||
| Median (Min-Max) | 0 (0–346) | 0 (0–352) | .179† | 0 to 0 | ||||
| No. of days in any institution during the follow-up year | ||||||||
| Mean (SD) | 125.7 (128.6) | 139.9 (138.2) | ||||||
| Median (Min-Max) | 61 (2–365) | 77 (2–365) | .688† | −13 to 23 | ||||
Note: *Chi-square test.
†Mann–Whitney U test.
SD = standard deviation.
Primary Endpoints and Use of Institutional Care.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | 95% Confidence Intervals for Differences Between Proportions or Medians . | ||||
|---|---|---|---|---|---|---|---|---|
| Admitted to permanent institutional care, % | 42.5 | 51.7 | .224* | −24.0 to 5.6 | ||||
| Deceased, % | 34.5 | 29.9 | .516* | −9.3 to 18.5 | ||||
| Institutionalized or deceased, % | 60.9 | 64.4 | .638* | −17.8 to 10.9 | ||||
| No. of days in acute hospital during the delirium | ||||||||
| Mean (SD) | 29.3 (25.6) | 22.4 (18.4) | ||||||
| Median (Min-Max) | 21 (2–110) | 16 (1–90) | .171† | −9 to 1 | ||||
| No. of days before permanent institutional care | ||||||||
| Mean (SD) | 236.5 (140.1) | 202.1 (153.5) | ||||||
| Median (Min-Max) | 354 (21–365) | 216.5 (9–365) | .060† | −39 to 0 | ||||
| No. of days before permanent institutional care or death | ||||||||
| Mean (SD) | 202.9 (147.2) | 182.2 (153.3) | ||||||
| Median (Min-Max) | 181 (3–365) | 99 (5–365) | .189† | −29 to 0 | ||||
| No. of acute hospital days during the follow-up year | ||||||||
| Mean (SD) | 52.5 (37.2) | 42.3 (35.5) | ||||||
| Median (Min-Max) | 47 (2–202) | 30 (2–176) | .032† | −19 to −1 | ||||
| No. of days in long-term care during the follow-up year | ||||||||
| Mean (SD) | 73.0 (112.6) | 97.6 (129.8) | ||||||
| Median (Min-Max) | 0 (0–346) | 0 (0–352) | .179† | 0 to 0 | ||||
| No. of days in any institution during the follow-up year | ||||||||
| Mean (SD) | 125.7 (128.6) | 139.9 (138.2) | ||||||
| Median (Min-Max) | 61 (2–365) | 77 (2–365) | .688† | −13 to 23 | ||||
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value . | 95% Confidence Intervals for Differences Between Proportions or Medians . | ||||
|---|---|---|---|---|---|---|---|---|
| Admitted to permanent institutional care, % | 42.5 | 51.7 | .224* | −24.0 to 5.6 | ||||
| Deceased, % | 34.5 | 29.9 | .516* | −9.3 to 18.5 | ||||
| Institutionalized or deceased, % | 60.9 | 64.4 | .638* | −17.8 to 10.9 | ||||
| No. of days in acute hospital during the delirium | ||||||||
| Mean (SD) | 29.3 (25.6) | 22.4 (18.4) | ||||||
| Median (Min-Max) | 21 (2–110) | 16 (1–90) | .171† | −9 to 1 | ||||
| No. of days before permanent institutional care | ||||||||
| Mean (SD) | 236.5 (140.1) | 202.1 (153.5) | ||||||
| Median (Min-Max) | 354 (21–365) | 216.5 (9–365) | .060† | −39 to 0 | ||||
| No. of days before permanent institutional care or death | ||||||||
| Mean (SD) | 202.9 (147.2) | 182.2 (153.3) | ||||||
| Median (Min-Max) | 181 (3–365) | 99 (5–365) | .189† | −29 to 0 | ||||
| No. of acute hospital days during the follow-up year | ||||||||
| Mean (SD) | 52.5 (37.2) | 42.3 (35.5) | ||||||
| Median (Min-Max) | 47 (2–202) | 30 (2–176) | .032† | −19 to −1 | ||||
| No. of days in long-term care during the follow-up year | ||||||||
| Mean (SD) | 73.0 (112.6) | 97.6 (129.8) | ||||||
| Median (Min-Max) | 0 (0–346) | 0 (0–352) | .179† | 0 to 0 | ||||
| No. of days in any institution during the follow-up year | ||||||||
| Mean (SD) | 125.7 (128.6) | 139.9 (138.2) | ||||||
| Median (Min-Max) | 61 (2–365) | 77 (2–365) | .688† | −13 to 23 | ||||
Note: *Chi-square test.
†Mann–Whitney U test.
SD = standard deviation.
Changes in Cognition and Physical Functioning During the Follow-Up Year.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Mean MMSE, baseline | 15.2 | 13.5 | |
| 3 months | 18.6 (N = 67) | 18.3 (N = 61) | |
| 6 months | 18.4 (N = 74) | 15.8 (N = 72) | .047 |
| Mean Barthel index, baseline | 79.0 | 79.1 | |
| 3 months | 64.4 (N = 70) | 65.1 (N = 68) | |
| 6 months | 70.2 (N = 77) | 63.8 (N = 78) | .144 |
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Mean MMSE, baseline | 15.2 | 13.5 | |
| 3 months | 18.6 (N = 67) | 18.3 (N = 61) | |
| 6 months | 18.4 (N = 74) | 15.8 (N = 72) | .047 |
| Mean Barthel index, baseline | 79.0 | 79.1 | |
| 3 months | 64.4 (N = 70) | 65.1 (N = 68) | |
| 6 months | 70.2 (N = 77) | 63.8 (N = 78) | .144 |
Notes: *Analysis of variance for repeated measures. The baseline scores were used as covariates.
MMSE = Mini-Mental State Examination.
Changes in Cognition and Physical Functioning During the Follow-Up Year.
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Mean MMSE, baseline | 15.2 | 13.5 | |
| 3 months | 18.6 (N = 67) | 18.3 (N = 61) | |
| 6 months | 18.4 (N = 74) | 15.8 (N = 72) | .047 |
| Mean Barthel index, baseline | 79.0 | 79.1 | |
| 3 months | 64.4 (N = 70) | 65.1 (N = 68) | |
| 6 months | 70.2 (N = 77) | 63.8 (N = 78) | .144 |
| Variable . | Intervention (N = 87) . | Control (N = 87) . | p Value* . |
|---|---|---|---|
| Mean MMSE, baseline | 15.2 | 13.5 | |
| 3 months | 18.6 (N = 67) | 18.3 (N = 61) | |
| 6 months | 18.4 (N = 74) | 15.8 (N = 72) | .047 |
| Mean Barthel index, baseline | 79.0 | 79.1 | |
| 3 months | 64.4 (N = 70) | 65.1 (N = 68) | |
| 6 months | 70.2 (N = 77) | 63.8 (N = 78) | .144 |
Notes: *Analysis of variance for repeated measures. The baseline scores were used as covariates.
MMSE = Mini-Mental State Examination.
This trial was supported by the Lions Organization (Punainen Sulka – Red Feather), Helsinki University Central Hospital, Helsinki City, and the Academy of Finland (Grant 48613).
We are grateful to statistician Hannu Kautiainen for his help during the revision of the manuscript.
References
Bucht G, Gustafson Y, Sandberg O. Epidemiology of delirium.
Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium.
McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality.
Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Prognostic significance of delirium in frail older people.
Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly.
Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients.
O'Keeffe S, Lavan. J. The prognostic significance of delirium in older hospital patients.
McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study.
Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients.
Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial.
Cole MG, Primeau FJ, Bailey RF, et al. Systematic intervention for elderly inpatients with delirium: a randomized trial.
Cole MG, McCusker J, Bellavance F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial.
Carnes M, Howell T, Rosenberg M, Francis J, Hildebrand C, Knuppel J. Physicians vary in approaches to the clinical management of delirium.
Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials.
Flacker JM, Lipsitz LA. Neural mechanisms of delirium: current hypotheses and evolving concepts.
Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine.
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician.
Wechsler D, ed. The Bellevue intelligence tests. The Measurement of Adult Intelligence. Baltimore, MD: Williams & Wilkins; 1944.
American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: APA; 1994.
Mahoney FI, Barthel DW. Functional evaluation: The Barthel index.
Lawton MP, Brody EM. Assessment of older people: self-monitoring and instrumental activities of daily living.
Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report.
Vellas BJ, Guigoz Y, Garry PJ. Facts Research and Intervention in Geriatrics: Nutrition in the Elderly. The Mini Nutritional Assessment (MNA), 3rd ed. Paris, France: Serdi Publishing; 1997.
Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia.
Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector.
Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review.
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials.
Gardner MJ, Altman DG. Statistics With Confidence. Confidence Intervals and Statistical Guidelines. London, U.K.: BMJ Books; 1989.
Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium.
Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting.
Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium-related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses.