-
PDF
- Split View
-
Views
-
Cite
Cite
Anna L. Petursdottir, Susan A. Farr, John E. Morley, William A. Banks, Gudrun V. Skuladottir, Effect of Dietary n-3 Polyunsaturated Fatty Acids on Brain Lipid Fatty Acid Composition, Learning Ability, and Memory of Senescence-Accelerated Mouse, The Journals of Gerontology: Series A, Volume 63, Issue 11, November 2008, Pages 1153–1160, https://doi.org/10.1093/gerona/63.11.1153
Close - Share Icon Share
Abstract
Animal studies have shown that a deficiency in brain of the n-3 polyunsaturated fatty acid (PUFA) docosahexaenoic acid (DHA) is associated with memory loss and diminished cognitive function. The senescence-accelerated prone 8 (SAMP8) mouse develops impairments in learning and memory at 8–12 months of age. The effect of diet supplemented with n-3 PUFA on brain phospholipid DHA status, learning, and memory ability in aged SAMP8 mice was investigated. At the age of 10 months, SAMP8 mice were fed either a low-DHA or a high-DHA diet for 8 weeks. In comparison to SAMP8 mice fed the low-DHA diet, those fed a high-DHA diet had improved acquisition and retention in a T-maze foot shock avoidance test and a higher proportion of DHA in hippocampal and amygdala phospholipids. This study demonstrates that, in mature animals, DHA is incorporated into brain phospholipids and that dietary n-3 PUFA is associated with delay in cognitive decline.
BRAIN tissue membrane phospholipids (PL) are rich in the n-3 polyunsaturated fatty acid (PUFA) docosahexaenoic acid (DHA or 22:6n-3). It has been shown that the balance between DHA and the n-6 PUFA arachidonic acid (20:4n-6) in brain membrane PL is crucial for normal functioning of the central nervous system (1–5). Humans and animals must obtain essential n-3 PUFA and n-6 PUFA from their diet. The sources for DHA and 20:4n-6 in brain membrane PL are derived from the diet or from conversion of their respective dietary precursors in liver (6,7). The precursors of DHA are α–linolenic acid from plant products and eicosapentaenoic acid (EPA or 20:5n-3) of marine origin and that of 20:4n-6 is linoleic acid (18:2n-6).
Studies demonstrate that lipid peroxidation in brain is an early event in Alzheimer's disease (AD), which strengthens the hypothesis that oxidative damage may play a role in the pathogenesis of AD (8,9). The double bonds in PUFA are highly susceptible to peroxidative damage, which is initiated by reactive oxygen species (10) and results in a decrease in content of n-6 PUFA and n-3 PUFA in membrane PL. Decrease in the DHA content of hippocampal tissue during aging has been demonstrated in patients with AD (11) and in 12-month-old senescence-accelerated prone 8 (SAMP8) mice (12). The SAMP8 strain of mice is a model of AD, because the mice develop age-related deficits in learning and memory that is associated with an age-related overexpression of amyloid precursor protein (APP) and elevated levels of amyloid-beta (Aβ) protein in brain (13–18). According to the amyloid hypothesis, accumulation of Aβ protein is central to the pathogenesis of AD (19–21). It has been suggested that DHA is a possible therapeutic agent for ameliorating learning deficiencies caused by AD, which could act by suppressing the increases in the levels of lipid peroxide, reactive oxygen species, and amyloid accumulation in AD models (22–24).
Epidemiological (25–27) and animal studies (28–31) have reported that there is an association between learning ability and dietary intake of n-3 PUFA. A study in an aged APPsw (Tg2576) transgenic mouse model has shown that dietary DHA intake late in life markedly reduced amyloid accumulation (22). A balance between dietary n-6 PUFA and n-3 PUFA affects the ratio between n-6 PUFA and n-3 PUFA in brain PL in 7-month-old SAMP8 mice. These studies suggest a mechanism by which dietary PUFA could influence learning and memory (32).
The hippocampus is an area of the brain that is important for recall of recent memory (33,34), and the amygdala is an area important for formation of long-term memory (35). It has been shown that the composition of fatty acids in the mouse brain PL is region-specific (36). Furthermore, it has been demonstrated that, in SAMP8 mice, PL classes in the hippocampus and amygdala differ in their fatty acid composition (12).
The purpose of the present study was to investigate the effects of dietary n-3 PUFA on the PUFA content in membrane PL classes and on antioxidant status, that is, vitamin E content, in hippocampus and amygdala of aged SAMP8 mice. Furthermore, the effect of dietary n-3 PUFA intake on acquisition and retention behavior seen in T-maze foot shock paradigms was investigated.
Materials and Methods
Animals, Diet, and Sampling
Animal experimental procedures were carried out in accordance with guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals. Naive 10-month-old male SAMP8 mice were obtained from a colony held at the laboratory of the Geriatric Research Education and Clinical Center (GRECC), Veterans Affairs Medical Center (St. Louis, MO). The colony is derived from siblings provided by Dr. Takeda of Kyoto University, Japan (37), and has been maintained as an inbred strain for 14 years under clean-room procedures (i.e., use of sterile gloves in handling mice, sterilized cages and bedding, restricted access to breeding area), and housed in microisolator HEPA filter units (Allentown Caging, Allentown, PA). The colony routinely undergoes serological testing for viral and bacterial contamination and has remained free of pathogens for more than 14 years. The SAMP8 mice were housed in rooms with a 12-hour light/dark cycle (lights on at 6 am) at 20–22°C with water and food available ad libitum. At 10 months of age, 20 SAMP8 mice were randomly assigned to one of two dietary groups. The first group received a low-DHA diet (Harlan Teklad Mouse Breeder Diet 8626; Indianapolis, IN) containing 4.4% total n-3 PUFA and 0.3% DHA. The second group received a high-DHA diet (F5069; Bio-Serv, Frenchtown, NJ) containing 30.3% total n-3 PUFA and 14.3% DHA. The fatty acid composition and the peroxidizability index (Peroxind), an index for polyunsaturation, of the diets are shown in Table 1. The Peroxind is calculated as follows: Peroxind = (% dienoic × 1) + (% trienoic × 2) + (% tetraenoic × 3) + (% pentaenoic × 4) + (% hexaenoic × 5). The Peroxind and amount of vitamin E (dl-α-tocopheryl acetate) were higher in the high-DHA diet than in the low-DHA diet (122.6 vs 42.1, and 150 IU/kg diet vs 103 IU/kg diet, respectively). The SAMP8 mice were maintained for 8 weeks on the experimental diets.
Animals were killed, and the hippocampus and amygdala were removed according to the method of Glowinski and Iversen (38) and prepared in the laboratory of the GRECC, immediately frozen in liquid nitrogen, and flown in dry ice to the Department of Physiology, Faculty of Medicine, University of Iceland, where they were stored at −80°C until analyzed.
T-Maze Training and Testing Procedures
The SAMP8 mice were 12 months of age at the start of behavioral testing and were maintained on their respective diets. The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The start box was separated from the alley by a plastic guillotine door, which prevented movement down the alley until training began. An electrifiable stainless steel rod floor ran throughout the maze to deliver scrambled foot shock. The mice were not permitted to explore the maze prior to training. A block of training trials began when a mouse was placed into the start box. The guillotine door was raised and a buzzer sounded simultaneously; 5 seconds later, foot shock was applied. The goal box entered on the first trial was designated “incorrect,” and the foot shock was continued until the mouse entered the other goal box, which in all subsequent trials was designated as “correct” for the particular mouse. At the end of each trial, the mouse was returned to its home cage until the next trial. The intertrial interval was 30 seconds with a foot-shock intensity of 0.30 mA. The buzzer intensity was 55 dB. The mice were trained until they made one avoidance. Retention was tested 1 week later by continuing training until the mice achieved the criterion of making five avoidances in six consecutive trials. The number of trials the mouse took to make one avoidance was the measure of acquisition. The number of trials needed to reach the criterion of making five avoidances in six consecutive trials was the measure of retention.
Lipid Extraction and Fatty Acid Analysis
Total lipids were extracted from the hippocampus and amygdala using methanol-chloroform (1:2, vol/vol; Merck, Darmstadt, Germany) by the method of Folch and colleagues (39). The antioxidant butylated hydroxytoluene (BHT, 5 mg/100 mL) was added to the extraction medium. Hippocampus and amygdala PL classes were separated by thin-layer chromatography (Adsorbosil H, soft layer; Alltech, Deerfield, IL) with the following solvent system: chloroform/ethanol/triethylamine/water (30:34:35:8, vol/vol/vol/vol). The migration was as follows: sphingomyelin < phosphatidylcholine (PC) < phosphatidylserine (PS) < phosphatidylinositol (PI) < phosphatidylethanolamine (PE) < cerebrosides. The fatty acids in each PL class were transmethylated for 45 minutes at 110°C using 14% boron trifluoride/methanol (Sigma Chemical Co., St. Louis, MO). The fatty acid methyl esters (FAME) were analyzed by use of a gas chromatograph (Hewlett Packard HP 7673; Palo Alto, CA) equipped with a Chrompack CP-WAX 52 CB polyethylene glycol column (25 m × 0.32 mm internal diameter × 0.2 μm; Varian B.V., Bergen op Zoom, the Netherlands). The injector and detector temperatures were maintained at 235°C and 250°C, respectively. The oven temperature was programmed for initial oven temperature of 90°C for 2 minutes, then rising at 30°C/min to 165°C, and at 3°C/min to 225°C, and held isothermal for 6 minutes. Hydrogen was used as the carrier gas. The FAME peaks were identified and calibrated against those of commercial standards (Sigma; Elysian, MN). The results are expressed as percentage of total fatty acids.
Quantitative Analysis of α-Tocopherol (Vitamin E)
Hippocampus and amygdala samples were homogenized in 0.9% saline and used for α-tocopherol analysis based on a method previously described by Su and colleagues (40). Care was taken to avoid substantial exposure of samples to light. Briefly, samples were extracted with hexane after precipitation of proteins with ethanol containing an internal standard of α-tocopherol acetate (10 μL/mL) and 0.01% BHT, evaporated under nitrogen, and redissolved in the mobile phase. The mobile phase consisted of methanol + 0.05% ammonium acetate, acetonitrile + 0.1% triethylamine, and chloroform. Three linear gradient steps were programmed. Samples were injected onto a high-performance liquid chromatography (system series 200; PerkinElmer, Buckinghamshire, UK) reversed-phase C18 5 μm-particle-diameter column. α-Tocopherol was monitored at 292 nm. The amount of α-tocopherol was determined by comparison of the peak areas against the standard curve. Concentration of protein was determined by a method based on the Lowry technique (41). The results are expressed as nmol α-tocopherol/mg protein.
Statistical Analysis
The effect of dietary n-3 PUFA intake on acquisition and retention of T-maze foot-shock avoidance, fatty acid composition of PL classes, and concentration of α-tocopherol in hippocampus and amygdala were analyzed with an independent sample t test. Differences were considered significant at p <.05. Statistical analysis was performed using the SPSS program (SPSS 11.5 for Windows; Chicago, IL).
Results
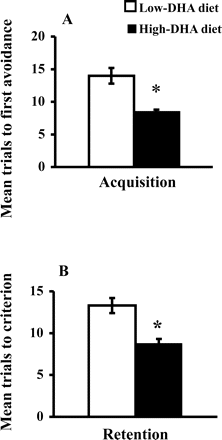
The 12-month-old SAMP8 mice fed the high-DHA diet took fewer trials than did those fed the low-DHA diet to make an avoidance (T(20) = 4.692; p <.0001) during acquisition (Figure 1A) and to make five avoidances in six consecutive trials (T(20) = 4.369; p <.0003) on the retention test (Figure 1B).
The fatty acid composition of PL classes in hippocampus and amygdala of the 12-month-old SAMP8 mice fed either the low-DHA or the high-DHA diet is shown in Table 2. The two groups did not differ in the proportions of total saturated fatty acids (SFA) and total monounsaturated fatty acids (MUFA) in any of the PL classes of hippocampus and amygdala, except in the PC of the amygdala. The high-DHA dietary intake led to lower proportions (p <.05) of total SFA and higher proportions (p <.05) of total MUFA in the PC of the amygdala than did the low-DHA dietary intake. The high-DHA dietary intake was reflected by a lower proportion (p <.05) of total n-6 PUFA and a lower ratio between proportions (p <.05) of n-6 and n-3 PUFA in PC, PS, PI, and PE of both hippocampus and amygdala. Furthermore, the high-DHA dietary intake was reflected in a higher proportion (p <.05) of total n-3 PUFA in PC, PI, and PE of both the hippocampus and amygdala, and in PS of the amygdala. The high-DHA dietary intake produced no significant effect on the peroxidizability index in any of the PL classes of either the hippocampus or amygdala (data not shown).
The high-DHA dietary intake led to higher proportions (p <.05) of DHA in PC (Figure 2A), PI (Figure 2C), and PE (Figure 2D) of both hippocampus and amygdala than the low-DHA dietary intake. The high-DHA dietary intake had no effect on the proportion of DHA in PS of both hippocampus and amygdala (Figure 2B). The elevated proportion of DHA was compensated by a lower proportion (p <.05) of 20:4n-6 in all the PL classes of both hippocampus and amygdala, except in PI of hippocampus (Figure 3, A–D).
No difference was found between the α-tocopherol concentrations in hippocampus or amygdala from the SAMP8 mice fed either the low-DHA or high-DHA diet (0.17 ± 0.02 (mean ± standard error of the mean) vs 0.23 ± 0.05 nmol/mg tissue protein, and 0.26 ± 0.05 vs 0.24 ± 0.03 nmol/mg tissue protein, respectively).
Discussion
High levels of DHA in brain tissue membrane PL have been associated with improved cognition in both animal and epidemiological studies. In this work, the effect of DHA in the diet of SAMP8 mice, animal model of AD, on cognition and membrane composition was studied. The dietary DHA was effectively incorporated into membrane PL classes in hippocampus and amygdala of aged SAMP8 mice. Furthermore, an improved acquisition and retention of T-maze foot-shock avoidance test was related to high-DHA dietary intake.
The high-DHA diet fed to the 10-month-old SAMP8 mice for 8 weeks was efficiently incorporated into PC, PI, and PE of both the hippocampus and amygdala. The reason why the proportion of DHA in PS was not affected by the high-DHA diet could be that PS contains the highest proportion of DHA compared to the other PL classes, and that an upper threshold for the incorporation of dietary n-3 PUFA has been reached (42). The present finding is consistent with studies (4,29,30) that have shown that dietary DHA competes with 20:4n-6, the main n-6 PUFA, for incorporation into brain PL classes, and restores the ratio between n-6 PUFA and n-3 PUFA of brain PL classes. Studies have indicated that the ratio of the dietary intake of n-6 and n-3 PUFA has a determining influence on the level of performance in learning and memory tasks (43–45). The improved acquisition and retention of T-maze foot-shock avoidance, demonstrated in the present study, could be related to the lower ratio between n-6 PUFA and n-3 PUFA found in the brain PL classes of the SAMP8 mice induced by the high-DHA diet.
The authors of the present study have demonstrated that 4-month-old SAMP8 mice fed a regular diet (containing 5.3% total n-3 PUFA and 1.3% DHA) took fewer trials to reach criterion in the T-maze foot-shock avoidance test than did 12-month-old SAMP8 mice fed the same diet (18). The hippocampus of these 12-month-old SAMP8 mice contained lower proportions of DHA in PS and PI, and a higher proportion of 20:4n-6 in PS than did the 4-month-old SAMP8 mice (12). The findings in the present study show that the high dietary DHA content leads to accumulation of DHA in membrane PL of hippocampus and amygdala. This accumulation of DHA is most likely achieved by replacement of lipid-peroxidized DHA in membrane PL and/or by the function of the high dose of the lipid-soluble chain-breaking antioxidant α-tocopherol (150 IU/kg diet) in the high-DHA diet, which is protective against peroxidative damage of DHA.
It has recently been shown that an increased intake of DHA may increase the production of SorLA/LR11, a protein known to destroy plaques associated with AD (46). In the present study, the hippocampal Aβ-peptide level of the SAMP8 mice was not investigated. However, in a number of previous studies we have demonstrated that the SAMP8 mice have elevated levels of APP and Aβ protein (15). The authors have also shown that lowering levels of Aβ protein in aged SAMP8 mice improves memory (17) and reduces oxidative protein damage (47). Therefore, in the present study it is indicated that the high-DHA diet may have prevented further amyloid accumulation and/or oxidative protein damage in the SAMP8 mice and thereby prevented decline in learning ability and memory.
A study in humans has demonstrated that a lower risk of developing AD is positively associated with dietary DHA, but not with dietary EPA (or 20:5n-3) (48). However, recent findings suggest that dietary EPA acts protectively against the damaging effects of Aβ because of the multiple antiinflammatory effects of EPA (49,50). Furthermore, it has been suggested that the distinct roles of DHA and its precursors α-linolenic acid (18:3n-3) and EPA in energy metabolism and brain function are complementary and that optimal retention of cognitive function in the elderly population depends on a blend of the roles of all three fatty acids (51). In the present study, EPA was not detected in the PL of hippocampus and amygdala, even though the high-DHA diet contained 11.7% EPA. It is likely that some of the EPA was converted to DHA in membrane PL or that some was used against damaging effects of Aβ-peptide.
In this study it has been demonstrated that, late in life, DHA of marine origin is incorporated into brain membrane PL, and that an n-3 PUFA–rich diet is associated with protection against a decline in learning and memory.
Decision Editor: Huber R. Warner, PhD
Effect of dietary n-3 polyunsaturated fatty acids (PUFA) on acquisition (A) and retention (B) of T-maze foot-shock avoidance. Number of trials to make one avoidance was the measure of acquisition, and the number of trials to reach the criterion of making five avoidances in six consecutive trials was the measure of retention. Values are mean ± standard error of the mean for 11 animals per group. *p <.01, compared to mice fed a low docosahexaenoic acid (DHA) diet (t test)
Effects of dietary n-3 polyunsaturated fatty acids (PUFA) on proportion (% of total fatty acids [TFA]) docosahexaenoic acid (DHA; 22:6n-3) in phospholipid classes of hippocampus and amygdala from senescence-accelerated prone 8 (SAMP8) mice. A, 22:6n-3 in phosphatidylcholine (PC); B, 22:6n-3 in phosphatidylserine (PS); C, 22:6n-3 in phosphatidylinositol (PI); D, 22:6n-3 in phosphatidylethanolamine (PE). Values are mean ± standard error of the mean for 8–10 preparations. *p <.05, compared to mice fed low-DHA diet (independent samples t test)
Effects of dietary n-3 polyunsaturated fatty acids (PUFA) on proportion (% of total fatty acids [TFA]) arachidonic acid (20:4n-6) in phospholipid classes of hippocampus and amygdala from senescence-accelerated prone 8 (SAMP8) mice. A, 20:4n-6 in phosphatidylcholine (PC); B, 20:4n-6 in phosphatidylserine (PS); C, 20:4n-6 in phosphatidylinositol (PI); D, 20:4n-6 in phosphatidylethanolamine (PE). Values are mean ± standard error of the mean for 8–10 preparations. *p <.05, compared to mice fed low-docosahexaenoic acid (DHA) diet (independent samples t test)
Fatty Acid Composition of Diets.
| Fatty Acids . | Low-DHA Diet* . | High-DHA Diet* . |
|---|---|---|
| Σ SFA | 25.1 | 29.3 |
| 14:0 | 1.0 | 7.3 |
| 16:0 | 17.2 | 17.0 |
| 18:0 | 6.0 | 3.1 |
| Σ MUFA | 34.7 | 14.4 |
| 16:1n-7 | 0.2 | 9.7 |
| 16:1n-9 | 1.9 | 0.3 |
| 18:1n-7 | 2.4 | 0.3 |
| 18:1n-9 | 29.7 | 3.0 |
| Σ n-6 PUFA | 32.4 | 2.8 |
| 18:2n-6 | 32.0 | 1.7 |
| Σ n-3 PUFA | 4.4 | 30.3 |
| 18:3n-3 | 3.7 | 1.3 |
| 20:5n-3 | 0.3 | 11.7 |
| 22:6n-3 | 0.3 | 14.3 |
| n-6/n-3 PUFA | 7.4 | 0.1 |
| Peroxind | 42.1 | 122.6 |
| Fatty Acids . | Low-DHA Diet* . | High-DHA Diet* . |
|---|---|---|
| Σ SFA | 25.1 | 29.3 |
| 14:0 | 1.0 | 7.3 |
| 16:0 | 17.2 | 17.0 |
| 18:0 | 6.0 | 3.1 |
| Σ MUFA | 34.7 | 14.4 |
| 16:1n-7 | 0.2 | 9.7 |
| 16:1n-9 | 1.9 | 0.3 |
| 18:1n-7 | 2.4 | 0.3 |
| 18:1n-9 | 29.7 | 3.0 |
| Σ n-6 PUFA | 32.4 | 2.8 |
| 18:2n-6 | 32.0 | 1.7 |
| Σ n-3 PUFA | 4.4 | 30.3 |
| 18:3n-3 | 3.7 | 1.3 |
| 20:5n-3 | 0.3 | 11.7 |
| 22:6n-3 | 0.3 | 14.3 |
| n-6/n-3 PUFA | 7.4 | 0.1 |
| Peroxind | 42.1 | 122.6 |
Notes: Values are expressed as percentage of total fatty acids and represent the average of three determinations. Fatty acids that were <1% of total fatty acids in both diets were excluded.
*10% dietary fat by weight.
DHA = docosahexaenoic acid; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; Peroxind = peroxidizability index = (% dienoic × 1) + (% trienoic × 2) + (% tetraenoic × 3) + (% pentaenoic × 4) + (% hexaenoic × 5).
Fatty Acid Composition of Diets.
| Fatty Acids . | Low-DHA Diet* . | High-DHA Diet* . |
|---|---|---|
| Σ SFA | 25.1 | 29.3 |
| 14:0 | 1.0 | 7.3 |
| 16:0 | 17.2 | 17.0 |
| 18:0 | 6.0 | 3.1 |
| Σ MUFA | 34.7 | 14.4 |
| 16:1n-7 | 0.2 | 9.7 |
| 16:1n-9 | 1.9 | 0.3 |
| 18:1n-7 | 2.4 | 0.3 |
| 18:1n-9 | 29.7 | 3.0 |
| Σ n-6 PUFA | 32.4 | 2.8 |
| 18:2n-6 | 32.0 | 1.7 |
| Σ n-3 PUFA | 4.4 | 30.3 |
| 18:3n-3 | 3.7 | 1.3 |
| 20:5n-3 | 0.3 | 11.7 |
| 22:6n-3 | 0.3 | 14.3 |
| n-6/n-3 PUFA | 7.4 | 0.1 |
| Peroxind | 42.1 | 122.6 |
| Fatty Acids . | Low-DHA Diet* . | High-DHA Diet* . |
|---|---|---|
| Σ SFA | 25.1 | 29.3 |
| 14:0 | 1.0 | 7.3 |
| 16:0 | 17.2 | 17.0 |
| 18:0 | 6.0 | 3.1 |
| Σ MUFA | 34.7 | 14.4 |
| 16:1n-7 | 0.2 | 9.7 |
| 16:1n-9 | 1.9 | 0.3 |
| 18:1n-7 | 2.4 | 0.3 |
| 18:1n-9 | 29.7 | 3.0 |
| Σ n-6 PUFA | 32.4 | 2.8 |
| 18:2n-6 | 32.0 | 1.7 |
| Σ n-3 PUFA | 4.4 | 30.3 |
| 18:3n-3 | 3.7 | 1.3 |
| 20:5n-3 | 0.3 | 11.7 |
| 22:6n-3 | 0.3 | 14.3 |
| n-6/n-3 PUFA | 7.4 | 0.1 |
| Peroxind | 42.1 | 122.6 |
Notes: Values are expressed as percentage of total fatty acids and represent the average of three determinations. Fatty acids that were <1% of total fatty acids in both diets were excluded.
*10% dietary fat by weight.
DHA = docosahexaenoic acid; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; Peroxind = peroxidizability index = (% dienoic × 1) + (% trienoic × 2) + (% tetraenoic × 3) + (% pentaenoic × 4) + (% hexaenoic × 5).
Effect of Dietary n-3 PUFA on Fatty Acid Composition of Phospholipid Classes in Hippocampus and Amygdala of 12-Month-Old SAMP8 Mice.
| . | SM . | . | PC . | . | PS . | . | PI . | . | PE . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acids . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | ||||||||||
| Σ SFA | ||||||||||||||||||||
| Hippocampus | 84.66 ± 1.06 | 84.13 ± 0.95 | 55.52 ± 0.42 | 54.05 ± 1.44 | 40.78 ± 0.38 | 40.51 ± 0.46 | 40.46 ± 0.65 | 39.81 ± 1.10 | 21.45 ± 1.86 | 22.95 ± 0.55 | ||||||||||
| Amygdala | 95.16 ± 0.84 | 93.47 ± 0.72 | 59.16 ± 0.21 | 58.41 ± 0.26* | 42.80 ± 0.24 | 42.32 ± 0.46 | 42.78 ± 0.50 | 42.55 ± 0.39 | 28.73 ± 0.54 | 28.97 ± 0.34 | ||||||||||
| Σ MUFA | ||||||||||||||||||||
| Hippocampus | 12.24 ± 1.10 | 13.08 ± 0.82 | 30.69 ± 0.49 | 32.54 ± 1.00 | 22.97 ± 1.11 | 22.99 ± 1.33 | 21.12 ± 0.76 | 23.09 ± 1.08 | 21.52 ± 2.53 | 19.07 ± 1.08 | ||||||||||
| Amygdala | 4.33 ± 0.55 | 5.64 ± 0.66 | 26.76 ± 0.26 | 28.76 ± 0.14* | 11.13 ± 0.91 | 11.35 ± 0.43 | 11.59 ± 0.91 | 12.54 ± 0.66 | 9.55 ± 0.79 | 10.22 ± 0.47 | ||||||||||
| Σ n-6 PUFA | ||||||||||||||||||||
| Hippocampus | 3.11 ± 0.24 | 2.79 ± 0.24 | 6.99 ± 0.35 | 4.07 ± 0.21* | 7.37 ± 0.15 | 4.81 ± 0.22* | 31.01 ± 1.52 | 25.49 ± 1.37* | 16.83 ± 0.28 | 10.35 ± 0.24* | ||||||||||
| Amygdala | 1.32 ± 0.29 | 0.85 ± 0.13 | 9.05 ± 0.29 | 5.74 ± 0.15* | 6.61 ± 0.33 | 3.53 ± 0.23* | 41.99 ± 1.46 | 36.11 ± 1.22* | 21.67 ± 0.89 | 13.71 ± 0.24* | ||||||||||
| Σ n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 5.37 ± 0.11 | 7.23 ± 0.10* | 27.50 ± 1.33 | 30.29 ± 1.40 | 4.64 ± 0.51 | 8.24 ± 0.79* | 24.82 ± 0.94 | 30.41 ± 1.10* | ||||||||||
| Amygdala | ND | ND | 4.22 ± 0.15 | 6.10 ± 0.09* | 39.44 ± 1.06 | 42.73 ± 0.69* | 3.32 ± 0.39 | 8.35 ± 0.80* | 27.81 ± 0.62 | 34.75 ± 0.69* | ||||||||||
| n-6/n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 1.31 ± 0.08 | 0.56 ± 0.03* | 0.27 ± 0.02 | 0.16 ± 0.01* | 7.36 ± 0.87 | 3.30 ± 0.33* | 0.68 ± 0.02 | 0.34 ± 0.01* | ||||||||||
| Amygdala | ND | ND | 2.18 ± 0.11 | 0.94 ± 0.02* | 0.17 ± 0.01 | 0.08 ± 0.01* | 13.95 ± 1.45 | 4.71 ± 0.47* | 0.78 ± 0.03 | 0.40 ± 0.01* | ||||||||||
| . | SM . | . | PC . | . | PS . | . | PI . | . | PE . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acids . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | ||||||||||
| Σ SFA | ||||||||||||||||||||
| Hippocampus | 84.66 ± 1.06 | 84.13 ± 0.95 | 55.52 ± 0.42 | 54.05 ± 1.44 | 40.78 ± 0.38 | 40.51 ± 0.46 | 40.46 ± 0.65 | 39.81 ± 1.10 | 21.45 ± 1.86 | 22.95 ± 0.55 | ||||||||||
| Amygdala | 95.16 ± 0.84 | 93.47 ± 0.72 | 59.16 ± 0.21 | 58.41 ± 0.26* | 42.80 ± 0.24 | 42.32 ± 0.46 | 42.78 ± 0.50 | 42.55 ± 0.39 | 28.73 ± 0.54 | 28.97 ± 0.34 | ||||||||||
| Σ MUFA | ||||||||||||||||||||
| Hippocampus | 12.24 ± 1.10 | 13.08 ± 0.82 | 30.69 ± 0.49 | 32.54 ± 1.00 | 22.97 ± 1.11 | 22.99 ± 1.33 | 21.12 ± 0.76 | 23.09 ± 1.08 | 21.52 ± 2.53 | 19.07 ± 1.08 | ||||||||||
| Amygdala | 4.33 ± 0.55 | 5.64 ± 0.66 | 26.76 ± 0.26 | 28.76 ± 0.14* | 11.13 ± 0.91 | 11.35 ± 0.43 | 11.59 ± 0.91 | 12.54 ± 0.66 | 9.55 ± 0.79 | 10.22 ± 0.47 | ||||||||||
| Σ n-6 PUFA | ||||||||||||||||||||
| Hippocampus | 3.11 ± 0.24 | 2.79 ± 0.24 | 6.99 ± 0.35 | 4.07 ± 0.21* | 7.37 ± 0.15 | 4.81 ± 0.22* | 31.01 ± 1.52 | 25.49 ± 1.37* | 16.83 ± 0.28 | 10.35 ± 0.24* | ||||||||||
| Amygdala | 1.32 ± 0.29 | 0.85 ± 0.13 | 9.05 ± 0.29 | 5.74 ± 0.15* | 6.61 ± 0.33 | 3.53 ± 0.23* | 41.99 ± 1.46 | 36.11 ± 1.22* | 21.67 ± 0.89 | 13.71 ± 0.24* | ||||||||||
| Σ n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 5.37 ± 0.11 | 7.23 ± 0.10* | 27.50 ± 1.33 | 30.29 ± 1.40 | 4.64 ± 0.51 | 8.24 ± 0.79* | 24.82 ± 0.94 | 30.41 ± 1.10* | ||||||||||
| Amygdala | ND | ND | 4.22 ± 0.15 | 6.10 ± 0.09* | 39.44 ± 1.06 | 42.73 ± 0.69* | 3.32 ± 0.39 | 8.35 ± 0.80* | 27.81 ± 0.62 | 34.75 ± 0.69* | ||||||||||
| n-6/n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 1.31 ± 0.08 | 0.56 ± 0.03* | 0.27 ± 0.02 | 0.16 ± 0.01* | 7.36 ± 0.87 | 3.30 ± 0.33* | 0.68 ± 0.02 | 0.34 ± 0.01* | ||||||||||
| Amygdala | ND | ND | 2.18 ± 0.11 | 0.94 ± 0.02* | 0.17 ± 0.01 | 0.08 ± 0.01* | 13.95 ± 1.45 | 4.71 ± 0.47* | 0.78 ± 0.03 | 0.40 ± 0.01* | ||||||||||
Notes: Values are expressed as percentage of total fatty acids (mean ± standard error of the mean) for 8–10 preparations.
*p <.05, compared to low-DHA diet (independent samples t test).
PUFA = polyunsaturated fatty acids; DHA = docosahexaenoic acid; SM = sphingomyelin; PC = phosphatidylcholine; PS = phosphatidylserine; PI = phosphatidylinositol; PE = phosphatidylethanolamine; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; ND = not detected.
Effect of Dietary n-3 PUFA on Fatty Acid Composition of Phospholipid Classes in Hippocampus and Amygdala of 12-Month-Old SAMP8 Mice.
| . | SM . | . | PC . | . | PS . | . | PI . | . | PE . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acids . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | ||||||||||
| Σ SFA | ||||||||||||||||||||
| Hippocampus | 84.66 ± 1.06 | 84.13 ± 0.95 | 55.52 ± 0.42 | 54.05 ± 1.44 | 40.78 ± 0.38 | 40.51 ± 0.46 | 40.46 ± 0.65 | 39.81 ± 1.10 | 21.45 ± 1.86 | 22.95 ± 0.55 | ||||||||||
| Amygdala | 95.16 ± 0.84 | 93.47 ± 0.72 | 59.16 ± 0.21 | 58.41 ± 0.26* | 42.80 ± 0.24 | 42.32 ± 0.46 | 42.78 ± 0.50 | 42.55 ± 0.39 | 28.73 ± 0.54 | 28.97 ± 0.34 | ||||||||||
| Σ MUFA | ||||||||||||||||||||
| Hippocampus | 12.24 ± 1.10 | 13.08 ± 0.82 | 30.69 ± 0.49 | 32.54 ± 1.00 | 22.97 ± 1.11 | 22.99 ± 1.33 | 21.12 ± 0.76 | 23.09 ± 1.08 | 21.52 ± 2.53 | 19.07 ± 1.08 | ||||||||||
| Amygdala | 4.33 ± 0.55 | 5.64 ± 0.66 | 26.76 ± 0.26 | 28.76 ± 0.14* | 11.13 ± 0.91 | 11.35 ± 0.43 | 11.59 ± 0.91 | 12.54 ± 0.66 | 9.55 ± 0.79 | 10.22 ± 0.47 | ||||||||||
| Σ n-6 PUFA | ||||||||||||||||||||
| Hippocampus | 3.11 ± 0.24 | 2.79 ± 0.24 | 6.99 ± 0.35 | 4.07 ± 0.21* | 7.37 ± 0.15 | 4.81 ± 0.22* | 31.01 ± 1.52 | 25.49 ± 1.37* | 16.83 ± 0.28 | 10.35 ± 0.24* | ||||||||||
| Amygdala | 1.32 ± 0.29 | 0.85 ± 0.13 | 9.05 ± 0.29 | 5.74 ± 0.15* | 6.61 ± 0.33 | 3.53 ± 0.23* | 41.99 ± 1.46 | 36.11 ± 1.22* | 21.67 ± 0.89 | 13.71 ± 0.24* | ||||||||||
| Σ n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 5.37 ± 0.11 | 7.23 ± 0.10* | 27.50 ± 1.33 | 30.29 ± 1.40 | 4.64 ± 0.51 | 8.24 ± 0.79* | 24.82 ± 0.94 | 30.41 ± 1.10* | ||||||||||
| Amygdala | ND | ND | 4.22 ± 0.15 | 6.10 ± 0.09* | 39.44 ± 1.06 | 42.73 ± 0.69* | 3.32 ± 0.39 | 8.35 ± 0.80* | 27.81 ± 0.62 | 34.75 ± 0.69* | ||||||||||
| n-6/n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 1.31 ± 0.08 | 0.56 ± 0.03* | 0.27 ± 0.02 | 0.16 ± 0.01* | 7.36 ± 0.87 | 3.30 ± 0.33* | 0.68 ± 0.02 | 0.34 ± 0.01* | ||||||||||
| Amygdala | ND | ND | 2.18 ± 0.11 | 0.94 ± 0.02* | 0.17 ± 0.01 | 0.08 ± 0.01* | 13.95 ± 1.45 | 4.71 ± 0.47* | 0.78 ± 0.03 | 0.40 ± 0.01* | ||||||||||
| . | SM . | . | PC . | . | PS . | . | PI . | . | PE . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acids . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | Low-DHA Diet . | High-DHA Diet . | ||||||||||
| Σ SFA | ||||||||||||||||||||
| Hippocampus | 84.66 ± 1.06 | 84.13 ± 0.95 | 55.52 ± 0.42 | 54.05 ± 1.44 | 40.78 ± 0.38 | 40.51 ± 0.46 | 40.46 ± 0.65 | 39.81 ± 1.10 | 21.45 ± 1.86 | 22.95 ± 0.55 | ||||||||||
| Amygdala | 95.16 ± 0.84 | 93.47 ± 0.72 | 59.16 ± 0.21 | 58.41 ± 0.26* | 42.80 ± 0.24 | 42.32 ± 0.46 | 42.78 ± 0.50 | 42.55 ± 0.39 | 28.73 ± 0.54 | 28.97 ± 0.34 | ||||||||||
| Σ MUFA | ||||||||||||||||||||
| Hippocampus | 12.24 ± 1.10 | 13.08 ± 0.82 | 30.69 ± 0.49 | 32.54 ± 1.00 | 22.97 ± 1.11 | 22.99 ± 1.33 | 21.12 ± 0.76 | 23.09 ± 1.08 | 21.52 ± 2.53 | 19.07 ± 1.08 | ||||||||||
| Amygdala | 4.33 ± 0.55 | 5.64 ± 0.66 | 26.76 ± 0.26 | 28.76 ± 0.14* | 11.13 ± 0.91 | 11.35 ± 0.43 | 11.59 ± 0.91 | 12.54 ± 0.66 | 9.55 ± 0.79 | 10.22 ± 0.47 | ||||||||||
| Σ n-6 PUFA | ||||||||||||||||||||
| Hippocampus | 3.11 ± 0.24 | 2.79 ± 0.24 | 6.99 ± 0.35 | 4.07 ± 0.21* | 7.37 ± 0.15 | 4.81 ± 0.22* | 31.01 ± 1.52 | 25.49 ± 1.37* | 16.83 ± 0.28 | 10.35 ± 0.24* | ||||||||||
| Amygdala | 1.32 ± 0.29 | 0.85 ± 0.13 | 9.05 ± 0.29 | 5.74 ± 0.15* | 6.61 ± 0.33 | 3.53 ± 0.23* | 41.99 ± 1.46 | 36.11 ± 1.22* | 21.67 ± 0.89 | 13.71 ± 0.24* | ||||||||||
| Σ n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 5.37 ± 0.11 | 7.23 ± 0.10* | 27.50 ± 1.33 | 30.29 ± 1.40 | 4.64 ± 0.51 | 8.24 ± 0.79* | 24.82 ± 0.94 | 30.41 ± 1.10* | ||||||||||
| Amygdala | ND | ND | 4.22 ± 0.15 | 6.10 ± 0.09* | 39.44 ± 1.06 | 42.73 ± 0.69* | 3.32 ± 0.39 | 8.35 ± 0.80* | 27.81 ± 0.62 | 34.75 ± 0.69* | ||||||||||
| n-6/n-3 PUFA | ||||||||||||||||||||
| Hippocampus | ND | ND | 1.31 ± 0.08 | 0.56 ± 0.03* | 0.27 ± 0.02 | 0.16 ± 0.01* | 7.36 ± 0.87 | 3.30 ± 0.33* | 0.68 ± 0.02 | 0.34 ± 0.01* | ||||||||||
| Amygdala | ND | ND | 2.18 ± 0.11 | 0.94 ± 0.02* | 0.17 ± 0.01 | 0.08 ± 0.01* | 13.95 ± 1.45 | 4.71 ± 0.47* | 0.78 ± 0.03 | 0.40 ± 0.01* | ||||||||||
Notes: Values are expressed as percentage of total fatty acids (mean ± standard error of the mean) for 8–10 preparations.
*p <.05, compared to low-DHA diet (independent samples t test).
PUFA = polyunsaturated fatty acids; DHA = docosahexaenoic acid; SM = sphingomyelin; PC = phosphatidylcholine; PS = phosphatidylserine; PI = phosphatidylinositol; PE = phosphatidylethanolamine; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; ND = not detected.
This work was supported by a VA Merit Award (W.A.B., S.A.F.); by grants R01NS41863 (W.A.B.), R01AA12743 (W.A.B.), and R21DA019386 (W.A.B.); by the Medical Research Service of the Department of Veterans Affairs, by the Icelandic Research Fund, and by The University of Iceland Research Fund.
References
Fernstrom JD. Effects of dietary polyunsaturated fatty acids on neuronal function.
Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat.
Champeil-Potokar G, Denis I, Goustard-Langelier B, Alessandri JM, Guesnet P, Lavialle M. Astrocytes in culture require docosahexaenoic acid to restore the n-3/n-6 polyunsaturated fatty acid balance in their membrane phospholipids.
Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function.
Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain.
Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver.
Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment.
Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in mild cognitive impairment and early Alzheimer's disease.
Söderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease.
Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Lipid peroxidation in brain during aging in the senescence-accelerated mouse (SAM).
Yagi H, Katoh S, Akiguchi I, Takeda T. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in resent memory.
Flood JF, Morley JE. Early onset of age-related impairment of aversive and appetitive learning in the SAM-P/8 mouse.
Morley JE, Kumar VB, Bernardo AE, et al. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory.
Kumar VB, Farr SA, Flood JF, et al. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice.
Morley JE, Farr SA, Flood JE. Antibody to amyloid beta protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice.
Farr SA, Banks WA, Uezu K, Gaskin FS, Morley JE. DHEAS improves learning and memory in aged SAMP8 mice but not in diabetic mice.
Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide.
Kanski J, Aksenova M, Schöneich C, Butterfield DA. Substitution of isoleucine-31 by helical-breaking proline abolishes oxidative stress and neurotoxic properties of Alzheimer's amyloid β-peptide (1-42).
Ikezu T, Luo X, Weber GA, et al. Amyloid precursor protein-processing products affect mononuclear phagocyte activation: pathways for sAPP- and Abeta-mediated neurotoxicity.
Hashimoto M, Hossain S, Shimada T, et al. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer's disease model rats.
Hashimoto M, Hossain S, Agdul H, Shido O. Docosahexaenoic acid-induced amelioration on impairment of memory learning in amyloid beta-infused rats relates to the decreases of amyloid beta and cholesterol levels in detergent-insoluble membrane fractions.
Lim GP, Calon F, Morihara T, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model.
Kalmijn S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies.
Kalmijn S, van Boxtel MP, Ocké M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age.
van Gelder BM, Tijhuis M, Kalmijn S, Kroumhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study.
Yamamoto N, Saitoh M, Moriuchi A, Nomura M, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on brain lipid compositions and learning ability of rats.
Suzuki H, Park SJ, Tamura M, Ando S. Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice: a comparison of sardine oil diet with palm oil diet.
Gamoh S, Hashimoto M, Sugioka K, et al. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats.
Carrié I, Guesnet P, Bourre J-M, Francès H. Diets containing long-chain n-3 polyunsaturated fatty acids affect behaviour differently during development than ageing in mice.
Umezawa M, Ohta A, Tojo H, Yagi H, Hosokawa M, Takeda T. Dietary α-linolenate/linoleate balance influences learning and memory in the senescence-accelerated mouse (SAM).
Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans.
Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information.
Cahill L, Haier RJ, Fallon J, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information.
Carrié I, Clément M, de Javel D, Francés H, Bourre J-M. Specific phospholipids fatty acid composition of brain regions in mice: effects of n-3 polyunsaturated fatty acid deficiency and phospholipids supplementation.
Takeda T, Hosokawa M, Takeshita S, et al. A new murine model of accelerated senescence.
Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain.
Folch J, Lees M, Sloane Stanley G. A simple method for the isolation and purification of total lipids from animal tissues.
Su Q, Rowley KG, O'Dea K. Stability of individual carotenoids, retinol and tocopherols in human plasma during exposure to light and after extraction.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent.
Venkatraman JT, Toohey T, Clandinin MT. Does a threshold for the effect of dietary omega-3 fatty acids on the fatty acid composition of nuclear envelope phospholipids exist?
Yehuda S, Carasso RL. Modulation of learning, pain thresholds, and thermoregulation in the rat by preparations of free purified alpha-linolenic and linoleic acids: determination of the optimal omega 3-to-omega 6 ratio.
Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. Fatty acids and brain peptides.
Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions.
Qiu-Lan M, Teter B, Ubeda OJ, et al. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer's disease (AD): relevance to AD prevention.
Poon HF, Farr SA, Banks WA, et al. Proteomic identification of less oxidized brain proteins in aged senescence-accelerated mice following administration of antisense oligonucleotide directed at the Abeta region of amyloid precursor protein.
Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease.
Lynch AM, Loane DJ, Minogue AM, et al. Eicosapentaenoic acid confers neuroprotection in the amyloid-β challenged aged hippocampus.
Minogue AM, Lynch AM, Loane DJ, Herron CE, Lynch MA. Modulation of amyloid-β-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid.



![Effects of dietary n-3 polyunsaturated fatty acids (PUFA) on proportion (% of total fatty acids [TFA]) docosahexaenoic acid (DHA; 22:6n-3) in phospholipid classes of hippocampus and amygdala from senescence-accelerated prone 8 (SAMP8) mice. A, 22:6n-3 in phosphatidylcholine (PC); B, 22:6n-3 in phosphatidylserine (PS); C, 22:6n-3 in phosphatidylinositol (PI); D, 22:6n-3 in phosphatidylethanolamine (PE). Values are mean ± standard error of the mean for 8–10 preparations. *p <.05, compared to mice fed low-DHA diet (independent samples t test)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biomedgerontology/63/11/10.1093/gerona/63.11.1153/2/m_grna-63-11-05-f02.gif?Expires=1716735110&Signature=T7OVzva3f~xMX-sAvheZGsQcklIu4NivS0CmoFbohM-QRw2OpOxpEfAdIMjDkt8HU1WuCUorNaR99hwhA-hJLUQEx0CBQtQJxUzWEY~zkqEHE7R9zEpfMzcURQEkOcZIfm8sVh-ZPmclgJh0Gd33TBnfIbBAoKX0jVNH6AtCqCVL49q3-cSpGwBzRnJDlzk8h6vm3wdRwY~mY~P4zERYDWDpqCMmTWNSA52mo~52mbCTL-DS93X6FIuZYdX5jbHgK2tVBrSe8h0OToJdVf9ZBoCfTc0-x5rk6NPvkbP3QZU~AvmIubIrmFMDKwMJBsfb7P64olJ2U6VTVdZxfUMmYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Effects of dietary n-3 polyunsaturated fatty acids (PUFA) on proportion (% of total fatty acids [TFA]) arachidonic acid (20:4n-6) in phospholipid classes of hippocampus and amygdala from senescence-accelerated prone 8 (SAMP8) mice. A, 20:4n-6 in phosphatidylcholine (PC); B, 20:4n-6 in phosphatidylserine (PS); C, 20:4n-6 in phosphatidylinositol (PI); D, 20:4n-6 in phosphatidylethanolamine (PE). Values are mean ± standard error of the mean for 8–10 preparations. *p <.05, compared to mice fed low-docosahexaenoic acid (DHA) diet (independent samples t test)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biomedgerontology/63/11/10.1093/gerona/63.11.1153/2/m_grna-63-11-05-f03.gif?Expires=1716735110&Signature=Ktd~kyUEr3Qc9x8bDT5oJWsFqGRR6ClNDyRsJoVvLZhFR3tUHBPsG~tpx1z8gDn9Dp9CLqZRDxgbDc3gSgoR0F7ucSJH2b69K8uy~H62yHLnUysDZ0F-Tdb6h7UaK7LY8IENugjhQgPCnOt32yfIi6RI9nK~90jJDmfKzBIxZOxLfvwQyTtU2SUCNpQx2OxOJLhVVUhPawqqt-PxQ2gw2nfaoIz-qVenih8q8jCM3uCLcHqbx7tKbJNii0Kw111iieE0ByX4y9-LX7CxOyOmAsDMHrWoFuLAxGSetA3fhfrBZkSXRyzyq5-E5lq9~MxeuDL23vGphCdk51A5D6EWtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
