-
PDF
- Split View
-
Views
-
Cite
Cite
Frederique Retornaz, Johanne Monette, Gerald Batist, Michèle Monette, Nadia Sourial, David Small, Stephen Caplan, Doreen Wan-Chow-Wah, Martine T. E. Puts, Howard Bergman, Usefulness of Frailty Markers in the Assessment of the Health and Functional Status of Older Cancer Patients Referred for Chemotherapy: A Pilot Study, The Journals of Gerontology: Series A, Volume 63, Issue 5, May 2008, Pages 518–522, https://doi.org/10.1093/gerona/63.5.518
Close - Share Icon Share
Abstract
Background. Older cancer patients seen in an oncology clinic seem to be healthier and less disabled than traditional geriatric patients. Choosing the most sensitive tools to assess their health status is a major issue. This cross-sectional study explores the usefulness of frailty markers in detecting vulnerability in older cancer patients.
Methods. The study included cancer patients ≥70 years old referred to an oncology clinic for chemotherapy. Information on comorbidities, disability in instrumental activities of daily living (IADL) and activities of daily living (ADL), and seven frailty markers (nutrition, mobility, strength, energy, physical activity, mood, and cognition) was collected. Patients were classified into four hierarchical groups: 1- No frailty markers, IADL, or ADL disability; 2- Presence of frailty markers without IADL or ADL disability; 3- IADL disability without ADL disability; 4- ADL disability.
Results. Among the 50 patients assessed, 6 (12.0%) were classified into Group 1, 21 (42.0%) into Group 2, 15 (30.0%) into Group 3, and 8 (16.0%) into Group 4. In Group 2, 7 patients (33.3 %) had one frailty marker, and 14 (66.7%) had two or more. The most prevalent of the frailty markers were nutrition, mobility, and physical activity.
Conclusion. The assessment of seven frailty markers allowed the detection of potential vulnerability among 42% of older cancer patients that would not have been detected through an assessment of IADL and ADL disability alone. A longitudinal study is needed to determine whether the use of frailty markers can better characterize the older cancer population and predict adverse outcomes due to cancer treatment.
BOTH the incidence and mortality of cancer increase with age (1,2). This increasingly older cancer population is heterogeneous. Therefore, it is crucial for clinicians to have the most accurate evaluation of the health and functional status to tailor treatment, as well as anticipate and possibly prevent complications. Geriatric assessment has been recommended for older cancer patients to identify those who may benefit from treatment and who are at higher risk of toxicity and complications (3–7).
Previous studies on older cancer patients seen in oncology have suggested that they are, for the most part, functionally independent (8–10). The usual geriatric assessment based on basic and instrumental activities of daily living (ADL and IADL, respectively) may therefore have a ceiling effect in detecting vulnerability in this population (11). Consequently, there is a need for more sensitive tools that will identify vulnerable patients who appear healthy but are susceptible to complications in response to aggressive treatments (4,6,11–13).
Frailty is a syndrome resulting from cumulative declines across multiple physiologic systems and represents a state of reduced homeostasis and resistance to stress leading to increased vulnerability and risk of adverse outcomes such as as falls, disability, hospitalization, and death (14–16). Fried and colleagues (14) identified five frailty characteristics: nutrition, mobility, strength, energy, and physical activity. Several authors have recently suggested that mood and cognition should also be included as characteristics of frailty (17–20). Regardless of the number of frailty markers, the presence of at least one of these markers confers an increased risk of adverse outcomes (14,21,22).
Therefore, in the assessment of the health and functional status of older cancer patients, the concept of frailty may be a useful approach to detect vulnerability. The primary objective of this study was to assess the prevalence of seven frailty markers in older cancer patients referred to oncology for chemotherapy. The secondary objective was to classify patients into hierarchical groups based on frailty markers as well as ADL and IADL disability.
Methods
Study Setting, Sample, and Design
This descriptive cross-sectional study was completed over a period of 4 months (from February to June 2006) at the Segal Cancer Centre of the Jewish General Hospital, Montreal, Canada. Inclusion criteria were: outpatients, 70 years old or older, diagnosed with a solid tumor or hematological malignancy, and referred to the oncology clinic for potential chemotherapy. Exclusion criteria were: patients with a life expectancy estimated to be <3 months, patients who did not speak English or French, patients unable to give informed consent, and patients previously treated with chemotherapy.
The geriatrician–oncologist (FR) regularly consulted the oncology database to identify all new patients, 70 years old or older, referred to the oncology clinic. Their charts were reviewed to identify eligible patients. The geriatrician–oncologist or a geriatric nurse clinician contacted the patient at the first oncologist's appointment to explain the study. After providing written informed consent, the patient was assessed either before or after the oncologist's visit, or at a later date, depending on the patient's wishes, but before the initiation of treatment. The protocol was approved by the Research Ethics Committee of the Jewish General Hospital.
Data Collection
The measurement tools were selected by a multidisciplinary team composed of geriatricians, oncologists, epidemiologists, and statisticians, based on a review of both the geriatric and oncology literature. The assessment was completed either by the geriatrician–oncologist or the geriatric nurse clinician using both self-report and performance-based measures, in addition to a medical chart review.
Age, sex, and oncology data, including type and stage of cancer, were collected from the patient's medical chart. Other demographic data, such as level of education, social support, and living arrangement were obtained using a self-report questionnaire. The total number of comorbidities was calculated for each patient using the classification in the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) (23). Performance status was assessed using the Eastern Cooperative Oncology Group Scale of Performance Status (ECOG-PS) (24).
IADL disability was assessed using the seven Older American Resources and Services (OARS) items (25): use of the phone, transportation, shopping, meal preparation, housework, medication, and finances. The denominator was adjusted to take into account patients who did not normally perform an activity such as cooking or laundry. ADL disability was assessed using six tasks of the Katz index (26): bathing, dressing, toileting, transferring, continence, and feeding. Disability in IADL or ADL was defined as the need for assistance to complete at least one IADL or ADL, respectively.
Nutritional status was assessed by body mass index (BMI) and two self-report questions: “In the last year, have you lost more than 10 pounds unintentionally?” (14) and “During the last 3 months, was food intake decreased for whatever cause?” (27). A BMI ≤ 18.5 was considered underweight and indicated undernutrition (28). An affirmative answer to one of the two questions or a low BMI indicated a positive marker of frailty for nutrition.
Mobility was assessed by the number of falls reported by the patient in the last 6 months and by the Timed Up and Go test (TUG) (29). Presence of one or more falls or a TUG time >10 seconds (29) was considered a positive marker of frailty for mobility.
Strength was assessed by three measurements of grip strength (in kilograms) in the dominant hand using a Jamar handheld dynamometer. The maximal grip strength was selected for the analysis. The lowest quintile by sex and BMI was considered a positive marker of frailty for strength (14).
Energy was assessed using a visual scale ranging from 0 (no energy) to 10 (full of energy). A score ≤ 3 indicated a positive marker of frailty for energy (16).
Physical activity was assessed by a validated self-report question from the Canadian Study of Health and Aging Risk Factor Questionnaire (RFQ) (30). No exercise or a low level of exercise was considered a positive marker of frailty for physical activity.
Mood was assessed by the Hospital Anxiety and Depression scale (HADS) (31). A total score ≥15 in the total scales was considered a positive marker of frailty for mood (32).
Cognition was assessed by the Mini-Mental State Examination (MMSE) (33) and the Montreal Cognitive Assessment (MoCA) (34). The MoCA is a test developed to identify patients who perform well on the MMSE but who present mild cognitive impairment (MCI). A score <26 on one or both measurement tools was considered a positive marker of frailty for cognition.
Data Analysis
Summary statistics of the demographics, tumor type and stage, health and functional status, and frailty markers were obtained. Patients were classified into four hierarchical groups based on the results of their assessment: 1- No frailty markers, IADL or ADL disability; 2- Presence of frailty markers without IADL or ADL disability; 3- IADL disability without ADL disability; 4- ADL disability.
Results
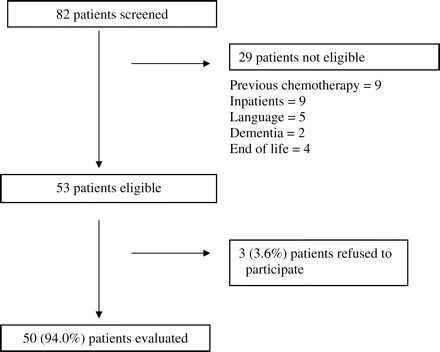
Eighty-two patients were screened during the study period. Based on the exclusion criteria (Figure 1), 29 were not eligible. Of the 53 eligible patients, 3 refused to participate. Overall, 50 patients were evaluated for a response rate of 94%.
Table 1 presents the demographics, oncology, and health and functional status data of the study group. The mean age of the patients was 76.8 years (standard deviation 5.2); 56.0% were women. For solid tumors, breast cancer was the most common diagnosis, followed by lung cancer and colorectal cancer; almost half had metastases. For hematological malignancies, leukemia was the most common diagnosis. Among the 50 patients, 13 (26.0%) had >3 comorbidities, 21 (42.0%) had IADL disability, and 8 (16.0%) had ADL disability. The most prevalent IADL and ADL disabilities were shopping (30.0%) and incontinence (12.0%) respectively.
Among the 50 patients, 88% had at least one frailty marker; 52% had three or more (Table 2). The most prevalent of the seven frailty markers were nutrition (62.0%), mobility (58.0%), physical activity (42.0%), and cognition (42.0%). More specifically, among the 38 patients with an MMSE score of ≥26, 12 (31.6%) had a MoCA score <26, suggesting MCI.
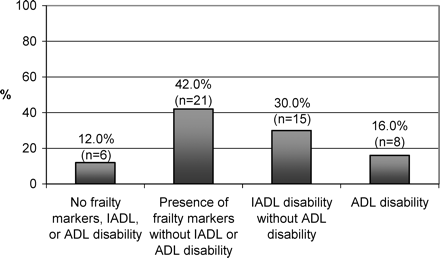
Figure 2 illustrates the distribution of the 50 patients among the four previously defined groups: Group 1: 6 (12.0%) patients with no frailty marker, IADL or ADL disability; Group 2: 21 (42.0%) patients with presence of frailty markers without IADL or ADL disability; Group 3: 15 (30.0%) patients with IADL disability but without ADL disability; and Group 4: 8 (16.0%) patients with ADL disability. All patients with ADL or IADL disability had at least one frailty marker.
Among the 21 patients (42.0%) in Group 2, 7 (33.3%) had one frailty marker; 6 (28.6%) had two; and 8 (38.1%) had three or more. The most prevalent frailty markers in this group were the same as in the whole study sample, with nutrition and mobility being the most common.
Discussion
To our knowledge, this is the first study to assess simultaneously seven frailty markers, IADL, ADL, and comorbidities in older cancer patients. Although more than half the patients in our study sample had no IADL or ADL disability, a large proportion had frailty markers, and only six (12.0%) had none. Moreover, 42.0% of patients presented at least one frailty marker without any IADL or ADL disability. Previous studies on health and functional status of older cancer patients (8–11), reporting primarily on IADL and ADL assessment but inconsistently on markers such as BMI, cognition, and mood, might not have identified a large proportion of those potentially vulnerable to the adverse outcomes of cancer treatment.
Our results are consistent with previous studies indicating that roughly 80% of older cancer outpatients are independent in ADL (9–11); more than half are independent in IADL (9–11); two-thirds are cognitively intact (10,35) and a majority have ≤3 comorbidities (9,10). These patients are generally more independent than older persons seen in specialized geriatric programs and home care services (8,36). The approach based on geriatric assessment has proven useful for patients experiencing multiple disabilities in ADL, interacting medical and social problems, and typical geriatric syndromes (falls, incontinence, dementia) (12,37). However, there is limited evidence of the effectiveness of this approach in independent older patients who are affected by a severe medical condition such as cancer (12,37). In a recent longitudinal study on women receiving adjuvant chemotherapy for breast cancer, Hurria and colleagues (11) found a high level of toxicity to treatment despite no change in IADL and ADL. These findings suggest that an approach based primarily on the assessment of IADL and ADL might have a potential ceiling effect in detecting vulnerability in the population of older cancer patients.
Using data from the Cardiovascular Health Study, which included community-dwelling American men and women 65 years old or older, Fried and colleagues (14) reported that older persons with at least three of five frailty markers are at a significantly increased risk of suffering from adverse outcomes such as falls, worsening mobility, ADL disability, hospitalization, and death within 3 years. Moreover, each of these frailty markers represents an independent predictor of adverse outcomes. Our results suggest that using frailty markers could help to better identify those highly functional independent older cancer patients who may be vulnerable to complications in response to aggressive treatments.
Choosing the most sensitive tools to assess health status is a major issue in geriatric oncology. For example, in the case of older cancer patients requiring chemotherapy, in particular adjuvant chemotherapy, a sensitive evaluation of the cognitive status is crucial. Patients with MCI are, in fact, at increased risk for developing delirium and eventually dementia (38,39). In our study, 12 of the 38 patients with a normal MMSE score had a MoCA suggestive of MCI. These patients would not have been identified as having a frailty marker for cognition had we relied solely on the MMSE score. This finding emphasizes the need to use more sensitive measurement tools in this population of older cancer patients.
Two important strengths of this study are the use of validated self-report and performance tests and the high mean age of our sample (77 years). This cross-sectional pilot study is the first step toward establishing an optimal approach to defining clinically relevant markers and measurement tools of health and functional status in this population.
There are limitations to this study. This is a cross-sectional study, restricted to a small number of patients from a single institution, which may not be representative of other centers. This study included only patients referred for chemotherapy who may be healthier than the general population of older cancer patients, owing to the potential referral bias. Although patients with various types of tumors and treatments were included in the sample, this design was selected to have a picture of the health and functional status of the older cancer patients referred to an oncology clinic.
Conclusion
Our results suggest that the usual geriatric assessment tools would have a ceiling effect and might not be intended for patients affected by only one severe medical condition such as cancer. Frailty markers may be a useful approach to detect potential vulnerability in older cancer patients. Longitudinal studies adequately powered to stratify by tumor and treatment type are needed to test the predictive validity of frailty markers in this population.
Decision Editor: Luigi Ferrucci, MD, PhD
Classification of patients into four hierarchical groups (N = 50). IADL = Instrumental Activities of Daily Living; ADL = Activities of Daily Living
Demographic, Oncology, and Health and Functional Status Data of the Study Group (N = 50).
| Measure . | Mean ± SD, [Min-Max]; N (%) . | |
|---|---|---|
| Age | 76.8 ± 5.2, [70–92] | |
| Female | 28 (56.0%) | |
| Cancer type | ||
| Breast | 12 (24.0%) | |
| Lung | 10 (20.0%) | |
| Colorectal | 8 (16.0%) | |
| Leukemia | 4 (8.0%) | |
| Prostate | 4 (8.0%) | |
| Other | 12 (24.0%) | |
| Metastatic tumor (n = 44)* | ||
| Present | 19 (43.2%) | |
| Absent | 20 (45.4%) | |
| Unknown | 5 (11.4%) | |
| Level of education (≥13 y) | 27 (54.0%) | |
| Living at home | 47 (94.0%) | |
| Presence of social support | 42 (84.0%) | |
| Source of social support (n = 42) | ||
| Spouse | 20 (47.6%) | |
| Children | 15 (35.7%) | |
| Other | 7 (16.7%) | |
| Comorbidities > 3 | 13 (26.0%) | |
| Functional status | ||
| ECOG-PS ≥ 2 | 9 (18.0%) | |
| IADL disability | 21 (42.0%) | |
| ADL disability | 8 (16.0%) | |
| Measure . | Mean ± SD, [Min-Max]; N (%) . | |
|---|---|---|
| Age | 76.8 ± 5.2, [70–92] | |
| Female | 28 (56.0%) | |
| Cancer type | ||
| Breast | 12 (24.0%) | |
| Lung | 10 (20.0%) | |
| Colorectal | 8 (16.0%) | |
| Leukemia | 4 (8.0%) | |
| Prostate | 4 (8.0%) | |
| Other | 12 (24.0%) | |
| Metastatic tumor (n = 44)* | ||
| Present | 19 (43.2%) | |
| Absent | 20 (45.4%) | |
| Unknown | 5 (11.4%) | |
| Level of education (≥13 y) | 27 (54.0%) | |
| Living at home | 47 (94.0%) | |
| Presence of social support | 42 (84.0%) | |
| Source of social support (n = 42) | ||
| Spouse | 20 (47.6%) | |
| Children | 15 (35.7%) | |
| Other | 7 (16.7%) | |
| Comorbidities > 3 | 13 (26.0%) | |
| Functional status | ||
| ECOG-PS ≥ 2 | 9 (18.0%) | |
| IADL disability | 21 (42.0%) | |
| ADL disability | 8 (16.0%) | |
Notes: *Patients with hematological malignancies (leukemia or lymphoma) were excluded (n = 6).
SD = standard deviation; ECOG-PS = Eastern Cooperative Oncology Group Scale of Performance Status; IADL = instrumental activities of daily living; ADL = activities of daily living.
Demographic, Oncology, and Health and Functional Status Data of the Study Group (N = 50).
| Measure . | Mean ± SD, [Min-Max]; N (%) . | |
|---|---|---|
| Age | 76.8 ± 5.2, [70–92] | |
| Female | 28 (56.0%) | |
| Cancer type | ||
| Breast | 12 (24.0%) | |
| Lung | 10 (20.0%) | |
| Colorectal | 8 (16.0%) | |
| Leukemia | 4 (8.0%) | |
| Prostate | 4 (8.0%) | |
| Other | 12 (24.0%) | |
| Metastatic tumor (n = 44)* | ||
| Present | 19 (43.2%) | |
| Absent | 20 (45.4%) | |
| Unknown | 5 (11.4%) | |
| Level of education (≥13 y) | 27 (54.0%) | |
| Living at home | 47 (94.0%) | |
| Presence of social support | 42 (84.0%) | |
| Source of social support (n = 42) | ||
| Spouse | 20 (47.6%) | |
| Children | 15 (35.7%) | |
| Other | 7 (16.7%) | |
| Comorbidities > 3 | 13 (26.0%) | |
| Functional status | ||
| ECOG-PS ≥ 2 | 9 (18.0%) | |
| IADL disability | 21 (42.0%) | |
| ADL disability | 8 (16.0%) | |
| Measure . | Mean ± SD, [Min-Max]; N (%) . | |
|---|---|---|
| Age | 76.8 ± 5.2, [70–92] | |
| Female | 28 (56.0%) | |
| Cancer type | ||
| Breast | 12 (24.0%) | |
| Lung | 10 (20.0%) | |
| Colorectal | 8 (16.0%) | |
| Leukemia | 4 (8.0%) | |
| Prostate | 4 (8.0%) | |
| Other | 12 (24.0%) | |
| Metastatic tumor (n = 44)* | ||
| Present | 19 (43.2%) | |
| Absent | 20 (45.4%) | |
| Unknown | 5 (11.4%) | |
| Level of education (≥13 y) | 27 (54.0%) | |
| Living at home | 47 (94.0%) | |
| Presence of social support | 42 (84.0%) | |
| Source of social support (n = 42) | ||
| Spouse | 20 (47.6%) | |
| Children | 15 (35.7%) | |
| Other | 7 (16.7%) | |
| Comorbidities > 3 | 13 (26.0%) | |
| Functional status | ||
| ECOG-PS ≥ 2 | 9 (18.0%) | |
| IADL disability | 21 (42.0%) | |
| ADL disability | 8 (16.0%) | |
Notes: *Patients with hematological malignancies (leukemia or lymphoma) were excluded (n = 6).
SD = standard deviation; ECOG-PS = Eastern Cooperative Oncology Group Scale of Performance Status; IADL = instrumental activities of daily living; ADL = activities of daily living.
Prevalence of Frailty Markers (N = 50).
| Measure . | N (%) . | |
|---|---|---|
| Number of frailty markers | ||
| 0 | 6 (12.0%) | |
| 1 | 8 (16.0%) | |
| 2 | 8 (16.0%) | |
| 3 | 10 (20.0%) | |
| 4 | 8 (16.0%) | |
| 5 | 5 (10.0%) | |
| 6 | 1 (2.0%) | |
| 7 | 1 (2.0%) | |
| Incomplete* | 3 (6.0%) | |
| Nutrition | ||
| Defined as: | 31 (62.0%) | |
| BMI ≤ 18.5 | 2 (4.0%) | |
| Unintentional weight loss > 10 pounds | 22 (44.0%) | |
| Decreased food intake | 25 (50.0%) | |
| Mobility | ||
| Defined as either: | 29 (58.0%) | |
| TUG > 10 s | 23 (46.0%) | |
| Falls in last 6 mo | 15 (30.0%) | |
| Strength | ||
| Grip strength ≤ lowest quintile by sex and BMI | 13 (26.0%) | |
| Energy | ||
| Visual scale ≤ 3 | 6 (12.0%) | |
| Unknown | 1 (2.0%) | |
| Physical activity | ||
| No exercise or low level of exercise | 21 (42.0%) | |
| Mood | ||
| HADS ≥ 15 | 11 (22.0%) | |
| Unknown | 1 (2.0%) | |
| Cognition | ||
| Defined as: | 21 (42.0%) | |
| MoCA < 26 | 19 (38.0%) | |
| MMSE < 26 | 9 (18.0%) | |
| Unknown | 3 (6.0%) | |
| Measure . | N (%) . | |
|---|---|---|
| Number of frailty markers | ||
| 0 | 6 (12.0%) | |
| 1 | 8 (16.0%) | |
| 2 | 8 (16.0%) | |
| 3 | 10 (20.0%) | |
| 4 | 8 (16.0%) | |
| 5 | 5 (10.0%) | |
| 6 | 1 (2.0%) | |
| 7 | 1 (2.0%) | |
| Incomplete* | 3 (6.0%) | |
| Nutrition | ||
| Defined as: | 31 (62.0%) | |
| BMI ≤ 18.5 | 2 (4.0%) | |
| Unintentional weight loss > 10 pounds | 22 (44.0%) | |
| Decreased food intake | 25 (50.0%) | |
| Mobility | ||
| Defined as either: | 29 (58.0%) | |
| TUG > 10 s | 23 (46.0%) | |
| Falls in last 6 mo | 15 (30.0%) | |
| Strength | ||
| Grip strength ≤ lowest quintile by sex and BMI | 13 (26.0%) | |
| Energy | ||
| Visual scale ≤ 3 | 6 (12.0%) | |
| Unknown | 1 (2.0%) | |
| Physical activity | ||
| No exercise or low level of exercise | 21 (42.0%) | |
| Mood | ||
| HADS ≥ 15 | 11 (22.0%) | |
| Unknown | 1 (2.0%) | |
| Cognition | ||
| Defined as: | 21 (42.0%) | |
| MoCA < 26 | 19 (38.0%) | |
| MMSE < 26 | 9 (18.0%) | |
| Unknown | 3 (6.0%) | |
Notes: *Two patients with two and four frailty markers recorded did not complete the MoCA or MMSE, so the total number of frailty markers could not be computed. One patient with two recorded frailty markers did not complete the MoCA, MMSE, HADS, or energy scale.
BMI = body mass index; TUG = Timed Up and Go; HADS = Hospital Anxiety and Depression Scale; MoCA = Montreal Cognitive Assessment; MMSE = Mini-Mental State Examination.
Prevalence of Frailty Markers (N = 50).
| Measure . | N (%) . | |
|---|---|---|
| Number of frailty markers | ||
| 0 | 6 (12.0%) | |
| 1 | 8 (16.0%) | |
| 2 | 8 (16.0%) | |
| 3 | 10 (20.0%) | |
| 4 | 8 (16.0%) | |
| 5 | 5 (10.0%) | |
| 6 | 1 (2.0%) | |
| 7 | 1 (2.0%) | |
| Incomplete* | 3 (6.0%) | |
| Nutrition | ||
| Defined as: | 31 (62.0%) | |
| BMI ≤ 18.5 | 2 (4.0%) | |
| Unintentional weight loss > 10 pounds | 22 (44.0%) | |
| Decreased food intake | 25 (50.0%) | |
| Mobility | ||
| Defined as either: | 29 (58.0%) | |
| TUG > 10 s | 23 (46.0%) | |
| Falls in last 6 mo | 15 (30.0%) | |
| Strength | ||
| Grip strength ≤ lowest quintile by sex and BMI | 13 (26.0%) | |
| Energy | ||
| Visual scale ≤ 3 | 6 (12.0%) | |
| Unknown | 1 (2.0%) | |
| Physical activity | ||
| No exercise or low level of exercise | 21 (42.0%) | |
| Mood | ||
| HADS ≥ 15 | 11 (22.0%) | |
| Unknown | 1 (2.0%) | |
| Cognition | ||
| Defined as: | 21 (42.0%) | |
| MoCA < 26 | 19 (38.0%) | |
| MMSE < 26 | 9 (18.0%) | |
| Unknown | 3 (6.0%) | |
| Measure . | N (%) . | |
|---|---|---|
| Number of frailty markers | ||
| 0 | 6 (12.0%) | |
| 1 | 8 (16.0%) | |
| 2 | 8 (16.0%) | |
| 3 | 10 (20.0%) | |
| 4 | 8 (16.0%) | |
| 5 | 5 (10.0%) | |
| 6 | 1 (2.0%) | |
| 7 | 1 (2.0%) | |
| Incomplete* | 3 (6.0%) | |
| Nutrition | ||
| Defined as: | 31 (62.0%) | |
| BMI ≤ 18.5 | 2 (4.0%) | |
| Unintentional weight loss > 10 pounds | 22 (44.0%) | |
| Decreased food intake | 25 (50.0%) | |
| Mobility | ||
| Defined as either: | 29 (58.0%) | |
| TUG > 10 s | 23 (46.0%) | |
| Falls in last 6 mo | 15 (30.0%) | |
| Strength | ||
| Grip strength ≤ lowest quintile by sex and BMI | 13 (26.0%) | |
| Energy | ||
| Visual scale ≤ 3 | 6 (12.0%) | |
| Unknown | 1 (2.0%) | |
| Physical activity | ||
| No exercise or low level of exercise | 21 (42.0%) | |
| Mood | ||
| HADS ≥ 15 | 11 (22.0%) | |
| Unknown | 1 (2.0%) | |
| Cognition | ||
| Defined as: | 21 (42.0%) | |
| MoCA < 26 | 19 (38.0%) | |
| MMSE < 26 | 9 (18.0%) | |
| Unknown | 3 (6.0%) | |
Notes: *Two patients with two and four frailty markers recorded did not complete the MoCA or MMSE, so the total number of frailty markers could not be computed. One patient with two recorded frailty markers did not complete the MoCA, MMSE, HADS, or energy scale.
BMI = body mass index; TUG = Timed Up and Go; HADS = Hospital Anxiety and Depression Scale; MoCA = Montreal Cognitive Assessment; MMSE = Mini-Mental State Examination.
This study was supported in part by grants from the French Ministry of Foreign Affairs (Bourse Lavoisier), the French National Society of Internal Medicine, and the Dr. Joseph Kaufman Chair in Geriatric Medicine, McGill University.
We thank the Solidage research group and the staff, nurses, and oncology clinic team members for their support in this project.
Research was carried out at the Division of Geriatric Medicine, McGill University, Jewish General Hospital, Montreal, Quebec, Canada.
References
Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives.
Canadian Cancer Society/National Cancer Institute of Canada. Canadian Cancer Statistics 2006. Toronto, Canada 2006.
Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG).
Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer.
Hurria A, Lachs MS, Cohen HJ, Muss HB, Kornblith AB. Geriatric assessment for oncologists: rationale and future directions.
Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of older patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the older study.
Repetto L, Venturino A, Vercelli M, et al. Performance status and comorbidity in older cancer patients compared with young patients with neoplasia and older patients without neoplastic conditions.
Serraino D, Fratino L, Zagonel V. Prevalence of functional disability among older patients with cancer.
Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in older cancer patients: an Italian Group for Geriatric Oncology Study.
Hurria A, Hurria A, Zuckerman E, et al. A prospective longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy.
Ferrucci L, Guralnik JM, Cavazzini C, et al. The frailty syndrome: a critical issue in geriatric oncology.
Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology.
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype.
Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study.
Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the Women's Health and Aging Studies.
Bergman H, Béland F, Karunananthan S, Hummel S, Hogan D, Wolfson C. Développement d'un cadre de travail pour comprendre et étudier la fragilité.
Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty.
Morley JE, Perry HM, III, Miller DK. Editorial: Something about frailty.
Bergman H, Ferrucci L, Guralnik J, et al. Frailty, an emerging research and clinical paradigm: issues and controversies.
Puts MT, Lips P, Deeg DJ. Static and dynamic measures of frailty predicted decline in performance-based and self-reported physical functioning.
Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JP. Old or frail: what tells us more?
Miller MD, Towers A. A manual of guidelines for scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). Pittsburgh, PA: University of Pittsburgh; 1991.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group.
Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire.
Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living.
Cornali C, Franzoni S, Frisoni GB, Trabucchi M. Anorexia as an independent predictor of mortality.
Cook Z, Kirk S, Lawrenson S, Sandford S. Use of BMI in the assessment of undernutrition in older subjects: reflecting on practice.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons.
Davis HS, MacPherson K, Merry HR, Wentzel C, Rockwood K. Reliability and validity of questions about exercise in the Canadian Study of Health and Aging.
Herrmann C. International experiences with the Hospital Anxiety and Depression Scale–a review of validation data and clinical results.
Ibbotson T, Maguire P, Selby P, Priestman T, Wallace L. Screening for anxiety and depression in cancer patients: the effects of disease and treatment.
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician.
Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment.
Extermann M, Chen H, Cantor AB, et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study.
Retornaz F, Seux V, Sourial N, et al. Comparison of the health and functional status between older inpatients with and without cancer admitted to a geriatric/internal medicine unit.
Wieland D, Rubenstein LZ. What do we know about patient targeting in geriatric evaluation and management (GEM) programs?
Author notes
1Division of Geriatric Medicine, 2Solidage Research Group on Integrated Services for Older Persons, Centre for Clinical Epidemiology and Community Studies, and 3Segal Cancer Centre, Jewish General Hospital, McGill University, Montreal, Quebec, Canada.