-
PDF
- Split View
-
Views
-
Cite
Cite
Diane R. Brown, Meral Topcu, Willingness to Participate in Clinical Treatment Research Among Older African Americans and Whites, The Gerontologist, Volume 43, Issue 1, February 2003, Pages 62–72, https://doi.org/10.1093/geront/43.1.62
Close - Share Icon Share
Abstract
Purpose: Using a health services utilization conceptual framework, the purpose of this analysis was to examine race differences in factors predictive of the behavioral intention of older persons to participate in a clinical treatment trial should they have a diagnosis of cancer. In addition, the analysis sought to determine if older African Americans were less likely than Whites to express willingness to participate, given knowledge of the Tuskegee syphilis study and greater fatalistic cancer beliefs.Design and Methods: Data were drawn from a community-based telephone survey of 216 African Americans and 222 Whites, 50 years of age and older.Results: Findings show that willingness to participate was significantly higher among males, persons of younger age, higher incomes, and with nonfatalistic cancer beliefs. Race differences were only apparent for the two significant interactions of race with age and high income. Neither knowledge of the Tuskegee study nor fatalistic cancer beliefs were more important for African Americans than for Whites.Implications: Study findings suggest that recruitment strategies need to be tailored to racial differences in factors affecting willingness to participate, particularly those related to age and income level.
Although rates of participation in health-related research are generally low, they are particularly low in racial/ethnic minority communities, medically underserved populations, and among older persons (Hall, 1999; Stallings et al., 2000). Despite the emerging consensus that there is a need to increase the participation of minorities and older persons in medical research (Dennis & Neese, 2000; Wrobel & Shapiro, 1999), the exact numbers of older minority persons who take part in medical research or who are willing to take part in health-related research are not known. In one of the few analyses that have attempted to understand rates of participation by race, age, and gender, Hutchins and colleagues (1999) examined data from research participants in trials conducted by the Southwest Oncology Group. Whereas women and African Americans were represented in proportion to their numbers in the population, persons over 65 years of age were significantly underrepresented. Findings from the Hutchins and colleagues' analysis, however, were limited in that they provided little insight into reasons for variability in rates of participation across studies and did not report participation by both age and race so that the number of older African Americans could be ascertained.
Participation in health-related research is particularly salient for older African Americans, given their higher rates of morbidity and mortality from various diseases. The health disparities between older African Americans and older Whites are well documented with regard to diabetes, hypertension, cardiovascular disease, stroke, and other conditions. Specifically with regard to cancer, older African Americans (age 55–74) have greater mortality than do younger persons and persons of other ethnic groups (Martin & Soldo, 1997). To eliminate these disparities and to develop appropriate interventions and treatments that will work best for older African Americans, it is essential that they take part in health-related research. Moreover, they need to participate in sufficient numbers for meaningful statistical analyses.
The impetus to recruit older persons and minorities into health-related research has resulted in a growing body of literature on recruitment issues and strategies. Most of this literature has focused on identifying personal, sociocultural, and socioeconomic barriers to research participation or has consisted of descriptions of successful and sometimes unsuccessful recruitment efforts used with older minority populations. Absent from much of literature has been a coherent conceptual framework within which to understand the range of factors that contribute to the participation of older persons in health-related research. Without a clear conceptual framework, it is difficult to elucidate the circumstances under which older African Americans would be willing to participate or to determine the extent to which they differ from similar older Whites. Further, in contributing to an emerging science of recruitment, it is important to consider how the different types of studies affect the willingness of older persons to participate in health-related studies. Specifically, research on recruitment needs to distinguish between treatment trials that are offered to individuals with illness symptomatology as opposed to prevention and nontherapeutic kinds of studies conducted on healthy populations. Clearly, willingness to participate in health-related research such as clinical treatment trials may be motivated by the presence or absence of illness or disease. To address this issue, this analysis focuses on factors affecting the willingness of older African Americans and Whites to participate in a clinical treatment study.
A Conceptual Framework
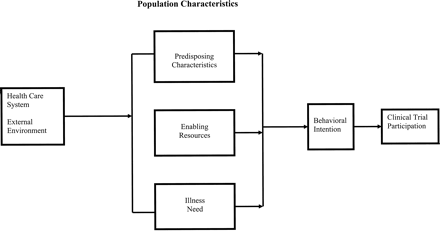
This analysis draws on a health services utilization framework that combines aspects of the theory of reasoned action (Azjen & Fishbein, 1980; Sutherland, da Cunha, Lockwood, & Till, 1998) and the health behavior model (Andersen, 1995). The conceptual framework is depicted in Figure 1. The theory of reasoned action focuses on cognitive factors that determine behavioral intention, whereas the health behavior model incorporates a greater range of individual and social structural factors. The theory of reasoned action has been used to explain behavior, particularly behavior that is volitional. Borrowing from the theory of reasoned action, it is an assumption of this conceptual framework that participation in a clinical treatment trial is preceded by a person's behavioral intention or willingness to take part in the study. In turn, the determinants of behavioral intention are a function of predisposing characteristics, enabling resources and illness need factors. Taken from Andersen's (1995) health behavior model, the predisposing and enabling components establish the conditions within which a person is or is not likely to express a behavioral intention to participate in clinical treatment research when stimulated by an illness need such as having a diagnosis of disease. The major components of the model—predisposing, enabling, and illness need factors—differentially predict willingness to participate in a clinical treatment study, and their effects may differ by race.
Predisposing Factors
According to the conceptual framework, some individuals will have a greater propensity to participate in a clinical treatment study than others. This propensity is likely to vary by demographic, social structural, and sociocultural predisposing factors. In one of the few studies that have examined demographic and social structural factors, African American men with higher levels of education appeared to be more likely to take part in health-related research than those with less education (Robinson, Ashley, & Haynes, 1996). In another study, Boult and colleagues (1998) found that among older high risk adults, those who were community dwelling, Medicare beneficiaries, women, and the oldest adults were less likely to express willingness to participate in a health-related study than were men and younger adults. Sociocultural factors such as health beliefs may also play a part in whether one is inclined to participate in health-related research (Champion, 1984). Among African Americans and other minorities, fear of cancer and fatalistic beliefs about the disease may influence volunteering for clinical research (Swanson & Ward, 1995). In addition, genetics and having a family history of disease as well as knowing someone who participated in a health-related study might affect one's inclination to participate in clinical treatment research (Champion, 1984).
Other predisposing factors that may facilitate or inhibit willingness to participate in clinical treatment studies include previous experiences with the health care system. Past experiences and perceptions of the health care system may be a particularly salient predisposing factor for African Americans in general and older African Americans in particular, given a history of negative experiences, neglect, and discrimination by the health care system (Corbie-Smith, Thomas, Williams, & Moody-Ayers, 1999). Distrust of medical institutions and cultural differences between the African American community and health care providers emerge as major barriers to African Americans being willing to participate in clinical research (Dennis & Neese, 2000; Zhu et al., 2000).
Among African Americans, the Tuskegee syphilis experiment is seen as the symbol of distrust toward the medical establishment. In investigating the effect of knowledge of Tuskegee on willingness to participate in clinical trials, Shavers and colleagues (2000) found that an overwhelming majority of African Americans (81%) in contrast to less than a third of Whites (28%) had heard of the Tuskegee study. Most important, irrespective of knowledge of the Tuskegee study, African Americans were less likely to express willingness to participate in clinical research than were Whites. The importance of racial differences in distrust was also evidenced in a study of African American and White women, 50 to 79 years of age, who refused to participate in the Women's Health Initiative (Mouton, Harris, Rovi, Solorzano, & Johnson, 1997). Although the majority of African Americans (89%) and Whites (86%) agreed that health-related research provides societal benefits, 32.1% of African American women felt that scientists cannot be trusted. In contrast, only 4.1% of White women agreed with this statement.
Enabling Factors
Enabling factors consist of resources that facilitate participation in health-related research. Inversely, the lack of these resources may contribute to the underrepresentation of African Americans in health-related research. Much of the literature on barriers to research participation has focused on enabling factors pertaining to awareness, eligibility, accessibility, and social support (Dennis & Neese, 2000; Shavers et al., 2000; Swanson & Ward, 1995). Awareness is an essential enabling factor. However, prior research indicates that physicians may be less likely to invite or refer African American patients to clinical trials (Pinto, McCaskill-Stevens, Wolfe, & Marcus, 2000), thereby limiting patients' awareness. In particular, physicians engaged in clinical research report that they do not feel confident to explain clinical trials in culturally appropriate terms to their African American patients (Stone, Mauch, & Steger, 1998).
Whereas some older African Americans may be excluded from studies because of comorbidities or poor health (Simon, Brown, Du, LoRusso, & Kellogg, 1998), accessibility to clinical treatment trials may also be limited because of low income or lack of insurance coverage for participation. In some health-related studies, the costs of medication, use of nonconventional therapies, and follow-up care may not be covered by health insurance or included as part of the trial itself. Accessibility may also be limited because participation in the research may conflict with family and job responsibilities. Further, accessibility issues may encompass the need for transportation, and in some instances require a telephone, a computer, or Internet access.
Social support from family members, friends, and others may also be enabling resources. From their experience in recruiting older African Americans (mean age 63 years), Gorelick and colleagues (1998) noted that the eligible participants who refused to participate or withdrew from the study reported that support of their family, friends, or elders in the community influenced their decision. Further, in some instances, older African Americans may not receive the endorsement of their physicians, especially if they are seeing community-based physicians who are not affiliated with an academic research medical center.
Illness Need
In extending the assumptions of the Andersen (1995) model, the conceptual framework suggests that illness need not only encompass disease symptomatology, but also one's beliefs about the disease and perceptions of severity. It would also include the perceived need and perceived benefit from participation in a clinical treatment trial. Illness need is often reflected in stage of disease, functional health status, subjective self-assessments of health status, and comorbidities. The conceptual model assumes that individuals with greater illness symptomatology would be more likely to take part in clinical treatment research than those with fewer or no symptoms.
Other Factors
The conceptual model also recognizes that the external environment, including its physical, political, and economic aspects may impact participation in health-related research. Specifically, the health care system, its organization, policies, and resources are likely to be important determinants of participation in clinical treatment research. As an example, recent changes in health care policy allow Medicare recipients to obtain coverage for clinical trial participation (Health Care Financing Administration, 2000).
In summation, current empirical knowledge is limited regarding an understanding of factors that predict the participation of older persons, specifically older African Americans in health-related research. The purpose of this analysis is to use this conceptual framework in an examination of factors predictive of behavioral intention to take part in clinical treatment research. Using clinical cancer treatment trials as the context, the analysis seeks to determine what older persons would do (behavioral intention) should they have a diagnosis of cancer (illness need) and then to determine if there are differences in behavioral intention between older African Americans and Whites. Second, the analysis seeks to examine the association between behavioral intention and selected predisposing and enabling factors and then to determine if there are differences by race. Of special concern to this analysis is the influence on behavioral intention of predisposing sociocultural factors of particular salience to older African Americans. These include knowledge of the Tuskegee syphilis study and fatalistic attitudes toward cancer reported to be higher among African Americans than Whites.
Methods
Data and Sampling Strategy
Data for the analysis come from a community-based survey of cancer awareness in the metropolitan Detroit area. A random digit-dialing selection process was used to select respondents for a 25-min telephone interview. The sampling strategy resulted in 1,225 respondents, 36% of whom were 50 years of age and older. The age and race distribution of the sample were similar to population estimates for the geographical area. After six attempts were made to reach respondents by telephone, the rate of response was 50%. Details on the methods are described elsewhere (Brown & Herskovitz, 2000). This analysis is based on the 438 respondents 50 years of age and older who participated in the interview. Slightly less than half (49.3%) were African American (n = 216); the remaining 50.7% were White (n = 222).
Measures
The dependent variable consisted of behavioral intention as defined by willingness to take part in a clinical cancer treatment trial. To ascertain behavioral intention, respondents were first read the following definition of a clinical trial:
A clinical trial is medical research in which scientists observe the course of a disease in human beings or evaluate the effectiveness of a therapy or treatment. Usually participants will receive some free medical care and may also receive the latest medical treatment.
Respondents were then asked about their willingness to take part in a clinical cancer treatment trial, based on the premise that they had an illness need, for example, a diagnosis of cancer. The question read, “If asked, would you consider participating in a clinical trial designed to test a medical treatment if you had a serious medical illness such as cancer?” Responses to this question were coded 1 for yes and 0 for no. Most respondents had no difficulty in answering the question. Where further clarity was needed, the interviewers were instructed to slowly reread the definition of a clinical trial and to repeat the question.
Predisposing variables included the demographic background characteristics of race, gender, age, employment status, and living arrangements. Gender was coded 1 for male and 0 for female, whereas self-identified race was coded as 1 for White and 0 for African American. Age was measured by date of birth and then calculated into actual age, whereas employment status was coded as 1 for employed and 0 for not employed. With regard to living arrangements, respondents were asked about the number of persons living with them in the household. This variable was coded 1 as living alone and 0 living with others. Respondents were asked about having relatives with cancer. For this variable, responses were coded 1 if respondents indicated that they had a blood relative who had been diagnosed with either prostate, breast, lung, or other cancer. Responses were coded 0 for no family members with cancer.
As a social structural predisposing measure, educational attainment was represented by the highest level of education completed. In addition, two sociocultural predisposing factors were included in the analysis. They pertained to knowledge of the Tuskegee syphilis experiment and having fatalistic cancer beliefs. Subsequent to the questions on willingness to participate in clinical cancer treatment trials, respondents were asked, “Have you heard of the Tuskegee syphilis experiment?” The responses were coded 1 for yes only if the respondents could demonstrate some knowledge regarding the Tuskegee experiment, such as a description of the subjects. Otherwise, the response was coded 0 for no knowledge. Fatalistic cancer beliefs were measured by asking respondents to indicate their extent of agreement or disagreement with six statements. Items included, “Everything causes cancer,” “Chances of being cured are not good,” “There is not much one can do to prevent cancer,” “Most people who have cancer die,” “Cancer is due to God's will or fate,” and “Getting cancer is a death sentence.” Responses ranged from 1 (strongly agree) to 5 (strongly disagree). Additive scale scores ranged from 5 to 30; items were reverse coded so that the higher scores reflected greater disagreement or less fatalistic attitudes.
In terms of enabling factors, family income was presented in terms of broadly scaled categories in order to maximize responses during the telephone interview. Health insurance was included as another enabling factor, given the importance of access to health care and having coverage for any possible out-of-pocket costs associated with research participation. Responses were coded 0 if respondents had health insurance and 1 for no coverage. For those respondents who indicated no willingness to participate in a clinical trial even if they had a diagnosis of cancer, they were asked a series of questions to ascertain under what circumstances they would consider participating. These included receipt of payment, having a doctor's recommendation, helping someone else, receipt of free medical exams, helping scientists learn more about the disease, knowing someone who had previously participated in a study, having no side effects, having no out-of-pocket costs, and having a family member or friend recommend participation. Responses were coded 1 for yes and 0 for not willing to participate under stated condition.
Analyses
The analyses began with an examination of the racial differences in predisposing and enabling characteristics. These were ascertained using chi-square analyses and t tests, depending upon level of measurement. Additional chi-square analyses were performed to assess the relationships between behavioral intention to participate in a clinical cancer treatment trial and the predisposing and enabling factors. These analyses were performed separately for each racial group. Based on the bivariate analyses, multivariate logistic regression analyses were subsequently conducted to examine the relative importance of predisposing and enabling factors. The continuous form of age was used, along with the ordinal Fatalistic Cancer Beliefs Scale. All other variables were dummy coded. As suggested by bivariate findings, interactions with race were constructed with age, income, and living arrangements. These interactions were entered as a group along with the basic model comprised of the predisposing and enabling variables. Race interactions were also constructed with knowledge of the Tuskegee study and with fatalistic cancer beliefs to assess race differences in these associations. These were also entered as a group into the basic model consisting of the predisposing and enabling variables.
Results
Table 1 gives the distribution of predisposing and enabling factors by race. As expected, there were significant differences in predisposing characteristics. Specifically, there were significant differences by race in the distribution of respondents by gender and living arrangements. African Americans over 50 years of age participating in the survey were more likely than Whites to be female, χ2(1, N = 438) = 8.76, p <.01, and to live alone, χ2(1, N = 438) = 3.95, p <.05. There were no significant differences in the age distribution or in employment status. As expected, older African Americans had significantly less education, χ2(2, N = 418) = 14.41, p <.001, than similar Whites. On the other hand, about half of both African Americans (51.4%) and Whites (50.9%) stated that they had at least one blood relative diagnosed with cancer. There was no statistically significant difference by race.
In terms of racial differences in enabling characteristics, African Americans reported significantly less family income, χ2(2, N = 438) = 23.27, p <.001, than Whites, although they did not differ in terms of health insurance coverage. With regard to sociocultural predisposing factors, older African Americans were significantly, χ2(1, N = 419) = 12.02, p <.001, more likely than Whites to indicate awareness of the Tuskegee syphilis experiment. Slightly more than half of African Americans (54.8%) and a little more than a third of Whites (37.9%) expressed awareness of the Tuskegee study. An analysis of responses to questions pertaining to fatalistic cancer beliefs indicated a significant difference, t(437) = −2.492, p <.01, between older African Americans and Whites. The mean for African Americans was 19.90 (SD = 5.14) and was 21.12 (SD = 5.13) for Whites, with African Americans more likely than Whites to express these beliefs.
Willingness to Participate in a Clinical Cancer Treatment Trial
When asked if they would take part in a clinical treatment trial if they had a serious medical illness such as cancer, African Americans and Whites had similar answers regarding their behavioral intention to do so. Specifically, 72.5% of African Americans and 78.2% of Whites responded positively; the racial difference was only marginally significant, χ2(1, N = 438) = 3.299, p <.07. Racial differences were also assessed to determine the circumstances under which unwilling respondents would agree to take part in a clinical cancer treatment trial should they have the disease. These findings are given in Table 2, along with racial comparisons. To be noted, African Americans were significantly less likely than Whites to express behavioral intention to take part in a clinical treatment trial even if they received some free medical care, t(110) = −2.134, p <.05, if it helped scientists learn more about the disease, t(111) =−2.310, p <.05, if they knew of someone who had participated, t(113) = −2.099, p <.05, or if there were no out-of-pocket costs, t(111) = −2.310, p <.05). There were no racial differences regarding circumstances such as if they received payment, if a doctor recommended it, if it might help cure someone of illness, if there were no side effects, or if a friend or family member recommended it. It is important to note that among those who initially were unwilling to participate, the two factors most likely to change behavioral intention for both older African Americans and Whites was having a doctor's recommendation and if participation might help cure someone of the illness.
Additional analyses were conducted to determine if there were differences by race in the bivariate associations between behavioral intention and the predisposing and enabling factors. These results are given in Table 3. In terms of predisposing factors, there was a significant difference in behavioral intention according to age for African Americans, χ2(2, N = 132) = 12.26, p <.01, but not for Whites. Among African Americans, behavioral intention appears to decline with increasing age. Those over 75 years of age were the least likely (40.5%) to indicate a willingness to participate in a clinical cancer treatment trial, whereas African Americans 50–60 years of age were the most willing (70.9%). Further, there were significant gender differences among both African Americans and Whites, with males being more willing to take part in a clinical trial than females. Level of educational attainment was not significantly related to behavioral intention for either African Americans or Whites. On the other hand, although living arrangements were not significantly associated with behavioral intention for African Americans, there was a significant association for Whites, χ2(1, N = 154) = 3.92, p <.05. Nearly three fourths (74.1%) of Whites who lived with others expressed behavioral intention to participate in a clinical cancer treatment trial in contrast to only 61.4% of Whites who lived alone. Also, having relatives with cancer was not significantly associated with behavioral intention to participate in clinical cancer treatment trial. With regard to enabling factors, level of income was significant for older Whites, χ2(2, N = 154) = 6.85, p <.05, but not for older African Americans. Among Whites, 78% of those with the highest incomes expressed a willingness to take part in a clinical cancer treatment trial, whereas Whites of the lowest incomes were the most reluctant (57.1%). Irrespective of race, having health insurance was not associated with behavioral intention to take part in a clinical cancer treatment trial. Interestingly however, although not significant, those without health insurance expressed a greater willingness to take part in a clinical cancer treatment trial than those who were insured.
In terms of sociocultural predisposing factors, knowledge about the Tuskegee syphilis study was not associated with willingness to participate in clinical cancer treatment trials for either African Americans or Whites. On the other hand, the relationship between fatalistic cancer beliefs and behavioral intention was significant for African Americans, χ2(2, N = 132) = 4.58, p <.05, but not for Whites. The findings in Table 3 show that slightly less than half (47.8%) of African Americans with the most fatalistic cancer beliefs were willing to take part in a clinical cancer treatment trial, whereas more than two thirds (68.6%) of those with the least fatalistic perspectives expressed a behavioral intention to do so.
Multivariate Analysis
A multivariate analysis was conducted to assess the relative importance of predisposing and enabling factors and to determine if the sociocultural factors of particular salience to African Americans were significant predictors of their behavioral intention to take part in a clinical cancer treatment trial. Table 4 gives the results of the logistic regression analyses. These results show that race is not a significant predictor of willingness to participate in a clinical cancer treatment trial although gender is. Specifically, males are more willing than females to participate in a clinical cancer treatment trial when other factors are controlled. Also, age is significant, with the oldest persons being less willing to participate than younger middle-aged persons. However, the Race × Age interaction is significant, suggesting that the relationship between age and willingness to participate in a clinical cancer treatment trial differs between older African Americans and older Whites. Specifically, although willingness to participate generally declines with increasing age, the oldest Whites are significantly more willing to participate in clinical treatment research than are similar African Americans. The logistic analyses also show that income is a significant predicator of willingness to participate in clinical cancer treatment research, specifically for those at the highest levels of income. Generally, higher income is associated with an increased inclination to participate in a clinical treatment trial. Further, the interaction of race with high income is significant, indicating that Whites of the highest incomes are not only significantly more willing to participate in a clinical treatment trial than are Whites of the lowest incomes, but are also more willing to participate than African Americans.
The findings were mixed with regard to the two sociocultural predisposing factors. Although the main effect of knowledge of Tuskegee was not significant, the presence of fatalistic cancer beliefs was a significant predictor of willingness to take part in a clinical treatment trial for both African Americans and Whites. Contrary to expectations, neither of the interactions by race with these two sociocultural predisposing factors were significant (not shown), thus indicating that these factors were not stronger predictors for African Americans than Whites with regard to willingness to take part in clinical treatment trial. To be noted, living arrangements was not significant nor was its interaction with race (not shown); having health insurance was also not significant when other factors are controlled.
Discussion
These findings show that the overwhelming majority—approximately three quarters of both older African Americans (73%) and older Whites (78%)—are willing to take part in clinical treatment research should they be diagnosed with cancer. Thus, when other factors are controlled, older African Americans and Whites with similar illness need are not significantly different in their willingness to take part in a clinical cancer treatment trial. This finding is in part reflected in national accrual data for cancer clinical treatment trials in which African American enrollment across all age groups generally parallels the incidence of cancer morbidity in the African American population (Tejeda et al., 1996).
Even though our findings show no significant difference by race in willingness to participate in a clinical trial, the conceptual framework allowed us to examine race differences in the predictors of behavioral intention. Specifically, the conceptual framework provided a basis for testing hypotheses related to two predisposing sociocultural factors: knowledge of the Tuskegee study and having fatalistic cancer attitudes. It was expected that knowledge of the Tuskegee study and fatalistic cancer attitudes would diminish willingness to participate among older African Americans. However, neither of these predisposing sociocultural factors were stronger predictors of behavioral intention for African Americans than for Whites. Even though older African Americans were more knowledgeable about the Tuskegee study than older Whites, this was not significantly associated with lack of willingness to participate in clinical treatment research. Similar to findings of Shavers and colleagues (2000), knowledge of the Tuskegee study appears to be a symbol of distrust among African Americans, but not necessarily a deterrent to research participation. At the same time, it may be useful in future studies to use another more direct measure of distrust of the health care system and medical research.
Having fatalistic cancer beliefs was a significant predictor of lack of willingness to participate in clinical treatment research, irrespective of race. However, although older African Americans expressed more fatalistic cancer beliefs than did older Whites, there was no evidence of a differential effect by race on willingness to participate in clinical treatment research. Thus, both African Americans and Whites who express fatalistic cancer beliefs may be more difficult to recruit into clinical treatment studies than those who do not have these perspectives.
In terms of other factors that predispose older persons to express willingness to participate in clinical cancer research, there were both differences and similarities by race. With regard to similarities, gender is a significant predictor, irrespective of race, with men being more willing than women to consider research participation. Men have historically had higher rates of participation in clinical trials than women (Brown, Fouad, Basen-Engquist, & Tortolero-Luna, 2000) and have a greater propensity to engage in risk-taking behavior (Cubbins & Tanfer, 2000). Age is also a significant predictor of willingness to participate in a clinical treatment trial, with a decline in willingness with increasing age. However, there is a significant difference by race with regard to age as a predictor of willingness to participate in a clinical treatment trial. The oldest Whites are significantly more willing to participate in clinical treatment research than are similar African Americans. Interpreted from another perspective, the oldest African Americans were significantly less inclined than the oldest Whites to express willingness to take part in clinical treatment trial. Perhaps this is a cohort factor, as the oldest African Americans in the survey are likely to have a longer history of first-hand experiences with racial discrimination, malpractice, and neglect from the health care system. On the other hand, many of the oldest African Americans may also have religious beliefs or worldviews that render them content with their longevity and inevitable mortality and therefore less willing to engage in experimental treatments to extend their life.
Having high income was associated with greater willingness to participate in a clinical treatment trial. Older Whites of higher income were more likely to do so than were lower income Whites or African Americans. It was surprising that health insurance was not a significant predictor of willingness to participate in a clinical treatment trial. However, the hypothetical nature of the question posed to survey respondents may be an underlying reason for the lack of significance.
These findings also indicate that approximately a fourth of both older African Americans and Whites would not be willing to take part in a clinical treatment trial, even if they were diagnosed with cancer. These are the persons who are apt to be the most difficult to recruit, if at all. It should be noted that of those persons who were initially unwilling to participate, 25–34% of older African Americans and older Whites indicated that they would consider participating if they received a doctor's recommendation or if it might help someone else be cured of the illness. These findings are important and may be particularly useful for developing recruitment strategies for both African American and White older persons, who are especially reluctant to participate. At the same time, there were race differences pertaining to other circumstances under which older persons could be motivated to participate. Older African Americans who initially were unwilling indicated they were significantly less likely than their White counterparts to be persuaded to participate even if they received free medical care, if it helped scientists learn more about the disease, if they knew of someone who had previously participated, or if there were no costs. These findings suggest that it may not be possible to recruit these individuals or that additional efforts are needed to ascertain reasons for unwillingness to participate.
Limitations of Study
We recognize that these analyses had several limitations. Behavioral intention or willingness to participate in a clinical treatment study needs to be corroborated with actual participation. Future studies should examine the extent to which behavioral intention predicts actual enrollment in clinical treatment research and to examine the circumstances under which participation does not occur. In addition, given the hypothetical nature of this analysis, we recognize that responses might be different under actual circumstances of illness need when older persons are confronted by a diagnosis of cancer and offered the opportunity to participate in a clinical cancer treatment study. Actual behavior may vary by stage of illness, the prognosis, the availability of treatment options, among numerous other factors. Nonetheless, findings from analyses of behavioral intentions can be useful in planning outreach and recruitment approaches and understanding the likelihood of research participation (Brown et al., 2000).
Future studies also need to test other aspects of the conceptual framework and to apply the model to different types of research. The model may require modification when applied to clinical prevention trials or to noninvasive studies involving surveys or personal interviews. In addition, future research needs to give consideration to specific characteristics of the health-related research such as participant burden, inconvenience, lack of appropriate incentives, lack of cultural competency of research staff, absence of Africans Americans on the research team, confusing and complex consent forms, among others. Finally, it is also important to recognize that findings may differ when the model is applied to other health conditions such as diabetes, heart disease, HIV, or even specific types of cancer.
Decision Editor: Laurence G. Branch, PhD
Meral Topcu is now with the Institute of Gerontology, Wayne State University, Detroit, MI.
1College of Urban, Labor, and Metropolitan Affairs, Wayne State University, Detroit, MI.
Distribution of Predisposing and Enabling Characteristics By Race
| . | African American (N=216) . | White (N=222) . | . | ||
|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | p . |
| Age | |||||
| 50–60 | 110 | 50.9 | 117 | 52.7 | ns |
| 61–75 | 64 | 29.6 | 63 | 28.4 | |
| >75 | 42 | 19.4 | 42 | 18.9 | |
| Gender | <.01 | ||||
| Female | 160 | 74.1 | 135 | 60.8 | |
| Male | 56 | 25.9 | 87 | 39.2 | |
| Employment Status | ns | ||||
| Employed | 70 | 33.5 | 81 | 37.3 | |
| Not employed | 139 | 66.5 | 136 | 62.7 | |
| Living Arrangements | <.05 | ||||
| Living alone | 101 | 46.8 | 83 | 37.4 | |
| Living with others | 115 | 53.2 | 139 | 62.6 | |
| Education | <.001 | ||||
| Less than 12 | 52 | 25.1 | 23 | 10.9 | |
| High school diploma | 59 | 28.5 | 74 | 35.1 | |
| >12 | 96 | 46.4 | 114 | 54.0 | |
| Income | <.001 | ||||
| Less than $25,000 | 94 | 43.5 | 56 | 25.2 | |
| $25,000–40,000 | 81 | 37.5 | 84 | 37.8 | |
| >$40,000 | 41 | 19.0 | 82 | 36.9 | |
| Health Insurance | ns | ||||
| Yes | 203 | 96.2 | 206 | 94.1 | |
| No | 8 | 3.8 | 13 | 5.9 | |
| Knowledge of Tuskegee | <.001 | ||||
| Yes | 114 | 54.8 | 80 | 37.9 | |
| No | 94 | 45.2 | 131 | 62.1 | |
| Relatives With Cancer | ns | ||||
| None | 105 | 48.6 | 109 | 49.1 | |
| 1 or more | 111 | 51.4 | 113 | 50.9 | |
| Cancer Fatalistic Beliefs | <.05 | ||||
| High (0–15) | 46 | 21.3 | 27 | 12.2 | |
| Moderate (16–23) | 135 | 62.5 | 147 | 66.2 | |
| Low(24–30) | 35 | 16.2 | 48 | 21.6 |
| . | African American (N=216) . | White (N=222) . | . | ||
|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | p . |
| Age | |||||
| 50–60 | 110 | 50.9 | 117 | 52.7 | ns |
| 61–75 | 64 | 29.6 | 63 | 28.4 | |
| >75 | 42 | 19.4 | 42 | 18.9 | |
| Gender | <.01 | ||||
| Female | 160 | 74.1 | 135 | 60.8 | |
| Male | 56 | 25.9 | 87 | 39.2 | |
| Employment Status | ns | ||||
| Employed | 70 | 33.5 | 81 | 37.3 | |
| Not employed | 139 | 66.5 | 136 | 62.7 | |
| Living Arrangements | <.05 | ||||
| Living alone | 101 | 46.8 | 83 | 37.4 | |
| Living with others | 115 | 53.2 | 139 | 62.6 | |
| Education | <.001 | ||||
| Less than 12 | 52 | 25.1 | 23 | 10.9 | |
| High school diploma | 59 | 28.5 | 74 | 35.1 | |
| >12 | 96 | 46.4 | 114 | 54.0 | |
| Income | <.001 | ||||
| Less than $25,000 | 94 | 43.5 | 56 | 25.2 | |
| $25,000–40,000 | 81 | 37.5 | 84 | 37.8 | |
| >$40,000 | 41 | 19.0 | 82 | 36.9 | |
| Health Insurance | ns | ||||
| Yes | 203 | 96.2 | 206 | 94.1 | |
| No | 8 | 3.8 | 13 | 5.9 | |
| Knowledge of Tuskegee | <.001 | ||||
| Yes | 114 | 54.8 | 80 | 37.9 | |
| No | 94 | 45.2 | 131 | 62.1 | |
| Relatives With Cancer | ns | ||||
| None | 105 | 48.6 | 109 | 49.1 | |
| 1 or more | 111 | 51.4 | 113 | 50.9 | |
| Cancer Fatalistic Beliefs | <.05 | ||||
| High (0–15) | 46 | 21.3 | 27 | 12.2 | |
| Moderate (16–23) | 135 | 62.5 | 147 | 66.2 | |
| Low(24–30) | 35 | 16.2 | 48 | 21.6 |
Notes: Employment status percentages based on 209 African Americans and 217 Whites. Education percentages based on 207 African Americans and 211 Whites. Health insurance percentages based on 211 African Americans and 219 Whites. Knowledge of Tuskegee percentages based on 208 African Americans and 211 Whites.
Distribution of Predisposing and Enabling Characteristics By Race
| . | African American (N=216) . | White (N=222) . | . | ||
|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | p . |
| Age | |||||
| 50–60 | 110 | 50.9 | 117 | 52.7 | ns |
| 61–75 | 64 | 29.6 | 63 | 28.4 | |
| >75 | 42 | 19.4 | 42 | 18.9 | |
| Gender | <.01 | ||||
| Female | 160 | 74.1 | 135 | 60.8 | |
| Male | 56 | 25.9 | 87 | 39.2 | |
| Employment Status | ns | ||||
| Employed | 70 | 33.5 | 81 | 37.3 | |
| Not employed | 139 | 66.5 | 136 | 62.7 | |
| Living Arrangements | <.05 | ||||
| Living alone | 101 | 46.8 | 83 | 37.4 | |
| Living with others | 115 | 53.2 | 139 | 62.6 | |
| Education | <.001 | ||||
| Less than 12 | 52 | 25.1 | 23 | 10.9 | |
| High school diploma | 59 | 28.5 | 74 | 35.1 | |
| >12 | 96 | 46.4 | 114 | 54.0 | |
| Income | <.001 | ||||
| Less than $25,000 | 94 | 43.5 | 56 | 25.2 | |
| $25,000–40,000 | 81 | 37.5 | 84 | 37.8 | |
| >$40,000 | 41 | 19.0 | 82 | 36.9 | |
| Health Insurance | ns | ||||
| Yes | 203 | 96.2 | 206 | 94.1 | |
| No | 8 | 3.8 | 13 | 5.9 | |
| Knowledge of Tuskegee | <.001 | ||||
| Yes | 114 | 54.8 | 80 | 37.9 | |
| No | 94 | 45.2 | 131 | 62.1 | |
| Relatives With Cancer | ns | ||||
| None | 105 | 48.6 | 109 | 49.1 | |
| 1 or more | 111 | 51.4 | 113 | 50.9 | |
| Cancer Fatalistic Beliefs | <.05 | ||||
| High (0–15) | 46 | 21.3 | 27 | 12.2 | |
| Moderate (16–23) | 135 | 62.5 | 147 | 66.2 | |
| Low(24–30) | 35 | 16.2 | 48 | 21.6 |
| . | African American (N=216) . | White (N=222) . | . | ||
|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | p . |
| Age | |||||
| 50–60 | 110 | 50.9 | 117 | 52.7 | ns |
| 61–75 | 64 | 29.6 | 63 | 28.4 | |
| >75 | 42 | 19.4 | 42 | 18.9 | |
| Gender | <.01 | ||||
| Female | 160 | 74.1 | 135 | 60.8 | |
| Male | 56 | 25.9 | 87 | 39.2 | |
| Employment Status | ns | ||||
| Employed | 70 | 33.5 | 81 | 37.3 | |
| Not employed | 139 | 66.5 | 136 | 62.7 | |
| Living Arrangements | <.05 | ||||
| Living alone | 101 | 46.8 | 83 | 37.4 | |
| Living with others | 115 | 53.2 | 139 | 62.6 | |
| Education | <.001 | ||||
| Less than 12 | 52 | 25.1 | 23 | 10.9 | |
| High school diploma | 59 | 28.5 | 74 | 35.1 | |
| >12 | 96 | 46.4 | 114 | 54.0 | |
| Income | <.001 | ||||
| Less than $25,000 | 94 | 43.5 | 56 | 25.2 | |
| $25,000–40,000 | 81 | 37.5 | 84 | 37.8 | |
| >$40,000 | 41 | 19.0 | 82 | 36.9 | |
| Health Insurance | ns | ||||
| Yes | 203 | 96.2 | 206 | 94.1 | |
| No | 8 | 3.8 | 13 | 5.9 | |
| Knowledge of Tuskegee | <.001 | ||||
| Yes | 114 | 54.8 | 80 | 37.9 | |
| No | 94 | 45.2 | 131 | 62.1 | |
| Relatives With Cancer | ns | ||||
| None | 105 | 48.6 | 109 | 49.1 | |
| 1 or more | 111 | 51.4 | 113 | 50.9 | |
| Cancer Fatalistic Beliefs | <.05 | ||||
| High (0–15) | 46 | 21.3 | 27 | 12.2 | |
| Moderate (16–23) | 135 | 62.5 | 147 | 66.2 | |
| Low(24–30) | 35 | 16.2 | 48 | 21.6 |
Notes: Employment status percentages based on 209 African Americans and 217 Whites. Education percentages based on 207 African Americans and 211 Whites. Health insurance percentages based on 211 African Americans and 219 Whites. Knowledge of Tuskegee percentages based on 208 African Americans and 211 Whites.
Circumstances by Race for Consideration of Treatment Trial for Those Who Initially Declined
| . | African American (N = 82) . | White (N = 67) . | . | ||
|---|---|---|---|---|---|
| Circumstance . | n . | % . | n . | % . | p . |
| If received payment | 6 | 7.3 | 3 | 4.5 | ns |
| If doctor recommended it | 22 | 26.8 | 20 | 29.9 | ns |
| If might help someone/cured of illness | 21 | 25.6 | 23 | 34.3 | ns |
| If received some free medical care/exams | 6 | 7.3 | 13 | 19.4 | <.05 |
| If it helped scientists learn more | 7 | 8.5 | 15 | 22.4 | <.05 |
| If knew someone who participated | 6 | 7.3 | 14 | 20.9 | <.05 |
| If there were no side effects | 10 | 12.2 | 14 | 20.9 | ns |
| If there was no cost | 7 | 8.5 | 14 | 20.9 | <.05 |
| If friend/family recommended it | 10 | 12.2 | 9 | 13.4 | ns |
| . | African American (N = 82) . | White (N = 67) . | . | ||
|---|---|---|---|---|---|
| Circumstance . | n . | % . | n . | % . | p . |
| If received payment | 6 | 7.3 | 3 | 4.5 | ns |
| If doctor recommended it | 22 | 26.8 | 20 | 29.9 | ns |
| If might help someone/cured of illness | 21 | 25.6 | 23 | 34.3 | ns |
| If received some free medical care/exams | 6 | 7.3 | 13 | 19.4 | <.05 |
| If it helped scientists learn more | 7 | 8.5 | 15 | 22.4 | <.05 |
| If knew someone who participated | 6 | 7.3 | 14 | 20.9 | <.05 |
| If there were no side effects | 10 | 12.2 | 14 | 20.9 | ns |
| If there was no cost | 7 | 8.5 | 14 | 20.9 | <.05 |
| If friend/family recommended it | 10 | 12.2 | 9 | 13.4 | ns |
Circumstances by Race for Consideration of Treatment Trial for Those Who Initially Declined
| . | African American (N = 82) . | White (N = 67) . | . | ||
|---|---|---|---|---|---|
| Circumstance . | n . | % . | n . | % . | p . |
| If received payment | 6 | 7.3 | 3 | 4.5 | ns |
| If doctor recommended it | 22 | 26.8 | 20 | 29.9 | ns |
| If might help someone/cured of illness | 21 | 25.6 | 23 | 34.3 | ns |
| If received some free medical care/exams | 6 | 7.3 | 13 | 19.4 | <.05 |
| If it helped scientists learn more | 7 | 8.5 | 15 | 22.4 | <.05 |
| If knew someone who participated | 6 | 7.3 | 14 | 20.9 | <.05 |
| If there were no side effects | 10 | 12.2 | 14 | 20.9 | ns |
| If there was no cost | 7 | 8.5 | 14 | 20.9 | <.05 |
| If friend/family recommended it | 10 | 12.2 | 9 | 13.4 | ns |
| . | African American (N = 82) . | White (N = 67) . | . | ||
|---|---|---|---|---|---|
| Circumstance . | n . | % . | n . | % . | p . |
| If received payment | 6 | 7.3 | 3 | 4.5 | ns |
| If doctor recommended it | 22 | 26.8 | 20 | 29.9 | ns |
| If might help someone/cured of illness | 21 | 25.6 | 23 | 34.3 | ns |
| If received some free medical care/exams | 6 | 7.3 | 13 | 19.4 | <.05 |
| If it helped scientists learn more | 7 | 8.5 | 15 | 22.4 | <.05 |
| If knew someone who participated | 6 | 7.3 | 14 | 20.9 | <.05 |
| If there were no side effects | 10 | 12.2 | 14 | 20.9 | ns |
| If there was no cost | 7 | 8.5 | 14 | 20.9 | <.05 |
| If friend/family recommended it | 10 | 12.2 | 9 | 13.4 | ns |
Factors Associated With Willingness to Participate in Clinical Cancer Treatment Trials By Race
| . | African American . | White . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | p . | n . | % . | p . |
| Age | <.01 | ns | ||||
| 50–60 | 78 | 70.9 | 86 | 73.5 | ||
| 61–75 | 37 | 57.8 | 42 | 66.7 | ||
| >75 | 17 | 40.5 | 26 | 61.9 | ||
| Gender | <.05 | <.001 | ||||
| Female | 91 | 56.9 | 83 | 61.5 | ||
| Male | 41 | 73.2 | 71 | 81.6 | ||
| Living Arrangements | ns | <.05 | ||||
| Living alone | 57 | 56.4 | 51 | 61.4 | ||
| Living with others | 75 | 65.2 | 103 | 74.1 | ||
| Education | ns | ns | ||||
| Less than 12 | 29 | 55.8 | 17 | 73.9 | ||
| High school diploma | 36 | 61.0 | 51 | 68.9 | ||
| >12 | 60 | 62.5 | 81 | 71.1 | ||
| Income | ns | <.05 | ||||
| Less than $25,000 | 59 | 62.8 | 32 | 57.1 | ||
| $25,000–40,000 | 47 | 58.0 | 58 | 69.0 | ||
| >$40,000 | 26 | 63.4 | 64 | 78.0 | ||
| Health Insurance | ns | ns | ||||
| Yes | 122 | 60.1 | 143 | 69.4 | ||
| No | 7 | 87.5 | 10 | 76.9 | ||
| Knowledge of Tuskegee | ns | ns | ||||
| Yes | 68 | 59.6 | 61 | 76.3 | ||
| No | 60 | 63.8 | 89 | 67.9 | ||
| Relatives With Cancer | ns | ns | ||||
| None | 65 | 61.9 | 76 | 69.7 | ||
| 1 or more | 67 | 60.4 | 78 | 69.0 | ||
| Cancer Fatalistic Beliefs | <.01 | ns | ||||
| High (0–15) | 22 | 47.8 | 20 | 74.1 | ||
| Medium (16–23) | 86 | 63.7 | 98 | 66.7 | ||
| Low (24–30) | 24 | 68.6 | 36 | 75.0 |
| . | African American . | White . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | p . | n . | % . | p . |
| Age | <.01 | ns | ||||
| 50–60 | 78 | 70.9 | 86 | 73.5 | ||
| 61–75 | 37 | 57.8 | 42 | 66.7 | ||
| >75 | 17 | 40.5 | 26 | 61.9 | ||
| Gender | <.05 | <.001 | ||||
| Female | 91 | 56.9 | 83 | 61.5 | ||
| Male | 41 | 73.2 | 71 | 81.6 | ||
| Living Arrangements | ns | <.05 | ||||
| Living alone | 57 | 56.4 | 51 | 61.4 | ||
| Living with others | 75 | 65.2 | 103 | 74.1 | ||
| Education | ns | ns | ||||
| Less than 12 | 29 | 55.8 | 17 | 73.9 | ||
| High school diploma | 36 | 61.0 | 51 | 68.9 | ||
| >12 | 60 | 62.5 | 81 | 71.1 | ||
| Income | ns | <.05 | ||||
| Less than $25,000 | 59 | 62.8 | 32 | 57.1 | ||
| $25,000–40,000 | 47 | 58.0 | 58 | 69.0 | ||
| >$40,000 | 26 | 63.4 | 64 | 78.0 | ||
| Health Insurance | ns | ns | ||||
| Yes | 122 | 60.1 | 143 | 69.4 | ||
| No | 7 | 87.5 | 10 | 76.9 | ||
| Knowledge of Tuskegee | ns | ns | ||||
| Yes | 68 | 59.6 | 61 | 76.3 | ||
| No | 60 | 63.8 | 89 | 67.9 | ||
| Relatives With Cancer | ns | ns | ||||
| None | 65 | 61.9 | 76 | 69.7 | ||
| 1 or more | 67 | 60.4 | 78 | 69.0 | ||
| Cancer Fatalistic Beliefs | <.01 | ns | ||||
| High (0–15) | 22 | 47.8 | 20 | 74.1 | ||
| Medium (16–23) | 86 | 63.7 | 98 | 66.7 | ||
| Low (24–30) | 24 | 68.6 | 36 | 75.0 |
Factors Associated With Willingness to Participate in Clinical Cancer Treatment Trials By Race
| . | African American . | White . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | p . | n . | % . | p . |
| Age | <.01 | ns | ||||
| 50–60 | 78 | 70.9 | 86 | 73.5 | ||
| 61–75 | 37 | 57.8 | 42 | 66.7 | ||
| >75 | 17 | 40.5 | 26 | 61.9 | ||
| Gender | <.05 | <.001 | ||||
| Female | 91 | 56.9 | 83 | 61.5 | ||
| Male | 41 | 73.2 | 71 | 81.6 | ||
| Living Arrangements | ns | <.05 | ||||
| Living alone | 57 | 56.4 | 51 | 61.4 | ||
| Living with others | 75 | 65.2 | 103 | 74.1 | ||
| Education | ns | ns | ||||
| Less than 12 | 29 | 55.8 | 17 | 73.9 | ||
| High school diploma | 36 | 61.0 | 51 | 68.9 | ||
| >12 | 60 | 62.5 | 81 | 71.1 | ||
| Income | ns | <.05 | ||||
| Less than $25,000 | 59 | 62.8 | 32 | 57.1 | ||
| $25,000–40,000 | 47 | 58.0 | 58 | 69.0 | ||
| >$40,000 | 26 | 63.4 | 64 | 78.0 | ||
| Health Insurance | ns | ns | ||||
| Yes | 122 | 60.1 | 143 | 69.4 | ||
| No | 7 | 87.5 | 10 | 76.9 | ||
| Knowledge of Tuskegee | ns | ns | ||||
| Yes | 68 | 59.6 | 61 | 76.3 | ||
| No | 60 | 63.8 | 89 | 67.9 | ||
| Relatives With Cancer | ns | ns | ||||
| None | 65 | 61.9 | 76 | 69.7 | ||
| 1 or more | 67 | 60.4 | 78 | 69.0 | ||
| Cancer Fatalistic Beliefs | <.01 | ns | ||||
| High (0–15) | 22 | 47.8 | 20 | 74.1 | ||
| Medium (16–23) | 86 | 63.7 | 98 | 66.7 | ||
| Low (24–30) | 24 | 68.6 | 36 | 75.0 |
| . | African American . | White . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | p . | n . | % . | p . |
| Age | <.01 | ns | ||||
| 50–60 | 78 | 70.9 | 86 | 73.5 | ||
| 61–75 | 37 | 57.8 | 42 | 66.7 | ||
| >75 | 17 | 40.5 | 26 | 61.9 | ||
| Gender | <.05 | <.001 | ||||
| Female | 91 | 56.9 | 83 | 61.5 | ||
| Male | 41 | 73.2 | 71 | 81.6 | ||
| Living Arrangements | ns | <.05 | ||||
| Living alone | 57 | 56.4 | 51 | 61.4 | ||
| Living with others | 75 | 65.2 | 103 | 74.1 | ||
| Education | ns | ns | ||||
| Less than 12 | 29 | 55.8 | 17 | 73.9 | ||
| High school diploma | 36 | 61.0 | 51 | 68.9 | ||
| >12 | 60 | 62.5 | 81 | 71.1 | ||
| Income | ns | <.05 | ||||
| Less than $25,000 | 59 | 62.8 | 32 | 57.1 | ||
| $25,000–40,000 | 47 | 58.0 | 58 | 69.0 | ||
| >$40,000 | 26 | 63.4 | 64 | 78.0 | ||
| Health Insurance | ns | ns | ||||
| Yes | 122 | 60.1 | 143 | 69.4 | ||
| No | 7 | 87.5 | 10 | 76.9 | ||
| Knowledge of Tuskegee | ns | ns | ||||
| Yes | 68 | 59.6 | 61 | 76.3 | ||
| No | 60 | 63.8 | 89 | 67.9 | ||
| Relatives With Cancer | ns | ns | ||||
| None | 65 | 61.9 | 76 | 69.7 | ||
| 1 or more | 67 | 60.4 | 78 | 69.0 | ||
| Cancer Fatalistic Beliefs | <.01 | ns | ||||
| High (0–15) | 22 | 47.8 | 20 | 74.1 | ||
| Medium (16–23) | 86 | 63.7 | 98 | 66.7 | ||
| Low (24–30) | 24 | 68.6 | 36 | 75.0 |
Logistic Regression Results of Predisposing and Enabling Factors Associated With Willingness to Participate in Clinical Cancer Treatment Trial (N = 438)
| Variable . | Beta Coefficient . | SE . | Odds Ratio . | p . |
|---|---|---|---|---|
| White | 1.313 | 1.791 | 3.965 | ns |
| Male | 0.953 | 0.262 | 2.593 | <.001 |
| Age | −0.112 | 0.034 | 0.894 | <.001 |
| Living arrangements | −0.308 | 0.237 | 0.735 | ns |
| Income | ||||
| High | 2.113 | 0.963 | 8.274 | <.05 |
| Moderate | 0.563 | 0.930 | 1.756 | ns |
| Health insurance | 0.598 | 0.606 | 1.818 | ns |
| Tuskegee | −0.156 | 0.241 | 0.856 | ns |
| Cancer fatalism | 0.047 | 0.024 | 1.048 | <.05 |
| Race × Age | 0.058 | 0.021 | 1.060 | <.01 |
| Race × High Income | 1.335 | 0.612 | 3.802 | <.05 |
| Race × Moderate Income | 0.324 | 0.601 | 1.383 | ns |
| χ2 = 49.198, p <.001 | ||||
| R2 =.112 |
| Variable . | Beta Coefficient . | SE . | Odds Ratio . | p . |
|---|---|---|---|---|
| White | 1.313 | 1.791 | 3.965 | ns |
| Male | 0.953 | 0.262 | 2.593 | <.001 |
| Age | −0.112 | 0.034 | 0.894 | <.001 |
| Living arrangements | −0.308 | 0.237 | 0.735 | ns |
| Income | ||||
| High | 2.113 | 0.963 | 8.274 | <.05 |
| Moderate | 0.563 | 0.930 | 1.756 | ns |
| Health insurance | 0.598 | 0.606 | 1.818 | ns |
| Tuskegee | −0.156 | 0.241 | 0.856 | ns |
| Cancer fatalism | 0.047 | 0.024 | 1.048 | <.05 |
| Race × Age | 0.058 | 0.021 | 1.060 | <.01 |
| Race × High Income | 1.335 | 0.612 | 3.802 | <.05 |
| Race × Moderate Income | 0.324 | 0.601 | 1.383 | ns |
| χ2 = 49.198, p <.001 | ||||
| R2 =.112 |
Logistic Regression Results of Predisposing and Enabling Factors Associated With Willingness to Participate in Clinical Cancer Treatment Trial (N = 438)
| Variable . | Beta Coefficient . | SE . | Odds Ratio . | p . |
|---|---|---|---|---|
| White | 1.313 | 1.791 | 3.965 | ns |
| Male | 0.953 | 0.262 | 2.593 | <.001 |
| Age | −0.112 | 0.034 | 0.894 | <.001 |
| Living arrangements | −0.308 | 0.237 | 0.735 | ns |
| Income | ||||
| High | 2.113 | 0.963 | 8.274 | <.05 |
| Moderate | 0.563 | 0.930 | 1.756 | ns |
| Health insurance | 0.598 | 0.606 | 1.818 | ns |
| Tuskegee | −0.156 | 0.241 | 0.856 | ns |
| Cancer fatalism | 0.047 | 0.024 | 1.048 | <.05 |
| Race × Age | 0.058 | 0.021 | 1.060 | <.01 |
| Race × High Income | 1.335 | 0.612 | 3.802 | <.05 |
| Race × Moderate Income | 0.324 | 0.601 | 1.383 | ns |
| χ2 = 49.198, p <.001 | ||||
| R2 =.112 |
| Variable . | Beta Coefficient . | SE . | Odds Ratio . | p . |
|---|---|---|---|---|
| White | 1.313 | 1.791 | 3.965 | ns |
| Male | 0.953 | 0.262 | 2.593 | <.001 |
| Age | −0.112 | 0.034 | 0.894 | <.001 |
| Living arrangements | −0.308 | 0.237 | 0.735 | ns |
| Income | ||||
| High | 2.113 | 0.963 | 8.274 | <.05 |
| Moderate | 0.563 | 0.930 | 1.756 | ns |
| Health insurance | 0.598 | 0.606 | 1.818 | ns |
| Tuskegee | −0.156 | 0.241 | 0.856 | ns |
| Cancer fatalism | 0.047 | 0.024 | 1.048 | <.05 |
| Race × Age | 0.058 | 0.021 | 1.060 | <.01 |
| Race × High Income | 1.335 | 0.612 | 3.802 | <.05 |
| Race × Moderate Income | 0.324 | 0.601 | 1.383 | ns |
| χ2 = 49.198, p <.001 | ||||
| R2 =.112 |
References
Andersen R. M.,
Azjen I., Fishbein M.,
Boult C., Boult L. M., Pirie P.,
Brown D. R., Fouad M. N., Basen-Engquist K., Tortolero-Luna G.,
Brown D. R., Herskovitz L.,
Champion V. L.,
Corbie-Smith G., Thomas S. B., Williams M. V., Moody-Ayers S.,
Cubbins L. A., Tanfer K.,
Dennis B. P., Neese J. B.,
Gorelick P. B., Harris Y., Burnett B., Bonecutter F.,
Hall W. D.,
Health Care Financing Administration.,
Hutchins L. F., Unger J. M., Crowley J. J., Coltman C. A., Albain K. S.,
Martin L. G., Soldo B. J., (Eds.)
Mouton C. P., Harris S., Rovi S., Solorzano P., Johnson M. S.,
Pinto H. A., McCaskill-Stevens W., Wolfe P., Marcus A. C.,
Robinson S. B., Ashley M., Haynes M. A.,
Shavers V. L., Lynch C. F., Burmeister L. F.,
Simon M. R., Brown D. R., Du W., LoRusso P., Kellogg C. M.,
Stallings F. L., Marvella E. F., Simpson N. K., Fouad M., Jernigan J. C., Trauth J. C., et al
Stone V. E., Mauch M. Y., Steger K. A.,
Swanson G. M., Ward A. J.,
Sutherland H. J., da Cunha R., Lockwood G. A., Till J. E.,
Tejeda H. A., Green S. B., Trimble E. L., Ford L., High J. L., Ungerleider R. S., et al
Wrobel A. J., Shapiro N. E. K.,