-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel Pakula, Aurélie Melchior, Agnès Denys, Christophe Vanpouille, Joël Mazurier, Fabrice Allain, Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis, Glycobiology, Volume 17, Issue 5, May 2007, Pages 492–503, https://doi.org/10.1093/glycob/cwm009
Close - Share Icon Share
Abstract
Many of the biological functions attributed to cell surface proteoglycans are dependent on the interaction with extracellular mediators through their heparan sulphate (HS) moieties and the participation of their core proteins in signaling events. A class of recently identified inflammatory mediators is secreted cyclophilins, which are mostly known as cyclosporin A-binding proteins. We previously demonstrated that cyclophilin B (CyPB) triggers chemotaxis and integrin-mediated adhesion of T lymphocytes mainly of the CD4+/CD45RO+ phenotype. These activities are related to interactions with two types of binding sites, CD147 and cell surface HS. Here, we demonstrate that CyPB-mediated adhesion of CD4+/CD45RO+ T cells is related to p44/42 mitogen-activated protein kinase (MAPK) activation by a mechanism involving CD147 and HS proteoglycans (HSPG). Although HSPG core proteins are represented by syndecan-1, -2, -4, CD44v3 and betaglycan in CD4+/CD45RO+ T cells, we found that only syndecan-1 is physically associated with CD147. The intensity of the heterocomplex increased in response to CyPB, suggesting a transient enhancement and/or stabilization in the association of CD147 to syndecan-1. Pretreatment with anti-syndecan-1 antibodies or knockdown of syndecan-1 expression by RNA interference dramatically reduced CyPB-induced p44/p42 MAPK activation and consequent migration and adhesion, supporting the model in which syndecan-1 serves as a binding subunit to form the fully active receptor of CyPB. Altogether, our findings provide a novel example of a soluble mediator in which a member of the syndecan family plays a critical role in efficient interaction with signaling receptors and initiation of cellular responses.
Introduction
Cyclophilins are ubiquitously distributed proteins, first identified as the main binding proteins for cyclosporin A (CsA), an immunosuppressive drug widely used in the prevention of graft rejection (Handschumacher et al. 1984; Schreiber 1991). They have also been shown to exhibit peptidyl-prolyl cis–trans isomerase activity (PPIase), which is thought to contribute to the role of these proteins in protein folding (Fischer et al. 1989; Takahashi et al. 1989). Cyclophilins share high sequence homology in the central core, which contains the catalytic and CsA-binding domains, while their C- and N-terminal extensions are structurally unrelated (Galat 1999). Although most studies have focused on the intracellular functions of cyclophilins, accumulating data suggest a role for these proteins as regulators of inflammation. The expression and release of cyclophilin A (CyPA) and B (CyPB) were found increased as a response to inflammatory stimuli, oxidative stress, and viral infection (Sherry et al. 1992; Gonzalez-Cuadrado et al. 1996; Tegeder et al. 1997; Endrich and Gehring 1998; Jin et al. 2000; Arora et al. 2005). Both cyclophilins trigger chemotaxis of polymorphonuclear leukocytes, monocytes, and T lymphocytes, supporting the hypothesis that they may contribute to the guidance of leukocyte populations to inflammatory sites (Sherry et al. 1992; Xu et al. 1992; Yurchenko et al. 2001; Allain et al. 2002; Arora et al. 2005).
We and others have demonstrated that the activities of secreted cyclophilins are related to interactions with CD147, a type I transmembrane protein also known as extracellular matrix metalloproteinase inducer (Pushkarsky et al. 2001; Yurchenko et al. 2001; Allain et al. 2002). By using site-directed mutated ligands, we demonstrated that the central core of CyPB is required for binding to T lymphocytes and consequent initiation of cell responses (Carpentier et al. 2002). Moreover, a proline residue located in an exposed loop of CD147 was found crucial to induce chemotaxis (Yurchenko et al. 2002). These data indicate that cyclophilins trigger common biological responses by a mechanism that involves prolyl isomerization of CD147, making this protein an essential component of the signaling receptor. Intriguingly, we found that only CyPB, but not CyPA, was capable to enhance integrin-mediated adhesion of some T lymphocytes, mainly of the CD4+/CD45RO+ phenotype, to the extracellular matrix. These findings have suggested that CyPB could interact with cell surface binding sites other than CD147, leading to the appearance of additional cell responses. In support of this idea, we found that CyPB is a high-affinity ligand for cell surface heparan sulphate (HS) present at the surface of T cells. Removal of HS by heparinase I treatment abolished the pro-adhesive activity of CyPB, and the addition of soluble HS could not restore CyPB-initiated adhesion of heparinase-treated T cells, indicating that HS must be present at the membrane of responsive cells (Allain et al. 2002; Vanpouille et al. 2004). Taken together, these data suggest that HS proteoglycans (HSPG) could serve as a second class of binding sites and participate for the unique proadhesive activity of CyPB.
Cell surface HSPG are anchored in the plasma membrane either by a transmembrane domain (syndecans, CD44 variants, and betaglycan) or by glycosylphosphatidylinositol linkage (glypicans) (Bernfield et al. 1999). Syndecans, which are represented by four members (syndecan-1 to -4), are the major source of cell surface HS chains, although syndecan-1 also contains chondroitan sulphate (CS) chains at the glycanation site nearest the plasma membrane. Betaglycan is a unique HSPG, also known as the type-III low-affinity receptor for transforming growth factor-beta (TGF-β). CD44 is a ubiquitous cell surface adhesion molecule. Different utilization of 10 variable exons, as well as variations in glycosylation, generate multiple isoforms of varying molecular weights. Among this family, only the splice variants CD44v3 are decorated with HS moieties (Jackson et al. 1995). Over the past several years, biochemical, cellular, and genetic studies have converged to reveal that HSPG are critical regulators for growth and inflammatory factors (Selleck 2000; Delehedde et al. 2002). Two scenarios have emerged for explaining the functions of these molecules. The first is a well-documented model, in which HS play a role in effective presentation of ligands to their cognate signaling receptor (Yayon et al. 1991; Tanaka et al. 1993; Kuschert et al. 1999). In the second scenario, proteoglycans may function as coreceptors. Interactions of cytosolic domains of syndecans and CD44 variants with cytoskeleton and neighboring signaling molecules have suggested that they may be involved in the regulation of molecular processes related to cell adhesion, migration, and proliferation (Rapraeger and Ott 1998; Ilangumaran et al. 1999; Woods and Couchman 2001).
In the current study, we have examined whether HSPG serve as a coreceptor for CyPB. Therefore, we analyzed the expression of HSPG on the plasma membrane of peripheral blood CD4+/CD45RO+ T lymphocytes. We then investigated the involvement of cell surface HSPG in the responses triggered by CyPB. Our findings demonstrate that syndecan-1 is a functional coreceptor for CyPB and acts in cooperation with CD147 to induce p44/42 mitogen-activated protein kinase (MAPK) activation and subsequent cell adhesion and migration of responsive T lymphocytes.
Results
Involvement of cell-surface HSPG and CD147 in CyPB-induced responses
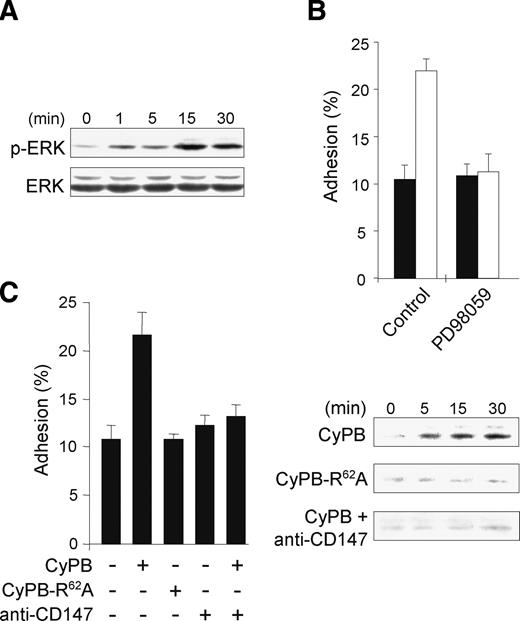
In the previous work, we demonstrated that removal of HS partially reduced Ca2 + flux and chemotaxis, but potently inhibited cell adhesion to fibronectin (Allain et al. 2002). To further investigate signaling pathways that might be involved in CyPB-induced adhesion of CD4+/CD45RO+ T cells, we analyzed the phosphorylation status of extracellular signal-regulated kinase (ERK)1/2 (p44/42 MAPK), Jun N-terminal kinase (JNK), and p38 MAPK in CyPB-treated cells. A characteristic increase in the level of phosphorylated ERK was observed after the addition of CyPB (Figure 1A, upper panel). The amount of total ERK was not changed (Figure 1A, lower panel), confirming that CyPB only stimulated the phosphorylation and activation of the preexisting proteins. No such increase in phosphorylation could be detected for JNK and p38 MAPK (data not shown). Time course analysis demonstrated that ERK phosphorylation reached a maximum at 15 min after stimulation. We also determined that fully enhanced adhesion was obtained at 30 min, indicating that ERK phosphorylation occurred before integrin activation (Figure 2B). Moreover, the optimal concentration of CyPB was estimated at 50 nM. Therefore, dose- and time-dependent responses to CyPB for activating ERK phosphorylation paralleled those observed for inducing cell adhesion, suggesting that both events are related. Pretreatment of responsive T lymphocytes with PD98059, a specific inhibitor of p44/42 MAPK pathway activation, inhibited CyPB-mediated cell adhesion to fibronectin, confirming the involvement of ERK activation in this process (Figure 1B). We then confirmed the role of CD147-mediated signaling in the responses triggered by CyPB. Addition of antibodies to CD147, but not isotype-matched antibodies, potently abolished ERK activation, which was correlated to the inhibition of CyPB adhesion of CD4+/CD45RO+ T cells to fibronectin. Consistent with the requirement of prolyl isomerization for CD147-mediated ERK activation, the mutant CyPBR62A, which is devoid of enzymatic activity (Carpentier et al. 2002), was unable to induce ERK phosphorylation and to enhance responsive T-cell adhesion to fibronectin (Figure 1C). Altogether, these data support the notion that CD147-mediated ERK activation by CyPB is necessary for triggering of CD4+/CD45RO+ T-cell adhesion.
Involvement of CD147 and p44/p42 MAPK pathway in CyPB-initiated adhesion of CD4+/CD45RO+ T lymphocytes. (A) CD4+/CD45RO+ T lymphocytes were either stimulated or not in the presence of 50 nM CyPB for various times and the activation of p44/p42 MAPK was analyzed by using antibodies to phosphorylated ERK1/2 (p-ERK). Parallel immunoblotting with anti-total ERKs confirmed equal loading of samples. (B) Involvement of p44/p42 MAPK in CyPB-initiated adhesion. Following pretreatment or not in the presence of PD98059 (50 µM), CD4+/CD45RO+ T lymphocytes were incubated either in the absence (filled bar) or the presence (open bar) of 50 nM of CyPB and then allowed to adhere into fibronectin-coated wells. (C) T cells were incubated in the absence or presence of either CyPB (50 nM), CyPB plus anti-CD147 antibodies (10 µg/mL) or CyPBR62A (50 nM), and thereafter used for analysis of cell adhesion to fibronectin or ERK1/2 phosphorylation. Data are representative of at least three separate experiments conducted with peripheral blood Tlymphocytes from different donors. Each bar of histograms represents the mean ± SD of triplicate determination of an individual experiment. Results of adhesion assays are expressed as percentages of initially added cells (106 per well) remaining associated to the coated well.
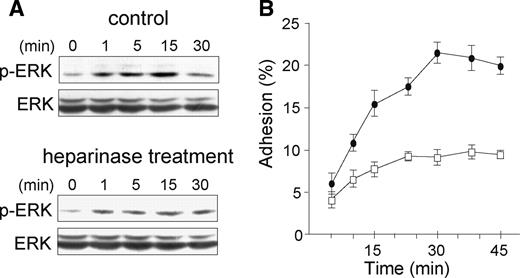
Involvement of cell surface HS in p44/p42 MAPK activation and adhesion induced by CyPB. (A) Untreated or heparinase I treated CD4+/CD45RO+ T lymphocytes were either stimulated or not with of 50 nM CyPB for various times and the phosphorylation status of ERK1/2 was analyzed by western blot. Representative results from at least three independent experiments conducted with T lymphocytes from different donors are shown. (B) Untreated (•) or heparinase I treated (○) cells were incubated in the presence of 50 nM CyPB and allowed to adhere into fibronectin-coated wells. Points represent means ± SD of triplicate and results are representative of three separate experiments.
We next investigated the involvement of HS in CyPB-mediated ERK activation. Pretreatment of CD4+/CD45RO+ T cells with heparinase I dramatically reduced CyPB-specific ERK phosphorylation (Figure 2A). In a model, in which CyPB binding to HS would be only required to complement low-affinity interactions with CD147, removal of HS should lead to a decrease in the time-course and/or amplitude of CyPB-induced responses, which could be restored by the addition of soluble HS. Here, we found that the effect of heparinase treatment did not cause a delay in ERK activation and subsequent adhesion to fibronectin (Figure 2B). In addition, complementation with heparin or soluble HS derived from T cells did not restore CyPB-induced ERK activation in heparinase-treated T cells, confirming that HS have to be present at the cell membrane of responsive cells. Finally, no more response was seen at higher concentrations of CyPB (data not shown), ruling out a role for HS in the presentation of low concentrations of ligand to signaling receptor. Taken together, these data indicate that CyPB induces ERK activation through CD147 and subsequent cell adhesion by a mechanism involving active participation of cell surface HSPG.
Analysis of HSPG of peripheral blood CD4+/CD45RO+ T lymphocytes
Data concerning the expression of HSPG on peripheral blood leukocytes are often controversial in the literature. However, low expression of these molecules on the cell surface of leukocytes and difficulties to detect their expression by classical immunochemical approaches may explain the variable detection of HSPG reported. To confirm the absence or presence of HSPG core proteins in peripheral blood CD4+/CD45RO+ T cells, we used complementary approaches, i.e. reverse transcription–polymerase chain reaction (RT-PCR), flow cytofluorimetry, western blot, and antibody-driven electrophoretic mobility shift assay (EMSA).
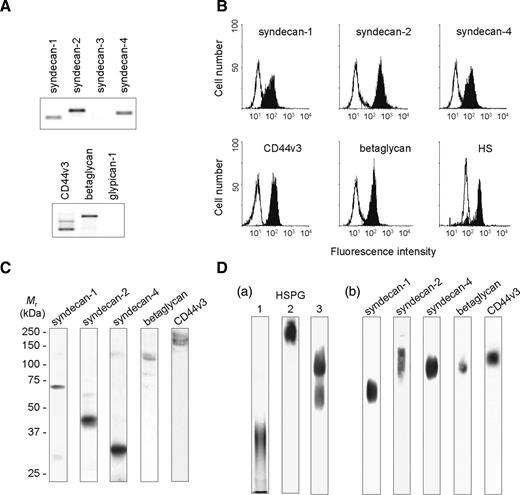
We first checked for the efficiency of PCR primers with control cells, which had been previously reported to express each HSPG. As expected, PCR products were visualized at the predicted sizes and their identity to HSPG core proteins was confirmed by further sequencing (data not shown). We then analyzed the expression of HSPG in peripheral blood CD4+/CD45RO+ T lymphocytes. As shown in Figure 3A, PCR products from syndecan-1, -2, -4, CD44v3 isoforms, and betaglycan could be visualized after 30 cycles of PCR amplification. In order to check for the absence of syndecan-3 and glypican-1, PCR amplification was conducted at higher number of cycles but did not show any significant expression of either HSPG (data not shown). As expected, flow cytofluorimetry analysis confirmed the presence of syndecan-1, -2, -4, CD44v3, and betaglycan on the cell membrane of CD4+/CD45RO+ T lymphocytes (Figure 3B). In contrast, no cell staining could be obtained with antibodies to syndecan-3 or glypican-1. To confirm the absence of members of the glypican family, T cells were treated with phosphatidyl-inositol phospholipase C, an enzyme used to remove proteins attached to the cell membrane via a GPI anchor. As expected, enzymatic treatment did not reduce either immunostaining of cells with anti-HS antibodies or binding of CyPB (data not shown), supporting the observations that HSPG of the glypican family are not expressed at the membrane of peripheral blood CD4+/CD45RO+ T cells. We then checked for the specificity of each anti-HSPG by western blot. As shown in Figure 3C, antibodies to syndecan-1, -2, -4, CD44v3, and betaglycan recognized distinct migrating species, without any cross-reactivity. The apparent molecular masses of deglycanated core proteins of HSPG were estimated to be approximately 70 kDa (syndecan-1), 43 kDa (syndecan-2), 32 kDa (syndecan-4), 115 kDa (betaglycan), and more than 150 kDa (CD44v3 variants). These values are in agreement with those previously observed (Jackson et al. 1995; Drzeniek et al. 1997; Charnaux et al. 2005), confirming the specificity of primary anti-HSPG antibodies. They are, however, higher than predicted ones, which might result from an incomplete deglycanation of HSPG.
Expression of HSPG on CD4+/CD45RO+ T lymphocytes: (A) Analysis of HSPG specific mRNA by semi-quantitative RT-PCR. GAPDH mRNA was also determined to normalize for input of total RNA. (B) Analysis of HSPG expression by flow cytofluorimetry. Reactivity of immunoreactive anti-HSPG antibodies (filled peaks) was compared to appropriate isotype-matched control antibodies (open peaks). (C) Analysis of HSPG expression by western blot. Cell surface HSPG were extracted and enriched by anion exchange chromatography on DEAE-sepharose. Following treatment with heparinase I and chondroitinase ABC, deglycanated HSPG were separated on 10% SDS-PAGE, transblotted onto nitrocellulose and immunostained with specific antibodies. (D) Analysis of HSPG expression by antibody-driven EMSA. (a) Lane 1: whole cell proteins were separated on 10% native PAGE for 3 h at 250 mA, transblotted onto nitrocellulose and HSPG were immunostained using a two-step procedure with anti-HS antibodies followed by secondary anti-mouse IgM; lane 2: cell lysate was preincubated in the presence of anti-HS antibodies (0.2 µg per lane) prior electrophoresis (3 h at 250 mA) and the immune complex was directly immunostained with peroxidase conjugated anti-mouse IgM antibodies; lane 3: cell lysate was preincubated with anti-HS antibodies and proteins were separated on native PAGE for 8 h at 250 mA prior immunostaining. (b) Cell lysates were preincubated in the presence of specific antibodies to HSPG (0.2 µg per lane). The proteins were then separated on native PAGE for 8 h at 250 mA, transblotted onto nitrocellulose and the immune complexes were directly immunostained with appropriate peroxidase conjugated secondary antibodies. Data are from an individual experiment and are representative of at least 10 separate experiments conducted with peripheral blood T lymphocytes from different donors.
Finally, we used an electrophoretic approach to further analyze the expression of HSPG in peripheral blood CD4+/CD45RO+ T lymphocytes. We assumed that gel mobility of HSPG could be based on the high negative charges of glycosaminoglycan (GAG) moieties, which make them migrate under native conditions. Indeed, HSPG could be detected as a broad smear after 3 h of electrophoretic migration at 250 mA, electroblotting and immunostaining with anti-HS antibodies. In contrast, preincubation of cell lysates with anti-HS antibodies led to a strong retention of HSPG at the top of the gel, which could be visualized as shifted immune complex after staining with secondary antibodies. To allow the complex to migrate into the gel, electrophoresis was then run for 8 h at 250 mA. Under these conditions, two major complexes formed by the association of HSPG with anti-HS antibodies could be visualized (Figure 3D, panel a). Such a differential migration is presumably linked to the nature of GAG modifications. Indeed, it could be hypothesized that anti-HS antibodies potently reduced the mobility of proteoglycans containing only HS moieties. On the contrary, syndecan-1, and CD44v3 variants also carry additional CS chains, which probably assisted them to migrate faster. We then repeated the experiments using primary antibodies to syndecan-1, -2, -4, betaglycan, and CD44v3. As expected, interaction of the core proteins of HSPG with their respective antibodies led to a delay in their migration, which could be visualized as shifted smears immunoreactive with secondary anti-IgG antibodies (Figure 3D, panel b). Under these conditions, we assumed that the number of GAG chains and the size of core proteins could have opposite influence on the mobility of HSPG. Indeed, the migration of betaglycan and CD44v3 variants, which are large HSPG, was potently reduced. In contrast, syndecan-1, which is modified with two CS and three HS chains, migrated faster than syndecan-2 and -4, which carry only three HS chains (Bernfield et al. 1999; Delehedde et al. 2002). Treatment of cell lysates with heparinase I and chondroitinase ABC prevented the migration of immune complexes into the gel, confirming that migration of the primary antibodies was dependent on interaction with and consequent carriage by intact HSPG. No immunoreactive smears were detected on the membrane with isotype-matched control antibodies, showing the specificity of the method. Finally, the use of anti-HSPG antibodies from different species did not modify the mobility of the immune complexes, indicating that the migration is solely dependent on the nature of immunoreactive HSPG (data not shown).
All experiments were conducted with peripheral blood T lymphocytes isolated from at least 10 different healthy donors. Even though some interindividual variation in the expression of HSPG could be observed, the general profiles obtained by RT-PCR and anti-HSPG immunoreactivity assays were similar and allowed us to conclude that CD4+/CD45RO+ T lymphocytes express syndecan-1, syndecan-2, syndecan-4, CD44v3, and betaglycan.
Role of CyPB in the association of syndecan-1 to CD147
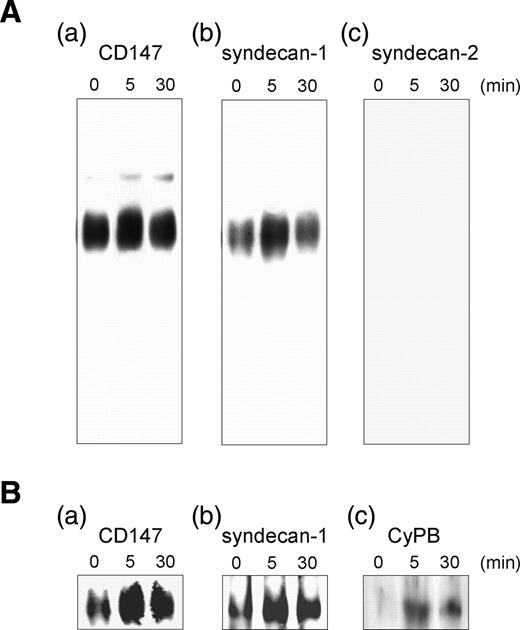
We then analyzed the possibility that CD147 could physically interact with HSPG and form an active complex at the membrane of responsive cells. Consistent with the findings that HS allowed electrophoretic migration of HSPG in native conditions and that interaction of HSPG with specific antibodies resulted in a shifted mobility of the immune complexes, we addressed the possibility that CD147/HSPG complexes could be also visualized by EMSA. To this end, CD4+/CD45RO+ T lymphocytes were incubated in the presence of CyPB for various times and thereafter used in EMSA with mouse anti-CD147 antibodies. This method allowed us to visualize immunoreactive shifted smears, which probably correspond to CD147 associated to HSPG (Figure 4A, panel a). To test this hypothesis, cell lysates were treated with heparinase and chondroitinase ABC prior addition of anti-CD147. As expected, the intensity of the immunoreactive smears was dramatically reduced (data not shown), confirming that migration of CD147 was dependent on the presence of GAG. In further experiments, the membrane was reprobed with goat antibodies to HSPG. The complex could be only immunostained with anti-syndecan-1 antibodies, indicating that syndecan-1 (Figure 4A, panel b), but neither syndecan-2 (Figure 4A, panel c) nor other HSPG (data not shown), were associated to CD147. The association of syndecan-1 to CD147 could be observed in the absence of CyPB, suggesting that CD147/HSPG complex preexists at the cell membrane of responsive T lymphocytes. Interestingly, the intensity of the complex increased after 5 min of incubation and decreased thereafter, indicating that CyPB transiently enhanced and/or stabilized the association between CD147 and HSPG. To further test this hypothesis, we reproduced the same experiments and the membrane was reprobed with antibodies to CyPB (Figure 4B). As expected, the protein was found associated to the complex retained with anti-CD147 antibodies. Moreover, we found that the presence of CyPB correlated with the transient increase in the intensity of CD147 and syndecan-1 immunostaining, indicating that binding of the protein to syndecan-1 led to the formation of a ternary complex with CD147. Altogether, these data indicate that the core protein of syndecan-1, but not of other HSPG, is physically associated to CD147 and that the association can be specifically increased and/or stabilized by CyPB.
Association of HSPG to CD147 on CD4+/CD45RO+ T lymphocytes. T cells were stimulated or not in the presence of CyPB (50 nM) for increasing times and thereafter lysed. Following preincubation with anti-CD147 antibodies (0.2 µg per lane), cell lysates were separated on 10% native PAGE for 8 h at 250 mA and transblotted onto nitrocellulose. (A) Membrane was either directly immunostained with peroxidase conjugated anti-mouse IgG secondary antibodies (a), or reprobed with goat antibodies to either syndecan-1 (b) or syndecan-2 (c). (B) In next experiments, the immune complexes formed by preincubation with anti-CD147 antibodies (a) were revealed with either goat antibodies to syndecan-1 (b) or rabbit antibodies to CyPB (c). Data are from an individual experiment and are representative of at least three separate experiments conducted with peripheral blood T lymphocytes from different donors.
Role of syndecan-1 in CyPB-induced responses
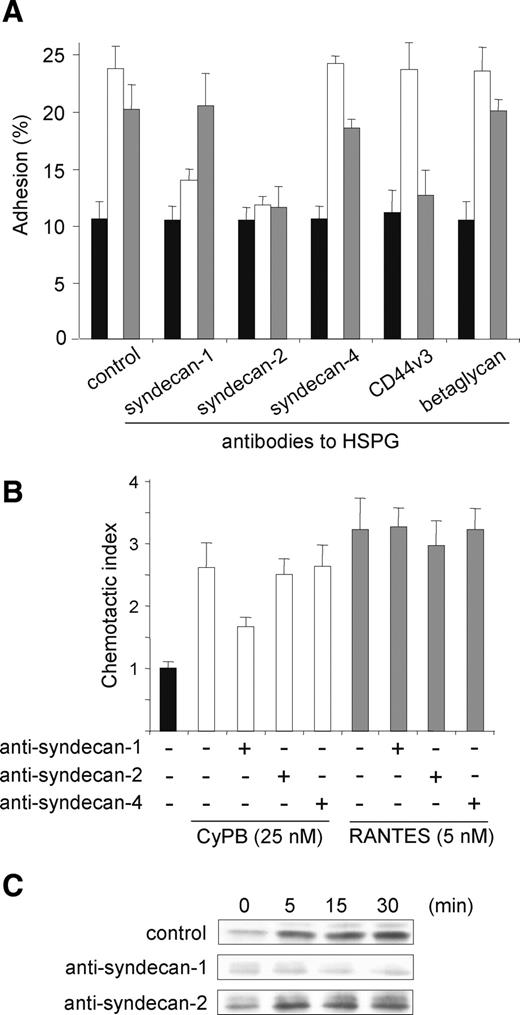
To extend our observations, we used an antibody neutralization strategy to assess the role of CD147/syndecan-1 complex in CyPB-mediated CD4+/CD45RO+ T-lymphocyte adhesion. The neutralizing antibodies have been checked for their ability to recognize the ectodomains of immunoreactive HSPG without inhibiting the binding of CyPB to HS moieties. Consequently, an inhibitory effect could be related to a blockade in the association between HSPG and CD147 through steric hindrance. Similar to the results obtained with anti-CD147, mouse antibodies to syndecan-1 potently reduced adhesion of responsive T cells to fibronectin (Figure 5A). As a control, goat antibodies to syndecan-1, which react with an intracellular epitope, were inefficient (data not shown). Antibodies to syndecan-4, CD44v3 and betaglycan had no inhibitory effect in the response triggered by CyPB, further supporting our hypothesis on the role of syndecan-1 in CyPB-induced cell responses. Surprisingly, we found that cell adhesion was strongly decreased in the presence of anti-syndecan-2 antibodies, suggesting that syndecan-2 also participates in T-cell adhesion to fibronectin (Figure 5A). These data were unexpected since we did not observe any association between syndecan-2 and CD147 in EMSA. Because syndecan-2 has been reported to cooperatively act with β1 integrins in cell adhesion, regulation of focal adhesion assembly, and stress fiber organization (Itano et al. 1996; Kusano et al. 2000; Munesue et al. 2002), we hypothesized that it was most probably involved in the regulation of adhesion to fibronectin rather than in CyPB-mediated signaling. To explore this hypothesis, we reproduce the same experiments with the pro-adhesive chemokine regulated upon activation, normal T-cell expressed, and secreted (RANTES)/CCL5 (Figure 5A). As expected, RANTES induced T-cell adhesion to fibronectin to a similar extent than CyPB. The addition of anti-syndecan-1 antibodies did not affect RANTES-mediated T-cell adhesion, indicating that the proteoglycan was not necessary for the responses induced by the chemokine. In contrast, anti-syndecan-2 and anti-CD44v3 antibodies strongly inhibited the adhesion induced by RANTES. The observation that CD44v3 participates in the responses triggered by RANTES was in agreement with the work of (Roscic-Mrkic et al. 2003), who described CD44v3 as a co signaling receptor for the chemokine. Moreover, the findings that anti-syndecan-2 antibodies were inhibitory for CyPB- and RANTES-mediated responses led us to conclude that this HSPG is not specifically required for CyPB/CD147 interaction but rather involved in the regulation of integrin activation and interaction with fibronectin. To validate this hypothesis, we analyzed the involvement of syndecan-1 and/or syndecan-2 in the capacity of CD4+/CD45RO+ T cells to migrate in response to CyPB or RANTES (Figure 5B). Antibodies to syndecan-1 dramatically reduced migration of T cells induced by CyPB, confirming that inhibition of the association of CD147 to syndecan-1 core protein renders the cells unresponsive to CyPB. Conversely, the addition of anti-syndecan-2 antibodies had no inhibitory effect on the in vitro migratory responses induced by either CyPB or RANTES. Finally, we analyzed the effects of antibodies to syndecan-1 or syndecan-2 in CyPB-induced p44/p42 MAPK activation (Figure 5C). Incubation of CD4+/CD45RO+ T cells with anti-syndecan-1 antibodies potently reduced CyPB-mediated ERK phosphorylation, while anti-syndecan-2 antibodies were ineffective, indicating that disruption of the association between syndecan-1 and CD147 led to neutralization of CyPB-mediated ERK activation. These data further support our hypothesis that syndecan-1 is specifically involved in the responses induced by CyPB, while syndecan-2 probably act as a regulator of cell adhesion to fibronectin.
Inhibitory effect of anti-HSPG antibodies on CyPB-induced cell responses. CD4+/CD45RO+ T lymphocytes were preincubated in the presence of antibodies to HSPG (2 µg/mL) and thereafter used for analysis of CyPB-induced migration, cell adhesion to fibronectin or p44/p42 MAPK activation. (A) T cells were incubated in the absence (filled bars) or the presence of 50 nM CyPB (open bars) or 15 nM RANTES (grey bars) and allowed to adhere into fibronectin-coated wells. Results are expressed as percentages of initially added cells remaining associated to the coated wells. (B) The chemotactic responses of T cells are expressed as the number of cells migrating to 25 nM CyPB (open bars) or RANTES 5 nM (grey bars) divided by the number of cells migrating to medium alone (filled bar). In both adhesion and migration assays, each bar of the histograms represent mean ± SD of triplicate from an individual experiment. (C) T cells were either stimulated or not with 50 nM CyPB for various times and the phosphorylation status of ERK1/2 was analyzed by western blot. Data are representative of at least three separate experiments conducted with peripheral blood T lymphocytes from different donors.
Effect of silencing syndecan-1 expression on CyPB-induced responses
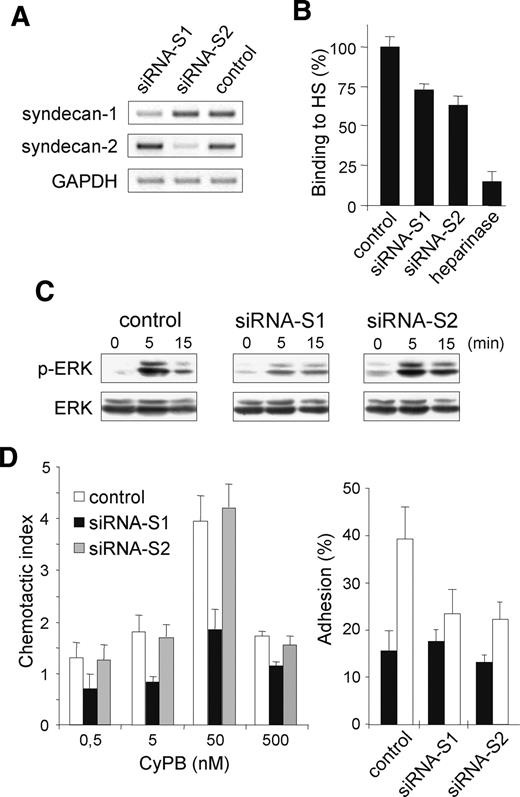
Finally, we used an RNA interference approach to confirm the involvement of syndecan-1 in CyPB-induced ERK activation and cell responses. To this end, we used THP-1 cells, which have been recently reported to be responsive to secreted cyclophilins (Kim et al. 2005). We first checked that CyPB bound to THP-1 cells and induced ERK phosphorylation, cell chemotaxis, and adhesion to fibronectin, by a mechanism dependent on the presence of CD147. The siRNA (small-interfering RNA) were then tested for their ability to suppress syndecan-1 or syndecan-2 specifically. Transfection of THP-1 cells with syndecan-1 siRNA resulted in a syndecan-1 mRNA down-regulation reaching 80% at day 2, by comparison with control cells. No significant decrease in the mRNA levels of syndecan-2 or other HSPG could be observed, confirming the specificity of the siRNA. Syndecan-2 siRNA was efficient to reduce syndecan-2 gene expression without affecting the levels of syndecan-1 or other HSPG (Figure 6A and data not shown). Moreover, cell immunostaining of CD147 was not modified, indicating that knockdown of syndecan-1 or syndecan-2 had no inhibitory effect on the expression of CD147 at the cell membrane (data not shown). We then analyzed the interaction between CyPB and cell-surface HS in transfected cells. As a control, we first checked that heparinase treatment reduced CyPB binding by more than 85%. Surprisingly, CyPB binding was only reduced by 27 and 36% in both the cells transfected with syndecan-1 siRNA and syndecan-2 siRNA, respectively (Figure 6B). These data indicate that CyPB interacts with HS independently of the nature of the core protein of HSPG. However, it can be hypothesized that HS generally serve to concentrate CyPB in the neighboring of responsive cells, while only the glycanic moieties of syndecan-1 are involved in the presentation of CyPB to CD147. When analyzing the intracellular events initiated by CyPB, we found, indeed, that syndecan-1 siRNA potently reduced the level of ERK phosphorylation. In contrast, knockdown of syndecan-2 was inefficient, further demonstrating that only syndecan-1 is required for inducing activation of p44/p42 MAPK pathway (Figure 6C). Finally, we tested the cell responses triggered by CyPB in transfected cells. As expected, CyPB-induced chemotaxis and cell adhesion were both reduced in THP-1 cells where syndecan-1 expression was down-regulated. Similar to the results obtained with anti-HSPG antibodies, syndecan-2 siRNA did not modify the chemotactic response to CyPB in THP-1 cells, while adhesion of transfected cells was potently reduced by comparison with control (Figure 6D). Altogether, these data further demonstrated that syndecan-1 is involved in the molecular events initiated by CyPB binding to responsive cells, whereas syndecan-2 probably functions as a regulator of T-lymphocyte adhesion to fibronectin.
Effect of syndecan-1 siRNA on CyPB-induced responses. Differentiated THP-1 cells were transfected with syndecan-1 siRNA, syndecan-2 siRNA, or GFP-siRNA (control). (A) The expression of specific RNA of syndecan-1 and syndecan-2 was analyzed by semi-quantitative RT-PCR 48 h post transfection. (B) Involvement of syndecan-1 and syndecan-2 in the interaction of CyPB with cell surface HS was analyzed by measuring the binding of transfected THP-1 cells (72 h posttransfection) to immobilized CyPB (1 µg/well). Heparinase-treated cells were used as a control to estimate the whole participation of HS in the interaction. (C) THP-1 cells (72 h posttransfection) were either stimulated or not in the presence of 50 nM CyPB for various times and the activation of p44/p42 MAPK was analyzed by using anti-phosphorylated ERK1/2 (p-ERK). Parallel immunoblotting with anti-total ERKs confirmed equal loading of samples. (D) CyPB-mediated adhesion and migration of THP-1 cells were analyzed 72 h posttransfection. Left panel: migratory response of THP-1 cells transfected with syndecan-1 siRNA (filled bars), syndecan-2 siRNA (grey bars) or GFP-siRNA (open bars) to increasing concentrations of CyPB. Right panel: cell adhesion to fibronectin in the absence (filled bars) or the presence of 50 nM CyPB. Each bar of histograms represents mean ± SD of triplicate. Representative results from three independent experiments are shown.
Discussion
CD147 was demonstrated to be essential for cyclophilin-induced cellular responses (Yurchenko et al. 2001; Allain et al. 2002; Yurchenko et al. 2002; Arora et al. 2005). In the current study, we further demonstrated that CyPB-mediated enhanced adhesion of T cells is dependent on CD147 and ERK phosphorylation. However, direct binding of cyclophilins to CD147 has not been formally demonstrated, suggesting a mode of activation where CD147 might transmit signaling events through an unusual mechanism. Interestingly, Pro180 and Gly181 residues in the extracellular loop of CD147 were found to be critical for inducing signaling events (Yurchenko et al. 2002). Moreover, defective PPIase cyclophilin mutants failed to induce cell adhesion (Carpentier et al. 2002). We further demonstrated here that CyPBR62A, a mutant deprived of PPIase activity, failed to initiate ERK phosphorylation, indicating that activation of signaling pathway would be consequent on the conformational rearrangement of the polypeptide chain of CD147. Such a PPIase-dependent mechanism of regulation has already been demonstrated for the tyrosine kinase Itk. According to the cis-trans isomerization of an exposed peptide bond by CyPA, the kinase could interact with either a SH3 domain or more commonly with a phosphotyrosine (Brazin et al. 2002).
CD147 is broadly expressed on hemopoietic and nonhemopoietic cells lines. It was initially identified on the surface of human cancer cells and has been proven to stimulate adjacent stromal cells to produce several matrix metalloproteinases. It is also involved in intercellular adhesion, via homophilic interaction (Guo et al. 1997; Sun and Hemler 2001; Toole 2003). Most CD147-expressing cells are, however, not responsive to secreted cyclophilins. Therefore, our previous findings that secreted CyPB binds to the membrane of responsive cells with high affinity have raised the possibility that CD147 is only a signaling molecule, which has to be associated to a binding subunit to form a fully active cyclophilin receptor. The present results strongly support this hypothesis. We demonstrated that CD147 is partially associated to syndecan-1 even in the absence of ligand. Binding of CyPB to the HS moieties of syndecan-1 induced an increase in the association between syndecan-1 and CD147, which probably led to initiation of signaling events and consequent cellular responses. Indeed, knockdown of syndecan-1 by siRNA or dissociation of the heterocomplex by anti-syndecan-1 antibodies potently reduced CyPB-induced ERK phosphorylation and subsequent chemotaxis and adhesion of responsive cells. These results support the model, in which binding to syndecan-1 is required to stably present CyPB to CD147 expressed on responsive cells.
Removal of cell surface HS by heparinase I treatment abolished CyPA-mediated signaling and chemotactic activity, indicating that the cell responses triggered by CyPA are also dependent on the presence of HSPG (Yurchenko et al. 2002). A mutant of CyPB in which 3KKK5 sequence was replaced by 3AAA5 did not increase T-cell adhesion to fibronectin but did induce reduced signaling and chemotactic responses, thus mimicking behavior of CyPA (Allain et al. 2002; Carpentier et al. 2002). One explanation for this discrepancy may be related to the affinity of both cyclophilins to cell surface HS. Indeed, the high-affinity interaction between CyPB and HS probably results in a more stable binding and may explain why CyPB is a more potent agonist than CyPA. Therefore, high-affinity binding of CyPB to HS moieties of syndecan-1 probably induces potent signaling events related to integrin-mediated adhesion of T cells, whereas low-affinity interaction of CyPA with the same HSPG is likely to be sufficient to initiate chemotaxis. Altogether, these data suggest that syndecan-1 could serve as the binding subunit to form the fully active receptor for both secreted CyPA and CyPB.
Surprisingly, we found that incubation of CD4+/CD45RO+ T lymphocytes with antibodies to syndecan-2 or knockdown of syndecan-2 by siRNA significantly inhibited cell adhesion to fibronectin, without modifying either ERK phosphorylation or cell migration. However, we found that anti-syndecan-2 antibodies also influenced RANTES-induced cell adhesion, thus suggesting that syndecan-2 is involved in the regulation of integrin-mediated cell adhesion to fibronectin rather than in CyPB-mediated signaling. These results are consistent with the findings demonstrating that syndecan-2 acts cooperatively with β1 integrins to regulate cell adhesion, focal adhesion assembly, and stress fiber organization (Itano et al. 1996; Kusano et al. 2000; Munesue et al. 2002), indicating that this HSPG is generally involved in the regulation of cell adhesion.
The demonstration that syndecans are involved in the responses induced by extracellular biologically active factor is not unprecedent. These HSPG contain a conserved motif through which they interact with regulatory proteins involved in organizing complexes at the plasma membrane and recruiting signaling proteins from the cytosol, suggesting that they participate in the modulation of intracellular events related to cell adhesion, growth, and differentiation (Woods and Couchman 2001). As examples, syndecan-1 mediates hepatocyte growth factor (HGF) binding and promotes Met signaling in multiple myeloma cells (Derksen et al. 2002). Most recently, syndecan-1 was demonstrated to be involved in the migration of peripheral blood monocytes induced by osteoprotegerin, a protein that regulates differentiation and activation of osteoclasts (Mosheimer et al. 2005). Syndecan-4 interacts with protein kinase C-α and PIP2 and plays a key role in the modulation of the responses initiated by basic fibroblast growth factor (FGF-2) and antithrombin III, for which it serves as a binding site and an intermediate molecule in signaling events (Volk et al. 1999; Kaneider et al. 2002). Interestingly, syndecan-4 from SDF-1/CXCL12 activated cells physically associates to CXCR4 and is required for p44/p42 MAPK activation, suggesting that the responses initiated by the chemokine is also dependent on the formation of a fully active syndecan/signaling receptor heterocomplex (Charnaux et al. 2005). In agreement with these data, the results presented here provide a novel example on the requirement of a member of the syndecan family in efficient binding of an extracellular biologically active factor to signaling receptor and initiation of cellular responses.
The presence of elevated levels of extracellular cyclophilins has been reported in several inflammatory diseases (Billich et al. 1997; Tegeder et al. 1997; Jin et al. 2000). In the case of rheumatoid arthritis, levels of CyPA within synovial fluid of patients were found to directly correlate with disease severity (Billich et al. 1997). Cartilage chondrocytes were shown to secrete CyPB in response to matrix metalloproteinases, providing an additional source of cyclophilins released during ongoing disease (De Ceuninck et al. 2003). Finally, Zhu et al. (2005) found increased expression of CD147 on monocytes/macrophages in rheumatoid arthritis and demonstrated that anti-CD147 antibodies or CD147 antagonistic peptides blocked migration to synovial fluids. Taken together, these data suggest an important contribution of CD147-cyclophilin interaction in inflammatory diseases. In addition to their involvement in inflammation, secreted cyclophilins have been implicated in the physiopathology of HIV-1. CyPA interaction with CD147 and syndecans has been demonstrated to play a role in HIV-1 infection and to contribute to AIDS pathogenesis (Saphire et al. 1999; Pushkarsky et al. 2001; Saphire et al. 2001). These data suggest two non-mutually exclusive mechanisms for explaining the activity of CyPA. By interacting with HS, CyPA might promote attachment of the virions to target cells and/or by interacting with CD147, could regulate viral entry. Our current findings suggest that binding to HS moieties of syndecan-1 might in fact facilitate subsequent interaction with CD147. Therefore, the signaling responses initiated by CD147/syndecan-1 engagement might contribute to HIV-induced cell activation, which is one of the crucial factors in AIDS pathogenesis.
Detailed analysis of the interaction of cyclophilins with CD147 and HS and characterization of the signaling pathways mediating cyclophilin activities would then provide an opportunity to manipulate inflammatory responses and to treat pathogenic conditions that depend on cyclophilins. In this context, the findings that HS bind and modulate the activity of secreted cyclophilins offer the opportunity to develop molecular mimetics with therapeutic applications to antagonize pathogenic interaction of cyclophilin with CD147.
Materials and methods
Antibodies and reagents
Recombinant human CyPB was produced and purified as described (Spik et al. 1991). The mutant CyPBR62A was engineered by site-directed mutagenesis and purified as described (Carpentier et al. 1999). This mutant was unable to promote adhesion of T cells, even though it retained the capability to interact with cell surface binding sites (Carpentier et al. 2002). Human fibronectin was a gift from Dr P. Delannoy (University of Lille, France). Cell surface HSPG were extracted and enriched by anion exchange chromatography on DEAE-sepharose (Amersham Pharmacia Biotech, Little Chalfont Buckinghamshire, UK), as described (Vanpouille et al. 2004). Rabbit polyclonal antibodies to CyPB were produced at home. Antibodies to human CD3 (X35), CD4 (13B.8.2), CD8 (B9.11), CD14 (RMO52), CD20 (B9E9), CD45RA (ALB11), CD45RO (UCHL1), and CD56 (T199) were purchased from Beckman Coulter (Fullerton, CA). Mouse monoclonal antibodies to CD147 (clone HIM6) were from BD Pharmingen (San Diego, CA). Goat polyclonal antibodies to human syndecan-1 (C-20), syndecan-2 (L-18), syndecan-4 (N-19), betaglycan (C-20), glypican-1 (S-16), rabbit polyclonal antibodies to syndecan-2 (M-140) and phospho-p38-MAPK (Tyr-182), mouse monoclonal antibodies to syndecan-1 (DL-101), syndecan-4 (5-G9), phospho-ERK1/2 (E-4), and JNK (D2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies to CD44v3 variant (AB2081) and mouse monoclonal anti-HS (MAB2040) were from Chemicon Int. (Temecula, CA). Rabbit polyclonal anti-phospho-JNK and mouse monoclonal anti-p38-MAPK (2F11) were from Upstate Biotechnology Inc. (Lake Placid, NY). Rabbit polyclonal anti-ERK1/2, fluorescein-conjugated antibodies to mouse, rabbit, goat IgG or mouse IgM, horseradish peroxidase-conjugated anti-goat IgG or anti-mouse IgM, and isotype-matched control antibodies were from Sigma Chemicals Co. (St Louis, MO). Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies were from Amersham Pharmacia Biotech. RANTES/CCL5, heparinase I (EC 4.2.2.7), chondroitinase ABC (EC 4.2.2.4), and phosphatidyl-inositol phospholipase C (EC 3.1.4.10) were obtained from Sigma. PD98059 was from Calbiochem (La Jolla, CA). Cell culture products were from Life Technologies (Paisley, Scotland, UK). Oligonucleotide primers and siRNA sequences were synthesized and purified by Eurogentec (Seraing, Belgium). All other chemicals, except where otherwise mentioned, were purchased from Sigma.
Isolation of T lymphocytes and cell culture
Human citrated venous blood samples from healthy donors were obtained from the Etablissement Français du Sang (Lille, France). Peripheral blood CD4+/CD45RO+ T lymphocytes were isolated by exhaustive negative selection (Allain et al. 2002). Purity of the T-cell population was assessed by flow cytofluorimetry and found more than 95%. Heparinase-treated T lymphocytes were obtained by incubating cells with 0.75 unit of heparinase I/106 cells for 2 h at 37 °C. Human promonocytic leukemia THP-1 cells (88081201; ECACC, Porton Down, Salisbury, UK) were routinely cultured in RPMI 1640 containing 10% FCS, 2 mM l-glutamine, 20 µM β-mercaptoethanol, 10 mM gentamycin in 5% CO2 enriched atmosphere at 37 °C. To induce responsiveness to CyPB, THP-1 cells were differentiated for 72 h with 50 nM 1,25-dihydroxy-vitamine D3 (Vey et al. 1992). Efficacy of the treatment was checked by analyzing CD14 expression by flow cytofluorimetry and found more than 90%.
Flow cytofluorimetry analysis
Analysis of the expression of CD markers was performed by incubating either peripheral blood T lymphocytes or THP-1 cells (5 × 105 cells per sample) in Dulbecco's phosphate buffered saline containing 0.5% bovine serum albumin (DPBS/BSA) supplemented with the appropriate monoclonal antibodies or the respective isotype-matched control IgG for 1 h at 4 °C (Denys et al. 1997). After washing, cells were labeled for 1 h at 4 °C with fluorescein-conjugated goat anti-mouse IgG (1/64). For the detection of HSPG, T cells were fixed for 30 min at 4 °C with 3% formaldehyde, pH 7.8 and treated with 0.1% saponin in DPBS/BSA supplemented with 1 mM ethylenediaminetetraacetic acid (EDTA) prior the addition of primary antibodies. Thereafter, T cells were incubated in the same buffer with goat polyclonal anti-syndecan-1, -2, -4 (1/100), goat polyclonal anti-betaglycan (1/100), rabbit polyclonal anti CD44v3 (1/500), mouse monoclonal anti-HS (1/100), or the respective control antibodies (1/2000) for 1 h at 4 °C. After washing, appropriate fluorescein-conjugated secondary antibodies were added for another 1 h-incubation. Cells were washed twice and immediately used for analysis. Data were monitored on a Becton Dickinson flow cytofluorimeter (FACScalibur) (Moutain View, CA) and analyzed with CellQuest software.
Western blot analysis
Cells (4 × 106 per sample) were washed in cold PBS and lysed in 100 µL of lysis buffer (20 mM phosphate buffer, pH 7.4, 350 mM NaCl, 10 mM KCl, 1 mM EDTA, 1% Triton X-100, 20% glycerol, 1 mM sodium orthovanadate, and 10 mM sodium fluoride) supplemented with protease inhibitor cocktail (Roche, Meylan, France) for 3 h at 4 °C. The lysates were clarified by centrifugation at 10 000g for 30 min at 4 °C. Proteins were then separated on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto nitrocellulose membranes (Sartorius, Göttingen, Germany). Blots were blocked for 1 h at room temperature in 150 mM NaCl, 20 mM Tris-HCl, and 1 mM EDTA, 0,1% Tween-20, pH 7.6 [Tris-buffered saline (TBS)–Tween], supplemented with 3% BSA. Membranes were then incubated for 2 h at room temperature with the appropriate primary antibodies in TBS-Tween supplemented with 1% BSA. After extensive washing with TBS-Tween, immunoblotted proteins were visualized with horseradish peroxidase-conjugated secondary antibodies (1 h-incubation, 1/5000) by using a chemiluminescence detection kit (ECL) (Amersham, Little Chalfont Buckinghamshire, England).
Electrophoretic mobility shift assay of HSPG
Cells (100 × 106 per mL) were washed in cold PBS and lysed in 20 mM phosphate buffer, pH 7.4 containing 350 mM NaCl, 10 mM KCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitor cocktail, overnight at 4 °C. The lysates were then clarified by centrifugation at 10 000g for 30 min at 4 °C and samples (70 µL) were incubated for 1 h at 4 °C in the absence or presence of antibodies immunoreactive to HSPG or CD147 (0.2 µg per sample). Thereafter, the samples were supplemented with 10 µL of 60% glycerol and subjected to electrophoresis on a 10% (w/v) native polyacrylamide gel (Bio-Rad Laboratories) in 40 mM TRIS/acetate, 1 mM EDTA, pH 7.8. A mixture of bromophenol blue was used as electrophoresis marker. After electrophoresis, the gel was incubated overnight in a 10 mM TRIS/HCl buffer, pH 7.4, containing 1% SDS and the proteins were transferred onto nitrocellulose. To check the position of HSPG-antibody complexes, the membrane was either directly immunostained with appropriate peroxidase-conjugated antibodies or used for a two-step immunostaining procedure, as described earlier. In the last case, primary antibodies were of different origin in order to avoid cross-reaction with the antibodies used for EMSA.
Semi-quantitative RT-PCR
Total RNA was extracted from 5 × 106 cells using a NucleoSpin RNA II kit, according to the instructions of the manufacturer (Macherey-Nagel, Hœrdt, France). Reverse transcription was performed from 2 µg of total RNA with an oligo-dT primer and M-MLV reverse transcriptase (Promega, Madison, WI). The PCR reactions were carried out using Readymix RedTaq PCR reaction mix. The synthetic primers for syndecan-2, CD44v3 and betaglycan were obtained from published data (Deng et al. 1999; Jones et al. 2000; Modrowski et al. 2000). The following primers for syndecan-1, -3, -4 and glypican-1 were designed according to the published cDNA sequences (accession numbers: NM_002997, NM_014654, NM_002999 and NM_002081, respectively). The primer sets for PCR and the size of the expected amplification products were as follows: syndecan-1, 5′- CCC CGT TTC TGG TGG TCT- 3′ (sense) and 5′- TGT CTG AAG GCT GAG TCC C -3′ (antisense), product size 175 bp; syndecan-3, 5′-CTG GAC AAT GCC ATC GAC TCG-3′ (sense) and 5′ -CTT CTG GTA TGT GAC GCT CGC -3′ (antisense), product size 219 bp; syndecan-4, 5′- GCT GCT GCT GTT CTT CGT -3′ (sense) and 5′- ACA ACT TCA GGG CCG ATC- 3′ (antisense), product size 213 bp; glypican-1, 5′- TGG TGG CTG CTA TGT GCG -3′ (sense) and 5′- TCC GCT CCG AGT CGT TCA-3′ (antisense), product size 340 bp. Optimum semi-quantitative PCR conditions were established to remain in the exponential phase of amplification. The following conditions were retained: 30 cycles of 94 °C for 1 min, 60 °C for 1 min and 74 °C for 1 min. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified in each sample to normalize for total mRNA input and confirm efficiency of cDNA synthesis. The amplification products were separated by electrophoresis in 2% agarose gel containing ethidium bromide and analyzed. Images were acquired with the Gel Doc 2000 Image analysis apparatus and analyzed with the supplied software Quantity-One (Bio-Rad, Richmond, CA). The sequence of each amplified product was confirmed by sequencing (Genoscreen, Lille, France).
RNA interference
Synthetic syndecan siRNA duplexes with symmetric 3′-deoxythymidine overhangs were chemically synthesized, purified by electrophoresis and annealed before use. The sequences used to inhibit mRNA expression of syndecan-1 and syndecan-2 were: 5′-GCA GGA CUU CAC CUU UGA ATT-3′, (nucleotides 923-941, geneBank Accession Number NM_002997), and 5′-CCA CGA CGC UGA AUA UAC ATT-3′ (nucleotides 881-899, geneBank Accession Number NM_002998), respectively. The oligonucleotide sequences were subjected to a basic local alignment search tool (BLAST) search analysis and no significant identity to other sequences could be detected. To check the sequence specificity of the RNA interference, a double-strand siRNA corresponding to the sequence 5′-GAA CGG CAU CAA GGU GAA CTT-3′ of GFP (Green Fluorescent Protein) was used as a negative control. THP-1 cells were transfected with 2 µg of siRNA duplexes in serum-free medium using DreamFect reagent (OZ-Biosciences, Marseille, France), according to the manufacturer's instructions. Efficiency of RNA interference was assessed in analyzing the respective expressions of mRNA from syndecan-1 and syndecan-2. The cell responsiveness to CyPB was analyzed 72 h posttransfection.
Cell adhesion and migration assays
In vitro chemotaxis was assayed in microchemotaxis chamber containing either 8 µm pore polycarbonate membrane for THP-1 cells or 5 µm pore polycarbonate membrane for T lymphocytes (Corning Costar Corp., Cambridge, MA), as described in Allain et al. (2002). Adhesion assays were performed in 96-well microtiter plates coated with fibronectin (1 µg per well), as described (Allain et al. 2002). Routinely, T lymphocytes (10 × 106 per mL) or THP-1 cells (7.5 × 106 per mL) were preincubated in DPBS/BSA with the appropriate agonist (CyPB, CyPBR62A or RANTES) for 10 min at 37 °C and the mixture was then distributed into the wells (100 µL) for an additional 20 min incubation at 37 °C. In time-course experiments, T cells were mixed with 50 nM CyPB and directly added to the fibronectin-coated plates for varying times. Thereafter, the plates were washed with DPBS and the remaining firmly attached cells were fixed with 3% formaldehyde, stained with 1% methylene blue and lysed with HCl 0.1 N. The absorbance was measured at 590 nM with a microplate BioRad reader Model 550 (Hercules, CA). Cell adhesion was estimated by using standard curves where absorbances were related to cell numbers, and results were expressed as a percentage of the initially added cells remaining fixed to the substrate. In some experiments, cells were pretreated for 1 h at 37 °C with 50 µM PD98059 or preincubated for 1 h at 20 °C in the presence of antibodies to cell surface HSPG (2 µg/mL) or to CD147 (10 µg/mL), and thereafter used for adhesion or migration assays. Neutralizing antibodies were checked for their inability to induce cell responses in the absence of agonist. Interaction between CyPB and cell-surface HS was determined as described (Carpentier et al. 2002).
Statistical analysis
Results are representative of at least three independent experiments conducted with either peripheral blood CD4+/CD45RO+ T lymphocytes from different donors or differentiated THP-1 cells. Statistical significance between the different values was analyzed by Student's t-test with a threshold of P < 0.05.
Acknowledgments
This investigation was supported by the Centre National de la Recherche Scientifique and by the Université des Sciences et Technologies de Lille, France.
Conflict of interest statement
None declared.
Abbreviations
- BLAST
basic local alignment search tool
- BSA
bovine serum albumin
- CS
chondroitan sulphate
- CsA
cyclosporine A
- CyPA
cyclophilin A
- CyPB
cyclophilin B
- DPBS
Dulbecco' phosphate buffered saline
- EDTA
ethylenediamine tetra-acetic acid
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- GAG
glycosaminoglycan
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HS
heparan sulphate
- HSPG
HS proteoglycan
- JNK
Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- PAGE
polyacrylamide gel electrophoresis
- PPIase
peptidyl-prolyl cis-trans isomerase
- RANTES
regulated upon activation, normal T-cell expressed, and secreted
- RT-PCR
reverse transcription polymerase chain reaction
- SDS
sodium dodecyl sulphate
- siRNA
small interfering RNA
- TBS
Tris-buffered saline.
References
Author notes
These authors contributed equally to this work.