-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew N. Round, Terence J. McMaster, Mervyn J. Miles, Anthony P. Corfield, Monica Berry, The isolated MUC5AC gene product from human ocular mucin displays intramolecular conformational heterogeneity, Glycobiology, Volume 17, Issue 6, June 2007, Pages 578–585, https://doi.org/10.1093/glycob/cwm027
Close - Share Icon Share
Abstract
Atomic force microscopy (AFM) has been used to show that human ocular mucins contain at least three distinct polymer conformations, separable by isopycnic density gradient centrifugation. In this work we have used affinity purification against the anti(mucin peptide core) monoclonal antibody 45M1 to isolate MUC5AC gene products, a major component of human ocular mucins. AFM images confirm that the affinity-purified polymers adopt distinct conformations that coidentify with two of those observed in the parent population, and further reveal that these two different conformations can be present within the same polymer. AFM images of the complexes formed after incubation of 45M1 with the parent sample reveal different rates of binding to the two MUC5AC polymer types. The variability of gene products within a mucin population was revealed by analyzing the height distributions along the polymer contour and periodicities in distances between occupied antibody binding sites. AFM analysis of mucin polymers at the single molecule level provides new information about the genetic origins of individual polymers and the contributions of glycosylation to the physicochemical properties of mucins, which can be correlated with information obtained from biochemistry, antibody binding assays, and molecular biology techniques.
Introduction
The eye, in common with other wet epithelial surfaces in the body, possesses a protective mucous layer, the tear film (Corfield et al. 1997). Mucin gene products, expressed in the conjunctiva and cornea and found in the tear film, include the membrane-associated mucins MUC1, MUC4, and MUC16 and the secreted mucins MUC2, MUC5AC, and MUC7 (Gipson and Inatomi 1998; Jumblatt et al. 1999, 2003; Pflugfelder et al. 2000; Argueso et al. 2003). The secreted gel-forming mucins define the rheological properties of mucous layers on epithelia. It is thought that MUC5AC gene products comprise the majority of mucins in the conjunctiva, although quantitative assessments of the relative amounts of these secretory mucin products are yet to be fully determined. Transcripts for MUC5AC are present at much higher levels than those for the secreted mucin MUC2 (McKenzie et al. 2000), and MUC5AC mucins and mRNA are decreased in dry eye patients (Argueso et al. 2002). Secreted, gel-forming mucins such as MUC2 and MUC5AC are multimeric glycoproteins with polypeptide chains that consist of variable numbers of tandem repeat sequences typically rich in serine, threonine (to which the O-glycan sidechains are attached), and proline, and are specific to each gene product. The polypeptide termini contain cysteine-rich domains and it is through these that the subunits assemble, via disulfide bonds, to form multimers (Dekker et al. 2002; Hollingsworth and Swanson 2004; Rose and Voynow 2006). Thus, the size, molecular weight, and properties of the multimeric mucins are determined not just by their peptide sequence, but by a series of posttranslational modifications.
The glycosylation of mucins can be highly variable, resulting in the production of glycoforms of the same gene product, as occurs for MUC5AC in the eye (Ellingham et al. 1999). After caesium chloride (CsCl) density gradient ultracentrifugation, Ellingham et al. found that MUC5AC reactivity was maintained across the range of buoyant densities containing mucins. That the diversity of mucin glycosylation reflects the regulation of individual cellular glycosyltransferase activity within tissues and has many origins is suggested by studies of the glycoforms of another multimeric mucin, MUC5B, which codes for the salivary mucin MG1 (Thornton et al. 1999; Veerman et al. 2003). Within the diverse repertoire of unique oligosaccharides found, a highly charged population of MUC5B mucins could be attributed to a particular glandular origin (Thornton et al. 1999), although a single salivary gland has been shown to synthesize different MUC5B glycoforms (Veerman et al. 2003).
While the details of how the existence of glycoforms of the same gene product entails functional consequences are not yet fully understood, diversity in glycosylation of a gene product can be expected to contribute directly to its physical as well as its biochemical properties. Steric and electrostatic considerations suggest that the glycosylation of mucins will confer an extended, stiffened conformation on the polymer (Jentoft 1990; Hanisch 2001; Gerken 2004), arising from negative charges contributed by, among others, sialyl and sulfate groups. An extended, stiff polymer occupies a large hydrodynamic volume and thus contributes to the rheological properties of a solution or gel of which it is part. The formation of a physicochemical barrier is an important functional role of mucins so the influence of glycosylation on the hydrodynamic properties of the mucin gel merits investigation.
Unusual patterns of O-glycosylation of secreted and membrane-associated mucins have been associated with changes in the properties of those mucins in diseases such as cystic fibrosis, ulcerative colitis, and inflammatory bowel disease (Brockhausen et al. 1995; Corfield et al. 2001; Rhodes and Campbell 2002).
Previously, we have used atomic force microscopy (AFM) to characterize individual mucin polymers from human conjunctivae (McMaster et al. 1999; Round et al. 2002, 2004). We have shown (Round et al. 2004) that the population of human ocular mucins contains at least three distinct polymer morphologies that have been labeled as Types I–III on the basis of their differentiation by visual inspection and analysis of AFM images. The criteria on which the three types are distinguished rely on comparative inspection of the polymers against each other and also against AFM images of polymers of well-known and characterized structures, in particular deoxyribonucleic acid (DNA) (Round and Miles 2004). With reference to DNA specifically, the three mucin types can be considered to be “DNA-like diameter and flexibility” (Type I), possessing polymer diameters and persistence lengths similar to those observed for DNA imaged under identical conditions (Round et al. 2002, 2004), “larger diameter than DNA” (Type II), and “smaller diameter and more flexible than DNA” (Type III). In our previous work, we also showed that the mucin Type I could be separated from the others on the basis of buoyant density in CsCl density gradient ultracentrifugation. As the charge on a mucin glycoprotein is carried largely by the sugars, this separation can be attributed to the structure of the glycosylated domains of the mucin polymers. Hence, because differences in conformation reflect differences in buoyant density, the conformations adopted by the mucins are shown to be influenced by the glycosylation of the peptide gene products.
The aim of this current work is to focus specifically on the products of one mucin gene, MUC5AC, whose glycoforms have been detected in ocular mucins (Ellingham et al. 1999). MUC5AC gene products were isolated from the largest hydrodynamic volume (size exclusion chromatography [SEC] excluded volume) population of conjunctival mucins by means of affinity chromatography against the antiMUC5AC monoclonal antibody 45M1. After removal of the bound antibodies, we then proceeded to compare by AFM imaging the conformations and dimensions of these MUC5AC polymers and those found in the parent mucin population. In a second, independent, experiment we incubated the parent conjunctival mucins with a low concentration of 45M1 antibody and directly observed by AFM imaging the binding of 45M1 to polymers in this sample.
Results
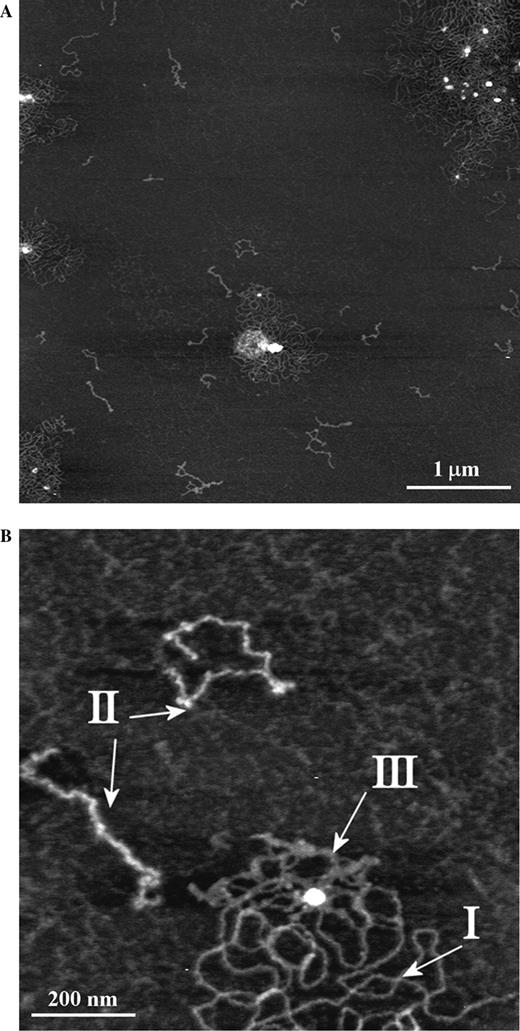
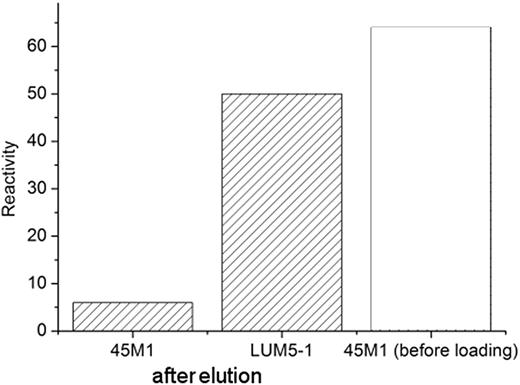
All mucin samples were determined to be free of nucleic acid contamination: AFM inspection only is not sufficient to unequivocally distinguish between DNA and some mucins (Round et al. 2002). Mucin polymer types and their classification are shown in Figure 1, which presents the largest intracellular conjunctival mucins. Figure 1A shows some examples of the variety of polymer conformations present, while the three polymer types are indicated in Figure 1B. Mucins of buoyant densities 1.35–1.45 g/mL and largest hydrodynamic volumes were then subjected to affinity chromatography against the antiMUC5AC peptide core monoclonal antibody 45M1. The affinity column eluents were probed with antibodies against MUC1, MUC2, MUC4, and MUC5AC peptide core. The eluants' reactivities with antibodies against MUC1, MUC2, and MUC4 were similar to their reactivities with secondary antibody only controls (Figure 2). Reactivity to 45M1 was very much reduced as expected for MUC5AC–45M1 complexes, while there was still strong reactivity to another antigen on MUC5AC, recognized by LUM5-1 (Figure 2). Antibody 45M1 recognizes an epitope in the peptide core encoded at the 5' terminal of the MUC5AC gene (Nollet et al. 2004), while LUM5-1 was raised against a synthetic peptide in the unique sequence flanking the gene tandem repeats (Hovenberg et al. 1996).
(A) AFM images of the largest human ocular mucins with mature glycosylation showing the range of polymer lengths, diameters, and conformations. 5000 × 5000 nm scan size, z-range 3 nm. (B) Detail of (A) highlighting examples of the polymer types I, II, and III referred to in the text. 1000 × 1000 nm scan size, z-range 2 nm.
The glycine eluent from 45M1-Protein G Sepharose column was probed with antibodies against mucin peptide epitopes. Reactivity, assessed by densitometry of dotblots, is expressed in arbitrary units. Reactivities with anti-MUC1, MUC2, and MUC4 antibodies were at the level of negative controls. For comparison we show the 45M1 reactivity of the parent mucin before loading onto the column (clear bar).
AFM imaging of mucins after affinity purification
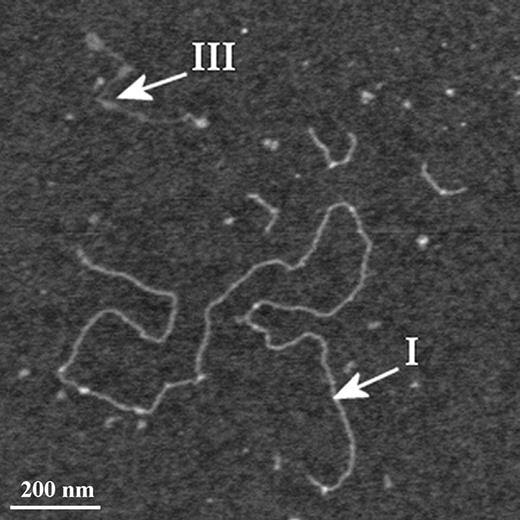
Affinity-purified mucins were imaged by AFM in intermittent contact mode in air. Figure 3 is representative of AFM images of these MUC5AC polymers. Applying the criteria described in the Introduction section, the polymers observed in this image, and throughout the sample, can immediately be split into two subsets; one, comprising the majority of polymers, includes linear, stiff, and extended polymers with contour lengths ranging from 100 nm to in excess of 2 µm. This subset of polymers closely resembles in their size, diameters, and conformations those seen in AFM images of the entire population of ocular mucins in the same buoyant density range (Round et al. 2002, 2004) and designated therein and above as Type I. The second subset consists of shorter, thinner, and more flexible polymers, similar to those designated Type III. Molecules resembling the Type II polymers described in the parent population were not found in the affinity-purified MUC5AC examined here.
AFM image of the affinity-purified mucins highlighting examples of the polymer types I and III referred to in the text. 1200 × 1200 nm scan size, z-range 3 nm.
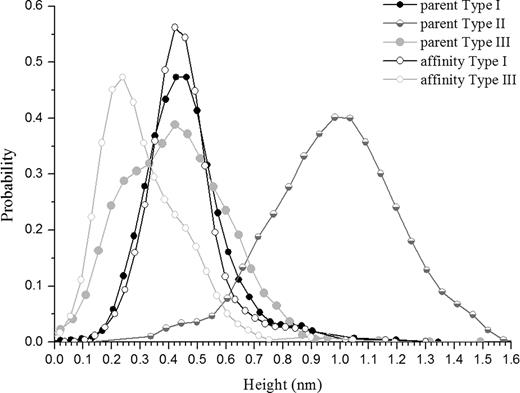
The Type I polymers seen in the affinity-purified sample were present as isolated, single molecules and no examples of the large looped aggregates surrounding central nuclei were observed, in contrast to the situation in parent mucins where the majority of Type I polymers seen were part of such an aggregate (compare Figures 1 and 3). This may be attributed to the additional purification steps this sample has gone through; the aggregates may either be lost on the affinity column or disrupted by the subsequent treatment with guanidine. We have examined heights (as measures of the polymer's diameter) of individual pixels along the contour of mucin molecules to determine whether there were any relationships between polymer type and the distribution of glycosylated domains. These are presented as kernel densities, which represent faithfully the structure of the data and remove dependence on bin width and end points that might affect the shape of a discrete histogram. The pixel heights along individual polymers show clear differences between the three populations identified as distinct polymer types (Figure 4). The distribution of heights in affinity-purified Type I polymers coincides with that of Type I polymers in the parent sample. The affinity-purified Type III polymer height distribution is included in that of the parent Type III polymers. They are both bimodal and possess an inflection point at 0.33 nm. The peak corresponding to larger diameters overlaps with the distribution of heights in Type I polymers. Type II polymers have a height distribution that is distinct from the other two types (protected least significance difference, PLSD; P < 0.001).
Kernel-estimated probability densities of heights of Type I, II, and III polymers in the parent and affinity-purified mucins. For parent mucins: Type I, n = 1468; Type II, n = 1203; Type III, n = 727. For affinity-purified polymers: Type I, n = 1092; Type III, n = 464. n refers to the number of pixels measured.
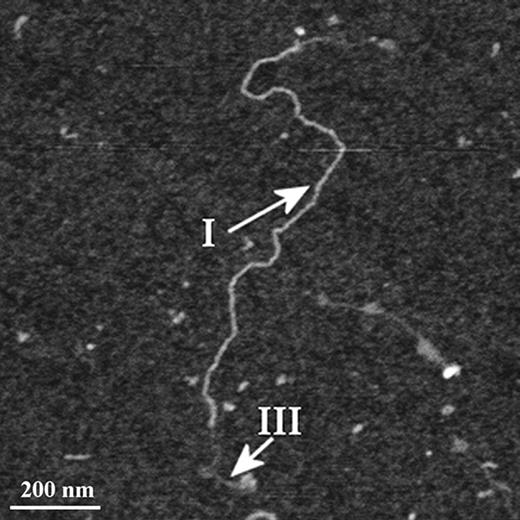
In some instances, of which Figure 5 is an example, single polymers were seen that contained domains of both types: a stiff, extended Type I region terminated at each end with thinner, flexible Type III strands (see arrows in Figure 5). The morphology observed here can be understood in light of the distribution of heights in Figure 4; both types of mucin are composed of varying proportions of two domains, each represented by one of the two peaks in the bimodal kernel density distribution. Although a distinct boundary between the two domains is only rarely observed, the ubiquity of the two domain model is revealed by the similar magnitudes of the two peaks. The significance of the observation that these distinct subunits are only easily resolved when they occur at the ends of a polymer is not determined here.
AFM image of affinity-purified MUC5AC mucins, showing detail of a single polymer containing two conformations. 1200 × 1200 nm scan size, z-range 3 nm.
AFM imaging of mucins exposed to 45M1 antibody
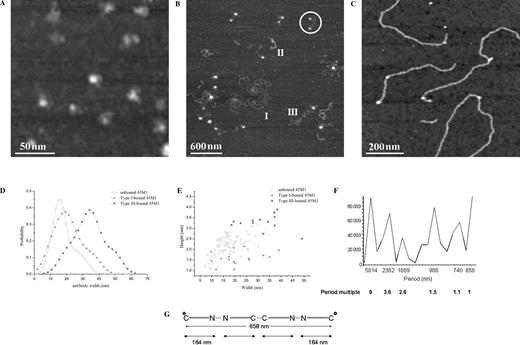
Parent mucins were incubated with 45M1 antibody at a dilution of 1:10 000, deposited onto mica and imaged by AFM in intermittent contact mode in air. To determine whether antibodies could be identified in AFM images, a sample of the antibody 45M1 was imaged in isolation under the same conditions (Figure 6A). Individual antibodies can be resolved here as rounded structures with a distribution of heights ranging from 1 to 3 nm with a mean of 2.19 nm (SD 0.46 nm) and a broad distribution of widths from 7 to 34 nm. Variations from a purely rounded structure are observed, some of which closely resemble the expected trilobed structure of immunoglobulin (Ig) G antibodies. The observed shapes and dimensions of the features in this image correspond closely to those observed for IgG molecules in previous work (Thomson 2005a, 2005b).
(A) AFM image of 45M1 antibodies on mica, showing the variety of conformations adopted. 200 × 200 nm scan size, z-range 2 nm. (B) AFM image of mature human ocular mucins after 1 h incubation with 45M1 antibody. All three polymer types are identified, and binding of 45M1 is observed only to Type III polymers. The ringed feature is an example of the bolas morphology, with an antibody bound at each end of a Type III polymer. 3000 × 3000 nm scan size, z-range 3 nm. (C) AFM image of 45M1 bound to Type I polymer after 24 h incubation. 840 × 840 nm scan size, z-range 2 nm. (D) Kernel-estimated probability densities of the widths of 45M1 antibodies. Unbound 45M1, n = 76; 45M1 bound to Type I polymers, n = 16; 45M1 bound to Type III polymers, n = 17. (E) Scatter plot of antibody heights versus widths for 45M1 antibodies, unbound, and bound to polymers Type I and III. (F) Fourier spectrum of contour distances between two consecutive antibodies. The spatial periods (total contour length/peak maximum) are indicated below the graph together with their ratios to the smallest period. (G) Proposed model of antibody binding corresponding to the bolas morphology. Asterisk represents observed 45M1 binding site. From this arrangement subunit size is calculated as 164 nm. The larger periods would represent a hexamer (1.5); a decamer (2.6); and a tetradecamer (3.6). The highest period, a polymer in excess of 5 µm, is a result of the concatenation of interantibody distances on a number of polymers.
Figure 6B shows an image of parent mucins after incubation with 45M1 for 1 h prior to deposition on mica. Examples of the three different conformations identified in previous images of this sample (Round et al. 2004) are also observed here (see polymers labeled I, II, and III). In addition, several rounded features are seen in association with the linear polymers. In particular, a frequently occurring morphology resembles 2-headed bolas, with a large rounded structure at each end of a flexible polymer (see ringed feature in Figure 6B). The flexible polymer can be identified according to the diameter and flexibility criteria used previously as a type III mucin polymer. Binding of 45M1 to Type I polymers is not observed in images of samples with 45M1 incubation times of 1 h. Images of samples of mucins and 45M1 obtained after incubation for 24 h at 4 °C did show binding of 45M1 to Type I polymers (Figure 6C), in the form of rounded features decorating the polymers. In the case of 45M1 bound to Type I polymers, the conformation of the polymer appears to be unaffected by the bound antibody whereas the conformations of Type III polymers bound by 45M1 show changes such as the aforementioned adoption of bolas morphology. In both cases, the observed features are present only in samples which contain both mucin and 45M1 antibody, and thus are interpreted as examples of antibody binding to mucin.
In order to provide evidence that the antibodies are binding to the mucin as opposed to simply codepositing on to the mica upon drying, we investigated the interaction of 45M1 with mucins lacking the epitope recognized by this antibody. The 45M1 recognizes an epitope in the peptide core encoded at the 5' terminal of the MUC5AC gene (Nollet et al. 2004), which is disrupted on reduction. An immunoblot assay confirms that 45M1 does not bind to ocular mucins subjected to reduction with dithiothreitol, in contrast to the unreduced mucin fraction. AFM images of this reduced mucin fraction with and without incubation with 45M1 show very few features of the shapes and dimensions commensurate with antibodies associated with native mucin polymers (data not presented).
A comparison of the widths of individual features interpreted as bound 45M1 antibodies and those of unbound 45M1 antibodies is presented in Figure 6D and E. Figure 6D presents kernel-estimated probability densities for the three sample populations (unbound 45M1, 45M1 bound to Type I mucins, and 45M1 bound to Type III mucins). The sample of antibodies bound to Type I polymers has a distribution of widths that overlaps with that of the sample of unbound 45M1, whereas the width distribution of 45M1 bound to Type III polymers is shifted to significantly larger values. Figure 6E represents these differences as a scatter plot showing the degree of overlap between the three samples.
A number of peaks in spatial frequencies were detected in the distribution of contour distances between consecutive antibodies within the same polymer (Figure 6F), each peak subtending a certain width. The shortest period detected in the Fourier spectrum, 658 nm, is too long to represent the distance between two C-termini of a mucin dimer, but could represent the next shortest polymer which is a tetramer, the bolas figure (see Figure 6G). The mucin molecules resembling bolas are thus tetramers or higher order polymers with C-termini at either end. This would imply a subunit length of approximately 164 nm. This value is consistent with subunit sizes suggested by the next three periods, which are 123, 168, and 156 nm. Fourier analysis of distances between antibodies and polymer ends reveals a different spectrum with the lowest frequency equivalent to a period of 674 nm.
Discussion
We have shown that three different polymer morphologies are common in the largest, human ocular mucins with mature glycosylation, two of which have overlapping height distributions. Mucins that were obtained after affinity chromatography against 45M1 coidentified with the latter two types only. The distribution of their heights is not different from that of polymers of the same type imaged from a mixed mucin population with the same buoyant density. Polymers obtained after affinity purification are recognized by LUM5-1 and not by antibodies against mucin peptide core of MUC1, 2, 4, or 5B. Directly after elution from the affinity column, the epitope recognized by 45M1 is unavailable for antibody binding as it is occupied by antibodies from the column, whereas the LUM5-1 reactivity is not affected. Extensive dialysis against guanidine hydrochloride (GuHCl) and then N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) buffer separated mucin–antibody complexes and restored reactivity with antibody 45M1, as observed by AFM in Figure 6. We conclude that these affinity-purified polymers are MUC5AC gene products.
Type II polymers have not only a different appearance but also a distinct height distribution, which we interpret as a distribution of glycosylated domains. They are not present in the affinity-purified MUC5AC population; no antibodies have been observed decorating this type of polymer. It follows that Type II polymers are not recognized by 45M1 antibodies, and are very likely products of a different mucin gene.
Some polymers contain domains that conform to Type I and others that fit the Type III distribution. If the differences between the polymer types that represent the same gene product follow differences in glycosylation, this observation suggests that not all subunits in a polymer are equally glycosylated, while the polymer itself would be placed by density centrifugation in accordance to its global properties. The presence of subunits with different glycosylation densities in the same polymer might also explain some of the charge ranges observed in a 0.5 g/mL isopycnic class (Ellingham et al. 1999).
Using a low antibody concentration, at least 10-fold lower than used for immunoasays, we have avoided antibody aggregation. A short incubation time of mucin and antibodies in solution resulted in binding mostly near the ends of the Type III polymers, the bolas morphology. Internal epitopes on stiffer and longer Type I polymers were labeled after a longer incubation in solution. The greater flexibility of the Type III polymers confers greater conformational freedom to the polymer, potentially including conformations that favor binding by 45M1. This difference emphasizes the effects of the neighborhood on antibody-antigen interactions. Furthermore, increased accessibility to the binding epitope may be conferred by sparser glycosylation. The sparser glycosylation is suggested by the presence of a peak in height at <0.33 nm in the Type III polymers. Recently, the question of the relationship between peptide sequence and glycosylation has been the subject of extensive research using recombinant chimeric mucin constructs (Silverman et al. 2001, 2003). These authors showed that the oligosaccharides found on the constructs were specific to the chimeric tandem repeat sequences. Expression of the same chimeric mucin construct in different cell lines resulted in different glycosylation patterns further underlining the individual cellular regulation of the glycosylation process (Parry et al. 2005).
The Fourier analysis we undertook on distances between consecutive antibody binding sites revealed that not all potential 45M1 epitopes were occupied. It is interesting to compare the shortest periods in the antibody–polymer end distances (674 nm) and antibody–antibody contour separation (658 nm), a difference which suggests that the 45M1 epitope is approximately 17 nm from the C end of the MUC5AC subunit.
Antibodies bound to Type III polymers have larger heights and widths than antibodies bound to Type I polymers or unbound. While some of this variability may be attributed to the different imaging conditions under which the three sets of data were obtained, the wrapping of this remaining C-terminal polymer around the bound 45M1 would increase the apparent dimensions of the antibody in the way we observe here.
Some of the variability observed in this spectrum is due to the uncertainty of AFM measurements. The pixel resolution limit of the images from which these measurements have been taken is a maximum of 6 nm, but the finite radius of the AFM probe tip means that the image records a convolution of tip and feature. This leads to a resolution limit of approximately 15 nm in the case of the images analyzed here. The widths of the peaks in the Fourier spectrum are also likely to follow from the variability in the MUC5AC gene in individual tissue donors from which the mucin sample has been obtained. By using techniques as described here, it is therefore possible to obtain measures of allellic variation in an individual, and a normal population, and to assess deviations in disease.
By isolating and characterizing individual polymers within mucin populations according to their physicochemical and biochemical properties, we demonstrate that the glycoforms of the MUC5AC gene product identified by Ellingham et al. (1999) are readily identifiable in AFM images. Characterization at the single molecule level by this method reveals intra- as well as intermolecular heterogeneity with respect to polymer conformation, diameter, and affinity for antibodies against peptide core epitopes. We attribute each of these properties to the presence of local regions of dense glycosylation.
Materials and methods
Isolation of mucin
Mucins were extracted from human cadaver conjunctivae in 4M GuHCl with protease inhibitors. The mucins were isolated on a CsCl gradient (buoyant density 1.3–1.5 g/mL) and refined by further CsCl density gradient centrifugation (with 0.5M GuHCl) into a population with the buoyant density 1.35–1.45 g/mL. This population was then separated into two fractions V0 (excluded volume) and Vi (included volume) by SEC and confirmed free of DNA contamination (Hoechst 33258 Dye, Turner BioSciences, CA). The V0 fraction was retained for imaging by AFM and is referred to throughout the paper as the parent mucin. Further details of the isolation and purification procedures employed can be found in Ellingham et al. (1999).
Affinity purification of MUC5AC
A volume of 250 µL of anti(MUC5AC peptide core) monoclonal antibody 45M1 (Abcam Ltd, Cambridge, UK) in 1.5 mL 20 mM sodium phosphate buffer (pH 7) was loaded onto a Protein G Sepharose 4 Fast Flow (Amersham Biosciences, Piscataway, NJ) column and incubated for 1 h at room temperature. The column was washed with 10 column volumes phosphate buffer to remove unbound antibody before loading the mucin solution. After 1 h incubation at room temperature, with mixing, the columns were washed again, as above, to dislodge any unbound material. The mucin–antibody complexes were eluted with 10 mL 0.1M glycine buffer (pH 2.7) applied as 2 mL aliquots. Elution samples were brought to pH 7.5 with 75 µL 2M Tris buffer (pH 8.5) predispensed in the collecting vessels. The presence of MUC5AC was confirmed by reaction with a polyclonal antibody to MUC5AC peptide core, antibody LUM5-1 (generous gift from Ingemar Carlstedt, University of Lund, Sweden). Reactivity with NCL-MUC-1 (Novocastra Laboratories, UK), LUM2-3 against MUC2 (gift from Ingemar Carlstedt), and 4F12 against MUC4 (DSHB, Iowa City, IA) was also tested. Reactivities were followed in dotblots on polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA) and visualized with horseradish peroxidase (HRP)-tagged secondary antibodies (Dako, Glostrup, Denmark) and diaminobenzidine (Sigma-Aldrich, Dorset, UK). Following affinity chromatography, the eluent was exhaustively dialyzed against 4M GuHCl.
Incubation of 45M1 antibody with mucin
Mucins were incubated with a 1:10 000 dilution of the stock antibody solution as received in 10 mM HEPES and 2 mM NiCl2 for either 1 h or 24 h at 4 °C prior to AFM imaging under the conditions described in AFM sample preparation and imaging.
AFM sample preparation and imaging
Mucins and antibodies were imaged using a Multimode/Nanoscope IIIa AFM (Digital Instruments/Veeco, Santa Barbara, CA). Olympus silicon cantilevers (Tokyo, Japan) with a nominal spring constant of 42 Nm−1 were used. Mucins stored in a solution containing 500 mM GuHCl were dialyzed against 10 mM HEPES and 2 mM NiCl2, deposited onto freshly cleaved mica in a 20 µL droplet, allowed to equilibrate for 5 min, rinsed in deionized water (Fluka Riedel-de-Haen, Seelze, Germany) and blown dry using dried nitrogen (N2) gas. All images were collected in the repulsive regime of intermittent contact mode in air (Round and Miles 2004). This procedure and the composition of the buffer have been demonstrated to allow mucins and other polymers to adopt equilibrium conformations at the mica surface (Hansma and Laney 1996; Rivetti et al. 1996; Round et al. 2002).
Analysis of AFM data
Lengths and heights of features in the AFM images were measured using the image processing software WSxM (Horcas I, Fernandez R, Gomez-Rodriguez JM, Colchero J, Gomez-Herrero J, Baro AM. 2007. Rev. Sci. Instrum. 78, 013705; http://www.nanotec.es Nanotec Electronica SL, Madrid, Spain). The precision of length measurements is limited by the convolution of the AFM probe tip with the feature being imaged and by the pixel resolution to approximately 15 nm. The height measurements were taken at each pixel along the contour of each polymer; depending on the length of the polymer there were between 100 and 1000 pixels per polymer. Kernel probability density estimates are calculated using software written by Professor J.R. Varma and released under GNU General Public License. The Fourier spectrum was calculated using Mathematica 5.2 (Wolfram Research Inc., Champaign, IL). Periods are the ratio of the frequency at peak maxima and the summed data lengths.
Acknowledgments
This project was supported by a grant from the BBSRC BioImaging Initiative. The authors thank Professor M.V. Berry for the Fourier routine in Mathematica.
Conflict of interest statement
None declared.
Abbreviations
- CsCl
caesium chloride
- DNA
deoxyribonucleic acid
- GuHCL
guanidine hydrochloride
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- HRP
horseradish peroxidase
- Ig
immunoglobulin
- N2
nitrogen
- PVDF
polyvinylidene difluoride
- PLSD
protected least significance difference
- SEC
size exclusion chromatography;