-
PDF
- Split View
-
Views
-
Cite
Cite
G. Valverde-Franco, J.S. Binette, W. Li, H. Wang, S. Chai, F. Laflamme, N. Tran-Khanh, E. Quenneville, T. Meijers, A.R. Poole, J.S. Mort, M.D. Buschmann, J.E. Henderson, Defects in articular cartilage metabolism and early arthritis in fibroblast growth factor receptor 3 deficient mice, Human Molecular Genetics, Volume 15, Issue 11, 1 June 2006, Pages 1783–1792, https://doi.org/10.1093/hmg/ddl100
Close - Share Icon Share
Abstract
Fibroblast growth factor (FGF) receptor 3 has been identified as a key regulator of endochondral bone development and of post-natal bone metabolism through its action on growth plate chondrocytes and osteoblasts, respectively. It has also been shown to promote chondrogenesis and cartilage production by cultured pre-chondrogenic cells in response to FGF18. In the current studies, we show that the absence of signaling through Fgfr3 in the joints of Fgfr3−/− mice leads to premature cartilage degeneration and early arthritis. Degenerative changes in cartilage matrix included excessive proteolysis of aggrecan core protein and type II collagen, as measured by neo-epitope immunoreactivity. These changes were accompanied by increased expression of metalloproteinase MMP13, type X collagen, cellular hypertrophy and loss of proteoglycan at the articular surface. Using a novel micro-mechanical indentation protocol, it was shown that articular cartilage in the humeral head of 4-month-old Fgfr3−/− mice was less resistant to compressive force and less stiff than that of littermate controls. These results identify Fgfr3 signaling as a potential target for intervention in degenerative disorders of cartilage metabolism.
INTRODUCTION
Hyaline cartilage is found in the developing growth plates and covering the articulating surfaces of long bones in the adult skeleton (1,2). Growth plate cartilage becomes mineralized and is partially resorbed to form a template on which the trabecular bone of the primary spongiosa is laid down during endochondral bone development (3). Articular cartilage remains un-mineralized to form a resilient, low friction surface that absorbs the shock caused by the high impact mechanical loading. It is composed primarily of an avascular type II collagen and proteoglycan (aggrecan) matrix that is secreted and maintained by relatively few chondrocytes in the adult. Articular cartilage therefore has a very limited capacity for repair. Focal lesions caused by trauma, infection or biochemical imbalance do not heal adequately and tend to progress rapidly to osteoarthritis (OA) (4,5). This is accompanied by increased proteolysis of proteoglycan and collagen, leading to cartilage matrix degradation. Many lines of investigation are thus focused on the development of improved technology for cartilage tissue engineering and assisted repair.
Assisted repair of focal defects in articular cartilage may be accomplished by ex vivo expansion of chondroprogenitor cells and subsequent transplantation in a scaffold with a source of growth factors to promote the differentiation and cartilage production (6–8). Growth factors that have been used with some degree of success include bone morphogenic proteins and transforming growth factor β (9–12), platelet-derived growth factor (13) and fibroblast growth factors (FGFs) (14–17). In chondrocytes, FGFs signal through three of four related receptors that undergo alternative splicing and are linked to the MEKK, phospholipase C γ and STAT signaling pathways (18). Targeted mutations of the gene encoding FGF receptor three (fgfr3) in mice resulted in developmental defects in endochondral bone that mimicked those seen in humans carrying the equivalent activating point mutations (19–22). Mice deficient in Fgfr3 signaling are phenotypically normal at birth but develop severe kyphosis, osteomalacia and osteopenia by 4 months of age (23,24). Additional in vivo and in vitro works have shown that these effects on growth plate cartilage and endochondral bone are mediated primarily through FGF2 and FGF18 interactions with Fgfr3 (25–27). Furthermore, mRNA encoding FGF18, FGFR2 and FGFR3 have been identified in articular cartilage explants removed from the talus joints of adult human donors (28).
Previous work has therefore identified Fgfr3 signaling as a key regulator of chondrocyte function in the developing growth plates and of osteoblast function in post-natal bone. FGF2 has also been identified as an important mitogen for articular chondrocytes ex vivo. The current work focused on defining the role of Fgfr3 signaling in post-natal articular cartilage. The shoulder and knee joints of Fgfr3+/+ and Fgfr3−/− mice were examined at 4 and 9 months of age for radiologic, histologic and molecular evidence of cartilage degradation. Compared with their wild-type littermates, Fgfr3−/− mice demonstrated narrowing of the humeral joint space and a reduction in sub-chondral bone volume as early as 4 months of age. These changes were accompanied by increased expression of type X collagen and increased cleavage of aggregan and type II collagen, as evidenced by immunochemical staining with neo-epitope antibodies. These results demonstrate an important role for FGFR3 in the maintenance of articular cartilage integrity and identify the FGF-FGFR3 signaling pathway as a potential target for the early intervention in degenerative cartilage disease.
RESULTS
In a previous work, signaling through Fgfr3 in chondrocytes was shown to play an important role in the development and maintenance of growth plate cartilage. An extensive literature document shows the similarities in the regulation of growth plate and articular chondrocyte biology. We therefore hypothesized that the congenital absence of Fgfr3 signaling would lead to altered cartilage development and metabolism of cartilage in the joints of Fgfr3−/− mice.
Radiologic evidence of joint degeneration in FGFR3−/− mice
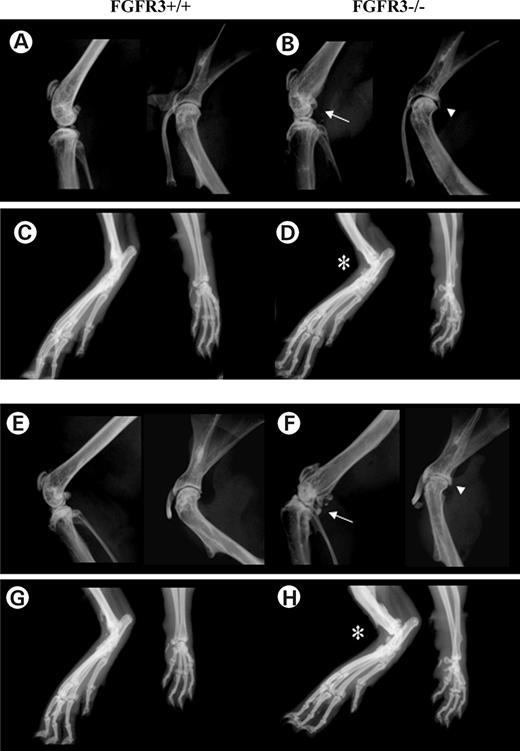
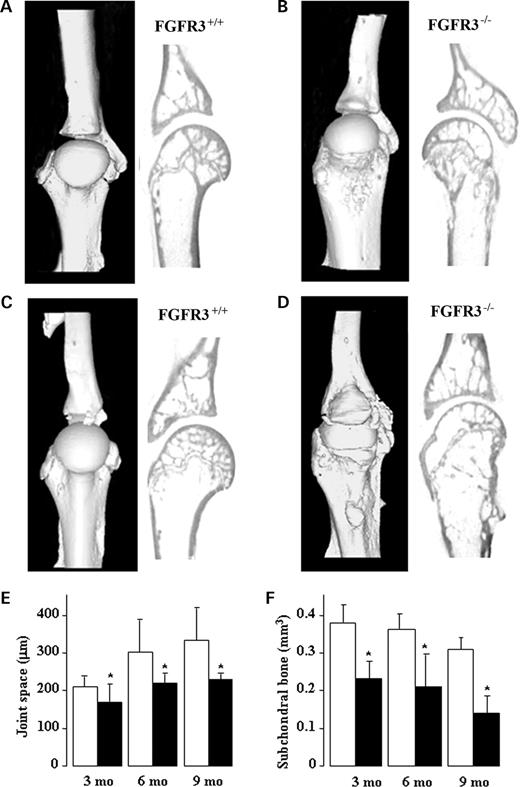
X-ray analysis of the knee (Fig. 1A, B, E and F left panel), shoulder (Fig. 1A, B, E and F right panel) and paws (Fig. 1C, D, G and H) of Fgfr3+/+ and Fgfr3−/− mice at 4 months (Fig. 1A–D) and 12 months (Fig. 1E–H) showed progressive joint deterioration (arrows), narrowing of the joint space (arrowheads) and soft tissue swelling (asterisk) in Fgfr3−/− compared with Fgfr3+/+ mice (Fig. 1). The micro-architecture of the shoulder joints was assessed and quantified using micro CT. Three-dimensional rendering and reconstruction of the scans from 4 month (Fig. 2A–B) and 9 month (Fig. 2C–D) mice showed progressive collapse of the humeral neck, roughened bone surfaces, numerous osteophytes protruding into the joint cavity and abnormal joint articulation in Fgfr3−/− mice compared with FGFR3+/+ mice. These changes were accompanied by the narrowing of the joint space (Fig. 2E) and a reduction in sub-chondral bone volume in the Fgfr3−/− mice (Fig. 2F).
Quantifiable, destructive changes in the joints of mice lacking FGFR3 signaling were therefore evident as early as 4 months of age. To characterize these changes at the molecular level, we performed histological analyses using molecular markers of cartilage matrix degradation.
Premature cartilage degradation and type X collagen in the knees of FGFR3−/− mice
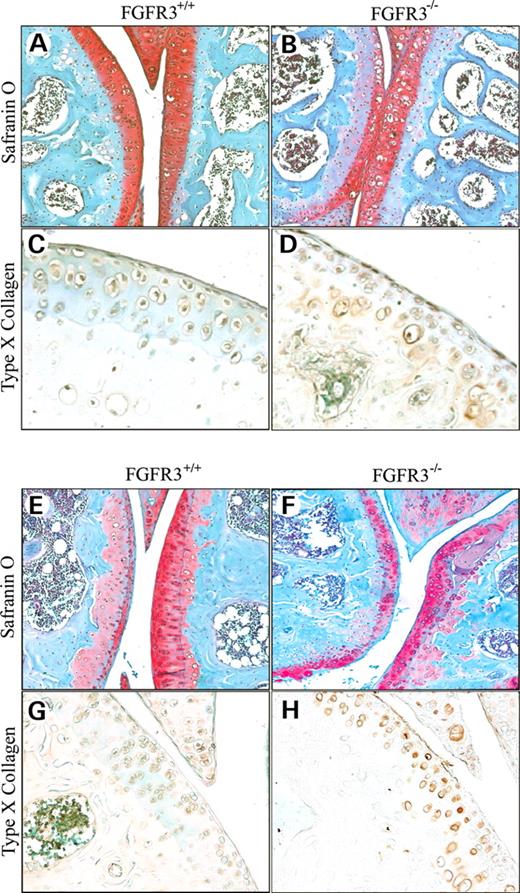
The knee joints of 4-month-old mice (Fig. 3A–D) stained with Safranin-O (Fig. 3A and B) appeared similar, except for an apparent increase in cell number and large lacunae and faint staining for proteoglycan in the underlying cartilage layer in Fgfr3−/− compared with FGFR3+/+ mice. Faint type X collagen immunoreactivity (Fig. 3C and D) that is associated with chondrocyte hypertrophy was also apparent at the articular surface in Fgfr3−/− but not in the Fgfr3+/+ mice. By 9 months of age (Fig. 3E–H), weak proteoglycan staining was also seen in the underlying mineralized cartilage in both Fgfr3+/+ and Fgfr3−/− mice (Fig. 3E and F). Fgfr3−/− mice also demonstrated a significant reduction in proteoglycan at the articular surface, fibrillation and meniscal hypertrophy, as well as an increase in type X collagen immunoreactivity (Fig. 3G and H), Taken together, these changes suggested progression of the articular chondrocytes toward a terminally differentiated phenotype, as seen in the transition zone of growth plate cartilage. It has been proposed that these hypertrophic changes require a concomitant increase in the cleavage of proteoglycan and type II collagen by locally active matrix metalloproteinases (MMPs), particularly MMP13 (29).
Increased cleavage of proteoglycan and type II collagen in the joints of Fgfr3−/− mice
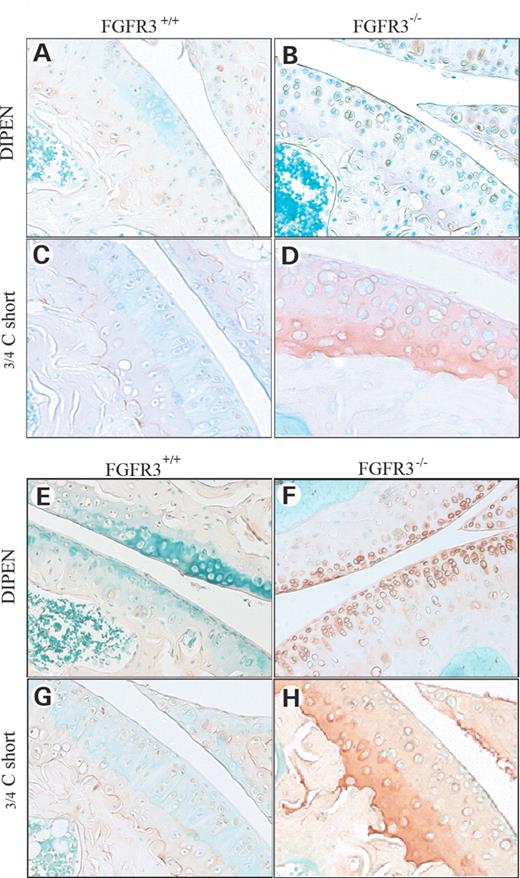
The DIPEN antibody was used to distinguish the fragments of aggregan generated by the specific cleavage of DIPEN341F342FGVG by MMP. The COL2-3/4Cs antibody was used to recognize a unique amino acid sequence neo-epitope in the COOH-terminus of the large 3/4 fragment of the a1(II) chain of type (II) collagen generated by the cleavage of collagenase. Figure 4 shows a modest increase in COL2-3/4Cs staining at 4 months in Fgfr3−/− joints (Fig. 4D) and a significant increase in both DIPEN (Fig. 4F) and COL2-3/4Cs (Fig. 4H) staining at 9 months compared with littermate controls (Fig. 4C, E and G). The increase in immunostaining in situ for type II collagen cleavage products was corroborated by an increase in the circulating level of type II collagen metabolites in Fgfr3−/− mice compared with age-matched controls (Table 1). In keeping with the reduction in sub-chondral bone, there was also an increase in the circulating C-telopeptide, a breakdown product of type I collagen, the major collagen present in bone.
Increased MMP13 in the joints of Fgfr3−/− mice
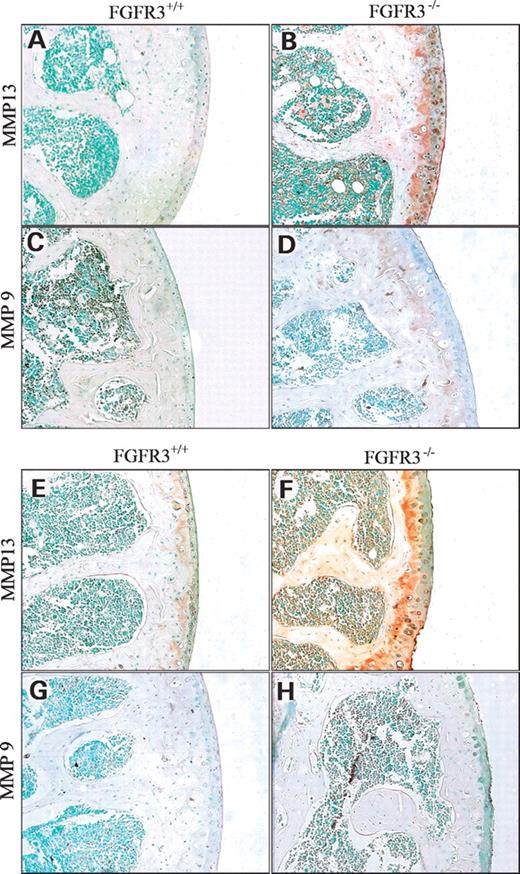
MMP13 and MMP9 are the principal enzymes responsible for the degradation of hyaline cartilage in the growth plates and the articular joints. Using antisera raised against MMP13 and MMP9, it was shown that MMP13 (Fig. 5A, B, E and F) was increased in the articular cartilage of the humeral head at 4 and 9 months in Fgfr3−/− mice (Fig. 5B, F) and at 9 months in Fgfr3+/+ mice (Fig. 5E). MMP9 immunoreactivity was not apparent in the articular cartilage of either of the genetic strain at any age tested (Fig. 5C, D, G and H). In contrast, MMP was detected in bone marrow cells, in trabecular bone and in growth plate chondrocytes (data not shown) in all mice at all ages.
Altered biomechanical properties of articular cartilage in Fgfr3−/− mice
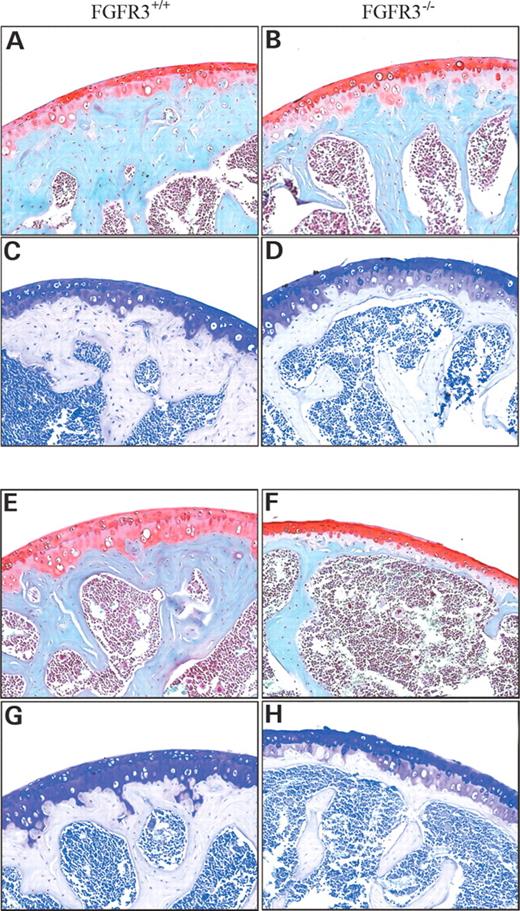
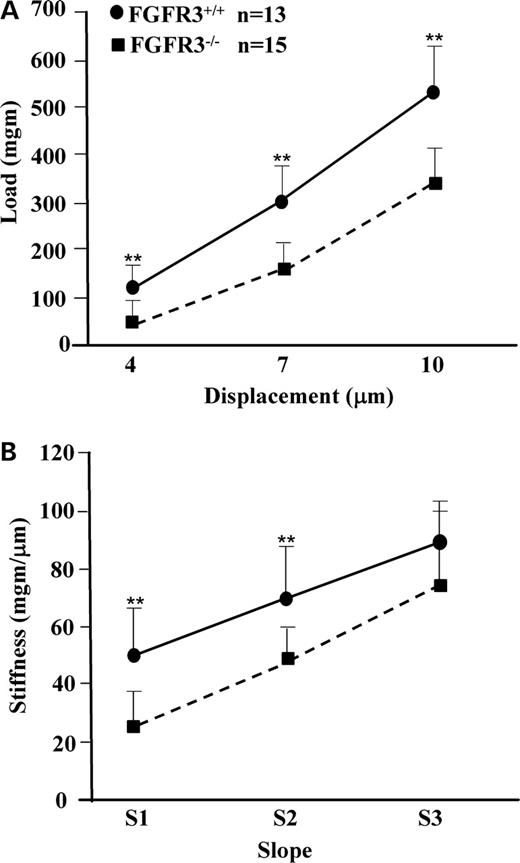
Cartilage stiffness and integrity are largely dependent on the presence of an intact cross-linked network of collagen entrapping the highly charged proteoglycan, aggrecan, which in turn effectively retains the interstitial water. Biomechanical properties of the cartilage are most accurately assessed using isolated cylindrical disks (30). However, the small size of the mouse joint precludes such an approach. We therefore developed a micro-indentation test that can be applied to intact small joints, similar to a previously published method (31). The mean thickness of articular cartilage at the head of the humerus, as shown in Figure 6, was 24.75±6.93 microns in Fgfr3+/+ mice and 38.61±7.65 microns in Fgfr3−/− mice (P<0.02). We then tested the resilience and stiffness of articular cartilage on freshly dissected left humeral heads from 4-month-old Fgfr3−/− and Fgfr3+/+ mice. Although there was no detectable increase in aggregan cleavage at 4 months in Fgfr3−/− mice, the loss of intact type II collagen resulted in a significant decrease in both cartilage resilience (Fig. 7A) and stiffness (Fig. 7B). Similar but more variable changes were seen when the right humerus was substituted for the left (data not shown).
DISCUSSION
Generalized destruction of articular cartilage, such as that seen in OA, is a common occurrence in people over 60. In many instances, it is believed to be a consequence of prior joint injury that does not heal due to the limited capacity of cartilage for self repair. Although the signaling pathways linked to receptors for FGFs have been identified as potential targets for intervention in assisted cartilage repair, little is known about their role in chondrogenesis and cartilage metabolism. In the previous work, we demonstrated a critical role for Fgfr3 signaling in cartilage production in vitro. In this study, we have found that the absence of signaling through Fgfr3 in chondrocytes in vivo leads to a similar degeneration of articular cartilage to that seen in human OA. Significant differences in proteoglycan content, expression of MMP13 and type X collagen, and cleavage of aggrecan and type II collagen were seen as early as 4 months in Fgfr3−/− mice compared with their littermate controls. This work identifies FGFR3 signaling as a critical regulator of articular cartilage metabolism and a potential pathway for the early intervention in degenerative cartilage disease.
Reduction in joint space in Fgfr−/− mice
Under physiological circumstances, the head of the humerus fits into a socket formed by the outer edge of the scapula where it is held in place by the rotator cuff. Micro CT analyses showed mis-alignment of the humerus with the scapula, collapse of the humeral neck and an apparent reduction in the joint space by 4 months of age in FGFR3−/− mice. The reduced space could not be accounted for by a reduction in the thickness of articular cartilage in the Fgfr3-deficient mice, in which the layer of uncalcified tissue was significantly thicker at 4 months of age. It did however contain more hypertrophic chondrocytes and less matrix than that of the wild-type mice that would have resulted in less resistance to compression when the joint was straightened for imaging (see discussion of biomechanical properties). These defects were seen in association with reduced sub-chondral bone volume and with the appearance of marginal osteophytes, a typical feature of late OA (32–34). The fact that C-telopeptide was elevated in the serum of Fgfr3−/− mice argues against focal bone loss due to inflammation, and for generalized bone loss as a consequence of Fgfr3 deficiency (35–37). The later conjecture is supported by previous work demonstrating increased MMP13, cortical thinning and osteopenia in 4-month-old Fgfr3−/− mice resulting from an intrinsic osteoblast defect (24).
Expression of type X collagen at the articular surface in Fgfr3−/− mice
The growth plates of long bones contain gradients of chondrocytes from committed chondro-progenitor cells to fully differentiated hypertrophic cells that undergo apoptosis at the chondro-osseous junction (3). The transition from immature matrix-producing cells to mature cells that mineralize their surrounding matrix, is accompanied by a switch from primarily type II to type X collagen. This transition is tightly regulated by coordinated signaling through growth factor receptors, including PTH1R and FGFR3 (38–40). Expression of type X collagen in articular cartilage is usually restricted to the layer of hypertrophic cells below the tidemark (41). Type X collagen immunoreactivity was apparent in a few cells at the articular surface in 4 mo old Fgfr3−/− mice and greatly increased in 9 mo mice deficient in Fgfr3 signaling. These results were consistent with the decrease in Safranin-O-positive matrix and the increase in large lacunae at the articular surface. These results support the hypothesis that Fgfr3 signaling is required to stabilize the phenotype of articular chondrocytes and prevent progression to terminal differentiation. Corroborating evidence comes from previous studies demonstrating Fgfr3 deficiency promoted type X collagen expression and hypertrophic differentiation of growth plate chondrocytes (23,40,42).
Increase in aggrecan and type II collagen cleavage in Fgfr3−/− mice
The tensile strength of articular cartilage is conferred by a healthy fibrillar network of type II collagen that traps aggrecan complexes which in turn confer stiffness. The initial cleavage of type II collagen by endogenous collagenase/s generates a 3/4 fragment that is recognized by the site-specific antibody Col2-3/4Cshort (43). Similarly, cleavage of aggrecan by MMP is site-specific between the G1 and G2 domains to generate a neo-epitope recognized by the VDIPEN antibody (44). The increase in type II collagen and aggrecan cleavage products in the articular cartilage of Fgfr3−/− mice resembled those seen in mice over-expressing MMP-13 (45). This predicted increase in protease activity in the articular cartilage of Fgfr3−/− mice is consistent with that shown previously in diaphyseal osteoblasts in 4-month-old mice (24). FGF2 is a universal ligand for all FGF receptors (46,47) and accumulates in the synovial fluid of arthritic joints, where it is believed to stimulate MMP-13 (collagenase 3) production by articular chondrocytes (48,49). Compensatory signaling by FGF2 interacting with FGFR1 or FGFR2, both up-regulated in the absence of Fgfr3 (24,27), could have resulted in an observed increase in MMP-13 that would result in increased type II collagen cleavage in the joints of Fgfr3−/− mice. The fact that circulating levels of the cleavage products of both type II (3/4Cshort) and type I (CTX-1) collagen were elevated in the circulation of Fgfr3−/− mice argues strongly for a generalized defect in MMP-13-mediated collagen metabolism. This conjecture is supported by the observed increase in MMP13 immunoreactivity in cartilage and bone over time. Consistent with the published literature, MMP9 expression was apparent in the growth plate but not in the articular cartilage (29,45,50). The increase in VDIPEN epitope is also consistent with an upregulation of MMP-13 activity, although proteoglycan cleavage could also occur as a remodeling and repair response to the loss of type II collagen integrity.
Impaired biomechanical properties of articular cartilage in Fgfr3−/− mice
The current literature indicates that the biomechanical properties and functional integrity of articular cartilage deteriorates prior to the appearance of visible signs of surface erosion. Using an arthroscopic indentation probe, it was shown in a cohort of 50 patients with chronic anterior cruciate ligament deficiency that cartilage stiffness decreased over time after injury and often preceded the appearance of visible cartilage defects at the time of reconstructive surgery (51). In other studies using focal indentation probes, cartilage stiffness correlated with proteoglycan content, as measured by gadolinium-enhanced magnetic resonance imaging but not with cartilage thickness (52,53). Using a micro-mechanical indentation tester, we showed a similar reduction in cartilage stiffness in the shoulders of 4-month-old Fgfr3−/− mice, in which the depth of articular cartilage was in fact greater than that seen in Fgfr3+/+ mice. The loss of stiffness could be attributed to the increased degradation of type II collagen, demonstrated by in situ immunostaining and ex vivo by ELISA assay of circulating levels of metabolites. Increased degradation of collagen type II is in turn related to the increase in type X collagen hypertrophic cells seen at the articular surface that produce less proteoglycan than non-hypertrophic cells and occupy a larger volume of the cartilage matrix. Further credence is given to this hypothesis by the observed increase in type II collagen metabolism, demonstrated by in situ immunostaining and ex vivo by ELISA assay of circulating levels of metabolites. This decrease in cartilage stiffness in association with impaired type II collagen metabolism is reminiscent of that seen in 15-month-old Col2a1+/− mice (31) that synthesize ∼50% less type II collagen than their wild-type littermates. Interestingly, these mice also exhibited a reduction in sub-chondral bone volume, as seen in our own Fgfr3−/− mice at 4 months of age. The reduction in sub-chondral bone volume no doubt reflects the defects in FGFR3−/− osteoblast function described previously (24) and most probably led to the obvious deformation of the articulating surfaces seen at 9 months of age. Another potential explanation for the loss of cartilage stiffness is joint mis-alignment, as shown by X-ray and micro CT imaging, which could lead to reduced mobility. In fact, it was shown that 11 weeks of joint immobilization in dogs resulted in a 42% decrease in cartilage stiffness (54). However, the morphological defects in the skeletons of 4-month-old Fgfr3−/− mice do not appear to inhibit their activity or their access to food. The combination of joint mis-alignment and reduced sub-chondral bone volume could however compromise the overall biomechanical properties of diarthrodial joints and combined with premature cartilage degradation could lead to global joint deterioration.
MATERIALS AND METHODS
Mice
All mouse procedures were performed in accordance with McGill University guidelines that are set by the Canadian Council on Animal Care. Mice homozygous for targeted inactivation of the gene encoding Fgfr3, and wild-type littermates, were obtained from intercross of FGFR+/− maintained on a C3H background (24). Cohorts of mice were anesthetized and euthanized by exsanguination at two, four, six, nine and 12 months for analyses. Representative data is shown for 4- and 9-month-old Fgfr3+/+ and Fgfr3−/− mice.
Radiologic imaging
High resolution X-rays of the shoulder joints, knees, forepaws and hindpaws were obtained on euthanized mice or the dissociated limbs using a Faxitron MX20, equipped with an FPX-2 Imaging system (Dalsa Medoptics, Waterloo, Ontario, Canada). Immediately after X-ray, the left shoulder was dissected free of soft tissue, fixed overnight in 4% paraformaldehyde, rinsed three times with PBS and scanned on a Skyscan1072 micro CT instrument (Skyscan, Antwerp, Belgium). Image acquisition was performed at 100 kV and 98 µA with a 0.9° rotation between frames to obtain two-dimensional images, which were used to generate three-dimensional reconstructions. The joint space and sub-chondral bone volume were calculated with the three-dimensional Creator software supplied with the instrument.
Histology, histochemistry and immunochemistry
All histology, histochemistry and immunochemistry procedures were performed essentially as described previously (24,55). The right knee and shoulder joints were dissected free of soft tissue, fixed for 16 h in 4% paraformaldehyde at 4°C and rinsed three times in PBS. Bones were decalcified in 4.13% EDTA for 14 days at 4°C, embedded in paraffin and 4 µm sections cut on a rotary microtome for staining with Safranin-O and fast green to identify proteoglycan. Left shoulder joints were embedded in LR white acrylic resin (London Resin Co. Ltd, London, UK) and 2 µ sections cut on an ultra microtome for staining with 0.2% toluidine blue for 1 min to identify mineralized and un-mineralized cartilage. Immunohistochemical analyses were performed on 4 µm sections of paraffin embedded tissue using the following primary antisera: rabbit anti-C-terminal peptide Val-Asp-Ile-Pro-Glu-Asn of aggrecan G1 domain, (DIPEN 1:800) (44); rabbit anti-peptide Gly-Pro-Hyp-Gly-Pro-Gln-Gly that represents a type II collagen primary cleavage site (COL2-3/4C short peptide 1:200) (43); mouse anti-type X collagen peptide Tyr-Asp-Pro-Arg-Thr-Gly-Ile-Phe-Thr (1:300) (56); rabbit anti-MMP9 peptide (Sigma Saint Louis, MO, USA, 1:200) and rabbit anti-MMP13 peptide ovalbumin conjugates kindly provided by Dr J.S. Mort (1:300).
Biomechanical testing
The left humeral heads of 4-month-old Fgfr3+/+ and Fgfr3−/− mice were used for testing the biomechanical properties of articular cartilage using a novel indentation test developed on a Mach-1® micro-mechanical tester (BioSyntech Canada Inc., Laval, Canada). Samples were dissected free of soft tissue and the distal metaphysis fixed with dental stone in a small plastic container before dissecting the joint capsule to expose the articular surface. The humeral head was immersed in PBS for 30 min at room temperature to equilibrate before controlled compression was applied by an actuator with 0.1 µm displacement precision that moved a cylindrical flat tip indentor of 60 µm diameter positioned perpendicular to the articular surface. Ramp compressions at 5 µm/s were applied until a 2 g load was reached at two different positions on each humeral head and the force response captured by a load cell with 7 mg precision. The point of contact was determined by visual inspection of the loading curve and cartilage stiffness was assessed by calculating load-displacement slopes in the displacement intervals of 2.5–5.5 µm (S1), 5.5–8.5 µm (S2) and 8.5–11.5 µm (S3). The results were analyzed by MANOVA, using the general linear model with Fgfr3+/+ versus Fgfr3−/− as a categorical predictor and displacement as a continuous predictor.
Biochemical analyses
A commercial ELISA assay was used to determine the circulating levels of the type I collagen degradation product CTX-I (Mouse C-telopeptide, Nordic Bioscience Diagnostics, USA). Circulating type II collagen cleavage products were measured with the DELFIA® ELISA assay (Perkin Elmer, Wellesley, MA, USA) that uses a monoclonal C2C antibody raised against the carboxyl-terminal 3/4 fragment of the degraded α1 (II) chain. This assay uses a time-resolved immunofluorometric technique that greatly reduces background fluorescence (57).
ACKNOWLEDGEMENTS
This work was supported by grants to J.E.H. from the Canadian Institutes of Health Research (CIHR), the Arthritis Society of Canada and the Canadian Arthritis Network Centres of Excellence, and to M.D.B. from CIHR. G.V.F. was supported by a studentship from the Fonds de la recherche en santé du Québec (FRSQ) and S.C., F.L. and N.T-K. by training awards from the CIHR Strategic Training Program in Skeletal Health Research. J.E.H. is a Chercheur Boursier Senior of the FRSQ and M.D.B. holds a Canada Research Chair in Cartilage Tissue Engineering. The authors acknowledge the expert technical assistance of Ailian Li (J.T.N. Wong Laboratories) and Miren Gratton (Centre for Bone and Periodontal Research).
Conflict of Interest statement. None declared.
Figure 1. X-ray analysis of joints at 4 and 12 months in Fgfr3+/+ and Fgfr3−/− mice. High resolution Faxitron X-rays of the knees (A, B, E and F left), shoulder joints (A, B, E and F right) and paws (C, D, G and H) of Fgfr3+/+ and Fgfr3−/− mice at 4 months (A–D) and 9 months (E–H) of age. Arrows and arrowheads show progressive joint destruction and asterisks show soft tissue swelling in the Fgfr3−/− mice. Images captured at ×2 magnification and are representative of 8–10 mice of each genotype at each age.
Figure 2. Quantitative micro CT analysis of the shoulder joints of Fgfr+/+ and Fgfr−/− mice. Representative three-dimensional reconstructions (A–D left panels) and mid-sagital two-dimensional sections (A–D right panels) of the shoulder joints of Fgfr3+/+ and Fgfr3−/− mice at 4 months (A and B) and 9 months (C and D) of age were captured on a Skyscan®1072 instrument. Significant deformation and mis-alignment of the joint was seen at 4 months and 9 months in Fgfr3−/− mice, in association with a reduction in the joint space (F) and significant thinning of the sub-chondral bone plate (G). Fgfr3+/+ (white bars) and Fgfr3−/− (black bars). Results in E and F are expressed as the mean±SD of 3–5 measurements made on 6–8 mice of each genotype at each age. *Significantly different from Fgfr3+/+P<0.01.
Figure 3. Histochemical and immunochemical staining for proteoglycan and type X collagen. The knee joints of Fgfr+/+ and Fgfr3−/− mice at 4 months (A–D) and 9 months (E–H) of age were decalcified, embedded in paraffin and stained with Safranin-O to identify proteoglycan (A, B, E and F) or immunostained to identify type X collagen (C, D, G and H). Articular cartilage in the distal femur and proximal tibia in Fgfr3−/− mice showed an increase in cell number and large lacunae at 4 months (B) and a reduction in cell number and proteoglycan content at 9 months (F). Type X collagen immunoreactivity was seen in the articular cartilage of Fgfr3−/− mice at 4 and 9 months (D and H) but not in that of Fgfr3+/+ mice (C and G). Results are representative of those obtained from five mice of each genotype at each age. Magnification at source ×20.
Figure 4. Immunochemical staining for neo-epitopes of aggrecan and type II collagen. The knee joints of Fgfr3+/+ and Fgfr3−/− mice at 4 months (A–D) and 9 months (E–H)) of age were decalcified, embedded in paraffin and stained with DIPEN, to identify aggrecan cleaved by MMPs (A, B, E and F) or with an antibody that recognizes the 3/4C terminal fragment of type II collagen cleaved by MMP13 (C, D, G and H). In Fgfr3−/− mice, aggrecan cleavage was increased at 9 months (F) compared with Fgfr3+/+ (E) mice. Type II collagen cleavage was significantly increased at 4 months (D) and 9 months (H) compared with wild-type control (C and G, respectively). Results are representative of those from five mice of each genotype at each age. Magnification at source ×20.
Figure 5. Immunochemical staining for MMP13 and MMP9 in Fgfr3+/+ and Fgfr3−/− mice. The humerus of Fgfr+/+ and Fgfr3−/− mice at 4 months (A–D) and 9 months (E–H) of age were decalcified, embedded in paraffin and immunostained with antisera that recognizes MMP13 (A, B, E and F) or MMP9 (C, D, G and H). Whereas MMP13 expression increased over time in both genetic strains, the staining was far more intense in Fgfr3−/− mice. Little difference was seen in MMP9 expression between Fgfr+/+ and Fgfr3−/−.
Figure 6. Identification of uncalcified cartilage in the humerus of Fgfr3+/+ and Fgfr3−/− mice. Specimens were prepared in the same manner as for Figure 5 and stained with Safranin-O (A, B, E and F) or Toluidine blue (C, D, G and H) to measure the depth of un-mineralized cartilage at the articular surface. Whereas the depth of un-mineralized cartilage appeared greater in Fgfr3−/− mice compared with Fgfr+/+, there was significantly less sub-chondral bone in the mutant mice at both ages.
Figure 7. Biomechanical properties of articular cartilage in Fgfr3+/+ and Fgfr3−/− mice. The freshly dissected heads of humeri from 4-month-old Fgfr3+/+ and Fgfr3−/− mice were subjected to loading by a flat tip indentor applied by an actuator moving at 5 µm/s with a 0.1 µm displacement precision (Mach-1® micro-mechanical tester). The load was measured continuously and is shown at displacements of 4, 7 and 10 µm (A), whereas cartilage stiffness was approximated as slopes of load versus displacement in the displacement intervals of 2.5–5.5 µm (S1), 5.5–8.5 µm (S2) and 8.5–11.5 µm (S3) (B). The response to loading (A) and resulting stiffness (B) were significantly decreased in the Fgfr3−/− mice (hatched line) compared with Fgfr3+/+ mice (solid line), as determined by MANOVA with Fgfr+/+/Fgfr3−/− and displacement as predictors. The results are representative of 25 measurements made on n=13 FGFR3+/+ mice and 24 measurements on n=15 FGFR3−/− mice.
Serum levels of type II (C2C) and type I (CTX-1) collagen metabolites
| Age . | C2C (ng/ml) . | CTX-1 (ng/ml) . | ||
|---|---|---|---|---|
| . | Fgfr3+/+ . | Fgfr3−/− . | Fgfr3+/+ . | Fgfr3−/− . |
| 2 months | 37.42±10.30 | 62.90±13.03* | 18.98±1.26 | 34.54±1.31* |
| 4 months | 39.89±8.87 | 54.80±10.10* | 16.70±3.38 | 27.28±10.69* |
| 6 months | 36.34±6.17 | 46.24±8.15* | 14.22±3.20 | 24.11±4.56* |
| 9 months | 45.43±7.30 | 55.75±5.71* | 16.90±3.96 | 35.69±7.30* |
| Age . | C2C (ng/ml) . | CTX-1 (ng/ml) . | ||
|---|---|---|---|---|
| . | Fgfr3+/+ . | Fgfr3−/− . | Fgfr3+/+ . | Fgfr3−/− . |
| 2 months | 37.42±10.30 | 62.90±13.03* | 18.98±1.26 | 34.54±1.31* |
| 4 months | 39.89±8.87 | 54.80±10.10* | 16.70±3.38 | 27.28±10.69* |
| 6 months | 36.34±6.17 | 46.24±8.15* | 14.22±3.20 | 24.11±4.56* |
| 9 months | 45.43±7.30 | 55.75±5.71* | 16.90±3.96 | 35.69±7.30* |
Values are expressed as the mean±SD of 20–25 serum samples from representative mice in each group. Significantly different from Fgfr3+/+.
*P<0.01.
Serum levels of type II (C2C) and type I (CTX-1) collagen metabolites
| Age . | C2C (ng/ml) . | CTX-1 (ng/ml) . | ||
|---|---|---|---|---|
| . | Fgfr3+/+ . | Fgfr3−/− . | Fgfr3+/+ . | Fgfr3−/− . |
| 2 months | 37.42±10.30 | 62.90±13.03* | 18.98±1.26 | 34.54±1.31* |
| 4 months | 39.89±8.87 | 54.80±10.10* | 16.70±3.38 | 27.28±10.69* |
| 6 months | 36.34±6.17 | 46.24±8.15* | 14.22±3.20 | 24.11±4.56* |
| 9 months | 45.43±7.30 | 55.75±5.71* | 16.90±3.96 | 35.69±7.30* |
| Age . | C2C (ng/ml) . | CTX-1 (ng/ml) . | ||
|---|---|---|---|---|
| . | Fgfr3+/+ . | Fgfr3−/− . | Fgfr3+/+ . | Fgfr3−/− . |
| 2 months | 37.42±10.30 | 62.90±13.03* | 18.98±1.26 | 34.54±1.31* |
| 4 months | 39.89±8.87 | 54.80±10.10* | 16.70±3.38 | 27.28±10.69* |
| 6 months | 36.34±6.17 | 46.24±8.15* | 14.22±3.20 | 24.11±4.56* |
| 9 months | 45.43±7.30 | 55.75±5.71* | 16.90±3.96 | 35.69±7.30* |
Values are expressed as the mean±SD of 20–25 serum samples from representative mice in each group. Significantly different from Fgfr3+/+.
*P<0.01.
References
Poole, A.R. (
Poole, A.R., Laverty, S. and Mwale, F. (
Hunziker, E.B. (
Solchaga, L.A., Goldberg, V.M. and Caplan, A.I. (
Korblin, M. and Estrov, Z. (
Dowthwaite, G.P., Bishop, J.C., Redman, S.N., Khan, I.M., Rooney, P., Evans, D.J.R., Haughton, L., Bayram, Z., Boyer, S., Thomson, B., Wolfe, M.S. and Archer, C.W. (
Ochi, M., Adachi, N., Nobutu, H., Yanada, S., Ito, Y. and Agung, M. (
Reddi, A.H. (
Issack, P.S. and DiCesare, P.E. (
Li, W.J., Tuli, R., Okafor, C., Derfoul, A., Danielson, K.G., Hall, D.J. and Tuan, R.S. (
Sekiya, I., Larson, B.L., Vuoristo, J.T., Reger, R.L. and Prockop, D.J. (
Barbero, A., Grogan, S., Schafer, D., Heberer, M., Mainil-Varlet, P. and Martin, I. (
Henson, F.M., Bowie, E.A. and Davies, M.E. (
Madry, H., Emkey, G., Zurakowski, D. and Trippel, S.B. (
Stevens, M.M., Marini, R.P., Martin, I., Langer, R. and Prasad Shastri, V. (
Veilleux, N. and Spector, M. (
Ornitz, D.M. and Marie, P.J. (
Naski, M.C., Colvin, J., Coffin, J. and Ornitz, D.M. (
Li, C., Chen, L., Iwata, T., Kitagawa, M., Fu, X.-Y. and Deng, C.-X. (
Bellus, G.A., Bamshad, M.J., Przylepa, K.A., Dorst, J., Lee, R.R., Hurko, O., Jabs, E.W., Curry, C.J.R., Wilcox, W.R., Lachman, R.S., Rimoin, D.L. and Francomano, C.A. (
Iwata, T., Li, C.-L., Deng, C.-X. and Francomano, C. (
Colvin, J.S., Bohne, B.A., Harding, G.W., McEwen, D.G. and Ornitz, D.M. (
Valverde-Franco, G., Liu, H., Davidson, D., Chai, S., Carvajal, H.V., Goltzman, D., Ornitz, D.M. and Henderson, J.E. (
Liu, Z., Xu, J., Colvin, J.S. and Ornitz, D.M. (
Ohbayashi, N., Shibayama, M., Kurotaki, Y., Imanishi, M., Fujimori, T., Itoh, N. and Takada, S. (
Davidson, D., Blanc, A., Filion, D., Wang, H., Plut, P., Pfeffer, G., Buschmann, M.D. and Henderson, J.E. (
Ellsworth, J.L., Berry, J., Bukowski, T., Claus, J., Feldhaus, A., Holderman, S., Holdren, M.S., Lum, K.D., Moore, E.E., Raymond, F. et al. (
D'Angelo, M., Yan, Z., Nooreyazdan, M., Pacifici, M., Sarment, D.S., Billings, P.C. and Leboy, P.S. (
Fortin, M., Soulhat, J., Sirazi-Adl, A., Hunziker, E.B. and Buschmann, M.D. (
Hyttinen, M.M., Toyras, J., Lapvetelainen, T., Lindblom, J., Prockop, D.J., Li, S.W., Arita, M., Jurvelin, J.S. and Helminen, H.J. (
Hunter, D.J., Zhang, Y., Niu, J., Tu, X., Amin, S., Goggins, J., Lavalley, M., Guermazi, A., Gale, D. and Felson, D.T. (
Scharstuhl, A., Glansbeek, H.L., van Beuningen, H., Vitters, E.L., van der Kraan, P. and van der Berg, W. (
Zhang, Y.W., Su, Y., Lanning, N., Swiatek, P.J., Bronson, R.T., Sigler, R., Martin, R.W. and vandeWoude, G.F. (
Walsh, N.C. and Gravallese, E.M. (
Lajeunesse, D. and Reboul, P. (
Garnero, P. and Delmas, P.D. (
Vortkamp, A., Lee, K., Lanske, B., Segre, G.V., Kronenberg, H.M. and Tabin, C.J. (
Kobayashi, T., Chung, U., Schipani, E., Starbuck, M., Karsenty, G., Katagiri, T., Goad, D., Lanske, B. and Kronenberg, H. (
Amizuka, N., Davidson, D., Liu, H., Valverde-Franco, G., Chai, S., Sasaki, T., Ozawa, H., Hammond, V., Ornitz, D.M., Goltzman, D. and Henderson, J.E. (
Poole, A.R., Kojima, T., Yasuda, T., Mwale, F., Kobayashi, M. and Laverty, S. (
Deng, C., Wynshaw-Boris, A., Zhou, F., Kuo, A. and Leder, P. (
Billinhurst, R.C., Dahlberg, L., Ionescu, M., Reiner, A., Bourne, R., Rorabeck, C., Mitchell, P., Hambor, J., Diekmann, O., Tschesche, H. et al. (
Hughes, C.E., Caterson, B., Fosang, A.J., Roughly, P.J. and Mort, J.S. (
Neuhold, L.A., Killar, L., Zhao, W., Sung, M.L.A., Warner, L., Kulik, J., Turner, J., Wu, W., Billinghurst, C., Meijers, T. et al. (
Ornitz, D.M. and Leder, P. (
Shimoaka, T., Ogasawara, T., Yonamine, A., Chikazu, D., Kawano, H., Nakamura, K., Itoh, N. and Kawaguchi, H. (
Orito, K., Koshino, T. and Saito, T. (
Wang, X., Manner, P.A., Horner, A., Shum, L., Tuan, R.S. and Nuckolls, G.H. (
Colnot, C., Thompson, X., Miclau, T., Werb, Z. and Helms, J.A. (
Vasara, A.L., Jurvelin, J.S., Peterson, L. and Kiviranta, I. (
Niederauer, G.G., Niederauer, G.M., Cullen, L.C., Athanasiou, K.A., Thomas, J.B. and Niederauer, M.Q. (
Samosky, J.T., Burstein, D., Grimson, W.E., Howe, R., Martin, S. and Gray, M.L. (
Jurvelin, J., Kiviranta, I., Tammi, M. and Helminen, J.H. (
Tran-Khan, N., Hoemann, C.D., McKee, M.D., Henderson, J.E. and Buschmann, M.D. (
vanderKraan, P.M., Vitters, E.L., Meijers, T.H., Poole, A.R. and vandenBerg, W.B. (