-
PDF
- Split View
-
Views
-
Cite
Cite
Gracy X. Rosario, Deepak N. Modi, Geetanjali Sachdeva, Dhananjay D. Manjramkar, Chander P. Puri, Morphological events in the primate endometrium in the presence of a preimplantation embryo, detected by the serum preimplantation factor bioassay, Human Reproduction, Volume 20, Issue 1, January 2005, Pages 61–71, https://doi.org/10.1093/humrep/deh534
Close - Share Icon Share
Abstract
BACKGROUND: Hormonal modulation of the endometrium towards receptivity is well established; however, the role of embryonic stimuli in modulation of the endometrium prior to implantation, especially in primates, is unknown. The aim of the present study was to evaluate the endometrial histology when the embryo was present in its vicinity prior to implantation. METHODS: Preimplantation factor (PIF) bioassay was used as a tool to detect the presence of an embryo in the uterine lumen of mated bonnet monkeys (Macaca radiata) (n=9). The control group comprised seven non-mated animals. The specificity of the PIF bioassay for the presence of an embryo was tested by studies in pregnant humans and monkeys. The effects of embryonic stimuli on the endometrial morphology were analysed by routine haematoxylin–eosin staining. The expressions of CD34, an endothelial cell marker, α-smooth muscle actin (α-SMA), a marker for blood vessel maturation, and prolactin, a marker of endometrial decidualization, were studied by immunohistochemistry. RESULTS: That PIF is embryo specific was established by its presence in sera of pregnant humans, monkeys and also in embryo culture media. Six mated bonnet monkeys were found to be PIF positive. Morphologically, the endometria from these PIF-positive animals showed the presence of the pre-epithelial plaque reaction, increased angiogenesis and stromal compaction. The significantly increased number of CD34- and α-SMA-positive blood vessels (P<0.05) in the endometria of PIF-positive animals indicated increased angiogenesis in response to embryonic stimuli. The endometrial expression of immunoreactive prolactin was also significantly increased (P<0.05) in the PIF-positive animals, indicating decidualization. CONCLUSIONS: Using PIF as a marker to detect early pregnancy in bonnet monkeys, we have shown that the embryo induces a pre-epithelial plaque type of reaction, increased angiogenesis and decidual reaction in the endometrium prior to implantation.
Introduction
Blastocyst implantation and successful establishment of pregnancy require a synchrony between two different spatial events directing embryo development and uterine receptivity. Enough data have been gathered over the last few years to suggest that uterine receptivity represents a distinct structural, biochemical and molecular profile of the endometrium during a specific period in the menstrual cycle. This profile results from multiple cellular events and their interactions, triggered in response to systemic signals especially steroid hormones (Frazor et al., 1999; Puri et al., 2000; Satyaswaroop and Tabibzadeh, 2000; Lessey, 2002; Spencer and Bazer, 2002; Gellersen and Brosens, 2003; Rosario et al., 2003).
Currently, studies are also being undertaken to understand the role of embryonic factors in remodelling of endometrium during the peri-implantation period. Distinct morphological changes have been observed at the early implantation sites in rhesus monkeys and marmosets (Enders et al., 1983; Enders and Lopata, 1999; Sengupta and Ghosh, 2002). Also several genes that show a differential pattern of expression in the implantation site versus inter-implantation site on day 4.5 of mouse pregnancy have been identified recently by microarray analysis (Reese et al., 2001).
Now there is increasing evidence that the embryo modulates endometrial physiology not only post-implantation but even in the absence of any direct physical contact, i.e. in the preimplantation period. In mice and rabbits, the morula stage embryos signal the endometrial epithelium and also the stroma to facilitate implantation (Harper et al., 1989; Shiotani et al., 1993; Wakuda et al., 1999). Human endometrial epithelial cells co-cultured with the preimplantation stage blastocysts showed modulation in the expression of chemokines, cytokines, growth factors and cell adhesion molecules (Kimber, 2000; Dominguez et al., 2002). However, sufficient data on the influence of embryonic stimuli on in vivo modulation of the preimplantation endometrial profile in humans are not yet available. The ethical constraints in obtaining biopsies in a conception cycle prior to implantation in humans have been the major obstacles in pursuing such studies.
Non-human primate models are being considered as an excellent option for pursuing the studies on endometrial physiology in the conception cycle (Enders et al., 1983; Ghosh et al., 1993; Frazor et al., 1999; Jones and Fazleabas, 2001; Niklaus et al., 2001; Sengupta and Ghosh, 2002). However, most of these studies have focused on endometrial changes during the later stages of pregnancy in non-human primates (Enders et al., 1983; Haluska et al., 1990, 2002; Pijnenborg et al., 1996; Enders and Lopata, 1999; Smith et al., 2001). Our interest has been in scanning the endometrium for histomorphological changes prior to implantation in bonnet monkeys. There are no reliable tools to ascertain the presence of preimplantation embryos in the uterine lumen in primates. Several attempts have been made to flush out embryos in non-human primate species. However, the procedures are rather difficult, invasive and cannot be applied to all species with similar efficacy (Seshagiri and Hearn, 1993; Seshagiri et al., 1994; Jayaprakash et al., 1997).
Amongst the available markers for pregnancy, the presence of chorionic gonadotrophin (CG) in the maternal serum is considered as a hallmark of pregnancy (Hsu et al., 1998). However, in non-human primate species, the presence of CG cannot be ascertained by the conventional procedures, i.e. enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay due to the inability of the antibodies against human CG (hCG) to cross-react (Baenziger, 1996). Significant levels of CG are detected in maternal serum only after implantation (Ghosh et al., 1997). Although CG in micro quantities can be detected using the Leydig cell bioassay, this assay is complex, time-consuming and difficult to perform (Wickings et al., 1979; Seshagiri and Hearn, 1993). Therefore, the use of CG to detect the preimplantation embryo has not been found to be very convenient in non-human primates.
Apart from CG, early pregnancy factor (EPF), platelet-activating factor (PAF) and preimplantation factor (PIF) have also been shown to be produced by preimplantation stage embryos, and significant levels of these markers have been detected in the maternal serum in the presence of a viable embryo in the uterine cavity (O'Neill et al., 1987; Harper et al., 1989; Nakatsuka et al., 1992; Lopata and Olivia, 1993; Shu-Xin and Zhen-Qun, 1993; Woodward et al., 1993; Roussev et al., 1995; Fan and Zheng, 1996; Jurisicova et al., 1999). Amongst these, PIF has been shown to be specifically associated with pregnancy and is reported to be present in maternal serum even in the preimplantation period. PIF is detectable in maternal serum from as early as day 2 of mouse pregnancy (Roussev et al., 1995) and on day 2 post-embryo transfer of human IVF cycles (Coulam et al., 1995). These observations prompted us to investigate the potential of PIF as a marker to detect viable preimplantation embryos in mated cycles of bonnet monkeys and also study the histological profiles of preimplantation phase endometria from PIF-positive mated animals.
Materials and methods
The present study was approved by the institutional ethics committees for human and animal research.
Human volunteers
Blood samples were collected from eight non-pregnant fertile women and from nine hCG-positive women in the first trimester of pregnancy. Serum was separated from these samples and stored at −20°C until use.
Bonnet monkeys (Macaca radiata)
Adult bonnet monkeys, housed singly under controlled conditions in the animal house facility, were fed with a diet composed of semi-formulated Indian bread, fresh seasonal fruits, eggs and sterile water.
Female bonnet monkeys were monitored for their cyclicity as detailed previously (Puri et al., 2000). Peripheral blood samples were collected for seven continuous days during the peri-ovulatory period and every third day thereafter. Serum was separated and stored at −20°C for hormonal estimations. Regularly cycling female bonnet monkeys (n=9) were mated with males of proven fertility for six consecutive days starting from 2 days prior to the expected estradiol peak.
Serum samples were also collected from two bonnet monkeys that were superovulated, mated and embryos retrieved by flushing (a kind gift from Professor Dr P.B.Sheshagiri, Indian Institute of Science, Bangalore, India). The protocol for superovulation and embryo retrieval has been detailed previously (Jayaprakash et al., 1997; Seshagiri et al., 2001)
Embryo culture media
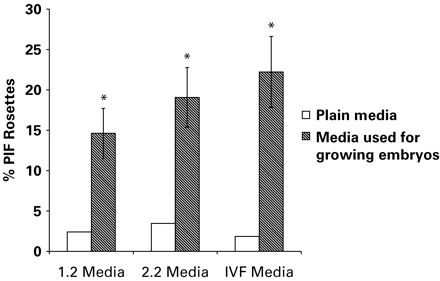
Embryo culture media (Sydney IVF, Australia) (Van Langen donckt et al., 2001) were collected from an IVF programme. This media was collected from Dr Indira Hinduja, Inkus Clinic, Mumbai. The culture media had been used to grow in vitro fertilized eggs up to different stages of development. The 1.2 medium is used for culturing embryos from fertilization to the 2-cell stage, 2.2 medium is used for culturing embryos to the 8-cell stage and IVF medium is used for blastocyst culture.
Radioimmunoassays for estradiol and progesterone
Serum estradiol and progesterone concentrations were measured by specific radioimmunoassays as described previously (Puri et al., 1987; Sachdeva et al., 2001). The inter- and intra-assay coefficients of variation were 14±2 and 9±1%, for the assays to detect estradiol and progesterone respectively.
PIF bioassay
Serum samples from first trimester pregnant women and mated bonnet monkeys collected on day 8 after the mid-cycle estradiol peak were used for the PIF bioassay. Serum samples from two superovulated monkeys were collected on days 3 and 6 post-hCG treatment, respectively. The serum samples from superovulated monkeys were diluted 1:2 with 0.01% human serum albumin (Sigma, MO) in phosphate-buffered saline, pH 7.4 (HSAPBS) prior to use. Sera from non-pregnant women and non-mated bonnet monkeys collected on day 8 post-estradiol peak were used as controls. Culture medium from four independent microdrops, each containing 2–3 embryos, were pooled and used to assay the PIF reaction. Medium without embryos was used as control.
The PIF bioassay described by Roussev et al. (1995) was used in this study with slight modifications. Briefly, lymphocytes and platelets were isolated using Ficoll density gradient centrifugation of blood samples from ‘O’ rhesus-positive males. The cell pellet was washed twice and resuspended in 0.01% HSAPBS to give a final concentration of 1 × 108 lymphocytes/ml. The serum samples were heat inactivated at 56°C for 30 min prior to use. Test serum (15 μl) was added to 15 μl of lymphocyte + platelet mixture and incubated at room temperature for 25 min with intermittent mixing. A 3 μl aliquot of T-cell CD2 antibody (Dako, Hamburg, Germany) was added and the solution incubated further at room temperature for 10 min. Further, 3 μl of rabbit complement (Sigma) was added and incubation was carried out at room temperature for 15 min. A 10 μl aliquot of the sample subsequently was placed on a glass slide and a total of 200 lymphocytes, for the number of rosettes, were counted. A positive rosette is a lymphocyte surrounded by three or more platelets as defined previously (Roussev et al., 1995). The percentage of positive rosettes was calculated and a value ≥9% was considered as a positive PIF reaction as recommended previously (Roussev et al., 1995).
All samples were counted in duplicate and in a blinded manner by two independent observers. The PIF values were represented as an average of the number counted by the two observers. Every PIF assay included a positive control (serum from a pregnant female at 8 weeks of gestation) and male serum as negative control. The inter- and intra-assay coefficient of variations were 1.36 and 4.4%, respectively. PIF-positive mated animals were referred to as fecund animals while PIF-negative mated and non-mated animals were considered as non-fecund animals.
Collection of endometrial biopsies
Endometrial biopsies were collected on day 8 post-estradiol peak from mated and non-mated animals as described previously (Katkam et al., 1995) and processed for routine paraffin embedding and sectioning. Haematoxylin and eosin-stained 5 μm thick sections were observed under an Olympus BX60 microscope (Tokyo, Japan).
Immunohistochemical localizations of CD34, α-SMA and prolactin
Immunohistochemical localizations were carried out on endometria from fecund and non-fecund animals. Briefly, endometrial sections from fecund (n=3) and non-fecund animals (n=3 for each CD34 and α-SMA, and n=5 for prolactin) were deparaffinized in xylene and rehydrated through various grades of alcohol. For CD34 immunolocalization, antigen retrieval was carried out by microwaving the sections in citrate buffer (pH 6.0) for 7 min. Endogenous peroxidase activity was quenched by incubating the sections in 0.3% H2O2 for 30 min. The tissues were then blocked with 1% normal goat serum in PBS for 30 min and incubated in respective primary antibodies diluted 1:50 for monoclonal CD34 (Zymed, CA) and polyclonal prolactin (prepared in-house at the institute) and diluted 1:40 for monoclonal α-SMA (Dako), at 4°C overnight. The primary antibodies were not added in the negative control sections. The next day, sections were washed twice in PBS for 10 min each and incubated in the respective biotinylated secondary antibodies [1:50 dilution of goat anti-mouse for CD34 and α-SMA (Dako) and 1:200 dilution of goat anti-rabbit for prolactin (Santacruz Biotechnology Inc., CA)] prepared in blocking solution for 2 h at room temperature. After washing, the sections were incubated with avidin–biotin–horseradish peroxidase complex (Vector Laboratories, Burlingham, CA for CD34 and α-SMA, and Santacruz Biotechnology Inc. for prolactin) for 30 min followed by PBS rinse and addition of the diaminobenzidine (Sigma) and 0.001% H2O2 in PBS for 10 min. The prolactin-immunostained sections were counterstained lightly with haematoxylin. The sections were dehydrated, cleared in xylene, mounted in DPX and viewed under the Olympus BX60 microscope.
Image analysis
The nuclear staining of the stromal cells in the functionalis and basalis region of endometrium from both groups of animals were quantified by the image analysis software Biovis 1.42. Stainings for immunoreactive CD34, α-SMA and prolactin were also quantified by the same software. Briefly, four areas from each section were randomly selected for each animal in the two groups. The integrated optical density (IOD) in each area for prolactin and percentage area stained for CD34 and α-SMA, and stromal cell count were calculated using the software. Negative control slides were also analysed in a similar fashion. The IOD and percentage area values of the negative control (without antibody) were subtracted from the IOD and percentage area values for each animal in the two groups. The mean, SD and SEM were calculated for each group. Statistical analysis was carried out using the Student's t-test.
Data analysis
The percentage areas in the luminal epithelium showing pre-epithelial plaque formation in endometria from fecund and non-fecund animals were counted. Also, the percentage number of glands showing secretory activity and pre-epithelial plaque formations and the number of gland cells showing adluminal vacuolation per 1000 cells were counted in these animals. The mean, SD and SEM were calculated for each group. Statistical analysis for all the studies was done by Student's t-test. A linear and exponential regression analysis was also applied to all the PIF values obtained for the two individual observers.
Results
Determination of bioactive PIF
A positive PIF reaction is one wherein a single lymphocyte is surrounded by three or more platelets. The coefficient of correlation between the values obtained by the two independent observers was 0.89.
The PIF bioassay was found to be positive in sera of pregnant woman. The percentage of rosettes detected in the hCG-positive women varied from 10.15 to 17.33, while it was 2.69–7.72 (P<0.01) in control non-pregnant women. Pregnancy was confirmed in all the hCG-positive women by ultrasonography on the day of blood collection for PIF bioassay. The percentage of lymphocytes detected as rosettes in sera samples (gift from Dr Seshagiri) from superovulated and pregnant bonnet monkey number 1 was 34.36, and it was 21.89 in pregnant animal number 2.
Figure 1 represents the results of assays used to detect PIF activity in IVF media used for growing embryos. Positive PIF activity was detected in culture medium used for culture of 2-cell embryos up to the blastocyst stage. Bioactive PIF was not detectable in control.
PIF activity was checked in the sera of nine mated bonnet monkeys and seven non-mated animals, and the individual values are shown in Table I. Out of nine serum samples from mated animals, six samples showed positive PIF activity, i.e. the percentage of rosettes was >9% (9.67–16.37%); the remaining three animals showed <9% rosettes. All the animals in the non-mated group showed PIF values <9% (4.25–7.66%).
Radioimmunoassay data
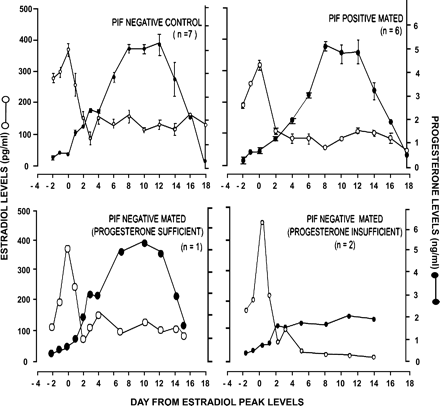
The levels of steroid hormones, i.e. estradiol and progesterone, in PIF-positive mated and PIF-negative non-mated and mated animals during the menstrual cycle are shown in Figure 2. The hormonal profiles of the PIF-negative non-mated and PIF-positive mated animals were similar, with peak estradiol concentrations being 370±43.05 pg/ml in PIF-negative non-mated animals, whereas they were 320±30.12 pg/ml in PIF-positive mated animals. The progesterone levels on the day of biopsy collection were 4.93±0.413 ng/ml in PIF-negative non-mated animals, whereas they were 5.18±0.522 ng/ml in the case of PIF-positive mated animals. No differences were noted in serum estradiol or progesterone levels of PIF-positive mated animals compared with PIF-negative non-mated animals on the day of biopsy (Figure 2).
Of the remaining three animals in the mated group that were not PIF positive, two animals had luteal insufficiency with progesterone levels being 1.81 ng/ml (control levels 4.93±0.413 ng/ml). The third animal had a hormonal profile similar to animals in the control group. The luteal-insufficient animals subsequently were excluded for all further analysis.
The PIF-positive mated animals are henceforth referred to as fecund animals, whereas PIF-negative non-mated animals are referred to as non-fecund animals for all other comparisons.
Histology
Histologically, the endometria from non-fecund animals showed typical luteal phase morphology. Distinct alterations were noted in the luminal epithelium, glandular epithelium, stroma and the pattern of angiogenesis in the endometria of fecund animals compared with those in non-fecund animals. However, the changes were not uniform over the entire area of the endometrium investigated. The representative photomicrographs are shown in Figures 3A and 4A.
Luminal epithelium
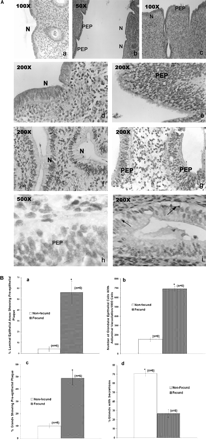
In non-fecund animals, the luminal epithelium of the mid-secretory phase endometrium showed characteristic features, i.e. the single layered columnar epithelial cells having oblong nuclei present at the base of the cells (Figure 3A, a and d). However, in the fecund animals, hyperproliferative activity with entero-reduplication of the nuclei was evident in some restricted areas of the luminal epithelium. In these areas, the nuclei increased in number and had formed groups (Figure 3A, b, c, e and h; PEP). These nuclei were rounded with condensed chromatin and the basement membranes at these sites were lost (Figure 3A, e and h). However, in adjacent areas, the epithelial cells were normal and indistinguishable from those seen in the non-fecund animals (Figure 3A, a, b, c and d; N). Semi-quantitative analysis of the number of areas showing plaque formation in both groups of animals is represented in Figure 3B (a).
Glands
The glands in the endometria from non-fecund animals were hypertrophied, with secretory activity seen in 71% of the glands (Figure 3A, f). However, in fecund animals, 73% of the endometrial glands did not show secretions (Figures 3A, g and 4A, b). In some areas, the glands were oedematous and showed adluminal vacuolation (Figure 3A, h). In the glands that showed vacuolation (Figure 3A, iFigure 3A, i, arrows), the nuclei had shifted to the centre, while in other cells they were basifixed. In some glands, the nuclei appeared like that of the luminal epithelium where the nuclei were rounded and in groups with no distinct cell boundary (Figure 3A, g). Semi-quantitative analysis of the number of glands showing secretions and plaque formation and the number of glandular epithelial cells showing adluminal vacuolation per 1000 cells in both groups of animals is shown in Figure 3B, b, c and d.
Stroma
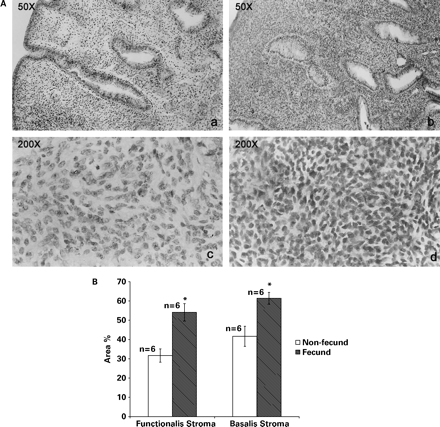
The stroma in the functionalis region of the endometria from non-fecund animals was oedematous, the cells staining pale and the nuclei being small and spindle shaped (a and c in Figure 4A). Morphologically, the stroma in the basalis was similar to that in the functionalis but the oedema was less pronounced and the stroma appeared dense. However, in fecund animals, the stroma of the entire endometrium was highly compact and hyperplastic, with compaction being more pronounced in the functionalis compared with the basalis in fecund animals. The stroma was densely packed with cells with little or no inter cellular spaces between them. The stromal cell cytoplasm stained more strongly with eosin and the nuclei were round with sparse chromatin (Figure 4A, b and d). The semi-quantitative analysis of the differences in the nuclear staining in the stromal compartments of fecund and non-fecund animals is shown in Figure 4B.
Blood vessels
A remarkable increase in angiogenesis was obvious in endometria from fecund animals. Small to medium sized blood vessels were detected throughout the stroma of the functionalis, with some blood vessels just below the luminal epithelium. In the basalis, the blood vessels were dilated and engorged with blood. In contrast, blood vessels were seen mainly in the basalis with few minor capillaries in the functionalis in non-fecund animals.
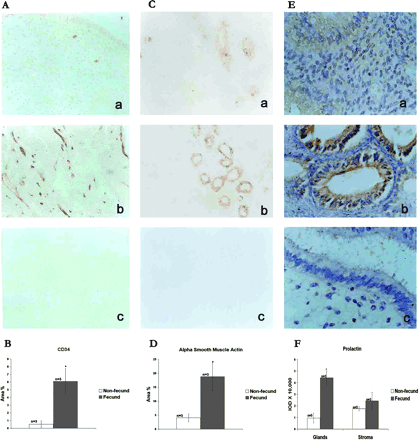
To quantitate the number of blood vessels in two groups of animals, CD34, an endothelial cell marker (Heimburg et al., 1999; Manconi et al., 2003), and α-SMA, a blood vessel maturation marker (Matsuzaki et al., 2001; Hickey et al., 2003), were immunolocalized in the endometria from fecund and non-fecund animals, and representative photographs are shown in Figure 5A and 5B. Semi-quantitative analysis of the immunoreactivity of CD34 and α-SMA is represented in Figure 5B and 5D, respectively. The numbers of CD34- and α-SMA-positive blood vessels were high in the basalis but barely detectable in the functionalis regions of the endometrium from non-fecund animals (Figure 5A, a, and 5C, a). In contrast, the number of CD34- and α-SMA-positive small to medium blood vessels were significantly high (P<0.05) in the basalis and functionalis regions of the endometrium from fecund animals (Figure 5A, b, and 5C, b).
Immunolocalization of prolactin
Prolactin was immunolocalized in the endometrium using polyclonal antibodies against human pituitary prolactin, and representative photographs are shown in Figure 5E. Semi-quantative analysis of the immunoreactivity of prolactin is shown in Figure 5F. Prolactin was barely detectable in the endometrial glandular cells from non-fecund animals (Figure 5E, a) whereas its expression was high in these cells in fecund animals (Figure 5F, b). The expression of prolactin in the stroma of fecund animals was comparable with that in non-fecund animals. Quantitative analysis of the immunostaining revealed almost a 4-fold induction in the prolactin expression in the glands of fecund animals and this difference in the staining of immunoreactive prolactin between two groups was statistically significant (P<0.05). The expression of prolactin was ∼1.5-fold higher in stroma of fecund animals compared with non-fecund animals. However, the difference was not statistically significant.
Discussion
Our knowledge about the very early events occurring in the peri-implantation phase primate endometrium, when exposed to embryonic stimuli, is still inconclusive. The paucity of studies in this direction may be attributed to lack of reliable tools to detect pregnancy in the very early stages (days 6–7 post-ovulation or days 6–7 post-fertilization in bonnet monkeys). Use of non-invasive procedures to retrieve preimplantation primate embryos has not met with great success (Jayaprakash et al., 1997).
An alternative approach of detecting embryonic antigens/products, such as EPF, CG and PAF, in the circulation to detect early pregnancy in primates has been employed by some investigators. There exists definitive evidence of CG being produced by preimplantation blastocysts in humans (Lopata and Olivia, 1993; Seshagiri and Hearn, 1993; Woodward et al., 1993; Seshagiri et al., 1994; Jurisicova et al., 1999). However, its utility in detecting very early pregnancy could not be exploited in non-human primates because of the non-availability of cross-reactive antibodies.
Amongst the other markers, PIF bioassay has been reported to detect the presence of an embryo in the uterine lumen prior to implantation (Roussev et al., 1995). Bioactive PIF has been detected in maternal serum on day 2 of mouse pregnancy and 2 days after embryo transfer in human IVF cycles (Coulam et al., 1995; Roussev et al., 1995). PIF is also detectable in the sera of women in the first trimester but not in women having undergone spontaneous abortions (Coulam et al., 1995). Consistent with these observations, in the present study too, PIF bioactivity was found to be high in sera of hCG-positive pregnant women. Moreover, the utility of PIF in detecting early pregnancy in non-human primates was confirmed by our studies demonstrating high PIF activity in sera of superovulated mated bonnet monkeys on day 3 of pregnancy where embryos were retrieved by flushing. However, PIF bioactivity was not detectable in sera of three mated bonnet monkeys that did not become pregnant. This indicated that PIF in maternal serum is not induced due to mating effects or the presence of semen.
After validating the presence of preimplantation embryos in mated bonnet monkeys, as assessed by PIF positivity, we next analysed the histological changes in different regions of the endometrium, i.e. luminal epithelium, glands and stroma, in animals during early pregnancy.
Few studies have been conducted to investigate histological changes in the luminal epithelium of post-implantation endometria in non-human primates (Enders et al., 1983; Enders and Lopata, 1999). An epithelial plaque reaction in the luminal epithelium had been observed in endometrium on day 15 of pregnancy in baboons and on day 10 of pregnancy in rhesus monkeys (Enders et al., 1983; Jones and Fazleabas, 2001). Histologically, the epithelial plaque is composed of mitotically active large, pale, round and multinucleated cells forming acinar clusters (Luckett, 1974; Enders et al., 1985; Ghosh and Sengupta, 1989). We also observed hyperproliferative activity in the luminal epithelium, though in certain focal areas, in PIF-positive fecund animals. In some areas, large clumps of nuclei with distinct entero-reduplication, poorly packed chromatin and loss of basement membrane were detected. However, we did not detect formation of the acinar clusters, a characteristic feature of an epithelial plaque. Since the other morphological features of these focal areas resemble at least in part, that of the epithelial plaque described on day 10 of rhesus pregnancy, it is likely that these changes are the events preceding an epithelial plaque reaction. These may thus be referred to as a ‘pre-epithelial plaque’ reaction. To the best of our knowledge, this is the first report that describes such changes in endometrium in response to embryonic stimuli, and we presume that these are the events preceding transformation of epithelial cells to plaque cells.
In addition to the pre-epithelial plaque reaction, the glandular epithelium of endometrium showed hypertrophy and adluminal vacuolation in many cells in the fecund animals. However, secretions were not evident in the lumen of most of the glands. The pseudo-stratified appearance of the glandular cells was also lost in some areas and the biopsies from fecund animals had slightly delayed maturational appearance. A similar histological delay of 2 days has been observed in human endometrial biopsies obtained from conception cycles (Colston et al., 1986).
The pre-epithelial plaque-like changes that were seen in the luminal epithelium were also observed in the neck region of many glands of the fecund animals. A focal increase in the number of nuclei with loss of basement membrane of the surrounding cells was seen. Such plaque-like changes were, however, not reported in the day 6 pregnant rhesus monkey endometrium (Ghosh et al., 1993) but have been observed in hCG-treated baboons (Jones and Fazleabas, 2001).
Enhanced neo-microvasculature was also evident in the endometrium of fecund bonnet monkeys. A large number of small blood vessels could be seen in the stroma underlying the luminal epithelium as indicated by the increase in the number of CD34-positive blood vessels in these animals. Also, the functionalis was filled with a number of microcapillaries and the blood vessels in the basalis had enlarged and were engorged with blood. Our observations are in agreement with the previous reports indicating increased vascularity and angiogenesis at the implantation site of rhesus monkeys (Sengupta and Ghosh, 2002). Expression of endothelial cell markers such as vimentin, and angiogenic factors such as placental growth factor and vascular endothelial growth factor has also been found to be altered at the implantation site on day 12 post-ovulation (Sengupta and Ghosh, 2002). However, no changes were detected in endometrial vasculature of day 6 pregnant rhesus monkeys where the degree of vasculature was estimated as a pooled estimate of changes in the fundal endometrium. But, as evident from our results, the changes occurring in response to the embryonic stimuli are not generalized and spatial proximity to embryonic signals may influence the extent of morphological changes in endometrium prior to implantation. It is arguable that blood supply in the endometrium is increased only in response to a physical interaction with the embryo (Sengupta and Ghosh, 2002) as physical deciduogenic stimuli in an artificial menstrual cycle also elicit a similar reaction (Ghosh and Sengupta, 1989). However, Jones and Fazleabas (2001) have elegantly demonstrated that infusion of physiological doses of hCG in the uterine lumen of baboon results in an increase in number of small blood capillaries in the stroma of the endometrial functionalis. These observations together with ours suggest that a physical interaction of the fetal and maternal tissues is not necessary to initiate neovascularization in the endometrium; embryonic molecules such as hCG influence the endometrium in a paracrine manner to commence an angiogenic reaction.
Endometrial stroma in the functionalis zone was more compact in fecund animals compared with that in non-fecund animals. This compaction was maximal in the stroma underlying the luminal epithelium where a pre-epithelial plaque-like reaction was seen. This compaction could occur due either to the loss of oedema or to hyperplasia of cells. Such extensive stromal changes have not been reported in the endometria of day 6 pregnant rhesus monkeys (Ghosh et al., 1993; Sengupta and Ghosh, 2002); marginal compaction of the stroma underlying the luminal epithelium has so far been reported in the endometria of day 10 pregnant rhesus monkeys (Enders et al., 1983). However, it is of interest to note that, in hCG-treated or day 15 pregnant baboons, dense stromal compaction below the surface epithelium has been observed (Jones and Fazleabas, 2001).
Decidualization, the first functional change in endometrium during pregnancy, involves a series of morphological and molecular changes in which stromal fibroblasts differentiate into secretory decidual cells (Enders, 1991; Frazor et al., 1999). In non-human primates, the presence of a conceptus is a prerequisite for the decidual reaction to occur (reviewed in Fazleabas et al., 1995). To test if decidualization has occurred in the endometria of the fecund animals, we studied the expression of prolactin, a known marker for the decidual reaction. The levels of immunoreactive prolactin were significantly raised in the glandular cells of the endometria from fecund animals as compared with non-fecund animals. Ours is the first report demonstrating a decidual reaction prior to implantation in a non-human primate model. However, this finding was intriguing as a decidual reaction is a stromal cell event and production of prolactin by stromal cells is a hallmark of initiation of this process (Tang et al., 1993). Frazor et al. (1999) have also observed an increase in glandular prolactin expression in the late secretory phase and on day 39 of baboon pregnancy. One possible reason for the glandular localization of prolactin could be that the stromal cell-derived prolactin is rapidly translocated to its site of action, i.e. the glandular cells. Indeed, prolactin receptors have been reported in the glandular epithelium of baboon endometrium (Frazor et al., 1999).
At present, the identities of the embryonic factors that trigger the changes in the endometrial morphology before implantation are not established. Extensive morphological and biochemical changes in the endometrium were observed in baboons infused with bioactive CG (Fazleabas et al., 1999; Jones and Fazleabas, 2001). There may also exist other unidentified embryonic factors that alter endometrial characteristics and facilitate implantation. Identification of such factors and deciphering their regulatory roles in implantation would be extremely valuable in understanding the intricacies underlying implantation in higher primates.
The utility of serum bioactive PIF as a marker for the detection of a preimplantation embryo as demonstrated in this study will prompt many investigators to undertake in vivo studies on embryonic modulation of endometrium in a fecund cycle using primate models. We anticipate that such studies would be useful in testing as well as devising newer targets for anti-implantation drugs and developing novel strategies for diagnosis and management of infertility.
Levels of bioactive PIF in human embryo culture media (*P<0.05).
Circulating levels of estradiol (○) and progesterone (•) in PIF-negative non-mated and mated and PIF-positive mated bonnet monkeys.
(A) Histomorphological analysis of peri-implantation phase endometria from non-fecund (a, d and f) and fecund (b, c, e and g–i) bonnet monkeys. PEP indicates the pre-epithelial plaque reaction, while N indicates the unaffected areas. Arrows in (i) indicate adluminal vacuolation. (B) Semi-quantitative comparisons of the percentage luminal epithelial areas showing pre-epithelial plaque changes (a), number of gland cells showing adluminal vacuolation per 1000 gland cells (b), percentage number of glands showing pre-epithelial plaque (c) and percentage number of glands showing secretions (d) in endometria from fecund and non-fecund animals.
(A) Stromal histomorphology of peri-implantation phase endometria from non-fecund (a and c) and fecund (b and d) bonnet monkeys. Note the compaction in the stromal compartment in the endometria from fecund animals. (B) Semi-quantitative comparison of nuclear staining in the stromal compartment of endometria from fecund and non-fecund animals.
Immunolocalizations of CD34 (A), α-SMA (C) and prolactin (E) in the peri-implantation phase endometria from non-fecund (a) and fecund (b) bonnet monkeys. Respective negative controls are shown in (c). Semiquantitative comparisons of immunoreactive CD34 (B), α-SMA (D) and prolactin (F) in endometria from fecund and non-fecund animals are shown lower panels (*P<0.05).
Serum PIF levels in bonnet monkeys
Each value represents the percentage of PIF rosettes for an individual animal.
PIF-positive values.
Serum PIF levels in bonnet monkeys
Each value represents the percentage of PIF rosettes for an individual animal.
PIF-positive values.
We thank Dr P.B.Seshagiri, Indian Institute of Science, Bangalore, for providing sera of superovulated pregnant bonnet monkeys, Dr I.Hinduja, Inkus IVF Centre, Mumbai, for providing the embryo culture media, Dr P.K.Meherji and Dr U.C.Hegde, NIRRH for providing the sera samples from pregnant females and the prolactin antibody, respectively, and Mr H.Karekar for the artwork. The work (NIRRH/MS/29/2003) is funded by the Indian Council for Medical Research. We also wish to thank the Lady Tata Memorial Trust and Council of Scientific and Industrial Research, India for fellowships to G.R.
References
Baenziger JU (
Colston A, Herbert C, Maxson W, Hill G and Pittaway D (
Coulam CB, Roussev RG, Tjomason EJ and Barnea ER (
Dominguez F, Pellicer A and Simon C (
Enders AC, Hendricx AG and Schlafke S (
Enders AC, Welsh AO and Schlafke S (
Enders AC (
Enders AC and Lopata A (
Fan XG and Zheng ZQ (
Fazleabas AT, Hild-Petito S and Verhage HG (
Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD and Miller JB (
Frazor J, Gaspar CA, Donnelly KM, Gibori G and Fazleabas AT (
Gellersen B and Brosens J (
Ghosh D and Sengupta J (
Ghosh D, Roy A, Sengupta J and Johannisson E (
Ghosh D, Stewart DR, Nayak NR, Lasley BL, Overstreet JW, Hendricx AG and Sengupta J (
Haluska GJ, West N, Novy MJ and Brenner RM (
Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW and Nov MJ (
Harper MJK, Kudolo GB, Alecozay AA and Jones MA (
Heimburg S, Oehler MK, Papadopoulos T, Caffier H, Kristen P and Dietl J (
Hickey M, Pillai G, Higham JM, Sullivan M, Horncastle D, Doherty D and Stamp G (
Hsu MI, Kolm P, Leete J, Dong KW, Muasher S and Oehninger S (
Jayaprakash D, Satish KS, Ramachandra SG, Ramesh V and Seshagiri PB (
Jones CJP and Fazleabas AT (
Jurisicova A, Antenos M, Kapasi K, Meriano J and Casper RF (
Katkam RR, Gopalkrishnan K, Chwalisz K, Schillinger E and Puri CP (
Kimber S (
Lopata A and Olivia K (
Luckett WP (
Manconi F, Kable E, Cox G, Markham R and Fraser IS (
Matsuzaki S, Canis M, Murakami T, Dechelotte P, Bruhat MA and Okamura K (
Nakatsuka M, Yoshida N and Kudo T (
Niklaus AL, Murphy CR and Lopata A (
O'Neill C, Gidley-Baird AA, Pike IL and Saunders DM (
Pijnenborg R, D'Hooghe T, Vercruysse L and Bambra C (
Puri CP, Elger WAG and Pongubala JMR (
Puri CP, Katkam RR, Sachdeva G, Patil V, Manjramkar DD and Kholkute SD (
Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN and Dey SK (
Rosario G, Sachdeva G, Okulicz WC, Ace CA, Katkam RR and Puri CP (
Roussev RG, Barnea ER, Thomason EJ and Coulam CB (
Sachdeva G, Patil V, Katkam RR, Manjramkar DD, Kholkute SD and Puri CP (
Satyaswaroop PG and Tabibzadeh S (
Sengupta J and Ghosh D (
Seshagiri PB and Hearn JP (
Seshagiri PB, Terasawa E and Hearn JP (
Seshagiri PB, Acharya KK, Jayaprakash KS and Shetty G (
Shiotani M, Noda Y and Mori T (
Shu-Xin H and Zhen-Qun Z (
Smith GC, Wu WX and Nathanielsz PW (
Spencer TE and Bazer FW (
Tang B, Guller S and Gurpide E (
Van Langendonckt A, Demylle D, Wyns C, Nisolle M and Donnez J (
Wakuda K, Takakura K, Nakanishi K, Kita N, Shi H, Hirose M and Noda Y (
Wickings EJ, Qazi MH and Nieschlag E (
Author notes
1Primate Biology Department and 2Experimental Animal Facility, National Institute for Research in Reproductive Health, Indian Council of Medical Research, Jehangir Merwanji Street, Parel, Mumbai-400012, Maharashtra, India