-
PDF
- Split View
-
Views
-
Cite
Cite
A.K. Sullivan, M. Marcus, M.P. Epstein, E.G. Allen, A.E. Anido, J.J. Paquin, M. Yadav-Shah, S.L. Sherman, Association of FMR1 repeat size with ovarian dysfunction, Human Reproduction, Volume 20, Issue 2, February 2005, Pages 402–412, https://doi.org/10.1093/humrep/deh635
Close - Share Icon Share
Abstract
BACKGROUND: Women who carry the FMR1 premutation allele have a significantly increased risk for ovarian dysfunction. We hypothesize that molecular characteristics of the FMR1 gene may explain this increased risk. METHODS: Thus, we examined the effect of FMR1 CGG repeat size and related factors on measures of ovarian dysfunction using data from 507 women with a wide range of repeat sizes. RESULTS AND CONCLUSIONS: We found a significant positive association of repeat size with ovarian dysfunction, but have preliminary evidence that this relationship is non-linear. We suggest that FMR1 repeat size in the lower range (<80 repeats) contributes to the variation in age at menopause; thus, FMR1 could be considered a quantitative trait locus. More importantly, when repeat size exceeds this threshold, the increase in risk for ovarian dysfunction is clinically significant. Intriguingly, this risk appears to plateau, or perhaps decrease, among women with very high repeats (≥100 repeats).
Introduction
Fragile X syndrome, a form of X-linked mental retardation, results from the hyperexpansion of a CGG trinucleotide repeat located in the 5′ untranslated region of the fragile X mental retardation (FMR1) gene (Fu et al., 1991; Oberle et al., 1991; Verkerk et al., 1991). Once the number of repeats exceeds 200, hypermethylation of a nearby CpG island is triggered. This leads to transcriptional silencing of the gene (Oberle et al., 1991; Sutcliffe et al., 1992). This ‘full’ mutation causes the absence of fragile X mental retardation protein (FMRP), an RNA-binding protein, and gives rise to fragile X syndrome phenotype (Pieretti et al., 1991).
Premutation alleles have been defined as those alleles ascertained from a family with fragile X syndrome, i.e. those known to expand to >200 repeats in several generations. These alleles range from the low 50s to 199 repeats. The smallest allele known to expand to the full mutation in one generation is 59 repeats (Nolin et al., 2003). Intermediate alleles have been defined as those alleles that have the potential for being unstable when transmitted from parent to child. These alleles are usually identified in the general population outside of the context of unstable transmissions and have been defined as those in the range of 41–60 repeats. However, this repeat size range clearly overlaps in repeat size with premutation alleles.
Scientists originally coined the term ‘premutation’ because male and female carriers did not have obvious symptoms of fragile X syndrome, but carried unstable alleles known to lead to the full mutation in one to three generations. Upon discovery of the molecular basis of the syndrome, the term was further justified when studies showed that the FMR1 gene was transcriptionally active and produced FMRP. This definition remains accurate; however, specific phenotypes, unrelated to fragile X syndrome, have been identified among premutation carriers. Here, we focus on the phenotype related to ovarian function.
To summarize the studies to date, ∼20% of FMR1 premutation carriers have premature ovarian failure (POF), or cessation of menstrual periods before the age of 40 years (reviewed in Sherman, 2000). Interestingly, women who carry the full mutation have the same risk as those without the premutation allele (referred to as ‘non-carriers’), ∼1%. Results from recent studies suggest that there may be a wide spectrum of ovarian dysfunction among premutation carriers, POF being the most extreme. Using survival analysis, two studies found that the average age at menopause occurred earlier for premutation carriers than for non-carriers (Hundscheid et al., 2000; Murray et al., 2000a). In addition, premutation carriers who were still cycling were found to have an increased level of FSH, an indicator of ovarian reserve, compared with non-carrier and full mutation relative controls (Murray et al., 1999; Hundscheid et al., 2001).
In addition to FMR1 premutation carrier status, several studies have examined the relationship between POF risk and other FMR1-related factors such as FMR1 repeat size, X-chromosome inactivation ratio and parental origin of the premutation. Murray et al. (2000a) found no significant effect of either FMR1 repeat size or X-chromosome inactivation pattern on risk of POF. Hundscheid et al. (2000) found a significant effect between POF and parental origin as 28% (23/82) of women with a paternally inherited premutation had POF, while only 3.7% (1/27) of women with a maternally inherited premutation had the phenotype. However, other studies have failed to confirm this finding (Murray et al., 2000b; Vianna-Morgante and Costa, 2000; Mallolas et al., 2001), although each study was relatively small.
The purpose of this study was to examine the association between FMR1 repeat size and various measures of ovarian function in a large sample of women with a wide range of repeat sizes. We found a significant association between increasing FMR1 repeat size and risk for ovarian dysfunction over the entire range of repeat sizes. Interestingly, we found preliminary evidence that the risk for ovarian dysfunction increased with increasing repeat size, but plateaued or even decreased among very high premutation carriers (≥100 repeats).
Materials and methods
Study population
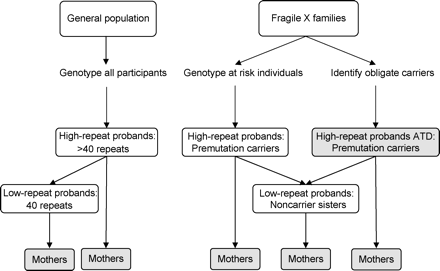
Our goal was to ascertain a large sample of intermediate and premutation allele carriers in order to thoroughly examine the entire range of repeat size alleles. To accomplish this, we ascertained women through two different sampling schemes (Figure 1): (i) through the general population to identify intermediate allele carriers and (ii) through families with a relative diagnosed with fragile X syndrome to identify premutation carriers. Non-carriers were drawn from both schemes. The protocols and consent forms for each scheme were approved by the Institutional Review Board at Emory University.
General population survey
We conducted the study at various venues throughout metropolitan Atlanta to include women of all ethnic groups between the ages of 18 and 50 years. The study, named Emory Study of Adult Learning and Reproduction, was described as a study to examine the potential effects of the FMR1 gene on these two traits. We obtained buccal brushes from 3069 women to determine FMR1 repeat size. We could not determine a final result for 288 women due to inadequate amounts of DNA. Of the 2781 women for whom we had a final result, we identified 196 as having >40 repeats and defined these subjects as high-repeat probands. We were able to contact 145 of these women and enrol 94 (65%). For each enrolled high-repeat proband with >50 repeats, we identified a woman with ≤40 repeats matched on site of ascertainment, age and ethnicity. These women were defined as low-repeat probands. We identified 105 women and were able to contact 81 of these women and enrol 71 (88%). We asked all high-repeat and low-repeat probands to complete a reproductive history questionnaire as well as a 4 h neuropsychological test battery as part of another study protocol.
To enrich the study population for high repeat alleles and for older women who had completed more of their reproductive lifespan, we asked all probands for the approval to contact their mothers and to send buccal brushes and a reproductive history questionnaire. We were able to contact 133 mothers who were ≤75 years old. Ninety-three mothers (70%) returned buccal brushes and a completed reproductive history questionnaire. We were able to determine repeat size for 88 mothers. Also, for any high-repeat proband or mother whose repeat size was >50 repeats, we asked approval to contact other extended family members to track the high-repeat allele. Of the 23 extended family members that we contacted, 18 (78%) returned buccal brushes and a completed reproductive history questionnaire. We were able to determine repeat size for 15 extended family members. We treated these individuals as probands, three of whom were already ascertained as mothers of probands.
Fragile X family survey
We screened families known to have a relative affected with the fragile X syndrome to determine eligible subjects. If carrier status was unknown, we collected buccal or blood samples and determined repeat size. We defined women between the ages of 18 and 75 years who carried the premutation (the allele that was shown to expand to the full mutation within the family) as high-repeat probands and defined their non-carrier sisters within the same age span as low-repeat probands. The participation rate was 100% (181/181) for high-repeat probands and 98% (43/44) for low-repeat probands. We identified many of the high-repeat probands through their affected or carrier offspring or descendents (i.e. obligate carriers). We recorded these women as those with known reproductive success [referred to as ‘ascertained through descendant’ (ATD)]. Similar to the protocol for the general population, we also recruited mothers of high-repeat and low-repeat probands. Of 59 mothers whom we were able to contact, 58 (98%) agreed to participate. Thirty-three women fell into two categories as outlined above: case proband and mother of a proband.
Data collection
Reproductive history questionnaire
We administered the reproductive history questionnaire in person, over the telephone, or through the mail. We obtained demographic information including age at interview, date of birth, ethnicity and education. We obtained information on potential confounders and effect modifiers including body mass index [BMI = weight in kilograms/(height in meters)2] and smoking (1= ever smoked on a regular basis, 0= otherwise). We obtained information on hormone medication use and defined three relevant variables: (i) a binary variable to define if a woman currently used hormone medication, (ii) a binary variable to determine if the hormone medication was prescribed for menopausal symptoms and (iii) the age the medication was administered. We obtained menstrual cycle history including age at menarche and age and date of last menstrual period. If the date of last menstrual period was >2 months previous to the interview, we identified the cause of menses cessation.
To determine whether the method that we administered the questionnaire altered the accuracy of reporting, we conducted regression analyses on two representative variables. We used age at menarche, and for those women who had gone through menopause, we used age at menopause as the outcome variable (adjusted for age at interview, ethnicity and smoking) and questionnaire administration (in person, over the telephone, or through the mail) as the predictor variable. Neither outcome variable differed significantly between the different administration types; thus, we combined data from all questionnaires.
Blood collection
We requested blood samples on all high-repeat and low-repeat probands ascertained through both sampling schemes and received blood on 219 (54%) out of 405 probands. We used these samples to confirm FMR1 CGG repeat size and determine the X-chromosome inactivation ratio. We collected serum FSH levels on a subset of women who were cycling, excluding those who had a hysterectomy, oophorectomy, or radiation or chemotherapy. The final sample of women with FSH measures included 154 women; 50 women who used oral contraceptives (OC) and 104 who did not. For women who did not use OC, we collected blood on the third day of the menstrual cycle. If the woman was unavailable on the third day, we took blood between days 2 and 5. For women on OC, we collected blood during the placebo pill week.
Study population characteristics
Our original data set contained 517 reproductive histories and 154 serum samples. We excluded 10 non-carrier women from the study: one woman had gonadal dysgenesis, two women did not specify the age of menses cessation, and seven women were ineligible after administering the questionnaire. For three premutation carriers and two non-carriers, we could not define a day for serum collection because of perimenopausal irregular cycle length, irregular length of bleeding, or irregular use of hormone medication. To be conservative, we defined these women as still cycling, but we excluded their FSH levels. Thus, in our final data set, we had a total of 507 reproductive histories and 149 serum samples.
The comparison of the general characteristics of premutation carriers (≥59 repeats) and non-carriers (<59 repeats) is listed in Table I according to ascertainment group: (i) probands ascertained without knowledge of reproductive success, (ii) probands ascertained through direct descendants (ATD) and (iii) mothers of probands. Mothers of probands were similar to probands ATD in that they too were selected through their offspring and therefore were known to be reproductively successful. We did separate analyses on probands ascertained without knowledge of reproductive success and on women ascertained through their offspring (probands ATD and mothers of probands). We found no differences in the conclusions of these data analyses (results not shown); thus, we present only analyses from the combined data set.
Table I shows that education level, BMI and hormone medication use did not differ between premutation carriers and non-carriers. However, the two groups differed significantly in age, smoking prevalence, and ethnicity. Relative to the non-carrier group, the premutation group was older at the time of interview by 3 years (t-test = 2.3, P=0.02), smoked more (χ21=7.0; P=0.008) and had a higher percentage of Caucasians (χ22=26.1; P<0.001). Thus, we adjusted all the appropriate analyses for age at interview, ethnicity and smoking.
Statistical analysis
We examined the effect of the FMR1 repeat size, X-chromosome inactivation ratio, and parental origin of the premutation on ovarian function as defined by the prevalence of POF and early menopause, age at menopause, and FSH level. We used repeat size defined as a continuous variable or defined by repeat size group (binary or ordinal categorical). As described above, we used repeat size to define high-repeat and low-repeat proband ascertainment (i.e. >40 repeats for high-repeat probands). However, for the analyses, we defined repeat size categories based on their potential risk for expansion to the full mutation. We considered these definitions less arbitrary than the historical definitions. We hypothesize that the property leading to expansion (e.g. chromatin structure, secondary structure of the repeat sequence) may be correlated with the risk for ovarian dysfunction. Thus, we defined premutation carriers as those with ≥59 repeats based on the observation that alleles in that range can expand to the full mutation in one generation (Nolin et al., 2003). Women with <59 repeats were designated as non-carriers. For some analyses, non-carriers were divided into those with common alleles (≤40 repeats) and those with intermediate alleles (41–58 repeats). Also premutation carriers were subdivided into those with low premutation alleles (59–79 repeats), medium premutation alleles (80–99 repeats), and high premutation alleles (≥100 repeats), these categories being associated with low (>50%), medium (50–80%) and high risk (>95%) to expand to the full mutation (Nolin et al., 2003). Table II shows the distribution of FMR1 CGG repeat size in the study population.
Statistical analyses for POF and early menopause
We defined POF as menopause before the age of 40 years and early menopause as menopause before the age of 45 years. To be comparable to previous published reports, we performed χ2 tests to examine the prevalence of POF and early menopause among premutation carriers and non-carriers. Such tests are not completely valid due to the dependency of related observations within families. Therefore, we assessed the effects of FMR1 premutation status and other associated risk factors on POF and early menopause using generalized-estimating-equation (GEE) methodology (Zeger and Liang, 1986) which accounts for the dependent nature of the relatives (primarily mother/daughter and sister/sister). We report the corresponding P values from the GEE analyses. We adjusted the POF and early menopause outcome variables for smoking and ethnicity (African American, Caucasian, or other/unknown) when examining the entire repeat size range.
Statistical analyses for age at menopause
Self-reported age at menopause defined as natural cessation of menses for ≥1 year is problematic, as many women are prescribed hormone medication as soon as symptoms of menopause appear. This complicates defining a specific age at menopause. Some women on HRT may have had the ability to continue cycling naturally and some may not; HRT use masks this distinction.
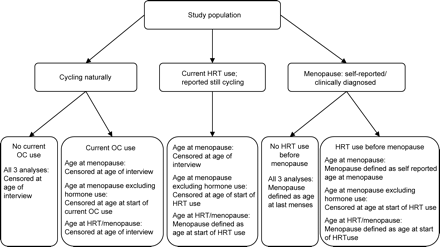
Thus, we explored the data under several definitions of age at menopause based on hormone medication use using survival analysis (Figure 2). For the initial analysis (termed ‘age at menopause’), we used a woman's self-reported age at her last menses as age at menopause. This included 13 women who reported a clinical diagnosis of menopause (i.e. their physician diagnosed them with ‘menopause’), but did not have cessation of menses for ≥1 year. For menopausal women, we considered the ‘failure’ time to be the self-defined age at menopause or age at the time of their last period. For all other women reported to be cycling, we censored their age of menopause at age of interview irrespective of hormone use. For this initial analysis, we excluded two non-carrier women who were still having periods while on HRT at ages 65 and 69 years, based on the assumption that their hormone medication induced their periods. Next, for a conservative analysis, we took any use of hormone medication into account. For women who began taking HRT before their self-reported age at menopause or the event that stopped their periods, we censored them at the age they started HRT use. For women who were currently using HRT or OC and were cycling, we censored them at the age they started hormone medication. Lastly, we included HRT use in the definition of age at menopause. For women who began taking HRT before their self-reported age at menopause, we used the age that the woman began HRT as her age at menopause. Similarly, for women who reported to be cycling but on HRT, we defined their age at menopause as their age at start of HRT use. For both of these analyses, we included the two women described above who were on HRT and still having periods at ages 65 and 69 years. We performed survival analyses using log-rank tests for three other comparison groups: common versus intermediate, non-carrier (including common and intermediate groups) versus low premutation, and low premutation versus medium/high premutation. To account for the dependency of observations within families for each survival model, we performed frailty-modelling analyses (Clayton and Cuzick, 1985). We adjusted all frailty models by age at interview, smoking and ethnicity (African American, Caucasian, or other/unknown).
Also, we used a linear model that regressed age at menopause on repeat size and other covariates to investigate a potential linear effect of repeat size. We used data from menopausal women only. Again, we accounted for the dependency of observations within families by applying GEE methodology.
Statistical analyses for FSH level
Increasing FSH level has been shown to be correlated with decreasing ovarian reserve (MacNaughton et al., 1992), which indicates that one can use FSH as an indicator of menopause. FSH levels ≤10 IU are recommended by IVF clinics to demonstrate an adequate ovarian reserve for achieving pregnancy. Women who are menopausal generally have FSH levels ≥40 IU. To examine FSH levels, we normalized FSH levels using a natural logarithm transformation after adding a constant of one. We accounted for the dependency of observations within families by performing analyses using GEE methodology. We adjusted FSH for age at blood draw, smoking, ethnicity (African American, Caucasian, or other/unknown) and current hormone medication use. We performed GEE analyses only on women who were still cycling.
For women ≥40 years old, many of the premutation carriers with potentially higher FSH levels had already undergone menopause and thus were not included in the analysis (Figure 4). To account for this situation, we conducted tobit analyses allowing us to incorporate both cycling and menopausal women (FSH levels for menopausal women defined as ≥40 IU). To do this, we applied the method described by Tobin (1958), who developed a regression-based method for the analysis of censored normal data. Tobin defined the likelihood of a censored observation y as the probability that the observed value is ≥y. By doing this, Tobin accounted for the possibility that the latent value of a censored observation is actually greater than the observed value y. Thus, we assigned menopausal women an FSH value of ≥40 IU, censored the distribution at that threshold of 40 IU, and accounted for this in the regression analysis. We accounted for dependent observations within families by introducing familial random effects into the tobit model (Epstein et al., 2003). We performed all statistical analyses on Statistical Analysis System (SAS) software for Windows (release 8.2).
Laboratory methods
FMR1 CGG repeat size
DNA was extracted from buccal samples or blood using Qiagen QiAmp DNA Blood Mini Kit. FMR1 CGG repeat sizes were determined by a fluorescent-sequencer method, as described elsewhere (Meadows et al., 1996), using the ABI Prism 377 DNA Sequencer. For results with only one band, we used a PCR-based hybridization technique as described elsewhere (Crawford et al., 2000) to identify a possible high band. If no high repeat allele was identified using this method, we concluded that the woman was homozygous for the smaller allele. The false-negative rate for repeat sizes >80 repeats (i.e. assuming a homozygote normal when the woman is a high-repeat heterozygote) is ∼5% with this protocol. The rate for those <80 repeats is <1%. Thus, based on the frequency of premutation carriers in the general population (<1/350 for repeat sizes >80 repeats) and the low false-negative rate, we can conclude that there will be very few missed premutation carriers among the common repeat size group.
X-chromosome inactivation assay
For the X-chromosome inactivation assay, DNA was digested overnight with HPAII at 37°C. The enzyme was then inactivated at 65°C for 10 min. After ethanol precipitation, the digested DNA and an aliquot of undigested DNA was PCR-amplified for the CAG trinucleotide repeat within the androgen receptor (AR) gene (or the microsatellite within the DXS6673E gene, if the individual was not heterozygous for AR) and FRAXA (the CGG repeat array within the FMR1 gene). Primers used for AR were: AR-Forward, 5′-Cy5GCTGTGAAGGTTGCTGTTCCTCAT-3′; and AR-Reverse, 5′-TCCAGAATCTGTTCCAGAGCGTGC-3′ (Tilley et al., 1989). Primers for FMR1 were C: 5′-Cy5GCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ and F: 5′-AGCCCCGCACTTCCACCAGCTCCTCCA-3′ (Fu et al., 1991). Primers for DXS6673E were: DXS-Forward, 5′-ATGCTAAGGACCATCCAGGA-3′; and DXS-Reverse, 5′-GGAGTTTTCCTCCCTCACCA-3′ (Beever et al., 2003). The PCR products were run on an ALF Express automated sequencer (Amersham Pharmacia). X-Chromosome inactivation ratios were calculated for the AR gene by normalizing the peak ratio for the digested sample to the peak ratio for the undigested sample. A male sample was run as a negative control. The X-chromosome inactivation ratios for the FMR1 locus were used to determine which FMR1 allele was inactivated more often.
FSH level
Serum was extracted from a fresh 10 ml blood sample and stored at −70°C until the assay was performed in batch. An immunoradiometric procedure (Diagnostic Products) was used to determine FSH levels. Fifty microlitres of thawed plasma of an assayed control along with 1 ml of radiolabelled antibody were incubated in labelled tubes in duplicate for 2 h on a flat bed rotator at 180 strokes/min at room temperature. Tubes were washed twice each time with 2 ml of the wash buffer provided with the reagents, and then decanted each time and drained for 10 min. Tubes are then counted in a 2-well LKB Gamma Counter for 5 min each. Assay sensitivities are <0.2 mIU/ml and within-assay coefficients of variation are 2–5%.
Results
CGG repeat size
Prevalence of premature ovarian failure (POF) and early menopause
We initiated our studies by investigating the increased prevalence of POF among all premutation carriers. As POF is defined as menopause before the age of 40 years, we calculated the prevalence of POF among those women who were aged ≥40 years at the time of interview. Of the 300 women aged ≥40 years, we excluded women whose menstrual cycles had stopped before the age of 40 years due to hysterectomy or oophorectomy (19 premutation carriers, 19 non-carriers). We also excluded women who reported that they were still cycling and on hormone medication (seven carriers and five non-carriers). Of the women included in the analysis (n=250, Table III), we found a significantly higher prevalence of POF among premutation carriers (12.9%, 12/93) compared with non-carriers (1.3%, 2/157) (χ21=14.9; P<0.001; GEE analysis, P<0.001).
We also calculated the prevalence of early menopause, defined as menopause before age 45 years, among those women ≥45 years at the time of interview (241 women). Again, we excluded women whose menstrual cycles had stopped before the age of 45 years for known reasons (22 premutation carriers, 29 non-carriers). All women were excluded due to a hysterectomy or oophorectomy, except two premutation carriers who reported other medical complications. We also excluded women who reported that they were still cycling and on hormone medication (nine carriers and seven non-carriers). Among all other women aged ≥45 years (n=174), the early menopause rate was 23.6% (13/55) among premutation carriers and 3.4% (4/119) among non-carriers (χ21=17.5; P<0.001; GEE analysis, P<0.001).
We then dissected the association of POF and early menopause by examining the entire range of repeats, first using repeat size as an ordinal categorical variable (common, intermediate, low premutation, medium premutation, high premutation) and then using repeat size as a continuous variable. We found a significant increase in the prevalence of POF with increasing repeat size over the entire repeat range assuming a linear model [odds ratio (OR) and 95% confidence limits (CI): 2.27 (1.57–3.28) and 1.03 (1.01–1.05) with repeat size as ordinal and continuous variables respectively; P<0.001]. Similar significance was found with early menopause [OR (95% CI): 2.78 (1.72–2.42; P<0.001) and 1.03 (1.00–1.06; P=0.03)] with repeat size as ordinal and continuous variables respectively). We then obtained OR for each repeat size group compared with the common size allele group to examine the assumption of linearity. The point estimate of the OR for the low premutation group was slightly increased compared to the OR for the intermediate group for POF, but neither was significantly different from 1. The OR for the medium and high premutation groups were significantly increased, but showed an interesting pattern when compared to each other: the OR for the highest premutation allele group was smaller than that for the medium premutation allele group. The pattern for the OR associated with early menopause were more linear in appearance; however, the OR were also not significant for the comparison between common and the intermediate and low premutation allele groups (Table III).
Age at menopause
To assess the effect of FMR1 premutation carrier status on age at menopause, we used survival log-rank tests that incorporated women of all ages. As defined in Materials and methods and outlined in Figure 2, we used three definitions for age at menopause based on use of hormone medication. Overall, we found that premutation carriers experienced menopause ∼5 years earlier compared with non-carriers, irrespective of the trait definition (P<0.0001, Table IV).
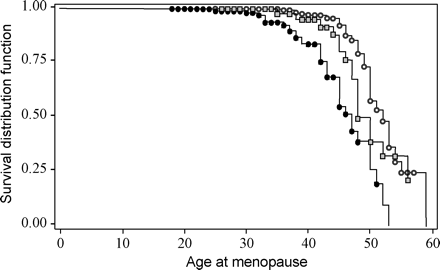
Next, we examined the effect of repeat size over the entire range of repeats. Over the entire repeat range using repeat size as a continuous variable, there was a highly significant association between increasing repeat size and earlier age at menopause (frailty model, P<0.0001 for all definitions of age at menopause). Again, we compared age at menopause among the different repeat size groups to better describe this relationship. It was necessary to collapse common and intermediate allele groups because the sample size limited this comparison (only 5/125 women with intermediate alleles had gone through menopause compared to 43/197 women with common alleles). We found that low premutation carriers (n=54) went through menopause ∼2.5 years earlier than non-carriers (Table IV). Using the frailty models and adjusting for the covariates, the significance levels for the association were affected by the definition of menopause with respect to hormone use (P=0.19 for ‘age at menopause’ and P=0.009 for the other two definitions, Table IV). Lastly, we compared the low premutation allele carriers to the medium/high carrier groups (collapsed due to small numbers). Age at menopause was ∼4 years earlier for the medium/high allele carriers (n=129) compared with the low premutation (P=0.01, P=0.04, P=0.02 for the three definitions of age at menopause, Table IV). Taken together, these findings indicate a strong association of age at menopause with increasing repeat size. Figure 3 shows the survival curves for non-carriers, low and medium/high premutation carriers to emphasize this association. The group differences (2.5 years between non-carrier and low premutations and 4 years between low and medium/high premutations) suggest that the association of repeat size is not linear, although more data are needed to determine if the repeat size effect exists in the lower repeat size ranges.
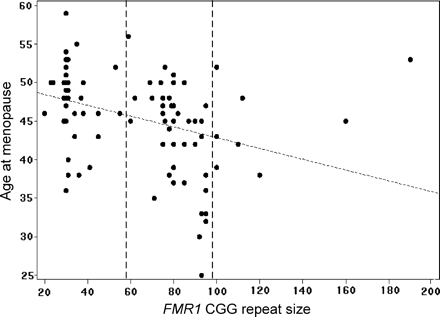
We used an alternative way to determine whether repeat size is associated with age at menopause and whether this association is linear. We performed linear regression restricting the analysis to women who had gone through menopause (n=99). We used the definition of the self-reported age at menopause (Figure 2). There was a marginally significant association of increasing repeat size with an earlier age at menopause over the entire repeat size range (GEE analysis: β = −0.05; 95% CI = −0.1 to 0.0004; P=0.05) (Figure 4). We conducted post hoc exploratory analyses to further examine the risk for early menopause among the high premutation carriers (n=9) as we had noticed that they had a later age at menopause and a lower prevalence of POF (Table III). Among premutation carriers (n=51), there was no linear effect of age at menopause with premutation size (P>0.10). When only low and medium premutation size groups (women with 59–99 repeats, n=42) were included in the analysis, the association of increasing repeat size with an earlier age at menopause was highly significant (GEE analysis: β = −0.33; 95% CI = −0.54 to −0.15; P=0.001). Although preliminary, these results suggest that women with ≥100 repeats may have a smaller risk for early menopause compared with women who carry medium size premutation alleles.
FSH as a marker for ovarian reserve
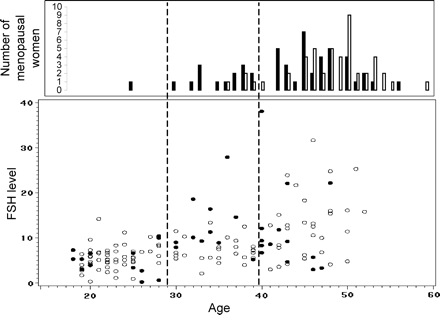
Next, we examined FSH levels of women who were still cycling (n=149: 38 premutation carriers and 111 non-carriers) as an indicator of ovarian reserve. Previous reports have shown that premutation carriers have higher FSH levels than non-carriers at the same age (Murray et al., 1999; Hundscheid et al., 2001), even those on OC (Hundscheid et al., 2001). To examine this association, we used GEE analysis and adjusted FSH level for age at blood draw, ethnicity, smoking and current hormone use. Overall, we found that premutation status was not significantly associated with FSH level in this initial analysis (P>0.10). Because of the known strong effect of age on FSH levels, we performed GEE analyses within age intervals [18–29 years (n=68), 30–39 years (n=37) and ≥40 years (n=44)]. Although sample sizes were small, we found that premutation carriers (n=11) had significantly increased FSH levels relative to non-carriers (n=26) among women 30–39 years old (β=0.55; 95% CI = 0.31 to 0.78; P<0.001) (Figure 5). In contrast, we found no significant differences in FSH level among premutation carriers and non-carriers within the other two age interval groups (P>0.10). Findings were similar when we analysed FSH values from only women who did not use hormone medication.
For women ≥40 years old, many of the premutation carriers with potentially higher FSH levels had already undergone menopause and thus were not included in the analysis (Figure 5). To account for this situation, we conducted tobit analyses allowing us to incorporate both cycling and menopausal women (FSH levels for menopausal women defined as ≥40 IU, see Materials and methods) giving a total data set of 248 women (99 menopausal and 149 cycling). Using this approach, we found that premutation carriers (n=89) had significantly increased FSH levels compared to non-carriers (n=159) (β = 0.41; 95% CI = 0.12 to 0.69; P=0.005) (Table V, All women).
We then modelled repeat size as an ordinal categorical variable and confirmed the increasing FSH levels with increasing repeat size (Table V). Interestingly, when we restricted the analyses to only premutation carriers (n=89), there was no significant association with repeat size and FSH level (P>0.10) (Table V). This result did not change if we restricted premutation carriers to those with <100 repeats (n=75) or to those ≥30 years old (n=66) (data not shown).
X-Chromosome inactivation ratio
The X-chromosome inactivation pattern could be hypothesized to be a risk factor for FMR1-associated ovarian dysfunction (see Introduction). Because we had blood on a smaller subset of individuals, we were unable to analyse the effect of X-chromosome inactivation patterns on ovarian dysfunction with respect to POF prevalence (only 5/78 with POF) and age at menopause (only 27 women who have gone through menopause). For descriptive purposes, the percentage of inactive X chromosomes carrying the larger allele (X-chromosome inactivation ratio) was 60, 56, 30, 40 and 63% among the five women with POF who had 41, 92, 95, 100 and 120 repeats respectively. Thus, there were no hints of skewed X-inactivation patterns among those with POF.
We did have an adequate sample size to examine FSH levels using tobit analyses among all women (n=161) and among all premutation carriers (n=54). We found no correlation between FSH and X-chromosome inactivation ratio defined as a continuous variable or as binary variable [skewed X-inactivation (≥80/≥20%) or not skewed] (P>0.10, Table V). We then examined the interaction term of repeat size and X-chromosome inactivation ratio [repeat size of the larger allele*(1–X-chromosome inactivation)] and found it to be significantly associated with increasing FSH level among all women (P=0.004). The significance level was similar to that using repeat size only among the reduced sample of women with X-chromosome inactivation results. This is consistent with the results that indicate no association with X-inactivation and FSH levels. When we restricted these analyses to premutation carriers only, we found no evidence for an association (P<0.10, Table V).
Parental origin
We did not find a difference in the prevalence of POF between women with a maternally inherited premutation (MIP) (5/33 = 15.2%) and women with a paternally inherited premutation (PIP) (6/52 = 11.5%) (χ21=0.23; P>0.10). To account for the dependency of observations within families, we also analysed the data using GEE models with parental origin of the premutation as the predictor variable and again found no significance (P>0.10). Similarly, using simple log-rank survival test, we did not detect a difference between age at menopause among 92 women with a PIP and 63 with a MIP (47.2±0.9 versus 47.1±1.3 respectively; P>0.10). Lastly, no association was found between FSH level and parental origin when we performed tobit analysis using all premutation carriers (n=78) (P>0.10).
Discussion
In this study, we confirmed previous findings that premutation carriers have an increased risk of ovarian dysfunction: they have a higher prevalence of POF and an earlier age at menopause than non-carriers. The estimate of the POF prevalence, 12.9%, is similar to the prevalence estimate of 12/98 in the study of Mallolas et al. (2001) that examined premutation women aged >45 years ascertained from families with fragile X syndrome. Although this estimate is somewhat lower than that from combined studies (20%; reviewed in Sherman, 2000), the estimated mean age at menopause of 47 years in our sample of premutation carriers was the same as that found by Murray et al. (2000a). POF prevalence may differ between studies for several reasons including the definition of POF, the definition of premutation, ascertainment of the study population, environmental factors and the genetic background. In addition, through this study, we have new evidence that the prevalence of POF increases with increasing repeat size; thus, the repeat size distribution among premutation carriers in a study sample may differ, leading to a different estimate of POF prevalence.
FSH elevation is the first measurable sign of reproductive ageing and is most prominent in the early follicular phase of the cycle. We obtained a single measure of FSH between days 2 and 5 of a woman's cycle. We did not see a statistically significant increase in adjusted FSH levels among premutation carriers who were cycling compared with non-carriers among all age groups. However, we did identify significantly increased levels of FSH among premutation carriers compared with non-carriers when the analysis was restricted to women aged 30–39 years. Hundscheid et al. (2001) observed a significant difference between all women in their data, but on closer examination, their sampled women were almost all in their 30s. Taken together, these data suggest that, on average, premutation carriers may not have clinical problems associated with ovarian function in their earlier reproductive years.
Among women in their 40s, premutation carriers who were cycling did not appear to have higher FSH levels than non-carriers. This may be due to the fact that premutation women move through menopausal transition faster than non-carriers. That is, women in their 40s enter menopause sooner than non-carriers and therefore are excluded from the sample of cycling women in this analysis. Using tobit analysis to account for these missing FSH levels of menopausal women, we found significantly increased FSH levels among premutation carriers compared with non-carriers when all age groups were included.
Once we confirmed ovarian dysfunction among premutation carriers in our study sample, we investigated FMR1-related risk factors that may predict risk or severity of this dysfunction. We found that repeat size significantly influenced the risk for ovarian dysfunction in our sample of women: the prevalence of POF and early menopause increased with repeat size (Table III), age at menopause decreased with increasing repeat size (Figures 4 and 5, Table IV) and FSH levels increased with repeat size (Table V). This association was observed over the entire range of repeat sizes. However, we have evidence that the effect of repeat size is not linear with risk. First, the OR for POF only increased slightly from those women with ≤40 repeats to those with up to 79 repeats (not statistically significant). The OR then increased significantly to 25 when repeat sizes were between 80 and 99 compared with those ≤40 repeats (Table III). More interestingly, the OR did not increase for those with ≥100 repeats compared with those with 80–99 repeats. The most parsimonious explanation of the data at this point is a threshold model. However, with more data from intermediate and high-repeat carriers, a more complex relationship may be identified. This complexity was most obvious with age at menopause: there was a linear effect of repeat size on age at menopause among carriers of low and medium repeat size alleles (59–99 repeats). However, premutation carriers with ≥100 repeats appear to go through menopause around the same age as non-carriers. Murray et al. (2003 and personal communication) observed this same phenomenon among high-repeat carriers. They suggested that women with >100 repeats may have ovarian target cells with full mutations and thus are not susceptible. An alternative explanation may be that the secondary structure of a repeat with >100 repeats at the DNA level or at the transcript level may not be recognizable to CGG binding proteins, whereas, under that size, there may be an increased affinity to binding with increased repeat size. Another explanation may be that the repeat size threshold for methylation may be reduced from >200 to >100 repeats in the target tissue (e.g. granulosa cells). Thus, such methylated alleles would have the same molecular characteristics and reduced risk for POF as full mutation alleles. Whatever the cause, a larger sample size of these women with ≥100 repeats is required before drawing conclusions about their reproductive profile.
Similar to age at menopause, we only identified a statistically significant repeat size effect with FSH levels when we included women with the entire range of repeats, not when we restricted the analysis to those in the premutation range. Excluding those women with ≥100 repeats and/or including only women >30 years of age did not change the conclusions. Perhaps obtaining only one measure of FSH is not sensitive enough to detect a difference among women who may be in menopause transition (Burger et al., 2002). Thus, a larger study of premutation women who are cycling with FSH measured during the follicular phase of two consecutive cycles may be needed to identify a repeat size effect.
We did not identify other FMR1-related risk factors including X-chromosome inactivation ratio or parental origin of the premutation. Our results on X-chromosome inactivation are similar to those of Murray et al. (2000a). For parental origin, we were unable to confirm the initial finding of Hundscheid et al. (2000) that there was a higher risk of POF among women with a paternally inherited premutation compared with a maternally inherited premutation. As our results are similar to those of other groups (Murray et al., 2000b; Vianna-Morgante and Costa, 2000; Mallolas et al., 2001), we conclude that parental origin does not play a role in the risk for ovarian dysfunction.
In conclusion, we found a significant association of repeat size and ovarian function. The repeat size polymorphism in the FMR1 gene could be considered a quantitative trait locus up through the intermediate or low premutation range, or one that contributes to reducing the age at menopause as repeat size increases. However, when repeat size exceeds ∼79 repeats, the increase in risk for ovarian dysfunction is clinically significant. That risk appears to plateau, or perhaps decrease, among women with very high repeats (≥100 repeats).
Our data also indicate that younger premutation women, on average, are similar to non-carriers with respect to their FSH levels. This suggests that women with the premutation should not have significant FMR1-related fertility problems in their younger reproductive years. It is interesting to put these results into context with recent findings of another phenotype associated with premutation carriers, namely a tremor and ataxia syndrome referred to as FXTAS. Studies have shown that this disorder is due to an RNA gain-of-function related to the large number of repeats found in the FMR1 transcripts and high levels of those transcripts in premutation carriers (Hagerman and Hagerman, 2002; Jin et al., 2003; Willemsen et al., 2003). The premutation-associated ovarian dysfunction could also be considered a late age-of-onset disorder. Perhaps the ovarian target tissue (e.g. oocytes, granulosa cells) accumulates the toxic effect of the FMR1 premutation transcripts, which eventually leads to increased atresia/apoptosis of follicles. Future studies of ovarian tissue from premutation carriers will help to determine if this hypothesis is true or if the early ovarian dysfunction is due to a reduction of the initial oocyte pool size.
Under either hypothesis, premutation carrier women will have a shortened reproductive life span. Thus, it is imperative to counsel premutation women accordingly so that they can incorporate this information into their family planning. Further large studies are needed to confirm the association of the repeat size effect among premutation carriers in order to better counsel these women about their risk. In addition, such studies with women spanning the entire range of repeat sizes would be useful to confirm our preliminary evidence that the FMR1 gene may be one genetic factor that plays a role in determining the multifactorial trait of age at menopause.
Ascertainment of the study populations. Those individuals with known reproductive success are shaded. ATD = ascertained through direct descendent.
Definition of the study populations for the survival analyses of age at menopause based on information taken from the reproductive history questionnaire. Women whose menstrual period stopped due to medical reasons are not included in the figure. They were censored at the age of that event. If they used HRT prior to that event, they were censored at the age at start of HRT use for the conservative analysis only.
Survival analysis results to compare age at menopause among low premutation (line with gray squares), medium/high premutation (line with black dots), and non-carrier women (line with white dots) using the ‘simple’ definition as defined in Figure 2. Dots and squares are censored values.
Scatter plot of FSH level (unadjusted for presentation purposes) by age at blood draw for all cycling women (bottom) and histogram of menopausal women by age at cessation of menses (top). Premutation carrier women are represented by black dots/bars and non-carrier women by white dots/bars.
Study population characteristics
| . | All . | Non-carriers (<59 repeats) . | . | . | . | Premutation carriers (≥59 repeats) . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | All . | All . | Probands . | Probands ATD . | Mothers of probands . | All . | Probands . | Probands ATD . | Mothers of probands . | |||||||||
| . | (n=507) . | (n=324) . | (n=214) . | (n=8) . | (n=108) . | (n=183) . | (n=92) . | (n=91) . | (n=30) . | |||||||||
| Age (years) at interview | ||||||||||||||||||
| Mean ± SD | 42.3±14.6 | 41.2±15.0 | 33.9±11.9 | 53.0±14.6 | 55.9±8.4 | 44.3±13.5 | 42.5±14.7 | 46.0±12.1 | 59.0±7.7 | |||||||||
| (range) | (18–75) | (18–75) | (18–65) | (28–71) | (40–75) | (18–75) | (18–75) | (25–75) | (47–71) | |||||||||
| Body mass index (kg/m2) | ||||||||||||||||||
| Mean ± SD | 26.8±6.6 | 26.8±6.8 | 26.4±6.8 | 25.3±5.8 | 27.9±7.0 | 26.8±6.0 | 27.4±6.0 | 26.2±6.0 | 28.6±7.2 | |||||||||
| (range) | (17–55) | (17–55) | (17–55) | (20–38) | (17–49) | (17–52) | (19–44) | (17–52) | (17–52) | |||||||||
| Ethnicity (%) | ||||||||||||||||||
| African American | 17.2 | 22.8 | 28.0 | 0 | 13.0 | 7.1 | 6.5 | 7.7 | 3.3 | |||||||||
| Caucasian | 76.3 | 69.1 | 63.6 | 100 | 79.6 | 89.1 | 89.1 | 89.0 | 96.7 | |||||||||
| Other/unknown | 6.5 | 8.0 | 8.4 | 0 | 7.4 | 3.8 | 4.4 | 3.3 | 0 | |||||||||
| Ever smoked (%) | 34.1 | 29.9 | 26.2 | 50 | 36.1 | 41.5 | 42.4 | 40.7 | 53.3 | |||||||||
| Education: completed college or more (%) | 45.8 | 47.8 | 47.2 | 62.5 | 49.1 | 42.1 | 41.3 | 42.9 | 30.0 | |||||||||
| Current hormone medication use (%) | 40.2 | 39.4 | 38.2 | 62.5 | 39.8 | 41.7 | 28.9 | 54.4 | 46.7 | |||||||||
| . | All . | Non-carriers (<59 repeats) . | . | . | . | Premutation carriers (≥59 repeats) . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | All . | All . | Probands . | Probands ATD . | Mothers of probands . | All . | Probands . | Probands ATD . | Mothers of probands . | |||||||||
| . | (n=507) . | (n=324) . | (n=214) . | (n=8) . | (n=108) . | (n=183) . | (n=92) . | (n=91) . | (n=30) . | |||||||||
| Age (years) at interview | ||||||||||||||||||
| Mean ± SD | 42.3±14.6 | 41.2±15.0 | 33.9±11.9 | 53.0±14.6 | 55.9±8.4 | 44.3±13.5 | 42.5±14.7 | 46.0±12.1 | 59.0±7.7 | |||||||||
| (range) | (18–75) | (18–75) | (18–65) | (28–71) | (40–75) | (18–75) | (18–75) | (25–75) | (47–71) | |||||||||
| Body mass index (kg/m2) | ||||||||||||||||||
| Mean ± SD | 26.8±6.6 | 26.8±6.8 | 26.4±6.8 | 25.3±5.8 | 27.9±7.0 | 26.8±6.0 | 27.4±6.0 | 26.2±6.0 | 28.6±7.2 | |||||||||
| (range) | (17–55) | (17–55) | (17–55) | (20–38) | (17–49) | (17–52) | (19–44) | (17–52) | (17–52) | |||||||||
| Ethnicity (%) | ||||||||||||||||||
| African American | 17.2 | 22.8 | 28.0 | 0 | 13.0 | 7.1 | 6.5 | 7.7 | 3.3 | |||||||||
| Caucasian | 76.3 | 69.1 | 63.6 | 100 | 79.6 | 89.1 | 89.1 | 89.0 | 96.7 | |||||||||
| Other/unknown | 6.5 | 8.0 | 8.4 | 0 | 7.4 | 3.8 | 4.4 | 3.3 | 0 | |||||||||
| Ever smoked (%) | 34.1 | 29.9 | 26.2 | 50 | 36.1 | 41.5 | 42.4 | 40.7 | 53.3 | |||||||||
| Education: completed college or more (%) | 45.8 | 47.8 | 47.2 | 62.5 | 49.1 | 42.1 | 41.3 | 42.9 | 30.0 | |||||||||
| Current hormone medication use (%) | 40.2 | 39.4 | 38.2 | 62.5 | 39.8 | 41.7 | 28.9 | 54.4 | 46.7 | |||||||||
Probands ATD = probands ascertained through an offspring or other direct descendent. Three women ascertained through the general population and 21 women ascertained through fragile X families fall into two categories: Probands ATD and Mothers. Twelve women ascertained through fragile X families fall into two categories: Probands and Mothers.
Study population characteristics
| . | All . | Non-carriers (<59 repeats) . | . | . | . | Premutation carriers (≥59 repeats) . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | All . | All . | Probands . | Probands ATD . | Mothers of probands . | All . | Probands . | Probands ATD . | Mothers of probands . | |||||||||
| . | (n=507) . | (n=324) . | (n=214) . | (n=8) . | (n=108) . | (n=183) . | (n=92) . | (n=91) . | (n=30) . | |||||||||
| Age (years) at interview | ||||||||||||||||||
| Mean ± SD | 42.3±14.6 | 41.2±15.0 | 33.9±11.9 | 53.0±14.6 | 55.9±8.4 | 44.3±13.5 | 42.5±14.7 | 46.0±12.1 | 59.0±7.7 | |||||||||
| (range) | (18–75) | (18–75) | (18–65) | (28–71) | (40–75) | (18–75) | (18–75) | (25–75) | (47–71) | |||||||||
| Body mass index (kg/m2) | ||||||||||||||||||
| Mean ± SD | 26.8±6.6 | 26.8±6.8 | 26.4±6.8 | 25.3±5.8 | 27.9±7.0 | 26.8±6.0 | 27.4±6.0 | 26.2±6.0 | 28.6±7.2 | |||||||||
| (range) | (17–55) | (17–55) | (17–55) | (20–38) | (17–49) | (17–52) | (19–44) | (17–52) | (17–52) | |||||||||
| Ethnicity (%) | ||||||||||||||||||
| African American | 17.2 | 22.8 | 28.0 | 0 | 13.0 | 7.1 | 6.5 | 7.7 | 3.3 | |||||||||
| Caucasian | 76.3 | 69.1 | 63.6 | 100 | 79.6 | 89.1 | 89.1 | 89.0 | 96.7 | |||||||||
| Other/unknown | 6.5 | 8.0 | 8.4 | 0 | 7.4 | 3.8 | 4.4 | 3.3 | 0 | |||||||||
| Ever smoked (%) | 34.1 | 29.9 | 26.2 | 50 | 36.1 | 41.5 | 42.4 | 40.7 | 53.3 | |||||||||
| Education: completed college or more (%) | 45.8 | 47.8 | 47.2 | 62.5 | 49.1 | 42.1 | 41.3 | 42.9 | 30.0 | |||||||||
| Current hormone medication use (%) | 40.2 | 39.4 | 38.2 | 62.5 | 39.8 | 41.7 | 28.9 | 54.4 | 46.7 | |||||||||
| . | All . | Non-carriers (<59 repeats) . | . | . | . | Premutation carriers (≥59 repeats) . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | All . | All . | Probands . | Probands ATD . | Mothers of probands . | All . | Probands . | Probands ATD . | Mothers of probands . | |||||||||
| . | (n=507) . | (n=324) . | (n=214) . | (n=8) . | (n=108) . | (n=183) . | (n=92) . | (n=91) . | (n=30) . | |||||||||
| Age (years) at interview | ||||||||||||||||||
| Mean ± SD | 42.3±14.6 | 41.2±15.0 | 33.9±11.9 | 53.0±14.6 | 55.9±8.4 | 44.3±13.5 | 42.5±14.7 | 46.0±12.1 | 59.0±7.7 | |||||||||
| (range) | (18–75) | (18–75) | (18–65) | (28–71) | (40–75) | (18–75) | (18–75) | (25–75) | (47–71) | |||||||||
| Body mass index (kg/m2) | ||||||||||||||||||
| Mean ± SD | 26.8±6.6 | 26.8±6.8 | 26.4±6.8 | 25.3±5.8 | 27.9±7.0 | 26.8±6.0 | 27.4±6.0 | 26.2±6.0 | 28.6±7.2 | |||||||||
| (range) | (17–55) | (17–55) | (17–55) | (20–38) | (17–49) | (17–52) | (19–44) | (17–52) | (17–52) | |||||||||
| Ethnicity (%) | ||||||||||||||||||
| African American | 17.2 | 22.8 | 28.0 | 0 | 13.0 | 7.1 | 6.5 | 7.7 | 3.3 | |||||||||
| Caucasian | 76.3 | 69.1 | 63.6 | 100 | 79.6 | 89.1 | 89.1 | 89.0 | 96.7 | |||||||||
| Other/unknown | 6.5 | 8.0 | 8.4 | 0 | 7.4 | 3.8 | 4.4 | 3.3 | 0 | |||||||||
| Ever smoked (%) | 34.1 | 29.9 | 26.2 | 50 | 36.1 | 41.5 | 42.4 | 40.7 | 53.3 | |||||||||
| Education: completed college or more (%) | 45.8 | 47.8 | 47.2 | 62.5 | 49.1 | 42.1 | 41.3 | 42.9 | 30.0 | |||||||||
| Current hormone medication use (%) | 40.2 | 39.4 | 38.2 | 62.5 | 39.8 | 41.7 | 28.9 | 54.4 | 46.7 | |||||||||
Probands ATD = probands ascertained through an offspring or other direct descendent. Three women ascertained through the general population and 21 women ascertained through fragile X families fall into two categories: Probands ATD and Mothers. Twelve women ascertained through fragile X families fall into two categories: Probands and Mothers.
Distribution of FMR1 CGG repeat size in study population
| Age at interview (years) . | Non-carrier range . | . | . | . | Premutation range . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Common range . | . | Intermediate range . | . | Low . | Medium . | High . | |||||
| ≤30 | 31–40 | 41–50 | 51–58 | 59–79 | 80–99 | ≥100 | ||||||
| All (n=507) | 117 (23.1) | 81 (16.0) | 98 (19.3) | 28 (5.5) | 54 (10.7) | 88 (17.4) | 41 (8.1) | |||||
| <40 years (n=207) | 47 (22.7) | 21 (10.1) | 60 (29.0) | 15 (7.3) | 10 (4.8) | 32 (15.5) | 22 (10.6) | |||||
| ≥40 years (n=300) | 70 (23.3) | 60 (20.0) | 38 (12.7) | 13 (4.3) | 44 (14.7) | 56 (18.7) | 19 (6.3) | |||||
| Age at interview (years) . | Non-carrier range . | . | . | . | Premutation range . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Common range . | . | Intermediate range . | . | Low . | Medium . | High . | |||||
| ≤30 | 31–40 | 41–50 | 51–58 | 59–79 | 80–99 | ≥100 | ||||||
| All (n=507) | 117 (23.1) | 81 (16.0) | 98 (19.3) | 28 (5.5) | 54 (10.7) | 88 (17.4) | 41 (8.1) | |||||
| <40 years (n=207) | 47 (22.7) | 21 (10.1) | 60 (29.0) | 15 (7.3) | 10 (4.8) | 32 (15.5) | 22 (10.6) | |||||
| ≥40 years (n=300) | 70 (23.3) | 60 (20.0) | 38 (12.7) | 13 (4.3) | 44 (14.7) | 56 (18.7) | 19 (6.3) | |||||
Distribution of FMR1 CGG repeat size in study population
| Age at interview (years) . | Non-carrier range . | . | . | . | Premutation range . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Common range . | . | Intermediate range . | . | Low . | Medium . | High . | |||||
| ≤30 | 31–40 | 41–50 | 51–58 | 59–79 | 80–99 | ≥100 | ||||||
| All (n=507) | 117 (23.1) | 81 (16.0) | 98 (19.3) | 28 (5.5) | 54 (10.7) | 88 (17.4) | 41 (8.1) | |||||
| <40 years (n=207) | 47 (22.7) | 21 (10.1) | 60 (29.0) | 15 (7.3) | 10 (4.8) | 32 (15.5) | 22 (10.6) | |||||
| ≥40 years (n=300) | 70 (23.3) | 60 (20.0) | 38 (12.7) | 13 (4.3) | 44 (14.7) | 56 (18.7) | 19 (6.3) | |||||
| Age at interview (years) . | Non-carrier range . | . | . | . | Premutation range . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Common range . | . | Intermediate range . | . | Low . | Medium . | High . | |||||
| ≤30 | 31–40 | 41–50 | 51–58 | 59–79 | 80–99 | ≥100 | ||||||
| All (n=507) | 117 (23.1) | 81 (16.0) | 98 (19.3) | 28 (5.5) | 54 (10.7) | 88 (17.4) | 41 (8.1) | |||||
| <40 years (n=207) | 47 (22.7) | 21 (10.1) | 60 (29.0) | 15 (7.3) | 10 (4.8) | 32 (15.5) | 22 (10.6) | |||||
| ≥40 years (n=300) | 70 (23.3) | 60 (20.0) | 38 (12.7) | 13 (4.3) | 44 (14.7) | 56 (18.7) | 19 (6.3) | |||||
Association of FMR1 repeat size with premature ovarian failure (POF) and early menopause (EM)
| . | Non-carrier . | . | Premutation . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Common . | Intermediate . | Low . | Medium . | High . | |||
| . | ≤40 . | 41–58 . | 59–79 . | 80–99 . | ≥100 . | |||
| POF prevalence (%) | 0.9 | 2.2 | 5.9 | 18.6 | 12.5 | |||
| (n=250 aged ≥40 years) | (1/112) | (1/45) | (2/34) | (8/43) | (2/16) | |||
| OR (95% CI) | Referent group | 2.51a (0.15–41.24) | 6.93a (0.63–75.94) | 25.27a (3.06–209.00) | 16.37a (1.49–179.85) | |||
| EM prevalence (%) | 3.4 | 3.0 | 13.4 | 30.8 | 33.3 | |||
| (n=174 aged ≥45 years) | (3/86) | (1/33) | (3/23) | (8/26) | (2/6) | |||
| OR (95% CI) | Referent group | 0.90 (0.09–8.92) | 5.82 (0.98–34.70) | 26.95 (3.25–221.67) | 31.33 (2.55–285.06) | |||
| . | Non-carrier . | . | Premutation . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Common . | Intermediate . | Low . | Medium . | High . | |||
| . | ≤40 . | 41–58 . | 59–79 . | 80–99 . | ≥100 . | |||
| POF prevalence (%) | 0.9 | 2.2 | 5.9 | 18.6 | 12.5 | |||
| (n=250 aged ≥40 years) | (1/112) | (1/45) | (2/34) | (8/43) | (2/16) | |||
| OR (95% CI) | Referent group | 2.51a (0.15–41.24) | 6.93a (0.63–75.94) | 25.27a (3.06–209.00) | 16.37a (1.49–179.85) | |||
| EM prevalence (%) | 3.4 | 3.0 | 13.4 | 30.8 | 33.3 | |||
| (n=174 aged ≥45 years) | (3/86) | (1/33) | (3/23) | (8/26) | (2/6) | |||
| OR (95% CI) | Referent group | 0.90 (0.09–8.92) | 5.82 (0.98–34.70) | 26.95 (3.25–221.67) | 31.33 (2.55–285.06) | |||
Odds ratios (OR) and 95% confidence intervals (CI) estimated from generalized-estimating-equation analysis. Analyses are adjusted by smoking and ethnicity unless otherwise stated.
Unadjusted for smoking and ethnicity due to sample size restrictions.
Association of FMR1 repeat size with premature ovarian failure (POF) and early menopause (EM)
| . | Non-carrier . | . | Premutation . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Common . | Intermediate . | Low . | Medium . | High . | |||
| . | ≤40 . | 41–58 . | 59–79 . | 80–99 . | ≥100 . | |||
| POF prevalence (%) | 0.9 | 2.2 | 5.9 | 18.6 | 12.5 | |||
| (n=250 aged ≥40 years) | (1/112) | (1/45) | (2/34) | (8/43) | (2/16) | |||
| OR (95% CI) | Referent group | 2.51a (0.15–41.24) | 6.93a (0.63–75.94) | 25.27a (3.06–209.00) | 16.37a (1.49–179.85) | |||
| EM prevalence (%) | 3.4 | 3.0 | 13.4 | 30.8 | 33.3 | |||
| (n=174 aged ≥45 years) | (3/86) | (1/33) | (3/23) | (8/26) | (2/6) | |||
| OR (95% CI) | Referent group | 0.90 (0.09–8.92) | 5.82 (0.98–34.70) | 26.95 (3.25–221.67) | 31.33 (2.55–285.06) | |||
| . | Non-carrier . | . | Premutation . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Common . | Intermediate . | Low . | Medium . | High . | |||
| . | ≤40 . | 41–58 . | 59–79 . | 80–99 . | ≥100 . | |||
| POF prevalence (%) | 0.9 | 2.2 | 5.9 | 18.6 | 12.5 | |||
| (n=250 aged ≥40 years) | (1/112) | (1/45) | (2/34) | (8/43) | (2/16) | |||
| OR (95% CI) | Referent group | 2.51a (0.15–41.24) | 6.93a (0.63–75.94) | 25.27a (3.06–209.00) | 16.37a (1.49–179.85) | |||
| EM prevalence (%) | 3.4 | 3.0 | 13.4 | 30.8 | 33.3 | |||
| (n=174 aged ≥45 years) | (3/86) | (1/33) | (3/23) | (8/26) | (2/6) | |||
| OR (95% CI) | Referent group | 0.90 (0.09–8.92) | 5.82 (0.98–34.70) | 26.95 (3.25–221.67) | 31.33 (2.55–285.06) | |||
Odds ratios (OR) and 95% confidence intervals (CI) estimated from generalized-estimating-equation analysis. Analyses are adjusted by smoking and ethnicity unless otherwise stated.
Unadjusted for smoking and ethnicity due to sample size restrictions.
Mean age at menopause (unadjusted) calculated using survival analysis for repeat size group
| Menopause definition (Figure 2) . | Non-carriers (common/intermediate) . | . | All premutation carriers . | . | Low premutation carriers (59–79 repeats) . | . | Medium/high premutation carriers (>80 repeats) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Mean ± SE . | n . | Mean ± SE . | N . | Mean ± SE . | n . | Mean ± SE . | ||||
| Age at menopause (years) | 322 | 52.2±0.6 | 183 | 47.5±0.7a | 54 | 49.7±1.1b | 129 | 45.9±0.8d | ||||
| Age at menopause excluding hormone use (years) | 324 | 53.1±0.7 | 183 | 47.7±0.8a | 54 | 49.6±1.1c | 129 | 45.9±0.8d | ||||
| Age at HRT/menopause (years) | 324 | 51.5±0.6 | 183 | 46.6±0.7a | 54 | 48.8±1.1c | 129 | 44.9±0.7d | ||||
| Menopause definition (Figure 2) . | Non-carriers (common/intermediate) . | . | All premutation carriers . | . | Low premutation carriers (59–79 repeats) . | . | Medium/high premutation carriers (>80 repeats) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Mean ± SE . | n . | Mean ± SE . | N . | Mean ± SE . | n . | Mean ± SE . | ||||
| Age at menopause (years) | 322 | 52.2±0.6 | 183 | 47.5±0.7a | 54 | 49.7±1.1b | 129 | 45.9±0.8d | ||||
| Age at menopause excluding hormone use (years) | 324 | 53.1±0.7 | 183 | 47.7±0.8a | 54 | 49.6±1.1c | 129 | 45.9±0.8d | ||||
| Age at HRT/menopause (years) | 324 | 51.5±0.6 | 183 | 46.6±0.7a | 54 | 48.8±1.1c | 129 | 44.9±0.7d | ||||
P-values evaluated using frailty models adjusting for family membership, smoking, race, and age at interview.
P<0.0001 for difference between non-carriers and premutation carriers.
P=0.19 for difference between non-carriers and low premutation carriers.
P<0.01 for difference between non-carriers and low premutation carriers.
P<0.05 for difference between low and medium/high premutation carriers.
Mean age at menopause (unadjusted) calculated using survival analysis for repeat size group
| Menopause definition (Figure 2) . | Non-carriers (common/intermediate) . | . | All premutation carriers . | . | Low premutation carriers (59–79 repeats) . | . | Medium/high premutation carriers (>80 repeats) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Mean ± SE . | n . | Mean ± SE . | N . | Mean ± SE . | n . | Mean ± SE . | ||||
| Age at menopause (years) | 322 | 52.2±0.6 | 183 | 47.5±0.7a | 54 | 49.7±1.1b | 129 | 45.9±0.8d | ||||
| Age at menopause excluding hormone use (years) | 324 | 53.1±0.7 | 183 | 47.7±0.8a | 54 | 49.6±1.1c | 129 | 45.9±0.8d | ||||
| Age at HRT/menopause (years) | 324 | 51.5±0.6 | 183 | 46.6±0.7a | 54 | 48.8±1.1c | 129 | 44.9±0.7d | ||||
| Menopause definition (Figure 2) . | Non-carriers (common/intermediate) . | . | All premutation carriers . | . | Low premutation carriers (59–79 repeats) . | . | Medium/high premutation carriers (>80 repeats) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | n . | Mean ± SE . | n . | Mean ± SE . | N . | Mean ± SE . | n . | Mean ± SE . | ||||
| Age at menopause (years) | 322 | 52.2±0.6 | 183 | 47.5±0.7a | 54 | 49.7±1.1b | 129 | 45.9±0.8d | ||||
| Age at menopause excluding hormone use (years) | 324 | 53.1±0.7 | 183 | 47.7±0.8a | 54 | 49.6±1.1c | 129 | 45.9±0.8d | ||||
| Age at HRT/menopause (years) | 324 | 51.5±0.6 | 183 | 46.6±0.7a | 54 | 48.8±1.1c | 129 | 44.9±0.7d | ||||
P-values evaluated using frailty models adjusting for family membership, smoking, race, and age at interview.
P<0.0001 for difference between non-carriers and premutation carriers.
P=0.19 for difference between non-carriers and low premutation carriers.
P<0.01 for difference between non-carriers and low premutation carriers.
P<0.05 for difference between low and medium/high premutation carriers.
Examination of potential FMR1-related risk factors associated with increased FSH level using tobit analysis
| FMR1 predictor variable . | All women . | . | . | Premutation carriers . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Sample size . | β coefficient (95% CI) . | P . | Sample size . | P . | |||
| Premutation status (binary) | 248 | 0.41 (0.12–0.69) | 0.005 | – | – | |||
| Repeat size (ordinal categorical) | 248 | 0.10 (0.00–0.20) | 0.05 | 89 | 0.90 | |||
| Repeat size (continuous) | 248 | 0.005 (0.001–0.010) | 0.03 | 89 | 0.75 | |||
| Repeat size (continuous)a | 161 | 0.006 (0.002–0.011) | 0.004 | 54 | 0.39 | |||
| X-inactivation ratio | 161 | – | 0.50 | 54 | 0.66 | |||
| Repeat size× X-inactivation ratio | 161 | 0.010 (0.004–0.017) | 0.003 | 54 | 0.41 | |||
| FMR1 predictor variable . | All women . | . | . | Premutation carriers . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Sample size . | β coefficient (95% CI) . | P . | Sample size . | P . | |||
| Premutation status (binary) | 248 | 0.41 (0.12–0.69) | 0.005 | – | – | |||
| Repeat size (ordinal categorical) | 248 | 0.10 (0.00–0.20) | 0.05 | 89 | 0.90 | |||
| Repeat size (continuous) | 248 | 0.005 (0.001–0.010) | 0.03 | 89 | 0.75 | |||
| Repeat size (continuous)a | 161 | 0.006 (0.002–0.011) | 0.004 | 54 | 0.39 | |||
| X-inactivation ratio | 161 | – | 0.50 | 54 | 0.66 | |||
| Repeat size× X-inactivation ratio | 161 | 0.010 (0.004–0.017) | 0.003 | 54 | 0.41 | |||
P-value is related to the coefficient of the predictor value being different from 0.
Analysis of repeat size using the reduced set of individuals who had X-inactivation results to ensure the same pattern as found for the larger sample.
Examination of potential FMR1-related risk factors associated with increased FSH level using tobit analysis
| FMR1 predictor variable . | All women . | . | . | Premutation carriers . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Sample size . | β coefficient (95% CI) . | P . | Sample size . | P . | |||
| Premutation status (binary) | 248 | 0.41 (0.12–0.69) | 0.005 | – | – | |||
| Repeat size (ordinal categorical) | 248 | 0.10 (0.00–0.20) | 0.05 | 89 | 0.90 | |||
| Repeat size (continuous) | 248 | 0.005 (0.001–0.010) | 0.03 | 89 | 0.75 | |||
| Repeat size (continuous)a | 161 | 0.006 (0.002–0.011) | 0.004 | 54 | 0.39 | |||
| X-inactivation ratio | 161 | – | 0.50 | 54 | 0.66 | |||
| Repeat size× X-inactivation ratio | 161 | 0.010 (0.004–0.017) | 0.003 | 54 | 0.41 | |||
| FMR1 predictor variable . | All women . | . | . | Premutation carriers . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | Sample size . | β coefficient (95% CI) . | P . | Sample size . | P . | |||
| Premutation status (binary) | 248 | 0.41 (0.12–0.69) | 0.005 | – | – | |||
| Repeat size (ordinal categorical) | 248 | 0.10 (0.00–0.20) | 0.05 | 89 | 0.90 | |||
| Repeat size (continuous) | 248 | 0.005 (0.001–0.010) | 0.03 | 89 | 0.75 | |||
| Repeat size (continuous)a | 161 | 0.006 (0.002–0.011) | 0.004 | 54 | 0.39 | |||
| X-inactivation ratio | 161 | – | 0.50 | 54 | 0.66 | |||
| Repeat size× X-inactivation ratio | 161 | 0.010 (0.004–0.017) | 0.003 | 54 | 0.41 | |||
P-value is related to the coefficient of the predictor value being different from 0.
Analysis of repeat size using the reduced set of individuals who had X-inactivation results to ensure the same pattern as found for the larger sample.
We would like to thank Johnnie Brown, Laesha Edwards, Krista Harkreader, Mary Leslie, Gloria Novak, Elizabeth Scott, Lisa Shubeck, Darlene Sowemimo and Lisa Taft for their tremendous efforts in enrollment of participants in this project. This project would not have succeeded without their commitment. We would also like to thank the volunteers and their families whose participation made this work possible as well as Chanley Small at Emory University for her support and generosity. We are also grateful to Milburn Emery, Bob Bonsall, and the General Clinical Research Center at Emory University for their technical assistance. This work was supported by the National Institutes of Health grants HD29909, HD35576, HD40637 and MO-1-RR00039.
References
Beever C, Lai BP, Baldry SE, Penaherrera MS, Jiang R, Robinson WP and Brown CJ (
Burger HG, Dudley EC, Robertson DM and Dennerstein L (
Clayton D and Cuzick J (
Crawford DC, Wilson B and Sherman SL (
Epstein MP, Lin X and Boehnke M (
Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST et al. (
Hagerman RJ and Hagerman PJ (
Hundscheid RD, Sistermans EA, Thomas CM, Braat DD, Straatman H, Kiemeney LA, Oostra BA and Smits AP (
Hundscheid RD, Braat DD, Kiemeney LA, Smits AP and Thomas CM (
Jin P, Zarnescu DC, Zhang F et al. (
MacNaughton J, Banah M, McCloud P, Hee J and Burger H (
Mallolas J, Duran M, Sanchez A, Jimenez D, Castellvi-Bel S, Rife M and Mila M (
Meadows KL, Pettay D, Newman J, Hersey J, Ashley AE and Sherman SL (
Murray A, Webb J, MacSwiney F, Shipley EL, Morton NE and Conway GS (
Murray A, Ennis S, MacSwiney F, Webb J and Morton NE (
Murray A, Ennis S and Morton N (
Murray A, Ware A and Jacobs P
Nolin SL, Brown WT, Glicksman A, Houck GE Jr, Gargano AD, Sullivan A, Biancalana V, Brondum-Nielsen K, Hjalgrim H, Holinski-Feder E et al. (
Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF and Mandel JL (
Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT and Nelson DL (
Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D and Warren ST (
Tilley WD, Marcelli M, Wilson JD and McPhaul MJ (
Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP et al. (
Vianna-Morgante AM and Costa SS (
Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, Van Unen L, Tassone F, Hoogeveen AT et al. (
Author notes
1Department of Human Genetics, Emory University School of Medicine, Atlanta, Georgia, USA