-
PDF
- Split View
-
Views
-
Cite
Cite
J.M.J. Smeenk, C.M. Verhaak, A.J.J.M. Vingerhoets, C.G.J. Sweep, J.M.W.M. Merkus, S.J. Willemsen, A. van Minnen, H. Straatman, D.D.M. Braat, Stress and outcome success in IVF: the role of self-reports and endocrine variables, Human Reproduction, Volume 20, Issue 4, April 2005, Pages 991–996, https://doi.org/10.1093/humrep/deh739
Close - Share Icon Share
Abstract
BACKGROUND: The aim of this study was to examine the associations between urinary levels of the stress hormones adrenaline, noradrenaline and cortisol during treatment with self reported stress, in order to investigate the mechanism for the previously observed negative association of anxiety and depression with the outcome of IVF/ICSI. METHODS: In a multicentre prospective cohort study, women entering their first cycle of IVF/ICSI treatment were asked to participate. From each participant nocturnal urine samples were collected; pre-treatment, before oocyte retrieval and before embryo-transfer (ET), to assess hormonal concentrations. Additionally, two questionnaires were administered before the start of the treatment to measure anxiety and depression. RESULTS: 168 women completed the questionnaires and collected at least two urine specimens. A significant positive correlation between urinary adrenaline concentrations at baseline and ET and the scores on depression at baseline were found. In women with successful treatment, lower concentrations of adrenaline at oocyte retrieval and lower concentrations of adrenaline and noradrenaline at ET, compared with unsuccessful women, were found. CONCLUSIONS: The significant positive association of adrenaline concentration with pregnancy and with depression suggested that this adrenal hormone could be one of the links in the complex relationship between psychosocial stress and outcome after IVF/ICSI.
Introduction
In vitro fertilization (IVF)/intra cytoplasmatic sperm injection (ICSI) is a stressful experience and its outcome may be influenced by many known and still unknown factors. In addition to biomedical factors (such as age and history of pregnancy), there is increasing evidence that psychological factors, for instance anxiety and depression, are also related to IVF/ICSI treatment outcome (Demyttenaere et al., 1998; Smeenk et al., 2001). However, there are also studies in which no relationship between the psychosocial status of women and treatment outcome has been found (Boivin and Takefman, 1995; Slade et al., 1997). This discrepancy may be explained by differences in population characteristics, study design and differences in the assessment of psychosocial factors.
In general, a distinction is made between direct and indirect effects of stress. The direct effects refer to the effects mediated by the autonomic nervous system, the (neuro) endocrine system and the immune system, whereas the indirect effects imply those health changes resulting from changes in health behaviour, for instance smoking. Modulation results in an integrated adaptive psychobiological reaction pattern to environmental challenges (Vingerhoets and Perski, 2000).
Although the effects of psychosocial stressors on the activity of the sympathetic medullar system and on the hypothalamic–pituitary–adrenal axis have been studied intensively (Sanders and Bruce, 1997; Woods et al., 1998; Gold et al., 2003; Schommer et al., 2003), no clear picture emerges on the exact relationship between the different types of stressors and release of stress hormones. Moreover, several authors reported low correlation coefficients between questionnaire based and hormonal indicators of stress (Sanders and Bruce, 1997; Woods et al., 1998).
So far, several reviews on stress and female reproduction have been published (e.g. Greil, 1997; Magiakou et al., 1997; Ferin, 1999; Dobson et al., 2003), but little is known about the relationship between psychosocial stress and the release of adrenal hormones in relation to IVF/ICSI treatment outcome. As the treatment itself most probably influences psychosocial factors, it is extremely difficult to separate cause and effect of psychosocial factors on the outcome in IVF (Boivin and Takefman, 1995). The complex relationship is mediated by an interplay of various systems; the underlying mechanisms of the relationship remain obscure so far. Even aspects of the treatment could influence the hormonal response. For instance Luppa et al. (1995) found an increased urinary excretion of cortisol metabolites after stimulation with a GnRH agonist.
In an earlier study we demonstrated that pre-treatment levels of anxiety and depression are significantly positively related to treatment outcome in IVF/ICSI (Smeenk et al., 2001). We hypothesized therefore, that pre-treatment levels of anxiety and depression could be reflected in higher concentrations of the stress hormones cortisol and catecholamines during treatment, which in turn could influence treatment outcome.
The aim of the present study was therefore to examine the association between the concentrations of the stress hormones during treatment and the self-reported stress in order to investigate the mechanism for the negative effect of anxiety and depression on the outcome of IVF/ICSI.
Materials and methods
Participants
Patients visiting the outpatient clinic of the department of Obstetrics and Gynaecology of the Radboud University, Nijmegen Medical Centre an academic tertiary referral centre, or visiting the Amphia hospital in Breda, a secondary referral centre, both in the Netherlands, were invited to participate in the study. They were scheduled for the first IVF/ICSI treatment cycle between January 1999 and March 2000. Details of the program and the protocol used have been described previously (Smeenk et al., 2001).
Between day 10 and 20 of the pre-medication cycle, i.e. before the start of GnRH-analogue administration (day 21), women were asked to complete questionnaires on psychological factors. In addition, the women were asked to provide urine samples. The first urine sample was obtained before treatment, so before the start of medication, at a random convenient day between day 10 and 20 of the pre-medication cycle. This day was not necessarily the day that the questionnaires were being filled out, but in the same period. The other urine samples were taken before the time of oocyte retrieval and before the embryo transfer (ET), two stressful moments during treatment, as indicated by studies from Merari et al. (1992) and Johnston et al. (1987).
Signed informed consent was obtained from all participants. This study was approved by the ethical committees of the institutions. All participants were guaranteed confidentiality, and only the principal investigator (J.S.) had full access to the questionnaires, concentrations of hormones in urine and the clinical data.
Sampling
Two psychological dimensions of stress were assessed in this study: anxiety and depression. Anxiety was measured by means of the Dutch version of the State Anxiety Inventory (STAI; Spielberger et al., 1970). Depression was measured using the Dutch version of the Beck Depression Inventory (BDI; Beck and Beamesderfer, 1974). Both questionnaires have shown satisfactory reliability and validity.
For endocrine assessments three nocturnal urine samples were collected. Initially, the urine sample was collected in a 2 litre polypropylene bottle containing 5 ml of 6 mol/l HCl as preservatives of the catecholamines to prevent oxidation; later we used sodium disulfite (Na2S2O5) and Na2EDTA as preservatives to enable us to measure cortisol in the same samples. Therefore, in the first 100 urine samples the concentration of cortisol could not be assessed.
Women were asked to collect all urine after midnight (00.00 hours) including the first morning sample, at three timepoints during their treatment cycle: T1: at baseline (before commencing the IVF/ICSI treatment); T2: on the day of oocyte retrieval; and T3: on the day of ET.
In the sample adrenaline, noradrenaline and cortisol levels were measured and expressed per mmol of creatinine to correct for urine sample size. Urinary creatinine concentrations were measured by a modified Jaffé method on a Hitachi 747 analyzer (Boehringer Mannhein, Germany).
Levels of adrenaline and noradrenaline were determined by fluorometric determination after high performance liquid chromatography (HPLC). In short, catecholamines were extracted and subsequently derivatized. The 2,3 diphenyl-quinoxaline derivatives of adrenaline and noradrenaline were measured (Willemsen et al., 1995). The inter-assay coefficient of variation for the noradrenalin assay amounted to 4.2% (n=21) and 5.7% (n=17) at concentrations of 160 ± 6.7 nmol/l and 403 ± 22 nmol/l, respectively. For adrenaline the coefficients of variation were 5.7% (n=21) and 6.2% (n=17) at levels of 63.1 ± 3.6 nmol/l and 67.4 ± 4.2 nmol/l, respectively.
Free cortisol was measured by radioimmunoassay (RIA) after extraction with dichloromethane and subsequent paper chromatography, according to the method described earlier for cortisol measurement in plasma and saliva (Meulenberg et al., 1987). To adapt this method for urinary assessment, [3H]cortisol was added as a recovery tracer to 0.1 ml of urine, before extraction by means of dichloromethane.
Successful treatment was defined as treatment resulting in a viable pregnancy, with a positive heartbeat on transvaginal ultrasound 5 weeks after ET.
Statistical analysis
Data analysis was performed by means of the SPSS-program (version 11.0 for windows, SPSS inc., Chicago, IL). The Spearman correlation coefficient was used to express the association between psychological and hormonal measurements (two-tailed).The distribution free Mann–Whitney test for two independent samples was used to compare hormonal findings at the various moments during treatment between pregnant and non-pregnant women (two-tailed). The nonparametric two-way ANOVA by ranks, or Friedman test, was used to detect a trend in the hormonal response during treatment (two-tailed).
Results
A total of 246 out of 291 participants (85%) returned the questionnaires and were willing to collect urine specimens. The remaining 45 patients did not participate because of hygienic or logistic reasons.
Only women for whom questionnaire data and at least two urine specimens (T1 and T2) were available, were included in the analysis. A dataset was obtained in 36 women (66% of the participants) coming from Breda and 132 (69%) coming from Nijmegen. The main reason for incomplete data was that a number of women forgot to collect the urine the morning before oocyte retrieval. The samples of one participant were discarded from analysis since the levels of adrenaline and noradrenaline at T1 showed extreme variation (>1000%) compared with the levels at T2 and T3.
Unfortunately, the first 100 urine samples were acidified directly after obtaining the sample and therefore the concentration of cortisol could not be assessed. Consequently, the sample size for cortisol is smaller than for the catecholamines and the sample size varies over the various stages of treatment.
Since no significant differences were found in anxiety (P=0.40) and depression (P=0.29) scores, and between adrenaline (P=0.24), noradrenaline (P=0.42) and cortisol (P=0.09) concentrations in urine, between the participants of the two participating centres, the statistical analyses were based on the combined dataset of both cities, resulting in a sample of 167 women (68% of all participants).
Table I shows characteristics on demographic and psychological variables of the participants.
The ongoing pregnancy rate after the cycle was 32%. During treatment, 16% (n=27) of the participants did not reach embryo transfer, due to cancellation by the patient, poor response, ovarian hyperstimulation syndrome or total fertilization failure. Women who forgot to collect urine samples on T3 did not show statistically significant differences on demographic, psychological or outcome variables compared with the women who did not forget to collect urine. No statistically significant differences were found between pregnant and non-pregnant women on age (P=0.12), duration of infertility (P=0.73), anxiety (P=0.28), depression (P=0.11), but also on the number of follicles (P=0.25), number of oocytes (P=0.45) and number of embryos (P=0.07).
A large variation between subjects was found for each of the hormones (Table II). Table III shows the correlation between the State Anxiety Inventory and the Beck Depression Inventory scores and endocrine variables. Depression scores were positively associated with concentrations of hormones. In particular, a significant positive association was found between adrenaline pre-treatment (0.17) and at the time of embryo transfer (0.25) and the pre-treatment findings on depression. An association for anxiety with adrenaline was found as well, although this did not reach statistical significance. Furthermore, a significant association was found between cortisol concentrations at T2 and the pre-treatment findings on anxiety (0.20) and depression (0.18). Exploratively, these analyses were repeated in subgroups comparing successfully (meaning pregnant), or unsuccessfully treated women. The correlation coefficients were stable.
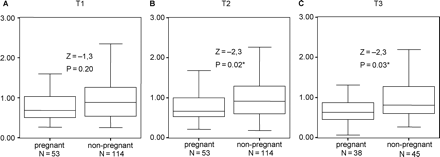
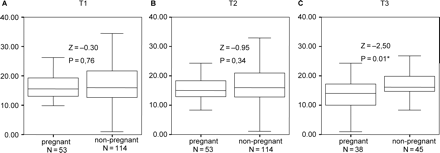
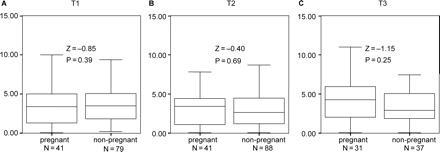
Next, hormonal levels between successfully and unsuccessfully treated women were compared. The figures show the concentrations of stress hormones during the various stages of treatment, in comparing pregnant and non pregnant women. Box and whisker plots were used to present the data, without displaying the outliers, which did not fit the scales of the figures. The outliers were used in the statistical analyses. Significantly higher levels of adrenaline at the time of oocyte retrieval (Figure 1B) and embryo transfer (Figure 1C) and on noradrenaline at the time of embryo transfer (Figure 2C) were found in unsuccessfully versus successfully treated women. No significant hormonal differences were found pre treatment (Figures 1A and 2A). In addition, no significant differences were found on cortisol (Figure 3A–C).
The concentration of adrenaline showed a downward trend during treatment (Chi = 10.51; P=0.005). In comparing the different stages of treatment, significantly lower levels of adrenaline were found at T3 in comparison with T1 (Z=−2.2; P=0.03) and T2 (Z=−2.5; P=0.01). No significant changes were found for the concentrations of noradrenaline (Chi = 0.68; P=0.71) or cortisol (Chi = 0.56; P=0.76) during treatment. No significant differences could be observed between unsuccessfully versus successfully treated women with regard to the pattern of hormonal response across treatment.
Discussion
The aim of the present study was to investigate the relationship between pre-treatment psychological findings and stress hormones during IVF/ICSI treatment. Additionally, the hormonal levels at various stages of treatment were compared between successfully and unsuccessfully treated women. Furthermore, the hormonal response during treatment was assessed.
A positive association between adrenaline levels during treatment and scores on pre-treatment depression was found. A similar, although not significant, association was found between adrenaline and pre-treatment anxiety. Cortisol levels at the time of oocyte retrieval were found to be associated with pre-treatment anxiety and depression scores, whereas no associations were found for noradrenaline. The correlation coefficients between questionnaire based and hormonal indicators of stress are comparable with those reported in previous publications (Sanders and Bruce, 1997; Woods et al., 1998).
There was a considerable inter-individual variation in baseline values of the hormones in this study. In a previous study, similar high variation in hormones were found and these could not be explained by menstrual cycle, behavioural, emotional or cognitive stress reactions (Hansen et al., 2001).
Overall, the concentration of adrenaline at the time of ET was found to be significantly lower than the concentrations of adrenaline at the time of oocyte retrieval or at baseline. A remarkable finding in our study was that successful treatment was associated with significantly lower levels of adrenaline at the time of oocyte retrieval and ET and lower levels of noradrenaline at the time of ET. The implantation phase seems to be involved, as other treatment variables were not found to differ between the outcome groups. Whether the effect of adrenaline is direct or indirect, however, needs further exploration. To our knowledge, no previous reports on the effect of adrenaline on treatment outcome in IVF exist.
The correlation of adrenaline concentrations with BDI was higher than with STAI. This could be due to the fact that the chronic nature of the threatening infertility was of more importance than the acute state stress, related to the treatment itself. During treatment a decrease in the concentration of adrenaline was found, which could be explained by high levels of anxiety in anticipation of treatment. No decrease was found in the concentrations of the other stress hormones. This finding is in contrast with Harlow et al. (1996) who found an increase in cortisol during IVF-treatment. However, some of the differences could be explained by the methodology. As most cortisol in blood is bound to a carrier protein, whereas in urine it is unbound, assays measuring cortisol in blood and urine are not necessarily measuring the same thing.
In our study, the stress response during treatment did not differ between women who became pregnant after treatment and those who did not become pregnant. This finding suggests that the response itself cannot account for the observed difference in treatment outcome. Furthermore, in this sample no differences were observed on pre-treatment psychological measures between the two outcome groups, possibly due to the smaller sample size.
Although the relationship between psychosocial stress and infertility has been studied intensively, so far little is known about the effects of catecholamines and cortisol on physiological processes involved in reproduction. Studies encompassing both catecholamines and cortisol in this particular field are extremely rare. Cortisol was studied far more often over the years, thus resulting in more hypotheses.
Catecholamines may affect fertility by altering uterine blood flow (Schenker et al., 1992). Cortisol, having immuno-suppressant properties, may affect immunological conditions needed for implantation. Furthermore, it was found that cortisol levels in follicular fluids in stimulated cycles were correlated with oocyte maturity and in vitro fertilizability (Fateh et al., 1989). A direct effect on granulosa cells affecting steroidogenesis and an influence on oocyte quality was proposed by Michael and Cooke (1994). However, in most studies merely an association is presented, as in our study, since optimal research is hardly possible under ‘in vivo’ circumstances in this field.
Recently, Lewicka et al. (2003) have shown that a higher serum cortisol/follicular cortisol ratio was associated with pregnancy. Csemiczky et al. (2000) found that infertile women have elevated stress levels in terms of circulating prolactin and cortisol levels compared to the fertile controls.
Stress was previously found to be associated with high amounts of activated T cells and reduced implantation rates in IVF-women (Gallinelli et al., 2001). Demyttenaere et al. (1992) indicated that women with high anticipatory state anxiety levels and high anticipatory cortisol concentrations have lower pregnancy rates in IVF. Facchinetti et al. (1997) demonstrated a negative correlation between stress susceptibility and outcome of IVF. Furthermore, Demyttenaere et al. (1991) suggested that personality dependent stress responses are important for conception rates in stimulated cycles. On the other hand, women undergoing IVF were found to respond biophysically differently to psychosocial stressors than controls (Lindheim et al., 1995).
A limitation of the study could be the possibly confounding relation between ovarian and stress hormones. The relation between ovarian hormones and the effect on catecholamines and cortisol was only scarcely investigated so far. Kerdelhue et al. (1997) concluded that the absence of changes in the activity of the corticotrophic axis during the hormonal stimulation of IVF suggests that there was no major stress component associated with the stimulation phase, although in this study cortisol wasn't assessed. Luppa et al. (1995) did find an increased urinary excretion of cortisol metabolites after stimulation with an GnRH agonist, but only in women with polycystic ovarian syndrome, and not in healthy premenopausal women. Hirshoren et al. (2002) found that hormonal changes during the normal menstrual cycle affect noradrenaline, although the correlations with ovarian hormones were very small. Sanders and Bruce (1999) concluded after studying hormonal and psychological measures of stress, that menstrual cycle quality does not account for the association between conception with more favourable moods. Ferin (1999) stated in a review on stress and the reproductive cycle that sex steroids may interact with both central and peripheral substrates of stress, thereby possibly modifying the hypothalamic–pituitary–adrenal axis.
Overall, hormonal treatment, and consequently the altered ovarian hormones, influence hormonal stress responses. The exact mechanism, however, remains unclear.
We conclude that anxiety and especially depression before IVF/ICSI treatment were positively associated with urine adrenaline levels during treatment. However, there was a large overlap in levels of this hormone between successfully and unsuccessfully treated women, which limits the applicability in daily clinical practice. A drop in adrenaline concentrations at the time of ET was found. Lower concentrations of adrenaline at the time of oocyte retrieval and ET and lower levels of noradrenaline at the time of ET were found in women with successful treatment. Adrenaline, therefore, might be an important factor in the complex relationship between psychosocial stress and outcome after IVF/ICSI.
We recommend that future studies relating stress to treatment outcome, should encompass all known aspects of stress, e.g. psychological aspects, the autonomic nervous system and the (neuro) endocrine system.
Adrenaline/creatinin (nmol/mmol of creatinin) during treatment, comparing pregnant and non-pregnant women.
Noradrenaline/creatinin during treatment, comparing pregnant and non-pregnant women.
Cortisol/creatinin during treatment, comparing pregnant and non-pregnant women.
Demographic and psychological characteristics of the participants (n=168)
| Measure . | Mean . | SD . | Range . | Median . |
|---|---|---|---|---|
| Age (years) | 34.3 | 3.5 | 25–42 | 34 |
| Duration of Infertility (years) | 3.7 | 2.1 | 1–13 | 3 |
| State anxiety score | 36.6 | 9.1 | 20–61 | 36 |
| BDI score | 5.4 | 4.7 | 0–27 | 5 |
| Measure . | Mean . | SD . | Range . | Median . |
|---|---|---|---|---|
| Age (years) | 34.3 | 3.5 | 25–42 | 34 |
| Duration of Infertility (years) | 3.7 | 2.1 | 1–13 | 3 |
| State anxiety score | 36.6 | 9.1 | 20–61 | 36 |
| BDI score | 5.4 | 4.7 | 0–27 | 5 |
BDI, Beck Depression Inventory.
Demographic and psychological characteristics of the participants (n=168)
| Measure . | Mean . | SD . | Range . | Median . |
|---|---|---|---|---|
| Age (years) | 34.3 | 3.5 | 25–42 | 34 |
| Duration of Infertility (years) | 3.7 | 2.1 | 1–13 | 3 |
| State anxiety score | 36.6 | 9.1 | 20–61 | 36 |
| BDI score | 5.4 | 4.7 | 0–27 | 5 |
| Measure . | Mean . | SD . | Range . | Median . |
|---|---|---|---|---|
| Age (years) | 34.3 | 3.5 | 25–42 | 34 |
| Duration of Infertility (years) | 3.7 | 2.1 | 1–13 | 3 |
| State anxiety score | 36.6 | 9.1 | 20–61 | 36 |
| BDI score | 5.4 | 4.7 | 0–27 | 5 |
BDI, Beck Depression Inventory.
Characteristics of the hormonal measures (nmol/mmol of creatinin)
| Measure . | n . | Median . | Minimum . | Maximum . | SD . |
|---|---|---|---|---|---|
| Adrenaline T1 | 167 | 0.82 | 0.25 | 6.49 | 0.89 |
| Adrenaline T2 | 167 | 0.81 | 0.18 | 5.90 | 0.81 |
| Adrenaline T3 | 83 | 0.72 | 0.07 | 4.63 | 0.84 |
| Noradrenaline T1 | 167 | 15.98 | 0.97 | 40.00 | 6.54 |
| Noradrenaline T2 | 167 | 15.62 | 0.24 | 44.87 | 6.87 |
| Noradrenaline T3 | 83 | 15.52 | 0.99 | 40.19 | 6.67 |
| Cortisol T1 | 120 | 3.50 | 0.08 | 30.48 | 4.24 |
| Cortisol T2 | 129 | 2.92 | 0.06 | 12.50 | 2.96 |
| Cortisol T3 | 68 | 3.18 | 0.04 | 36.36 | 5.45 |
| Measure . | n . | Median . | Minimum . | Maximum . | SD . |
|---|---|---|---|---|---|
| Adrenaline T1 | 167 | 0.82 | 0.25 | 6.49 | 0.89 |
| Adrenaline T2 | 167 | 0.81 | 0.18 | 5.90 | 0.81 |
| Adrenaline T3 | 83 | 0.72 | 0.07 | 4.63 | 0.84 |
| Noradrenaline T1 | 167 | 15.98 | 0.97 | 40.00 | 6.54 |
| Noradrenaline T2 | 167 | 15.62 | 0.24 | 44.87 | 6.87 |
| Noradrenaline T3 | 83 | 15.52 | 0.99 | 40.19 | 6.67 |
| Cortisol T1 | 120 | 3.50 | 0.08 | 30.48 | 4.24 |
| Cortisol T2 | 129 | 2.92 | 0.06 | 12.50 | 2.96 |
| Cortisol T3 | 68 | 3.18 | 0.04 | 36.36 | 5.45 |
Characteristics of the hormonal measures (nmol/mmol of creatinin)
| Measure . | n . | Median . | Minimum . | Maximum . | SD . |
|---|---|---|---|---|---|
| Adrenaline T1 | 167 | 0.82 | 0.25 | 6.49 | 0.89 |
| Adrenaline T2 | 167 | 0.81 | 0.18 | 5.90 | 0.81 |
| Adrenaline T3 | 83 | 0.72 | 0.07 | 4.63 | 0.84 |
| Noradrenaline T1 | 167 | 15.98 | 0.97 | 40.00 | 6.54 |
| Noradrenaline T2 | 167 | 15.62 | 0.24 | 44.87 | 6.87 |
| Noradrenaline T3 | 83 | 15.52 | 0.99 | 40.19 | 6.67 |
| Cortisol T1 | 120 | 3.50 | 0.08 | 30.48 | 4.24 |
| Cortisol T2 | 129 | 2.92 | 0.06 | 12.50 | 2.96 |
| Cortisol T3 | 68 | 3.18 | 0.04 | 36.36 | 5.45 |
| Measure . | n . | Median . | Minimum . | Maximum . | SD . |
|---|---|---|---|---|---|
| Adrenaline T1 | 167 | 0.82 | 0.25 | 6.49 | 0.89 |
| Adrenaline T2 | 167 | 0.81 | 0.18 | 5.90 | 0.81 |
| Adrenaline T3 | 83 | 0.72 | 0.07 | 4.63 | 0.84 |
| Noradrenaline T1 | 167 | 15.98 | 0.97 | 40.00 | 6.54 |
| Noradrenaline T2 | 167 | 15.62 | 0.24 | 44.87 | 6.87 |
| Noradrenaline T3 | 83 | 15.52 | 0.99 | 40.19 | 6.67 |
| Cortisol T1 | 120 | 3.50 | 0.08 | 30.48 | 4.24 |
| Cortisol T2 | 129 | 2.92 | 0.06 | 12.50 | 2.96 |
| Cortisol T3 | 68 | 3.18 | 0.04 | 36.36 | 5.45 |
Correlations of questionnaire findings and endocrine measurements (nmol/mmol of creatinin) Spearman's ρ
| . | STAI-State . | BDI score . |
|---|---|---|
| Adrenaline T1 | 0.12 (P=0.12) | 0.17 (P=0.04)* |
| Adrenaline T2 | 0.14 (P=0.08) | 0.14 (P=0.08) |
| Adrenaline T3 | 0.21 (P=0.06) | 0.25 (P=0.02)* |
| Noradrenaline T1 | 0.06 (P=0.56) | 0.09 (P=0.28) |
| Noradrenaline T2 | 0.03 (P=0.74) | 0.01 (P=0.86) |
| Noradrenaline T3 | 0.11 (P=0.34) | 0.16 (P=0.14) |
| Cortisol T1 | 0.06 (P=0.52) | 0.13 (P=0.16) |
| Cortisol T2 | 0.20 (P=0.02)* | 0.18 (P=0.04)* |
| Cortisol T3 | −0.08 (P=0.52) | −0.10 (P=0.42) |
| . | STAI-State . | BDI score . |
|---|---|---|
| Adrenaline T1 | 0.12 (P=0.12) | 0.17 (P=0.04)* |
| Adrenaline T2 | 0.14 (P=0.08) | 0.14 (P=0.08) |
| Adrenaline T3 | 0.21 (P=0.06) | 0.25 (P=0.02)* |
| Noradrenaline T1 | 0.06 (P=0.56) | 0.09 (P=0.28) |
| Noradrenaline T2 | 0.03 (P=0.74) | 0.01 (P=0.86) |
| Noradrenaline T3 | 0.11 (P=0.34) | 0.16 (P=0.14) |
| Cortisol T1 | 0.06 (P=0.52) | 0.13 (P=0.16) |
| Cortisol T2 | 0.20 (P=0.02)* | 0.18 (P=0.04)* |
| Cortisol T3 | −0.08 (P=0.52) | −0.10 (P=0.42) |
*Observation significant at P<0.05.
Correlations of questionnaire findings and endocrine measurements (nmol/mmol of creatinin) Spearman's ρ
| . | STAI-State . | BDI score . |
|---|---|---|
| Adrenaline T1 | 0.12 (P=0.12) | 0.17 (P=0.04)* |
| Adrenaline T2 | 0.14 (P=0.08) | 0.14 (P=0.08) |
| Adrenaline T3 | 0.21 (P=0.06) | 0.25 (P=0.02)* |
| Noradrenaline T1 | 0.06 (P=0.56) | 0.09 (P=0.28) |
| Noradrenaline T2 | 0.03 (P=0.74) | 0.01 (P=0.86) |
| Noradrenaline T3 | 0.11 (P=0.34) | 0.16 (P=0.14) |
| Cortisol T1 | 0.06 (P=0.52) | 0.13 (P=0.16) |
| Cortisol T2 | 0.20 (P=0.02)* | 0.18 (P=0.04)* |
| Cortisol T3 | −0.08 (P=0.52) | −0.10 (P=0.42) |
| . | STAI-State . | BDI score . |
|---|---|---|
| Adrenaline T1 | 0.12 (P=0.12) | 0.17 (P=0.04)* |
| Adrenaline T2 | 0.14 (P=0.08) | 0.14 (P=0.08) |
| Adrenaline T3 | 0.21 (P=0.06) | 0.25 (P=0.02)* |
| Noradrenaline T1 | 0.06 (P=0.56) | 0.09 (P=0.28) |
| Noradrenaline T2 | 0.03 (P=0.74) | 0.01 (P=0.86) |
| Noradrenaline T3 | 0.11 (P=0.34) | 0.16 (P=0.14) |
| Cortisol T1 | 0.06 (P=0.52) | 0.13 (P=0.16) |
| Cortisol T2 | 0.20 (P=0.02)* | 0.18 (P=0.04)* |
| Cortisol T3 | −0.08 (P=0.52) | −0.10 (P=0.42) |
*Observation significant at P<0.05.
We gratefully thank all women for their cooperation. We would also like to thank the co-workers of all participating hospitals and Dr Marianne ten Kate-Booy for her contribution to the study. This study was supported by the Dutch Praeventiefonds (grant no. 28-3012).
References
Beck AT and Beamesderfer A (
Boivin J and Takefman J (
Csemiczky G, Landgren BM and Collins A (
Demyttenaere K, Nijs P, Evers-Kiebooms G and Koninckx PR (
Demyttenaere K, Nijs P, Evers-Kiebooms G and Koninckx PR (
Demyttenaere K, Bonte L, Gheldof M, Vervaeke M, Meuleman C, Vanderschuerem D and D'Hooghe T (
Dobson H, Ghuman S, Prabhakar S and Smith R (
Fateh M, Ben-Rafael Z, Benadiva CA, Mastroianni L Jr and Flickinger GL (
Facchinetti F, Matteo ML, Artini GP, Volpe A and Genazzani AR (
Ferin M (
Gallinelli A, Roncaglia R, Matteo ML, Ciaccio I, Volpe A and Facchinetti F (
Gold SM, Zakowski SG, Valdimarsdottir HB and Bovbjerg DH (
Greil AL (
Hansen AM, Garde AH, Skovgaard LT and Christensen JM (
Harlow CR, Fahy UM, Talbot WM, Wardle PG and Hull MG (
Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J and Jaco G (
Johnston M, Shaw R and Bird D (
Kerdelhue B, Lenoir V, Kolm P, Seltman HJ, Jones JW Jr and Jones GS (
Lewicka S, von Hagens C, Hettinger U, Grunwald K, Vecsei P, Runnebaum B and Rabe T (
Lindheim SR, Legro RS, Morris RS, Vijod MA, Lobo RA, Paulson RJ and Sauer MV (
Luppa P, Muller B, Jacob K, Kimmig R, Strowitzki T, Hoss C, Weber MM, Engelhardt D and Lobo RA (
Magiakou MA, Mastorakos G, Webster E and Chrousos GP (
Merari D, Feldberg D, Elizur A, Goldman J and Modan B (
Meulenberg PMM, Ross HA, Swinkels LMJW and Benraad ThJ (
Michael AE and Cooke BA (
Sanders KA and Bruce NW (
Sanders KA and Bruce NW (
Schenker JG, Meirow D and Schenker E (
Schommer NC, Hellhammer DH and Kirschbaum C (
Slade P, Emery J and Lieberman BA (
Smeenk JMJ, Verhaak CM, Eugster A, van Minnen A, Zielhuis GA and Braat DDM (
Spielberger CD, Gorsuch RL and Lushene RE (
Vingerhoets AJJM and Perski A (
Willemsen JJ, Ross HA, Jabobs MC, Lenders JW, Thien T, Swinkels LM and Benraad TJ (
Author notes
Departments of 1Obstetrics and Gynaecology, 2Medical Psychology, 4Chemical Endocrinology and 6Epidemiology and Biostatistics, Radboud University Nijmegen Medical Center, PO Box 9101, NL-6500 HB Nijmegen, 5Department of Clinical Psychology, Radboud University Nijmegen Medical Center, PO Box 9104, NL-6500 HE Nijmegen and 3Department of Clinical Health Psychology, Tilburg University, PO Box 90153, NL-5000 LE Tilburg, The Netherlands