-
PDF
- Split View
-
Views
-
Cite
Cite
A. Fazeli, C. Bruce, D.O. Anumba, Characterization of Toll-like receptors in the female reproductive tract in humans, Human Reproduction, Volume 20, Issue 5, 1 May 2005, Pages 1372–1378, https://doi.org/10.1093/humrep/deh775
Close - Share Icon Share
Abstract
BACKGROUND: Rapid innate immune defences against infection involve the recognition of invading pathogens by specific pattern recognition receptors recently attributed to the family of Toll-like receptors (TLR). Little is known about the in vivo protein expression or distribution of TLR in the female reproductive tract in humans. It is likely that TLR distribution in the female reproductive tract reflects the immunological tolerance to the commensal organisms in lower parts of the tract (vagina, ectocervix and, partially, endocervix) and the intolerance to commensal microbial flora in the upper tract (the uterus and uterine tubes). METHODS: Using immunohistochemistry techniques, distribution of TLR1–6 was studied in surgical sections from the vagina, ecto- and endocervix, endometrium and uterine tubes, obtained from patients undergoing abdominal hysterectomy for benign gynaecological conditions. RESULTS: TLR1, 2, 3, 5 and 6 were present in the epithelia of different regions of female reproductive tract. However, TLR4 was only present in the endocervix, endometrium and uterine tubes and absent in vagina and ectocervix. In addition, a secretory form of TLR4 seems to be produced by the endocervical glands. CONCLUSION: TLR4 may play an important role in modulation of immunological tolerance in the lower parts of the female reproductive tract, and in host defence against ascending infection.
Introduction
In health the vagina is colonized by several hundred bacterial commensal species which have a protective function against infection by pathogenic organisms. Organisms such as the Lactobacillus species serve to maintain vaginal acidity by glycogen cleavage in epithelial cells to release lactic acid (Kasprowicz and Bialecka, 1993). Vaginal acidity appears to play a key role in determining the vaginal microbial flora and preventing ascending infection by pathogenic organisms that thrive under more alkaline conditions (Hillier, 1999). The mucosal surface of the reproductive tract provides a physical barrier against infection, and has adapted to a dynamic non-sterile environment challenged by several antigenic and inflammatory stimuli associated with sexual intercourse and the endogenous vaginal microbial flora. In contrast to the lower parts of the female reproductive tract (vagina, ectocervix and to some extent endocervix), the upper parts of the female reproductive tract, including the uterine cavity and uterine tubes, are virtually free of organisms, with little by way of commensal microbial activity (Heinonen et al., 1985). The mechanisms which account for this contrasting distribution of organisms between the upper and lower parts of the female reproductive tract are not known. It is likely that the divergent immunological tolerance of the tract towards microorganisms is modulated by an alert innate immune system in the upper regions of the female reproductive tract, and a tolerant system in the lower parts of the tract.
Rapid innate immune defences against infection usually involve the recognition of invading pathogens by specific pattern recognition receptors recently attributed to the family of Toll-like receptors (TLR). Originally identified in the early Drosophila larvae (Stein et al., 1991), 10 structurally related mammalian receptor proteins have now been identified (Akira, 2003). Consistent with their role in pathogen recognition, TLR family members are expressed by cells involved in the first line of host defence, including neutrophils, macrophages, dendritic cells, dermal endothelial cells and mucosal epithelial cells. Collectively, TLR function to alert the immune system to the presence of microorganisms. The different members of the TLR family are expressed on different cell organelles and appear to mediate signal transduction to different antigenic stimuli by engaging with specific ligands leading to the production of various pro-inflammatory cytokines, chemokines and effector molecules, depending on the cell type that is activated (Hirschfeld et al., 2001; Jones et al., 2001).
The role and signal transduction mechanisms for the various members of the Toll receptor family are increasingly becoming recognized. Although they have been shown to participate in the recognition of pathogens by the innate immune system, it is not clear how a restricted family of receptors has the capacity to recognize the wide spectrum of TLR stimuli known to exist. For example, it has been shown that two members of the TLR family, TLR2 and TLR6, together coordinate macrophage activation by Gram-positive bacteria and the yeast cell-wall particle, zymosan. They are both required for macrophage recognition of peptidoglycan, a Gram-positive pathogen component. But only TLR2 is required for the recognition of bacterial lipopeptide (Ozinsky et al., 2000). It seems the cytoplasmic domain of TLR2 can form functional heteromeric complexes with TLR6 or TLR1, ultimately leading to cytokine induction. In contrast, some other TLR are active as homomeric complexes. Double-stranded RNA and mRNA from cells killed by invading pathogens seem to stimulate TLR3 (Alexopoulou et al., 2001; Kariko et al., 2004). The major component of the outer membrane of Gram-negative bacteria, lipopolysaccharide (LPS), is recognized by TLR4 in association with CD14 and MD-2 (Akashi et al., 2001; da Silva Correia et al., 2001; Nagai et al., 2002). TLR5 recognizes bacterial flagellin (Hayashi et al., 2001). TLR7 and TLR8 have not been shown to recognize a naturally occurring microbial product, although small antiviral molecules, such as imiquimod and resiquimod, stimulate cells through TLR7 and TLR8 (Jurk et al., 2002). Bacterial unmethylated DNA binds TLR9 (Hemmi et al., 2000). The ligand for the more recently discovered TLR10 is not known (Chuang and Ulevitch, 2001).
It has previously been reported that some human reproductive tract epithelial cell lines (endocervical, ectocervical, vaginal and uterine cell lines) express several TLR genes (Fichorova et al., 2002; Schaefer et al., 2004). However, nothing is known about the in vivo protein expression or distribution of TLR in the female reproductive tract in humans. In addition the potential role of TLR in mediating innate immunity in the female reproductive tract is not understood.
In the current investigation, we hypothesized that distribution of TLR1–6 in the epithelial surface of the female reproductive tract is likely to reflect the immunological tolerance to the commensal organisms in the lumen of the vagina, ectocervix and to some extent endocervix, and the intolerance to commensal microbial flora in the upper tract (the uterus and uterine tubes). We therefore investigated the presence of TLR1–6 molecules in surgical sections from the vagina, ecto- and endocervix, endometrium and uterine tubes obtained from patients undergoing abdominal hysterectomy for benign gynaecological conditions. Our results showed that except for TLR4, TLR1, 2, 3, 5 and 6 were present in the epithelia of different regions of female reproductive tract. We also demonstrated a unique distribution of TLR4 in the cervix which may suggest a key role in preventing ascending bacterial infections of the genital tract.
Materials and methods
Patients and samples
Tissue from nine (eight Caucasians and one African) patients undergoing hysterectomy for benign gynaecological conditions were studied. The median age (range) of the women was 45 (33–56) years. Two of the subjects were post-menopausal, two were in the secretory whilst four were in the proliferative menstrual phase. Menstrual phases were consistent with endometrial histological dating. Women on hormonal therapy at the time of their operation and those with a recent history of genital tract infection were excluded. Written informed consent was obtained prior to the collection of samples, and the study was approved by the local institutional Research Ethics Committee. Full thickness wedge biopsies including epithelial and stromal tissue were freshly taken post-operatively from five sites: uterine tubes, endometrium, endocervix, ectocervix and vagina. Small sections of tissue (5 × 5 mm) were fixed in 10% formalin. Tissue sections (3 mm) were prepared from these samples. In addition, tissue sections (∼5 × 5 mm) from the same samples were embedded in optimal cutting temperature compound (VWR, UK) and snap-frozen in hexane (Sigma–Aldrich, UK) suspended in liquid nitrogen. The cryosections (5 mm) were prepared and stored at −80 °C until use.
Antibodies and peptides
Antibodies and peptides used in the experiments were obtained from Santa Cruz Biotechnology Inc. (USA). These were goat polyclonal antibodies specific for N-terminal domains of TLR1, TLR2, TLR3, TLR5, TLR6 (catalogue nos., sc8687, sc8689, sc8691, sc8695, sc5657 respectively) and goat polyclonal antibody specific for C-terminal domains of TLR4 (catalogue no. sc8694). Blocking peptides specific for the respective antibodies were used to detect non-specific staining.
Immunostaining
Cryosections were air-dried before use for 30 min, fixed in acetone at −20 °C for 10 min, air-dried again for 30 min, and then washed in phosphate-buffered saline (PBS).
Formalin-fixed sections were dewaxed in xylene twice for 5 min, followed by rehydration in graded ethanol. Endogenous peroxidase activity was quenched in the paraffin sections by incubating in 3% v/v hydrogen peroxidase in methanol for 20 min. Antigen retrieval on these sections was performed by microwave irradiation for 12 min in 10 mmol/l sodium citrate pH 6.0. Sections were allowed to cool for 15 min and then washed in PBS.
Both formalin-fixed and cryopreserved slides were then stained using a Vectastain Elite ABC peroxidase kit (Vector Laboratories Ltd, UK). In addition, to avoid non-specific binding, an avidin/biotin blocking kit (Vector) was used. Briefly, slides were blocked for 1 h at room temperature in PBS containing 0.2% v/v horse serum and 25% v/v avidin supplied in blocking kit. The block was removed and slides were incubated for 2 h at room temperature in primary antibody at an appropriate dilution using antibody diluent media (Dakocytomation Ltd, UK) and 250 ml biotin per ml of diluted antibody. Binding was visualized by incubation with peroxidase substrate AEC (3-amino-9-ethylcarbazole) (Vector) for 10 min, washed in distilled water for 3 min and counterstained in 10% haematoxylin for 10 min. Slides were washed in tap water for 2 min and mounted with Aquamount (VWR).
Optimum staining was achieved by incubating tissue sections with different concentrations of TLR antibodies (TLR1, TLR2, TLR3, TLR4, TLR5 and TLR6, with 4, 4, 10, 10, 4 and 10 μg/ml respectively). Control sections were obtained by omission of the primary antibody. Specific TLR antibody stainings were blocked by coincubation of diluted TLR antibody with 20-fold concentration of the corresponding specific peptide overnight at 4 °C. The blocked primary antibody was then used in immunoperoxidase staining as described above. Immunostained sections were examined using an Olympus BH2 microscope at ×125, ×250 and ×500 magnification (Olympus, UK).
Results
Formalin-fixed sections provided the best immunological and morphological staining results with nearly all antibodies against TLR except anti-TLR2. In the case of anti-TLR2 antibody the best results were obtained by performing staining on cryosections.
TLR1
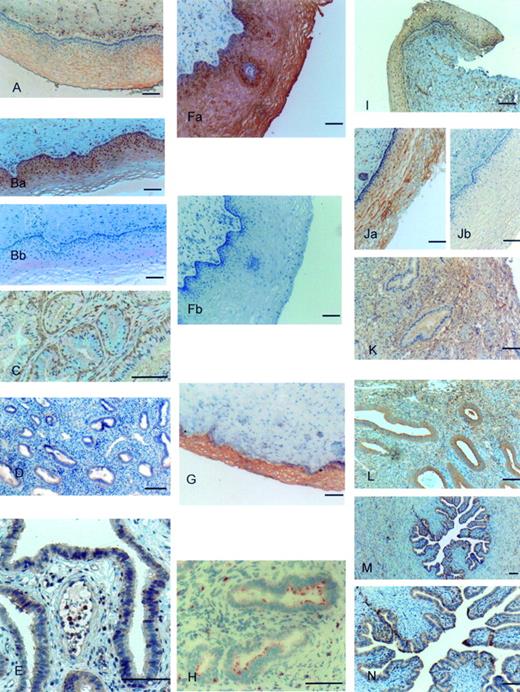
Vaginal epithelium was strongly stained for TLR1 (Figure 1A). Staining was mainly limited to the cytoplasm of epithelial cells. In contrast in ectocervix, TLR1 staining was intense at the basal layer of the epithelium compared to apical layers (Figure 1Ba). The smooth muscle cells in stroma and those surrounding blood vessels were also stained positive for TLR1. There was slight staining of endothelial cells lining the blood vessels. No staining, however, was found in the cytoplasm of secretory glands of endocervix (Figure 1C). In these sections staining was limited to basal layers of cells lining these glands (Figure 1C). Both endometrial glands (Figure 1D) and epithelium of uterine tubes (Figure 1E) were intensively stained for TLR1 as well as muscle cells within the myometrium. Blocking of the anti-TLR1 antibody with specific peptide markedly decreased the staining (Figure 1Bb).
TLR2
TLR2 was highly expressed in the epithelium of vagina (Figure 1Fa). There was some weak staining of muscle cells within the stroma of the vagina. However, no staining with anti-TLR2 antibody was observed in the endothelial cells lining the blood vessels of vaginal sections. Epithelium of ectocervix was very intensively and uniformly stained with anti-TLR2 antibody (Figure 1G) and some staining was also present on the endothelial cells lining the blood vessels. Epithelia of uterine tubes and endometrium were positive for TLR2. In endocervix, anti-TLR2 staining was observed on small patches of the apical part of the glands (Figure 1H). Blocking of the anti-TLR2 antibody with specific peptide completely abolished the staining (Figure 1Fb).
TLR3
Vaginal epithelium was positively stained with anti-TLR3 antibody. There was marked staining of the subepithelial stroma (Figure 1I). Strong cytoplasmic staining was present in ectocervical epithelium (Figure 1Ja). Anti-TLR3 antibody staining was not present in the cytoplasm of the endocervical glands but was well presented on the apical and basal plasma membranes of these cells (Figure 1K). There was strong anti-TLR3 antibody staining present in the stromal layers of endocervix (Figure 1K). Cytoplasm of the endometrial glands showed intense staining of anti-TLR3 antibody (Figure 1L). Epithelia of uterine tubes were strongly positive for anti-TLR3 antibody (Figure 1M and N). Blocking of the anti-TLR3 antibody with specific peptide completely abolished the staining (Figure 1Jb).
TLR4
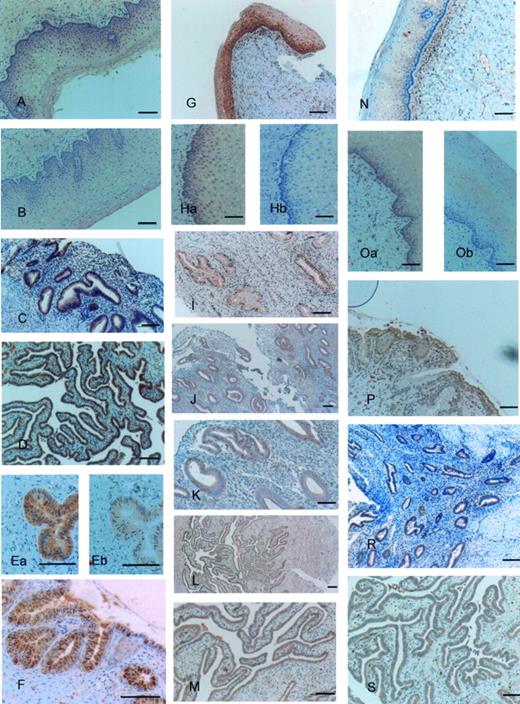
Epithelia of vagina and ectocervix were both negative for anti-TLR4 antibody. There was a very weak staining just present on the apical parts of the epithelium (Figure 2A and B). In contrast intense staining by anti-TLR4 antibody was present in the epithelium and cells lining the endocervical glands (Figure 2Ea and F). Anti-TLR4 antibody stained vacuole-like structures in the endocervical glands. These structures seemed to be destined for secretion from the endocervical glands and, in some sections, seemed to be secreted out of the endocervical glands (Figure 2Ea and F). Endometrial glands (Figure 2C) and epithelia of uterine tubes (Figure 2D) were positive for TLR4. Blocking of the anti-TLR4 antibody with specific peptide markedly decreased the staining (Figure 2Eb).
TLR 5
There was intensive staining by anti-TLR5 antibody in basal sections of vaginal (Figure 2G) and ectocervical epithelium (Figure 2Ha). It seemed that a gradient of staining existed from the apical to the basal regions of the epithelium (Figure 2G). Endothelial cells lining the blood vessels and muscle cells were positive for anti-TLR5 antibody in both tissues. Strong staining was present in endocervical (Figure 2I) and endometrial glands (Figure 2J and K), and in the epithelium of the uterine tubes (Figure 2L and M). Blocking of the anti-TLR5 antibody with specific peptide completely abolished the staining (Figure 2Hb).
TLR 6
Staining with anti-TLR6 antibody was mainly observed in the basal part of the epithelium and adjacent stroma of the vaginal sections (Figure 2N). Ectocervical epithelium was uniformly stained with anti-TLR6 antibody (Figure 2Oa). Endocervical glands were also positive for anti-TLR6 antibody with intense staining of the apical epithelium (Figure 2P). The epithelium of the endometrial glands (Figure 2R) and uterine tubes (Figure 2S) were both stained positive for anti-TLR6 antibody. Blocking of the anti-TLR6 antibody with specific peptide markedly decreased staining (Figure 2Ob).
Discussion
TLR constitute a major part of the innate immune system and several reports exist on determination and characterization of TLR in different tissues and organs (Bsibsi et al., 2002; Zarember and Godowski, 2002; Backhed and Hornef, 2003; Basu and Fenton, 2004). However, little has been done to identify TLR in the female reproductive tract. To the best of our knowledge our study is the first to demonstrate the in vivo distribution of TLR in the human female reproductive tract. The TLR molecules 1, 2, 3, 5 and 6 were present in the epithelium of all the tissues studied in the current investigation. Only TLR4 showed a differential expression, being present in endocervix, endometrium and uterine tubes but absent in vagina and ectocervix. Irrespective of the age and status of reproductive cycle, the TLR picture observed was the same in all the patients. Although the number of patients involved in the current investigation was small, the finding of no obvious cyclic changes in TLR immunoreactivity in the pre-menopausal reproductive tissue, and, more importantly, its persistence in the post-menopausal reproductive tissue, may indicate that TLR are expressed in the reproductive tissue constitutively, independently of ovarian hormones. Because of the limited number of patients involved in the current investigation, future studies involving a larger cohort of patients (preferably in their reproductive years), in combination with tissue laser dissection techniques, quantitative genomic and proteomic technologies are needed to examine hormonal influences on the expression of TLR genes and proteins to verify this point.
Like Fichorova et al. (2002) we can only speculate about the possible evolutionary pressure that would lead to such differential expression of TLR4 in upper and lower parts of female reproductive tract in humans. It has been suggested that the lack of TLR4 expression in intestinal epithelial cells might prevent the constant pro-inflammatory gene activation that could occur with exposure to normal enteric flora and might account for some of the chronic intestinal inflammation associated with disorders such as inflammatory bowel disease (Cario and Podolsky, 2000; Cario et al., 2000; Abreu et al., 2001). The lower female genital tract also has a complex ecosystem, and it is possible that a similar model might exist in this compartment. Although the activation of the innate immune response is critical to control infection caused by pathogenic microorganisms, excessive cytokine production without proper regulation is harmful to the host and may lead to microcirculatory dysfunction, tissue damage, shock, or even death in extreme cases (Beutler et al., 1985; Danner et al., 1991). Certainly, a threshold of sensitivity to bacterial components in the genital tract is required to avoid unnecessary inflammation. While inflammation is essential for clearing bacterial infections, excessive inflammation would be particularly detrimental to the defence function of the mucosal surface.
Two previous studies have looked at TLR gene expression in reproductive cell cultures in humans. Schaefer et al. (2004), employing RT–PCR, demonstrated the expression of TLR1–9, but not TLR10 genes in a uterine epithelial cell line (ECC-1), generated from an adenocarcinoma of the human endometrium. In another study, Fichorova et al. (2002) studied the presence of TLR1–6 genes in immortalized endocervical, ectocervical and vaginal epithelial cell lines and primary endocervical and ectocervical epithelial cells. They demonstrated mRNA for TLR1, 2, 3 and 6 in all specimens. In contrast no PCR product was detected for TLR4 in either of the cell populations. Furthermore, they reported that a discrepancy in the expression of TLR5 existed between the immortalized cell lines and the primary cell cultures: the immortalized cell lines expressed TLR5 whilst the primary cells failed to express TLR5.
We found TLR1–6 in the human endometrial biopsies in agreement with the observations of Schaefer et al. (2004). However, our results are in disagreement with those of Fichorova et al. (2002) who did not observe TLR4 expression in endocervix. We found strong immune staining of endocervical epithelium by anti-TLR4 antibody. This observation is unlikely to be due to a non-specific reaction of the anti-TLR4 antibody employed in our studies. The anti-TLR4 antibody reaction that we observed was specific and the staining markedly decreased when the antibody was preincubated with the corresponding specific peptide (Figure 2Eb). In addition the use of this antibody has been validated by several other studies for localization of TLR4 in human tissue and cell cultures (Bsibsi et al., 2002; Baker et al., 2003; Pivarcsi et al., 2003). The discrepancy between our results and that of Fichorova et al. (2002) may arise because we have studied in vivo human tissue as against the in vitro cell culture and neoplastic cell lines used for some of the studies reported by Fichorova et al. A similar discrepancy between in vivo and in vitro cell cultures in the localization of pattern recognition receptors has been observed in gut epithelium in mouse. For example CD14 and TLR4 were positive in the small intestine in vivo sections (Ortega-Cava et al., 2003) and in cell line m-ICcl2 (Hornef et al., 2002), while the mouse rectum carcinoma cell line CMT93 was CD14 negative (Cario et al., 2000). Although these observations in mouse cell lines may represent differential expressions of CD14 and TLR4, caution needs to be exercised in making conclusions about in vivo receptor expression or activity based on ex vivo observations.
An interesting observation in the current investigation was the presence of secretory granule-like objects in endocervical glands positive for TLR4. In some cases, it seemed that these secretory granules were transported out of the cells and were secreted out of the endocervical glands. If true, this may represent a secretory form of TLR4 in humans. TLR2 (LeBouder et al., 2003) and TLR4 (Iwami et al., 2000) secretory forms have been previously described in humans and mice respectively. The secretory form of TLR4 appears to be the product of alternative splicing of the mouse TLR4 gene. However, the significance and biological function of soluble TLR4 in mice is not completely understood. In mice, soluble TLR4 appears to inhibit LPS-mediated signals but at the same time soluble TLR4 mRNA appears to be induced by LPS (Iwami et al., 2000). It is reasonable to presume that soluble TLR4 in mice works as a feedback mechanism to inhibit the excessive LPS responses in macrophages and T cells. It is therefore plausible that any soluble TLR4 observed in human endocervix may have a down-regulatory function. Such a role would be consistent with the specific and unique localization of this molecule in the endocervix which defines the boundary between the upper and lower female reproductive tract. It is possible that soluble TLR4 plays a role in suppression of the innate immune system in the female reproductive tract, preventing unnecessary activation of the inflammatory process. Future investigations should be directed towards understanding the nature and significance of this secretory form of TLR4 in the human reproductive tract.
In conclusion, our study for the first time demonstrates the in vivo localization of TLR1–6 in the female reproductive tract of humans. These TLR were widely expressed in female reproductive tract tissue. The only uniquely localized receptor was TLR4 which was absent from ectocervical and vaginal epithelium but demonstrated strong immune staining in the endocervical, endometrial and tubal epithelium. Interestingly a secretory form of TLR4 appears to be actively produced by human endocervical glands. The absence of TLR4 in vaginal and endocervical epithelium, as well as the presence of a secretory form of TLR4 in endocervical glands, may have important implications in modulation of immunological tolerance in the lower parts of the female reproductive tract, and may play a unique role in host defence against ascending infection.
Toll-like receptor (TLR) 1, 2 and 3 expression in human female reproductive tract tissues. Immunostaining was performed using the avidin–biotin–peroxidase technique and polycolonal antibodies specific for the N-terminal domains of TLR1, TLR2 and TLR3. TLR1 expression was mainly visible in epithelia of vagina (A), ectocervix (Ba), basal part of endocervical glands (C), endometrial glands (D) and uterine tube epithelium (E). TLR2 was highly expressed in vaginal epithelium (Fa) and ectocervix epithelium (G) as well as endometrial glands and uterine tube epithelium. TLR2 stained small patches on the apical parts of endocervical glands (H). Vaginal epithelium (1I), ectocervical epithelium (Ja) and uterine tube epithelia (M and N) were all stained with anti-TLR3 antibody. Apical and basal plasma membranes of endocervical glands (K) as well as cytoplasm of endometrial glands (L) showed strong staining with anti-TLR3 antibody. Blocking of the anti-TLR1 (Bb), TLR2 (Fb) and TLR3 (Jb) antibodies with respective specific peptides markedly decreased or totally abolished the staining.
Toll-like receptor (TLR) 4, 5 and 6 expression in human female reproductive tract tissues. Immunostaining was performed using the avidin–biotin–peroxidase technique and polycolonal antibodies specific for the C terminal domain of TLR4 and the N-terminal domains of TLR5 and TLR6. Epithelia of vagina (A) and ectocervix (B) were both negative for anti-TLR4 antibody and only very weak staining was present on the apical parts of epithelium. Intense staining of anti-TLR4 was present in the endocervical glands (Ea and F), endometrial glands (C) and uterine tube epithelia (D). TLR5 expression was visible in epithelia of vagina (G), ectocervix (Ha), endocervical glands (I), endometrial glands (J and K) and uterine tubes epithelium (L and M). TLR6 was highly expressed in vaginal epithelium (N) and ectocervix epithelium (Oa) as well as endocervix (P), endometrial glands (R) and uterine tube epithelium (S). Blocking of the anti-TLR4 (Eb), TLR5 (Hb) and TLR6 (Ob) antibodies with respective specific peptides markedly decreased or totally abolished the staining.
We gratefully acknowledge funding support for this study by the Sheffield Teaching Hospitals Trust.
References
Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET and Arditi M (
Akashi S, Nagai Y, Ogata H, Oikawa M, Fukase K, Kusumoto S, Kawasaki K, Nishijima M, Hayashi S, Kimoto M et al. (
Alexopoulou L, Holt AC, Medzhitov R and Flavell RA (
Backhed F and Hornef M (
Baker BS, Ovigne JM, Powles AV, Corcoran S and Fry L (
Basu S and Fenton MJ (
Beutler B, Milsark IW and Cerami AC (
Bsibsi M, Ravid R, Gveric D and van Noort JM (
Cario E and Podolsky DK (
Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker H-C and Podolsky DK (
Chuang T and Ulevitch RJ (
da Silva Correia J, Soldau K, Christen U, Tobias PS and Ulevitch RJ (
Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM and Parillo JE (
Fichorova RN, Cronin AO, Lien E, Anderson DJ and Ingalls RR (
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM and Aderem A (
Heinonen PK, Teisala K, Punnonen R, Miettinen A, Lehtinen M and Paavonen J (
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K et al. (
Hillier SL (
Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM and Vogel SN (
Hornef MW, Frisan T, Vandewalle A, Normark S and Richter-Dahlfors A (
Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T and Yoshikai Y (
Jones BW, Heldwein KA, Means TK, Saukkonen JJ and Fenton MJ (
Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G and Bauer S (
Kariko K, Ni H, Capodici J, Lamphier M and Weissman D (
Kasprowicz A and Bialecka A (
LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP et al. (
Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M and Miyake K (
Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y and Kinoshita Y (
Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L and Aderem A (
Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, Beer Z, Bata-Csorgoo Z, Magocsi M, Rajnavolgyi E et al. (
Schaefer TM, Desouza K, Fahey JV, Beagley KW and Wira CR (
Stein D, Roth S, Vogelsang E and Nusslein-Volhard C (