-
PDF
- Split View
-
Views
-
Cite
Cite
Benoît Schubert, Michel Canis, Claude Darcha, Christine Artonne, Jean-Luc Pouly, Pierre Déchelotte, Daniel Boucher, Geneviève Grizard, Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation, Human Reproduction, Volume 20, Issue 7, 1 July 2005, Pages 1786–1792, https://doi.org/10.1093/humrep/dei002
Close - Share Icon Share
Abstract
BACKGROUND: The scarcity of human ovarian tissue is a major problem in developing research on ovarian cryopreservation. We were interested in ovarian cortex surrounding benign ovarian cysts harvested during their requisite operations. METHODS: Ovarian tissue was collected from 25 women (mean age = 27.7 ± 1.0 SEM) and frozen in serum-free cryoprotective medium. Histological and viability analysis were performed on fresh and frozen–thawed slices of tissue. RESULTS: Dermoid (n=7), endometriosis (n=13) and serous (n=5) cysts were observed. Follicular densities (expressed per mm3) in ovarian cortex surrounding dermoid cysts were higher than in endometriosis and serous cysts for both histological (median of follicular densities: 13.04, 0.31 and 0.89 respectively) and viability analysis (2.93, 0.05 and 0.71 respectively). Freezing–thawing did not result in gross abnormality of follicle population either in number or morphology (80% of follicles preserved a normal pattern). However, a slight decrease of the density of living follicles (expressed per mm2) was reported. CONCLUSIONS: Ovarian cortex surrounding ovarian cysts, especially dermoid cysts, could be considered a source of ovarian tissue for future research. In our study, the cryopreservation procedure resulted in high follicular survival assessed by both histological and viability analysis. Nevertheless, further studies of in vivo and in vitro follicular maturation are needed to strengthen this model.
Introduction
Ovarian tissue cryopreservation is now performed for young women and even for very young girls (Poirot et al., 2002) prior to having treatments with high gonadotoxic risks. This emerging assisted reproductive technology has become widely used and is increasingly proposed to these patients. Great expectations were held following the work of Oktay and Karlikaya (2000), Callejo et al. (2001), Radford et al. (2001) and Schmidt et al. (2004). These gave the first evidence that cryopreserved human ovarian autologous grafts had endocrine and exocrine functions. Furthermore, embryos were not only obtained (Oktay et al., 2004), but the birth of a healthy girl was recently reported after cryopreservation and transplantation (Donnez et al., 2004).
Much work has been done in animal studies. This had an ulterior purpose as it was also motivated by the desire to preserve endangered species. Pregnancies and births have been reported in different animal species after ovarian tissue cryopreservation and autologous grafts, such as mice (Gunasena et al., 1997; Candy et al., 2000), and larger animals such as ewes (Gosden et al., 1994; Salle et al., 2002) and rhesus monkeys (Lee et al., 2004). The animal model is an important and common source of tissue for research. Human tissue, however, has its own characteristics not necessarily shared by animal models. We believe research work performed with human tissue is superior in order to understand and improve cryopreservation, grafting and in vitro maturation techniques. There remains the delicate problem of the source of this human ovarian tissue. It has endocrine and exocrine functions that decrease throughout life, and oocytes do not regenerate. Moreover, it is well known that age is a key factor concerning follicular content (Faddy, 2000). Therefore, the best tissue to use in a research programme would be that which could be harvested from young women.
Retrieving ovarian cortical tissue is commonly done through an invasive procedure, and most units working on human ovarian tissue have retrieved specimens during operations performed for patients who have faced medical problems that would affect their fertility, e.g. breast cancer and acute leukaemia (Gook et al., 2001; Kim et al., 2002), during Caesarean section (Newton et al., 1996; Oktay et al., 1997, 1998; Oktay and Karlikaya, 2000; Hreinsson et al., 2002; Scott et al., 2004), or during gynaecological or infertility evaluation (Hovatta et al., 1999; Nisolle et al., 2000).
According to the work from Maneschi et al. (1993), normal morphological patterns have been observed in the cortex surrounding ovarian benign cysts. Thus, we were interested in that particular tissue, since ovarian cysts often occur in young women. Indeed, from 1980 to 1996, we reported 1140 patients in our centre with benign ovarian cysts (Canis et al., 2000), and the mean age of the patients who underwent laparoscopy for an adnexal cystic mass was 35.8 ± 12.6 years (Canis et al., 1994).
The aim of this study was to define whether the use of human ovarian cortex surrounding benign cysts was an appropriate model to study ovarian tissue, and particularly the effect of cryopreservation. We describe these cysts and report the results of the histology and viability analyses for fresh and frozen–thawed tissue for each piece of ovarian tissue harvested.
Material and methods
Ovarian tissue collection
This study was performed under a protocol approved by our regional research ethics committee (Comité Consultatif des Personnes se Prêtant à la Recherche Biomédicale d'Auvergne). Ovarian tissue was collected from 25 women aged 19 to 36 years (mean ± SEM: 27.7 ± 1.0) during the operation treating their ovarian cyst, after giving informed consent to participate in the study. The size of the cyst, estimated by ultrasonography the day before surgery, was >40 mm. Using strict guidelines previously published, pre- and peri-operative evaluation and laparoscopic diagnosis of the cyst was undertaken (Canis et al., 1994, 2000). The ovarian cystectomy was performed as previously described (Canis et al., 1992). After the procedure, the surgeon cut a 1–2 cm2 piece of ovarian cortex overlying the cyst with scissors and without coagulation. The pathologist performed histological examination and only benign ovarian cysts were included in our study.
The specimen was immediately plunged in ‘medium A’ and transported at room temperature. Medium A was composed of: NaCl (94.7 mmol/l), KCl (4.8 mmol/l), MgSO4 (0.8 mmol/l), NaH2PO4 (1.0 mmol/l), NaHCO3 (25.0 mmol/l), CaCl2 (1.8 mmol/l), sodium lactate (21.3 mmol/l), sodium pyruvate (0.3 mmol/l), glucose (5.5 mmol/l), L-glutamine (25.0 mmol/l), taurine (0.5 mmol/l), and 0.5% of human serum albumin (HSA). All products were purchased from Sigma (France) except HSA, which was provided by LFB (France). Generally, there was little or no medulla that had to be removed in order to obtain a 1 mm thick slice of ovarian cortex. The ovarian cortex was cut into slices (two to nine) of ∼10–75 mm2, keeping the tissue immersed in medium A. At least one slice was used for each of the histological and viability analyses.
Tissue cryopreservation
The cryopreservation medium (medium B) consisted of medium A without CaCl2 and supplemented with HEPES (21.8 mmol/l), glycine (50.0 mmol/l), propanediol (3.0 mol/l) and raffinose (0.05 mol/l) (Sigma). The slices were transferred to a 50 ml vial (Falcon; Fisher Bioblock Scientific, France) containing medium A; then the solution was diluted 1:1 (v/v) by medium B at room temperature. In order to ensure a uniform exposure of the tissue to the cryoprotectants, the vials were gently shaken and medium B was added in three steps. After dilution, the mixture was maintained for 15 min at room temperature for equilibration. Each slice immersed in this cryoprotective solution was then transferred to a 1.8 ml cryovial (Nunc, Fisher Bioblock Scientific) and loaded in a programmable freezer (minicool 40 PC; Air liquide, France). The freezing procedure was based on the technique used for ewes' ovarian tissue by Demirci et al. (2001) that had excellent results. A thermal probe placed in a cryovial containing the cryoprotective solution free of tissue recorded the freezing program. The cooling rate was 2°C/min from room temperature to −11°C, at which nucleation was induced by semi-automatic seeding, i.e. injection of a high amount of liquid nitrogen into the freezer. The temperature was lowered to −40°C at 2°C/min and then at 10°C/min to −150°C. Finally, the cryovials were plunged into liquid nitrogen for storage for between 4 days to 4 months prior to usage.
The cryovials were rapidly thawed in an incubator at 37°C for 5 min. In order to limit the osmotic stress, the cryoprotective solution was progressively diluted by medium A with gentle shaking to promote efflux of cryoprotectant from the tissue. Then the tissue slice was transferred to medium A and washed twice with this medium, before viability analysis or fixation for histological study.
Histology
We calculated the number of follicles per unit of volume in the cortex surrounding benign ovarian cysts. Primordial and primary follicles (diameters: 35 ± 6 and 46 ± 6 μm respectively; Gougeon and Lefèvre, 1997) were the most represented.
The slices of ovarian cortex were fixed in Bouin's solution, embedded in paraffin wax, cut into 4 μm sections and stained with haematoxylin and eosin. These were performed at 120 μm intervals to avoid double counting of follicles. All oocytes with a large cytoplasmic area, with or without a visible nucleus, were counted on each section. Ten to 20 sections were analysed for each patient. All sections were analysed using an Olympus microscope (model BX 40; Olympus, Germany) at ×400 magnification. The developmental stages of the follicles were evaluated as defined by Gougeon (1986): in a primordial follicle the oocyte is surrounded by flattened granulosa cells (GC) or a mixture of flattened and cuboidal GC; in the primary follicle, the oocyte is surrounded by a single layer of cuboidal GC; in the secondary follicle, the oocyte is surrounded by two or more layers of GC; in the preantral follicle, small fluid-filled cavities appear between GC; and in antral follicles, these fluid-filled cavities are aggregated to form the antrum.
To estimate the follicular density, the histological sections were digitized via a Matrox Meteor MC/4 card (Samba technologies, France) in conjunction with a Sony 3CCD colour camera DXC 950P (Sony Corp., Japan). The area of the sections was determined by delineation of the tissue boundary using IPS 32 version 4.27 software (Samba technologies). The volume of the analysed ovarian tissue (V) was then calculated by the formula: V (mm3)=S×0.06, where S is sum of all the sections areas (mm2) and 0.06 mm is the estimated thickness of the tissue analysed. Considering the average follicular size, the same number of follicles was presumed to be present 30 μm above and below the tissue section. This gave an estimated tissue thickness of 60 μm.
The density of primordial and primary follicles in the ovarian cortex was calculated as the total number of follicles divided by the volume of the cortex analysed and expressed as the number of follicles per mm3 of ovarian cortex.
In addition, a qualitative analysis of the follicles was performed. In this view, the cytoplasm and nucleus of GC and oocyte were carefully examined. Only follicles containing an oocyte with a clearly visible nucleus and surrounded by GC were considered. Contraction of cytoplasm, pyknotic nucleus, and large empty spaces between GC and the follicle's basal membrane were considered as signs of atresia (Gougeon, 1986). Other follicles were considered as morphologically normal.
Viability
The Live/Dead® Viability Cytoxicity kit (Molecular Probes, France) was used. The slice of ovarian cortex was cut into tiny pieces (<1 × 1 mm) and incubated with 2 μmol/l calcein AM and 4 μmol/l ethidium homodimer for 45 min at 37°C. Esterase activity of living cells converts the non-fluorescent cell–permeant calcein AM into an intensely fluorescent calcein. The polyanionic dye calcein is well retained within cytoplasm of living cells, producing a uniform green fluorescence (excitation/emission 495/515 nm). Ethidium homodimer enters cells with damaged membranes and counterstains the nuclei of all dead cells in red (excitation/emission 495/635 nm). After exposure to the dyes according to the manufacturer's instructions, the specimens were washed twice in phosphate-buffered saline (Sigma) and placed on slides and covered with a cover-slip before analysis under an epifluorescence microscope (Nikon, France) at ×100 magnification. A conventional fluorescein longpass filter, by which calcein AM and ethidium homodimer could be viewed simultaneously, was used. The green dye stained the whole cytoplasm of the living cells and, as the oocyte is the largest cell of the cortex, follicles appeared clearly as bright green spherical structures. The ethidium homodimer stained the nucleus of the dead cells. The size of the oocyte's nucleus was not greatly different from that of the other cells; consequently non-viable oocytes could not be distinguished from other dead cells. It would have been useful to distinguish living and dead follicles, but only the living follicular density could be estimated. This was done according to the surface of the slice measured before analysis and expressed per mm2 of ovarian cortex. In order to try to ascertain whether these spherical structures were follicles, histological analysis was performed on the piece of tissue used in the assay. However, the quality of the tissue was poor due to the prior treatment procedure for the viability test. Nonetheless, the presence of primordial and primary follicles was confirmed on this histological staining.
Statistical analysis
Statistical analyses were performed with Statview for windows, version 5.0, SAS Institute Inc. The Mann–Whitney U-test was used to investigate the differences in the age of the patients. The χ2-test was used for statistical analysis of the difference in the proportion of morphologically normal follicles in fresh and frozen–thawed tissue. The relationship between the follicular densities in fresh tissue and the size of the cyst or the age of the patients was estimated using Spearman's correlation coefficient. P<0.05 was considered significant.
Results
After cystectomy, histology revealed three different kinds of cysts: dermoid (n=7), endometriotic (n=13) and serous (n=5) cysts. The sizes ranged from 40 to 170 mm (Table I) measuring highest axis length of the cysts. No significant difference was observed in the ages of the women between the three groups.
Histological analysis
We used the ovarian cortex of a 26 year old patient's cyst in order to assess the intra-individual variation of the follicular density. The follicular densities, i.e. number of follicles per mm3, were 5.9, 5.3, 7.6 and 11.0 respectively (coefficient of variation, CV: 35%).
Extensive fibrosis surrounding the cysts was observed in 12 samples, of which nine belonged to the endometriosis group.
We performed a follicle classification for 10 patients (205 follicles): 87.8% were primordial, 10.2% were primary and 2% were secondary follicles (Figure 1a). No antral follicles were found. Finally, as follicular densities concerned mostly primordial and primary follicles, only these were tabulated and considered in Table I.
In fresh tissue, the follicular densities were higher for dermoid than for endometriotic and serous cysts. After cryopreservation and thawing, the same trend was observed.
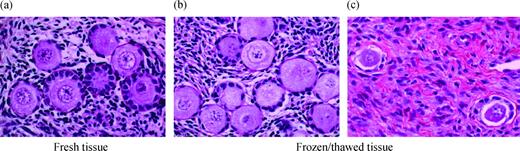
A morphological analysis of the follicles was also performed to assess the effect of the freezing–thawing procedure. Taking into account the follicles from all three groups, 657 complete follicles (i.e. the nucleus of the oocyte appeared clearly in the section) were analysed in the fresh tissue and 568 in the frozen–thawed tissue. Ninety-seven per cent of the follicles from the fresh tissue, and 77% after freezing–thawing, did not show any sign of atresia (P<0.05). Therefore, after the freezing–thawing procedure, the normal morphology of the follicles was preserved in 79% of the cases (Figure 1b). Furthermore, after cryopreservation, a specific pattern was observed among atretic follicles: about three-quarters of them were characterized by the dissociation of the GC from the basal membrane and the retraction of the oocyte (Figure 1c).
Viability analysis
The intra-individual variation in density of viable follicles was estimated from the cortex of the 26 year old patient's cyst from four slices of 50 mm2. The mean densities of living follicles, i.e. number of follicles per mm2, were: 0.3, 0.7, 0.9 and 1.3 respectively (CV: 52%).
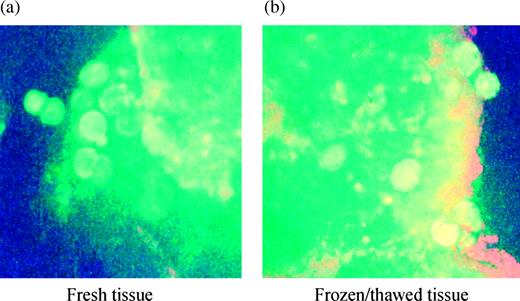
In the frozen–thawed tissue, the green fluorescence (viable follicles) did not appear as uniform as that observed in fresh tissue (Figure 2). However, as the global structure of a healthy follicle was retained, they were considered as living follicles.
In both fresh and frozen–thawed tissue, the density of viable follicles was higher for dermoid cysts than for endometriotic and serous cysts.
After freezing and thawing, a slight decrease of the density of viable follicles was observed, though not systematically, in each group (Table I).
Relationship between the follicular density and the age of the patients or the size of the cysts
In each group, the follicular density estimated in fresh tissue, both by histological analysis and the Live/Dead® viability test, correlated neither with the age of the patients nor with the size of the cysts.
Discussion
Few studies have been performed on ovarian cortical tissue surrounding benign cysts. In cortical tissue surrounding dermoid cysts, cystadenomas and endometriomas, normal morphological patterns, similar to those of the normal ovarian cortex, were observed in 92, 77 and 19% and with a regular vascular network in 84, 78 and 22% of the samples respectively (Maneschi et al., 1993). Thus, endometriosis appeared to be a different disease from the other kind of cysts. It did not seem to be restricted only to the cyst, but rather invaded the surrounding cortex, and was responsible for the fibrotic reaction. Indeed, in our study, fibrosis on the histology of the endometriotic cysts was frequently observed (9/13). For the dermoid and serous cysts analysed, however, we observed a clear limit between the cyst and the ovarian cortex. Thus in these cases, the ovarian cortex only seemed to be stretched by the cyst and did not appear damaged.
It could be expected that the greater the size of the cyst, the more the ovarian cortex would be expanded, resulting in a low density. However, regardless of the nature of the cyst, no correlation was found between its size and the follicular density observed. In fact, there was a significant variability (CV = 35% for histological analysis and 52% for viability analysis), suggesting a heterogenic distribution of follicles that could have a stronger impact than the size of the cyst. Indeed, we have frequently observed that follicles situated in the cortex surrounding benign cysts appeared grouped in clusters rather than homogeneously distributed. Such an observation has previously been described in normal ovarian cortex (Smitz and Cortvrindt, 2002). Additionally, heterogeneity between patients has been described. The follicular density in ovarian biopsies obtained from 60 infertile women ranged from 0 to 160 follicles/mm3, with a median of 8 follicles/mm3 (Lass et al., 1997). Webber et al., (2003) also reported in normal ovarian cortex, a median density of preantral follicles of 11.4 follicles per mm3, with a wide range from 4 to 34. The distribution of the follicles, according to their stage of maturation, was also very similar to that found in normal ovarian cortex. The proportion of primordial, primary and secondary follicles in normal tissue was 73, 26 and 2% respectively (Hovatta et al., 1999) and 86, 12 and 2% respectively (Qu et al., 2000) in the ovarian cortex obtained from infertile patients.
A significant correlation has previously been described between follicular density and the ages of the patients (Faddy, 2000). In our study, no such correlation was found. This may be due to the small number of patients and the narrow interval of the patient's age in our study. However, Poirot et al. (2002) studied a larger group of patients, and, if only patients between 19 and 36 were considered, as in our study, there was no correlation between follicular density and patient age (data not shown).
Hence, in this study, we have clearly demonstrated that the ovarian cortical tissue surrounding benign cysts shows many similarities with normal ovarian cortex. The results regarding ovarian cortical tissue surrounding dermoid cysts are particularly hopeful for research purposes. The results regarding the cortical tissue surrounding serous and endometriosis cysts are less certain.
We developed a procedure for human ovarian tissue cryopreservation using serum-free medium containing propanediol as a penetrating cryoprotective agent and raffinose as a non-permeating sugar. Propanediol was chosen because it is popularly used for human embryo cryopreservation, without any severe deleterious effects (Mandelbaum et al., 1998; Aytoz et al., 1999). In our cryopreservation medium, the antioxidant agents (taurine and l-glutamine) may be beneficial through the oxidative stress to further follicular maturation (Nugent et al., 1998; Gosden, 2000). Further, the effectiveness of HSA has also been demonstrated by the comparison of results obtained with solution containing either HSA or serum (Hreinsson et al., 2003).
Our freezing–thawing procedure had good results in the recovery of morphologically normal follicles (∼80%). This result matches that obtained in normal human ovarian tissue. About 80% of viable follicles were obtained using cryoprotectant solutions containing either dimethylsulphoxide (1.5 mol/l) or propanediol (1.5 mol/l) and sucrose (0.1 mol/l) (Hovatta et al., 1996). Hreinsson et al. (2003) observed that cryopreservation in a medium containing propanediol (1.5 mol/l), sucrose (0.1 mol/l) and HSA or serum induced a significant reduction of follicles judged to be viable, but the proportion of these follicles remained ∼70%. In addition, in these two experiments, the freezing procedure was different from ours and especially after the seeding: the temperature decrease rate was 0.3 versus 2°C/min.
After the freezing–thawing, the follicles showed a green fluorescence (calcein AM staining) less homogeneous than the fluorescence of the fresh follicles. Therefore, it could be hypothesized that the freezing–thawing procedure damaged some of the cells of the follicles and could be responsible for the pattern observed. Hence, if only very few granulosa cells were dead within a follicle, their nucleus (a little red spot) likely could not be seen through the green coloured cytoplasm of the other cells and especially through the large cytoplasm of the oocyte. However, as the morphology of the follicles was apparently maintained and, as no red spot could be observed, these follicles were considered as living follicles in our experience.
The Live/Dead® test was previously used for tissue pieces (Cortvrindt and Smitz, 2001). It has also been used for mechanically and chemically isolated follicles. This latter experimental procedure allowed the view of living but also dead follicles and the percentage of viable follicles was either unchanged (Oktay et al., 1997) or decreased (Hreinsson et al., 2003) after cryopreservation. Compared to these studies, the disadvantage of our technique was that it was unable to calculate the exact ratio of living to dead follicles. However, it offered some advantages. It was easy to perform since no particular pre-treatment was necessary, and no follicular destruction was expected. Thus, our technique can be used to rapidly screen the density of living follicles in ovarian cortex specimens.
The results of this study show that ovarian cortical tissue surrounding ovarian cysts, especially dermoid cysts, is a promising source of ovarian tissue for research purposes. Because it can be performed quickly, the viability test (Live/Dead® assay) is very useful to select the tissues containing living follicles, and is complementary to histological analysis.
The use of a serum-free medium containing propanediol and raffinose as cryoprotectants, combined with a faster freezing program, still resulted in a satisfactory and comparable rate of follicular survival after cryopreservation. Nevertheless, further studies of in vivo and in vitro follicular maturation in tissue cultures and animal studies are needed to support the feasibility of these models. We have therefore commenced such studies and hope to demonstrate the appropriateness of this model in the future.
Haematoxylin–eosin staining. Fresh (a) and frozen–thawed (b, c) human ovarian cortex surrounding a dermoid cyst (patient no. 5; a, b), and an endometriosis cyst (patient no. 19; c) (original magnification ×400). Primordial and primary follicles are observed. After cryopreservation the morphology of the follicles is well preserved (b); however, atretic follicles are also observed with a specific pattern which is never seen in fresh tissue: a dissociation of the granulosa cells from the basal membrane and retraction of the oocyte is noted (c).
Live/Dead® Viability Cytoxicity kit (Molecular Probes) used with fresh (a) and frozen–thawed (b) ovarian cortex surrounding a dermoid cyst (patient no. 5; original magnification ×100). The green discs within the tissue are living follicles. Note that in frozen–thawed tissue the green fluorescence is not as homogeneous as in fresh tissue.
Age of the patients, size of the cysts and follicular densities observed by histological and viability analysis performed on cortex surrounding benign ovarian cysts
| . | Patient no. . | Age (years) . | Size of the cyst (mm) . | Fresh tissue . | . | Frozen–thawed tissue . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Primordial and primary follicles . | Viable follicles . | Primordial and primary follicles . | Viable follicles . | ||
| (no./mm3) | (no. of follicles /mm2) | (no./mm3) | (no. of follicles/mm2) | ||||||
| Dermoid cysts | 1 | 21 | 50 | 38.67 | 3.28 | 3.16 | 1.78 | ||
| 2 | 26 | 41 | 13.04 | 2.51 | nd | nd | |||
| 3 | 31 | 60 | 13.75 | 2.93 | 17.42 | 3.12 | |||
| 4 | 35 | nd | 0.66 | 22.20 | nd | nd | |||
| 5 | 28 | 40 | 53.70 | 5.10 | 52.85 | 12.00 | |||
| 6a | 31 | 54 | 0.00 | 0.02 | 0.16 | 0.30 | |||
| 7 | 20 | 42 | 4.50 | 0.52 | 38.55 | 0.12 | |||
| 27.4±2.1 | 47.8±3.3 | 13.04 | 2.93 | 17.42 | 1.78 | ||||
| Endometriosis cysts | 8a | 34 | 42 | 2.47 | 1.67 | 1.26 | 0.07 | ||
| 9a | 33 | 55 | 0.64 | 0.05 | 0.95 | 0.20 | |||
| 10a | 36 | 100 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 11 | 30 | 51 | 0.12 | 3.00 | nd | nd | |||
| 12a | 32 | 50 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 13 | 26 | 40 | 0.00 | 2.17 | nd | nd | |||
| 14a | 24 | 90 | 0.31 | 0.00 | 0.13 | 0.00 | |||
| 15 | 30 | 48 | 2.78 | 1.44 | 10.23 | 0.29 | |||
| 16a | 23 | 45 | 4.21 | 0.97 | 3.32 | 1.80 | |||
| 17a | 27 | 76 | 0.00 | 0.01 | 0.00 | 0.00 | |||
| 18a | 29 | 40 | 13.37 | 0.00 | 0.00 | 0.00 | |||
| 19 | 24 | 60 | 3.06 | 0.56 | 2.05 | 0.56 | |||
| 20a | 32 | 43 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 29.2±1.1 | 56.9±5.4 | 0.31 | 0.05 | 0.13 | 0.00 | ||||
| Serous cysts | 21 | 19 | 100 | 0.89 | 5.44 | 5.51 | 0.05 | ||
| 22a | 27 | 62 | 0.00 | 0.71 | 0.00 | 0.10 | |||
| 23 | 31 | 63 | 0.13 | 0.00 | 0.00 | 0.00 | |||
| 24 | 23 | 70 | 3.13 | 0.22 | 3.10 | 0.04 | |||
| 25a | 21 | 170 | 1.47 | 1.00 | 3.02 | 0.06 | |||
| 24.2±2.1 | 93.0±20.4 | 0.89 | 0.71 | 3.02 | 0.05 | ||||
| . | Patient no. . | Age (years) . | Size of the cyst (mm) . | Fresh tissue . | . | Frozen–thawed tissue . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Primordial and primary follicles . | Viable follicles . | Primordial and primary follicles . | Viable follicles . | ||
| (no./mm3) | (no. of follicles /mm2) | (no./mm3) | (no. of follicles/mm2) | ||||||
| Dermoid cysts | 1 | 21 | 50 | 38.67 | 3.28 | 3.16 | 1.78 | ||
| 2 | 26 | 41 | 13.04 | 2.51 | nd | nd | |||
| 3 | 31 | 60 | 13.75 | 2.93 | 17.42 | 3.12 | |||
| 4 | 35 | nd | 0.66 | 22.20 | nd | nd | |||
| 5 | 28 | 40 | 53.70 | 5.10 | 52.85 | 12.00 | |||
| 6a | 31 | 54 | 0.00 | 0.02 | 0.16 | 0.30 | |||
| 7 | 20 | 42 | 4.50 | 0.52 | 38.55 | 0.12 | |||
| 27.4±2.1 | 47.8±3.3 | 13.04 | 2.93 | 17.42 | 1.78 | ||||
| Endometriosis cysts | 8a | 34 | 42 | 2.47 | 1.67 | 1.26 | 0.07 | ||
| 9a | 33 | 55 | 0.64 | 0.05 | 0.95 | 0.20 | |||
| 10a | 36 | 100 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 11 | 30 | 51 | 0.12 | 3.00 | nd | nd | |||
| 12a | 32 | 50 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 13 | 26 | 40 | 0.00 | 2.17 | nd | nd | |||
| 14a | 24 | 90 | 0.31 | 0.00 | 0.13 | 0.00 | |||
| 15 | 30 | 48 | 2.78 | 1.44 | 10.23 | 0.29 | |||
| 16a | 23 | 45 | 4.21 | 0.97 | 3.32 | 1.80 | |||
| 17a | 27 | 76 | 0.00 | 0.01 | 0.00 | 0.00 | |||
| 18a | 29 | 40 | 13.37 | 0.00 | 0.00 | 0.00 | |||
| 19 | 24 | 60 | 3.06 | 0.56 | 2.05 | 0.56 | |||
| 20a | 32 | 43 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 29.2±1.1 | 56.9±5.4 | 0.31 | 0.05 | 0.13 | 0.00 | ||||
| Serous cysts | 21 | 19 | 100 | 0.89 | 5.44 | 5.51 | 0.05 | ||
| 22a | 27 | 62 | 0.00 | 0.71 | 0.00 | 0.10 | |||
| 23 | 31 | 63 | 0.13 | 0.00 | 0.00 | 0.00 | |||
| 24 | 23 | 70 | 3.13 | 0.22 | 3.10 | 0.04 | |||
| 25a | 21 | 170 | 1.47 | 1.00 | 3.02 | 0.06 | |||
| 24.2±2.1 | 93.0±20.4 | 0.89 | 0.71 | 3.02 | 0.05 | ||||
Individual values are reported and below each group medians are given, except for the age of the patients and the size of the cysts (mean ± SEM).
Note that follicular densities are expressed by mm3 whereas the densities of viable follicles are expressed by mm2 (see Materials and methods section).
Extensive fibrosis of the tissue was observed.
nd = not determined.
Age of the patients, size of the cysts and follicular densities observed by histological and viability analysis performed on cortex surrounding benign ovarian cysts
| . | Patient no. . | Age (years) . | Size of the cyst (mm) . | Fresh tissue . | . | Frozen–thawed tissue . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Primordial and primary follicles . | Viable follicles . | Primordial and primary follicles . | Viable follicles . | ||
| (no./mm3) | (no. of follicles /mm2) | (no./mm3) | (no. of follicles/mm2) | ||||||
| Dermoid cysts | 1 | 21 | 50 | 38.67 | 3.28 | 3.16 | 1.78 | ||
| 2 | 26 | 41 | 13.04 | 2.51 | nd | nd | |||
| 3 | 31 | 60 | 13.75 | 2.93 | 17.42 | 3.12 | |||
| 4 | 35 | nd | 0.66 | 22.20 | nd | nd | |||
| 5 | 28 | 40 | 53.70 | 5.10 | 52.85 | 12.00 | |||
| 6a | 31 | 54 | 0.00 | 0.02 | 0.16 | 0.30 | |||
| 7 | 20 | 42 | 4.50 | 0.52 | 38.55 | 0.12 | |||
| 27.4±2.1 | 47.8±3.3 | 13.04 | 2.93 | 17.42 | 1.78 | ||||
| Endometriosis cysts | 8a | 34 | 42 | 2.47 | 1.67 | 1.26 | 0.07 | ||
| 9a | 33 | 55 | 0.64 | 0.05 | 0.95 | 0.20 | |||
| 10a | 36 | 100 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 11 | 30 | 51 | 0.12 | 3.00 | nd | nd | |||
| 12a | 32 | 50 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 13 | 26 | 40 | 0.00 | 2.17 | nd | nd | |||
| 14a | 24 | 90 | 0.31 | 0.00 | 0.13 | 0.00 | |||
| 15 | 30 | 48 | 2.78 | 1.44 | 10.23 | 0.29 | |||
| 16a | 23 | 45 | 4.21 | 0.97 | 3.32 | 1.80 | |||
| 17a | 27 | 76 | 0.00 | 0.01 | 0.00 | 0.00 | |||
| 18a | 29 | 40 | 13.37 | 0.00 | 0.00 | 0.00 | |||
| 19 | 24 | 60 | 3.06 | 0.56 | 2.05 | 0.56 | |||
| 20a | 32 | 43 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 29.2±1.1 | 56.9±5.4 | 0.31 | 0.05 | 0.13 | 0.00 | ||||
| Serous cysts | 21 | 19 | 100 | 0.89 | 5.44 | 5.51 | 0.05 | ||
| 22a | 27 | 62 | 0.00 | 0.71 | 0.00 | 0.10 | |||
| 23 | 31 | 63 | 0.13 | 0.00 | 0.00 | 0.00 | |||
| 24 | 23 | 70 | 3.13 | 0.22 | 3.10 | 0.04 | |||
| 25a | 21 | 170 | 1.47 | 1.00 | 3.02 | 0.06 | |||
| 24.2±2.1 | 93.0±20.4 | 0.89 | 0.71 | 3.02 | 0.05 | ||||
| . | Patient no. . | Age (years) . | Size of the cyst (mm) . | Fresh tissue . | . | Frozen–thawed tissue . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Primordial and primary follicles . | Viable follicles . | Primordial and primary follicles . | Viable follicles . | ||
| (no./mm3) | (no. of follicles /mm2) | (no./mm3) | (no. of follicles/mm2) | ||||||
| Dermoid cysts | 1 | 21 | 50 | 38.67 | 3.28 | 3.16 | 1.78 | ||
| 2 | 26 | 41 | 13.04 | 2.51 | nd | nd | |||
| 3 | 31 | 60 | 13.75 | 2.93 | 17.42 | 3.12 | |||
| 4 | 35 | nd | 0.66 | 22.20 | nd | nd | |||
| 5 | 28 | 40 | 53.70 | 5.10 | 52.85 | 12.00 | |||
| 6a | 31 | 54 | 0.00 | 0.02 | 0.16 | 0.30 | |||
| 7 | 20 | 42 | 4.50 | 0.52 | 38.55 | 0.12 | |||
| 27.4±2.1 | 47.8±3.3 | 13.04 | 2.93 | 17.42 | 1.78 | ||||
| Endometriosis cysts | 8a | 34 | 42 | 2.47 | 1.67 | 1.26 | 0.07 | ||
| 9a | 33 | 55 | 0.64 | 0.05 | 0.95 | 0.20 | |||
| 10a | 36 | 100 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 11 | 30 | 51 | 0.12 | 3.00 | nd | nd | |||
| 12a | 32 | 50 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 13 | 26 | 40 | 0.00 | 2.17 | nd | nd | |||
| 14a | 24 | 90 | 0.31 | 0.00 | 0.13 | 0.00 | |||
| 15 | 30 | 48 | 2.78 | 1.44 | 10.23 | 0.29 | |||
| 16a | 23 | 45 | 4.21 | 0.97 | 3.32 | 1.80 | |||
| 17a | 27 | 76 | 0.00 | 0.01 | 0.00 | 0.00 | |||
| 18a | 29 | 40 | 13.37 | 0.00 | 0.00 | 0.00 | |||
| 19 | 24 | 60 | 3.06 | 0.56 | 2.05 | 0.56 | |||
| 20a | 32 | 43 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 29.2±1.1 | 56.9±5.4 | 0.31 | 0.05 | 0.13 | 0.00 | ||||
| Serous cysts | 21 | 19 | 100 | 0.89 | 5.44 | 5.51 | 0.05 | ||
| 22a | 27 | 62 | 0.00 | 0.71 | 0.00 | 0.10 | |||
| 23 | 31 | 63 | 0.13 | 0.00 | 0.00 | 0.00 | |||
| 24 | 23 | 70 | 3.13 | 0.22 | 3.10 | 0.04 | |||
| 25a | 21 | 170 | 1.47 | 1.00 | 3.02 | 0.06 | |||
| 24.2±2.1 | 93.0±20.4 | 0.89 | 0.71 | 3.02 | 0.05 | ||||
Individual values are reported and below each group medians are given, except for the age of the patients and the size of the cysts (mean ± SEM).
Note that follicular densities are expressed by mm3 whereas the densities of viable follicles are expressed by mm2 (see Materials and methods section).
Extensive fibrosis of the tissue was observed.
nd = not determined.
We gratefully acknowledge Dr W.C.Ang for extensive revision of the manuscript. This work was supported by a local hospital grant (PHRC).
References
Aytoz A, Van den Abbeel E, Bonduelle M, Camus M, Joris H, Van Steirteghem A and Devroey P (
Callejo J, Salvador C, Miralles A, Vilaseca S, Laila JM and Balasch J (
Candy CJ, Wood MJ and Whittingham DG (
Canis M, Bassil S, Wattiez A, Pouly JL, Manhes H, Mage G and Bruhat MA (
Canis M, Mage G, Pouly JL, Wattiez A, Manhes H and Bruhat MA (
Canis M, Botchorishvili R, Manhes H, Wattiez A, Mage G, Pouly JL and Bruhat MA (
Cortvrindt R and Smitz EJ (
Demirci B, Lornage J, Salle B, Frappart L, Franck M and Guérin J-F (
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B and Van Langendonckt A (
Gook DA, McCully BA, Edgar DH and McBain JC (
Gosden RG (
Gosden RG, Baird DT, Wade JC and Webb R (
Gougeon A (
Gougeon A and Lefèvre B (
Gunasena KT, Villines PM, Critser ES and Critser JK (
Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A and Winston RM (
Hovatta O, Wright C, Krausz T, Hardy K and Winston RML (
Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ and Hovatta O (
Hreinsson J, Zhang P, Swahn ML, Hultenby K and Hovatta O (
Kim SS, Soules MR and Battaglia DE (
Lass A, Silye R, Abrams D-C, Krausz T, Hovatta O, Margara R and Winston RML (
Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW and Wolf DP (
Mandelbaum J, Belaisch-Allart J, Junca AM, Antoine JM, Plachot M, Alvarez S, Alnot MO and Salat-Baroux J (
Maneschi F, Marasa L, Incandela S, Mazzarese M and Zupi E (
Newton H, Aubard Y, Rutherford A, Sharma V and Gosden RG (
Nisolle M, Casanas-Roux F, Qu J, Motta P and Donnez J (
Nugent D, Newton H, Gallivan L and Gosden RG (
Oktay K and Karlikaya G (
Oktay K, Nugent D, Newton H, Salha O, Chatterjee P and Gosden RG (
Oktay K, Newton H, Mullan J and Gosden RG (
Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M and Rosenwaks Z (
Poirot C, Vacher-Lavenu M-C, Helardot P, Guibert J, Brugières L and Jouannet P (
Qu J, Godin PA, Nisolle M and Donnez J (
Radford JA, Lieberman BA, Brison DR, Smith ARB, Critchlow JD, Russel SA, Watson AJ, Clayton JA, Harris M, Gosden RG et al. (
Salle B, Demirci B, Franck M, Rudigoz RC, Guérin JF and Lornage J (
Schmidt KLT, Andersen CY, Starup J, Loft A, Byskov AG and Andersen AN (
Scott JE, Zhang P and Hovatta O (
Smitz JE and Cortvrindt RG (
Author notes
1Biology of Development and Reproduction–CECOS Auvergne, 2Department of Obstetrics, Gynecology and Human Reproduction, 3Pathology Laboratory, Hôtel-Dieu, CHU Clermont-Ferrand, BP 69, 63058 Clermont-Ferrand Cedex 1, 4EA 975, Biology of Reproduction, Faculty of Medicine, BP 38, Clermont-Ferrand, France