-
PDF
- Split View
-
Views
-
Cite
Cite
E. Tuckerman, S.M. Laird, A. Prakash, T.C. Li, Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage, Human Reproduction, Volume 22, Issue 8, August 2007, Pages 2208–2213, https://doi.org/10.1093/humrep/dem141
Close - Share Icon Share
Abstract
Studies in mice suggest that CD56 + uterine natural killer (uNK) cells play an important role in implantation. Studies in humans have described an increase in the number of uNK cells in the non-pregnant mid-secretory endometrium of women with unexplained recurrent miscarriage (RM). However, the predictive value of uNK cell number in the maintenance of pregnancy is controversial.
A blind retrospective study was undertaken. The percentage of stromal cells positive for CD56 was identified by immunocytochemistry in endometrial biopsies from 10 normal control women and 87 women with unexplained RM, of whom 51 became pregnant following biopsy. Biopsies were obtained on days LH + 7 to LH + 9.
As in previous studies, the number of uNK cells in the 87 women with RM (mean 11.2% range 1.1–41.4%) was significantly higher (P = 0.013) than in the control women (mean 6.2% range 2.2–13.9%). No significance difference in uNK numbers was observed between 19 women who miscarried (mean 9.6% range 1.7–25.0%) and 32 women who had a live birth (mean 13.3% range 1.1–41.4%) in a subsequent pregnancy.
In this study numbers of uNK cells in the peri-implantation endometrium of women with unexplained recurrent miscarriage did not predict subsequent pregnancy outcome.
Introduction
The endometrial leukocyte population consists mainly of uterine natural killer cells (uNK), macrophages and T cells, (Bulmer, 1995; Bulmer, 1996; Johnson et al., 1999) and is distinctly different to that of peripheral blood. In contrast to NK cells found in the peripheral blood, uNK cells express CD56 and CD38, but not the classical T cell or NK cell markers CD3, CD4, CD8, CD16 and CD57 (Bulmer, 1991). An approximate 10% of endometrial uNK cells are CD56 + CD16+, similar to peripheral NK cells, and show a minimum expression of CD56. These CD56 + CD16 + NK cells are sometimes referred to as CD56dim NK cells, in contrast to the major endometrial population of uNK cells, which are referred to as CD56 + CD16- or CD56bright.
The number of uNK cells varies through the menstrual cycle, with a dramatic increase between days 6 and 7 after the LH surge, the putative time of implantation. The number of uNK cells remains high during early pregnancy and composes 70% of the lymphocytes at the interface between maternal decidua and the invading trophoblast.
The uNK cell numbers decline after the first trimester and are absent at term.
Previous investigations on the expression of uNK cells in women with recurrent miscarriage (RM) show controversial results. First, while two studies showed an increase in the numbers of CD56 + cells in the non-pregnant endometrium of women with RM (Clifford et al., 1999; Quenby et al., 1999), a subsequent study (Michimata et al., 2002) was unable to show any difference in the number of endometrial CD56 + cells between women with RM and controls. Second, the prognostic value of the measurement of uNK cells is also uncertain. In 12 women with RM, Quenby et al. (1999) observed significantly higher numbers of uNK cells in four women who miscarried in a subsequent pregnancy when compared with eight women who had a live birth in a subsequent pregnancy, which suggests that high numbers of pre-pregnancy uNK cells may predict miscarriage in the next pregnancy. However Michimata et al. (2002) could not confirm this association in 6 of 17 RM women who miscarried. Both these studies are limited by their small sample size. The Michimata et al. study also does not conform to the accepted criteria for RM as it includes women who had two miscarriages. In addition, there is no currently agreed method for assessing numbers of uNK cells, and methods adopted by various investigators vary from the mean numbers of uNK cells (Clifford et al., 1999), the number of uNK cells as a percentage of total stromal cells (Quenby et al., 1999; Tuckerman et al., 2004) and the number of uNK cells as a percentage of CD45 + cells (Michimata et al., 2002).
The aim of this study was to investigate whether or not the number of pre-pregnancy endometrial CD56 + cells in women with unexplained RM is able to predict outcome in a subsequent pregnancy. We have analysed the percentage of immunostained CD56 + cells in 51 LH timed archived wax-embedded endometrial biopsies from women diagnosed with unexplained RM, who became pregnant following biopsy. Numbers of CD56 + cells were calculated in a blinded manner and then correlated with pregnancy outcome.
Materials and Methods
Subjects
Local ethical committee's approval plus informed written patient consent was obtained for this study. Unexplained RM was defined as a history of three consecutive miscarriages in the first trimester in which all the following results were normal: parental karyotypes, thyroid function, anticardiolipin antibodies, antiphospholipid antibodies, lupus anticoagulant, FSH, prolactin, progesterone, estrogen, testosterone, free androgen index, pelvic ultrasonography and hysterosalpingogram (Li, 1998). Daily measurement of LH in either serum or urine was used to identify the LH surge and the endometrial biopsies were collected with a Pipelle sampler (Prodimed, France) on day LH + 7–LH + 9, fixed overnight in formalin and automatically wax embedded.
Biopsies were collected from 87 women with unexplained RM, of whom 51 conceived again after biopsy. Of these 51 women, 32 had a subsequent live birth (live birth group) and 19 miscarried again (miscarriage group) in the pregnancy following the biopsy. The mean age of the women in the live birth group was 34 years (range 23–41), and the mean number of miscarriages was 3 (range 3–5), whereas the mean age of the women in the miscarriage group was 35 years (range 23–44), and the mean number of miscarriages was 4 (range 3–8).
Biopsies were collected in an identical way from 10 normal control women. All the control women had regular menstrual cycles and had not used oral contraception or an intrauterine contraceptive device in the two months preceding the biopsy. Seven of the control women were of proven fertility, and all the control biopsies were collected at day LH + 7. The mean ages of both control and RM women with a known pregnancy outcome was 34 years (range: control 29–39; RM 23–44 years).
Immunocytochemistry
The 5 um sections were dewaxed in xylene, rehydrated through the alcohols to Tris-buffered saline (TBS) (pH 7.6) and quenched in 0.3% hydrogen peroxide in methanol for 20 min. After washing, unmasking was performed in an 800 W microwave oven in 10 mmol/l citrate buffer (pH 6.0). Buffer was heated in the microwave oven until boiling. Slides were added to the buffer and left covered at high heat for 3 min. Slides were further incubated for 12 min on medium heat and allowed to cool for 20 min. An ABC kit (Vector Laboratories, UK) was used according to the manufacturer’s instructions and the following adaptations. Slides were washed in TBS and blocked in blocking buffer containing 250 ul avidin/ml (Vector Laboratories) for 1 h at room temperature, and incubated overnight at + 4°C with a mouse monoclonal primary anti-CD56 antibody (NCL-CD56-504; Novacastra Laboratories Ltd, UK) diluted 1:50 in antibody buffer containing 250 ul/ml biotin. Slides were washed in TBS throughout, and after application of secondary antibody and Vectorstain, binding was visualized by incubation with peroxidase substrate DAB (3.3′diaminobenzidene terahydrochloride; Vector Laboratories). Slides were washed in distilled water and counterstained with 20% haematoxylin for 30 min, differentiated, dehydrated through the alcohols, cleared in xylene and mounted in Vectormount (Vector Laboratories).
Analysis
Statistics were computed using SPSS 11. The numbers of CD56 positive and CD56 negative stromal cells were counted in 10 × 400 magnification microscope fields from each biopsy (without knowledge of reproductive outcome). The percentage of positive stromal cells was calculated. The non-parametric Mann–Whitney test was used to compare (i) numbers of CD56 + cells in women with RM and in controls, (ii) numbers of CD56 + cells in women who miscarried compared with CD56 + numbers in women who had a live birth and (iii) numbers of CD56 + cells in women with primary RM, secondary RM and controls. Pearson's correlation test was used to investigate the relationship between numbers of CD56 + cells and age, or previous numbers of miscarriages. Pearson's correlation was also used to compare two types of CD56 + cell analysis, i.e. total CD56 + cell number and CD56 + as a percentage of total stromal cells. We used the chi-square test to see if the ratio of live births to miscarriages in our RM study population differed from that previously reported for other populations of women with unexplained RM (Clifford et al., 1997; Brigham et al., 1999). A P-value of < 0.05 was considered significant.
Results
Numbers of CD56 + cells in women with RM and controls
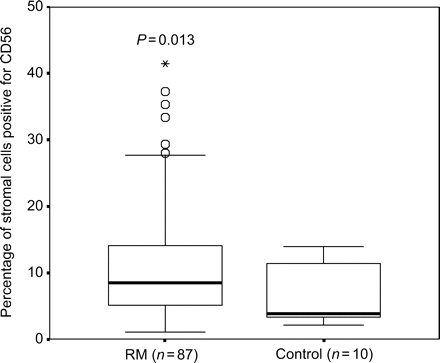
The numbers of endometrial CD56 + cells in the 87 women with RM (mean ± SEM 11.2 ± 0.9% range 1.1–41.4) were significantly higher (P = 0.013) than that of the control subjects (mean ± SEM 6.2 ± 1.4% range 2.2–13.9) (Fig. 1). Examples of immunohistochemical staining are illustrated in a previous paper (Tuckerman et al., 2004).
Boxplot showing the percentage of CD56 positive cells in timed endometrial biopsies from women with RM and control women (open circle indicates outlier and asterisk indicates far outlier)
Numbers of CD56 + cells in women who miscarried and in women who had a live birth
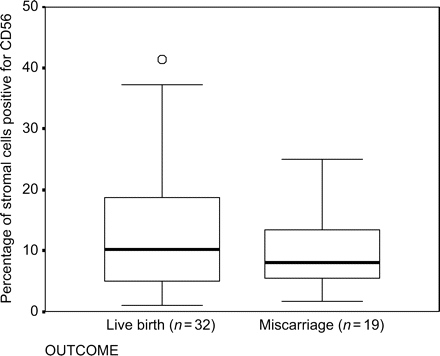
No significance difference (P = 0.44) was observed between the number of endometrial CD56 + cells in the women with RM who had live births (n = 32, mean ± SEM 13.3 ± 1.9%, range 1.1–41.4) and those who subsequently miscarried (n = 19, Mean ± SEM 9.6 ± 1.4%, range 1.7–25.0) (Fig. 2).
Boxplot showing the percentage of CD56 positive cells in timed endometrial biopsies and pregnancy outcome following biopsy in 51 women with unexplained RM (opern circle indicates outlier)
The live birth rate (63%) in our study population of 51 women, although slightly lower, was not significantly different from that of the 75 and 71% reported in previous studies on future pregnancy outcome in women with unexplained RM (Clifford et al., 1997; Brigham et al., 1999). 15 out of the 51 women with RM had numbers of CD56 + cells that were above the 90th percentile of the control group (90th percentile = 13.8%). Among these women, 11 had a live birth and 4 miscarried. The live birth rate in women with RM who had numbers of CD56 + cells above the 90th percentile (11/15 or 73%) was also not significantly different from those who had numbers of CD56 + cells below the 90th percentile (21/36 or 58%).
Numbers of CD56 + in women with primary RM and secondary RM
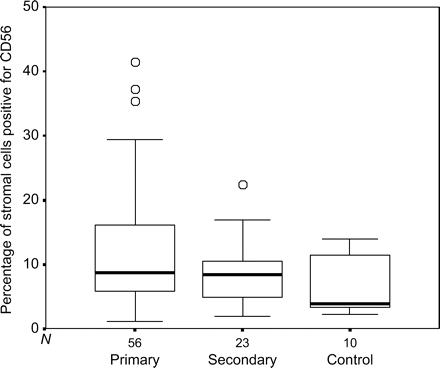
In the 79 women for which primary or secondary RM status was known there was no significant difference (P = 0.25) between numbers of CD56 + cells in women with primary RM (n = 56) when compared with women with secondary RM (n = 23). However, when considered separately, only the women with primary RM had significantly higher (P = 0.019) numbers of CD56 + cells when compared with the numbers in the control women (n = 10) (Fig. 3).
Boxplot showing the percentage of CD56 positive cells in timed endometrial biopsies from women with primary RM, secondary RM and control women (open circle indicates outlier)
Different methods of scoring CD56 + cells
A significant correlation (Pearson's correlation P < 0.01) was observed between the total number of CD56 + cells and the ratio of CD56 + cells to the total number of stromal cells in each biopsy.
Relationship between numbers of CD56 + cells and age or previous numbers of miscarriages
No relationship was observed between the number of CD56 + cells and either age or previous number of miscarriages (Pearson's correlation: age, P = 0.290; number of miscarriages, P = 0.287).
Discussion
The prognostic value of counting the number of pre-pregnancy uNK (CD56 + ) cells
This study counted the number of CD56 + cells in precisely timed endometrial biopsies from 51 women with RM, and found no significant difference in the numbers of CD56 + uNK cells between women with RM who had a subsequent live birth and those who miscarried once again in a subsequent pregnancy. A previous study (Quenby et al., 1999) has reported an association between high numbers of pre-pregnancy uNK cells and miscarriage in a subsequent pregnancy after biopsy. However, the numbers included in that study were small; of 16 women who became pregnant, only 4 miscarried and the result was just significant (P = 0.04). Our study included a larger sample (n = 51) and suggests that the number of CD56 + uNK cells measured in a pre-pregnacy study cycle does not predict reproductive outcome in a subsequent pregnancy.
The numbers of uNK cells rapidly increase in the early secretory phase of the menstrual cycle (Bulmer et al., 1996) and this may be a significant confounding variable affecting the results of different studies. Therefore in our study all the endometrial samples were precisely timed according to the LH surge and obtained within a well-defined period (LH + 7–9), in order to minimize variation due to changes in the menstrual cycle.
Additional strengths of our study are the relatively large number of women studied in a well-defined group of women with RM. All the women with RM in this study had at least three consecutive miscarriages within the first trimester. A possible weakness is the unknown karyotype of the conceptus which miscarried, with the result that in our study, we do not know whether or not the subsequent miscarriages were due to endometrial or fetal causes. Analysis showing an abnormal karyotype may have indicated an alternative reason for miscarriage in the women with high numbers of uNK cells. However, karyotype of the conceptus is irrelevant when considering the RM women with high numbers of uNK cells that went on to have a live birth, as the phenotype of all the babies delivered was recorded as normal.
Although we found no differences in the numbers of CD56 + cells in the endometrium of the RM women who had a live birth and those that did not, there may be differences in CD56 + subpopulations that relate to reproductive outcome. It is unclear if subclassification of the CD56 + cells will help to improve the prognostic value of the measurement. Further work is required to address this question. For example, we do not know the proportion of uNK CD56 + cells, which are also CD16 + or CD68 + (activation marker). Subclassification based on double immunostaining may show a more distinct relationship between high uNK cell numbers and unexplained RM and may perhaps improve the prognostic value of uNK cell analysis.
Prednisolone has been suggested as a suitable treatment for RM women with high numbers of CD56 + cells (Quenby et al., 1999) and has been shown to decrease the numbers of CD56 + cells (Quenby et al., 2005). However, as the relevance of high numbers of uNK cells is not clear and predisolone treatment is not without risk or adverse effects, treatment with predisolone may need to be considered carefully. In a recent review on the role of NK cells and reproductive failure, Rai et al. (2005) has pointed out that glucocorticoid treatment during pregnancy may be associated with a number of complications including gestational diabetes, pre-eclampsia and rupture of membranes resulting in preterm delivery (Laskin et al., 1997).
Non-conception and conception cycle
In this study, the uNK cell measurement was made on an endometrial biopsy from a non-conception cycle. It is unclear if the results in a non-conception cycle are similar to those in conception cycles. It is possible that the presence of an implanting embryo may significantly alter the number and function of the uNK cells. Ideally therefore, the measurement should be made in a conception cycle but ethical considerations make it difficult to obtain endometrial biopsies in conception cycles.
Definition of normality
In this study, we observed significantly higher numbers of uNK cells in the endometrium of women with unexplained RM than in the normal control women, and our results are in accordance with previous reports (Clifford et al., 1999; Quenby et al., 1999). However, there is still a lack of consensus on what is a high level of uNK cells, since the normal number of uNK cells in a fertile population is yet to be defined. In this study, using the 90th percentile of a relatively small control group to define the upper limit of normality, the cut off is 13.8% (proportion of stormal cells stained positive for CD56). Other studies have used different upper limits, Quenby et al. (2005) used the 75th percentile of the controls as the cut off and only women with < 5% of stromal cells positive for CD56 were defined as normal. However, if we compare the mean and range of uNK cell numbers in the control women in our study (mean 6.2, range 2.2–13.9) with those of the original study by Quenby et al. (1999) (mean 4.7, range 0.2–9.5) the numbers of uNK cells are within a similar range. Furthermore, the slightly higher numbers of CD56 + cells in our study may be a consequence of the different method of tissue preparation: we have analysed uNK cells in wax-embedded specimens, whereas Quenby et al. (1999, 2005) analysed CD56 + cells in frozen tissue.
While this study showed that the measurement of CD56 + cells in the endometrium of women with unexplained RM did not have a significant prognostic value in the outcome of a subsequent pregnancy, it does not rule out the possibility that uNK cells have an important role in implantation and that alterations in uNK cell populations may be responsible for recurrent pregnancy loss. It may be that measurement of CD56 + cell numbers is not an appropriate method to define normality of uNK cells and that other methods should be used such as activation or receptor expression. Whatever alternative method is considered, the definition of ‘normality’ depends on recruiting a sufficient number of control subjects as well as women with RM. Recruitment of a sufficiently large sample of control subjects may not be easy, but collaboration among different centres to establish a tissue bank of control subjects should overcome such a difficulty.
Normal variation between cycles and the degree of variation in uNK cell number between different areas of the endometrium also requires further investigation. It is important that a clear baseline is established, so that accurate determination of normal and abnormal becomes meaningful.
uNK cell numbers in primary and secondary RM
In order to investigate the effect of a live birth on uNK cell number, we compared the numbers of uNK cells in women with primary RM, secondary RM and control women. When women with primary or secondary RM are considered together as a group the number of CD56 + cells are significantly higher than the control subjects. When they are considered separately, women with primary unexplained RM also had a significantly (P = 0.019) higher number of CD56 + cells than the controls, but women with secondary RM did not. The number of subjects in the secondary RM group is smaller than the primary RM group which suggests that a possible explanation for the lack of significant difference between the secondary RM group and the controls was a lack of sufficient power to detect the difference. Alternatively, it is possible that the numbers of uNK cells in women with primary RM and secondary RM are different, but this is less likely as our analysis showed no significant difference between these two groups. It seems appropriate therefore, for the purpose of subsequent analysis, to group the primary and secondary RM groups together. In this respect our finding is consistent with previous reports that the number of uNK cells in women with unexplained RM is significantly higher than controls.
Methods used to count CD56 + cells
Various methods have been used to assess the numbers of immunostained CD56 + in endometrial tissue sections. Most investigators count the number of CD56 + cells in a total of 10 × 400 magnification microscope fields. In some studies (Quenby et al., 1999; Tuckerman et al., 2004), both negative and positive stromal cells in each microscope field were counted and the percentage of CD56 + cells expressed as a function of the total numbers of stromal cells. This method is believed to reduce inter-patient variability. In other studies (Clifford et al., 1999), only the positive cells were counted in order to give the mean number of positive cells. In our study, we found close correlation when CD56 + cells were expressed as either the percentage of positive cells or the total number, therefore either method would give similar results.
Function of CD56 + cells in the endometrium
The precise function of CD56 + cells in the endometrium and decidua remains speculative. Experiments with mice have shown that activated uNK cells secrete interferon gamma and that this is involved in the development of the spiral arteries (Croy et al., 2003). The uNK cells are abundant during the first three months of pregnancy, which is a time of maximum blood vessel development. However, in the mouse, uNK cells are not essential for successful pregnancy outcome (Barber et al., 2003), although female mice with a null mutation for interleukin (IL) 15 and no uNK cells have smaller offspring, consistent with reduced placental blood vessel development. The uNK cells also express receptors for HLA-G (King et al., 1998). HLA-G expression is unique to the invading cytotrophoblast. The position of uNK cells in early pregnancy and their ability to express killer inhibitory receptors (KIRs) for HLA-G suggests that these cells are also involved in the regulation of trophoblast invasion and maternal/trophoblast signalling during early pregnancy. However, HLA-C is the dominant ligand for uNK cell KIRs and recent evidence suggests that combinations of maternal KIRs on uNK cells combined with specific polymorphisms for fetal HLA-C may be unfavourable to trophoblast cell invasion (Moffett and Hiby, 2007). In contrast to CD56 + CD16 + NK cells, CD56 + CD16 − cells are potent secretors of cytokines but have low cytolytic ability and cytokine production is therefore another function of these cells.
Peripheral blood CD56 + cells originate in the bone marrow and are activated by IL-15; uNK cells may traffic into the endometrium from the peripheral blood (van den Heuval et al., 2005) although the presence of Ki67 (Pace et al., 1989) suggests that they also multiply within the endometrium where they come under the influence of various stromal cell populations, such as monocytes and dendritic cells. The uNK cells express glucocorticoid receptor and estrogen receptor β but not progesterone receptors or estrogen receptor α (Henderson et al., 2003). They may, however, be under the control of progesterone, possibly through the action of IL-15, as progesterone enhances IL-15 production in cultured human endometrial stromal cells (Okada et al., 2000a). IL-15 is essential for NK cell maintenance in blood and endometrium, and the production of endometrial IL-15 is increased during the secretory phase of the cycle and in first trimester decidua (Okada et al., 2000b). Maintenance of CD56 + cells within the endometrium may also be dependant on various other cytokines, such as IL-12 and IL-18 (Ledee-Bataille et al., 2005).
Conclusions
This study confirms previous reports that the number of endometrial CD56 + (NK) cells in women with RM is higher than those in control subjects. However, in this study, the number of endometrial CD56 + cells in peri-implantation endometrium did not appear to have any useful prognostic value on the outcome of a subsequent pregnancy. This observation questions the usefulness of the measurement of endometrial uNK cell number and the introduction of measurement of uNK cell numbers into routine clinical practice.
Acknowledgements
We would like to thank Staff Nurse Barbara Anstie and Staff Nurse Kath Wood for maintaining the recurrent miscarriage database.