-
PDF
- Split View
-
Views
-
Cite
Cite
M. Seppälä, H. Koistinen, R. Koistinen, P.C.N. Chiu, W.S.B. Yeung, Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations, Human Reproduction Update, Volume 13, Issue 3, May/June 2007, Pages 275–287, https://doi.org/10.1093/humupd/dmm004
Close - Share Icon Share
Abstract
Glycodelin is an example of a glycoprotein whose complex-type glycans mediate biological actions in human reproduction and immune reactions. Being attached to an identical protein backbone, glycodelin oligosaccharides vary significantly from one reproductive tissue to another and have an effect on its own secretion and role in cell communication. For instance, uterine glycodelin-A inhibits sperm–oocyte interaction by binding on the sperm head. This is a glycosylation-dependent phenomenon, in which fucosyltransferase-5 plays a key role. Glycodelin-S from seminal plasma binds evenly around the sperm head and maintains an uncapacitated state in the spermatozoa, until the isoform is detached during sperm passage through the cervix. Glycodelin-F from follicular fluid and Fallopian tube binds to the acrosomal region of the sperm head, thereby inhibiting both the sperm–oocyte binding and premature progesterone-induced acrosome reaction. The cumulus cells surrounding the oocyte can capture glycodelin-A and -F from the surrounding environment and convert these isoforms to a cumulus cell isoform, glycodelin-C. It differs by glycosylation from the other isoforms, and it too attaches on the sperm head, with the highest density in the equatorial region. Glycodelin-C is capable of detaching the sperm-bound inhibitory isoforms so that the sperm–oocyte binding is enhanced. Glycodelin-A also has immunosuppressive actions directed to cellular, humoral and innate immunity. Although these actions depend mainly on the protein backbone, glycosylation also plays a part. Glycosylated glycodelin may be involved in the protection of spermatozoa against maternal immune reactions, and glycodelin also has apoptogenic activity. Some glycosylation patterns of glycodelin may mask its apoptogenic domain. This review updates the recent research and clinical associations of glycodelin, highlighting the role of glycosylation.
Immunological and structural background
Nomenclature, antibodies and immunodetection
The name ‘glycodelin’ was proposed by the Helsinki team to their collaborators who had resolved the glycan structures and biological actions of purified glycoprotein ( Riittinen et al. , 1991 ), previously known as placental protein 14 or progesterone associated endometrial protein ( Dell et al. , 1995 ; Kämäräinen et al. , 1996 ; Koistinen et al. , 1996 ; Morris et al. , 1996 ). The name ‘glycodelin’ was subsequently agreed by the pioneers who had launched these and a variety of other names for this protein into the literature (reviewed in Seppälä et al. , 1998 ). The name ‘glycodelin’ highlights the importance of glycosylation for the biological activity of the glycoprotein that is formed in various sites of the body.
Glycodelin is sugar-rich, comprising 17.5 wt.% carbohydrate ( Bohn et al. , 1982 ). On the basis of the differences in glycosylation, the isoforms so far characterized are designated as glycodelin-A (amniotic fluid, endometrium/decidua and maternal serum) ( Dell et al. , 1995 ; Koistinen et al. , 2003 ), glycodelin-S (seminal plasma and seminal vesicles) ( Morris et al. , 1996 ), glycodelin-F (follicular fluid and the oviduct) ( Tse et al. , 2002 ) and glycodelin-C (cumulus oophorus) ( Chiu et al. , 2006 ). Polyclonal and monoclonal anti-glycodelin antibodies generated using purified glycodelin protein from amniotic fluid and seminal plasma show similar immunoreactivity against these four glycodelin isoforms. Although carbohydrates play an active role in protein folding and participate in the formation of discontinuous epitopes, the differentially glycosylated glycodelin isoforms, such as glycodelin-A and glycodelin-S, share similar thermodynamic parameters of reversible denaturation, suggesting that their native folding is not influenced by different glycosylation patterns ( Koistinen et al. , 1999 ). This may explain why the generation of antibodies specific for either glycodelin-A or glycodelin-S has turned out to be difficult, and all the four glycosylated isoforms react in the same way with anti-glycodelin antibodies.
In addition to the conventional monoclonal and polyclonal antibodies generated with the use of native glycosylated glycodelin that has been purified from biological materials, polyclonal antibodies have been produced against a synthetic linear femtopeptide consisting of amino acids 69–83 of the glycodelin sequence –NH 2 -Lys-Lys-Val-Leu-Gly-Glu-Lys-Thr-Glu-Asn-Pro-Lys-Lys-Phe-Lys-COOH ( Poddar et al. , 1998 ). Not unexpectedly, the results obtained with the use of this peptide antibody ( Horowitz et al. , 2001 ; Song et al. , 2001 ) are different from those obtained with the anti-glycodelin antibodies ( Julkunen et al. , 1986a ; Waites et al. , 1990 ; Mandelin et al. , 2003 ). The difference is seen both in normal tissues and in tumours, and cross-testing of the two types of antibodies (R. Koistinen and M. Seppälä, unpublished observation) revealed that, while both react with glycodelin, the femtopeptide antibody shows a wider spread of immunostaining in tumours, normal tissues and, notably, in blood vessels in which the glycodelin antibodies show no reactivity. Most importantly, not all immunoreactivity of the femtopeptide antibody can be abolished by absorption with purified glycodelin-A, demonstrating that its additional reactivity is glycodelin unrelated. Given that about one half of the sequence of the linear femtopeptide used for immunization ( Poddar et al. , 1998 ) is similar to the sequences present in many human proteins, such as SCP-1 peptide (100% identity with the first seven amino acids of the femtopeptide), cutaneous T cell Iymphoma (CTCL) tumour antigen se2-1, bullous pemphigoid antigen 1, dynein heavy chain domain 3, KIAA1503 protein, dystonin isoform 1eA precursor and a number of other proteins, any of these proteins/peptides may potentially cross-react with the femtopeptide antibody. Therefore, until the nature of the glycodelin unrelated additional immunoreactivity of the peptide antibody has been specified, the name ‘glycodelin’ is misleading in this context. Perhaps, a name ‘glycodelin-derived peptide’ should more accurately describe this immunoreactivity to distinguish it from glycodelin.
Glycodelin gene
The Human Genome Organization (HUGO) has registered progestagen-associated endometrial protein (PAEP) as the official symbol of the glycodelin gene ( Kämäräinen et al. , 1991 ). The gene is 5.05 kb long and, like many other lipocalin genes, it is divided into seven exons ( Vaisse et al. , 1990 ). The nucleotide sequence encoding the retinol-binding motif of β-lactoglobulins is conserved in the glycodelin gene that comprises four putative glucocorticoid/progesterone response elements (PRE) in the promoter region ( Vaisse et al. , 1990 ). The presence of PREs is compatible with the observations that progesterone is involved in the regulation of glycodelin synthesis.
Primary structure
Glycodelin is a member of the lipocalin family of proteins. Its primary sequence of 180 amino acid residues ( Julkunen et al. , 1988 ) has significant similarity with β-lactoglobulins from several species. The crystal structure of glycodelin is not known, and information on its tertiary structure rests on the Swiss model deduced from the crystal structure of bovine β-lactoglobulin ( Koistinen et al. , 1999 ).
Carbohydrate moieties
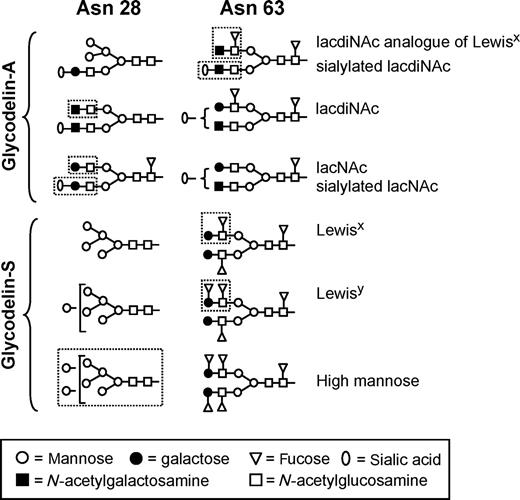
The amino acid sequence of glycodelin comprises three potential N -glycosylation sites, at positions 28, 63 and 85 ( Julkunen et al. , 1988 ). Two of them (Asn 28 and Asn 63) are glycosylated in uterine glycodelin-A and seminal plasma glycodelin-S. Nevertheless, there are striking differences in the glycosylation patterns between these two isoforms (Figure 1 ) ( Dell et al. , 1995 ; Morris et al. , 1996 ). Among the complex-type glycans of uterine glycodelin-A, the major non-reducing epitopes are lacNAc, lacdiNAc, sialylated lacNAc, sialylated lacdiNAc, blood group Lewis x and the lacdiNAc analogue of Lewis x ( Dell et al. , 1995 ) (Figure 1 ). A substantial subset of the glycans are fucosylated on the penultimate GlcNAc, yielding the fucosylated lacdiNAc type sequence (GalNAcβ1-4[Fucα1-3]GlcNAc). In addition to their physicochemical and immunological similarities, purified glycodelin from amniotic fluid, endometrium, decidua and pregnancy serum is similar in respect of their glycans and may be subgrouped as glycodelin-A ( Koistinen et al. , 2003 ). The high degree of sialylation and fucosylation are important characteristics of this isoform. Glycodelin-S differs from glycodelin-A in that it contains no sialylated glycans. Glycosylation of glycodelin-S is highly site-specific, as Asn 63 carries only complex-type structures, whereas only high mannose structures are found at Asn 28. Moreover, glycodelin-S glycans are unusually fucose-rich, so that more than 80% of the complex-type glycans at Asn 63 have 3–5 fucose residues per glycan. Each biantennary glycan in glycodelin-S is core fucosylated and the remaining fucoses are attached to lacNAc antennae, resulting in Lewis x and Lewis y blood group epitopes ( Morris et al. , 1996 ). The third well-defined isoform, glycodelin-F, from follicular fluid also has the same protein core as glycodelin-A and glycodelin-S, but it too has its own specific glycosylation, as indicated by studies employing fluorophore-assisted carbohydrate electrophoresis and lectin binding characteristics ( Chiu et al. , 2003b ). The fourth isoform, glycodelin-C, is modified from glycodelin-A and -F by the cumulus cells. Compared with its parent isoforms, its molecular size is smaller and the isoelectric point is higher, and their lectin binding properties are different. Glycodelin-C reacts strongly with concanavalin-A, Wisteria floribunda agglutinin, Ricinus communis agglutinin, Ulex europaeus agglutinin and Dolichos biflorus agglutinin but, unlike its parent isoforms, not with Sambucus nigra bark agglutinin that reacts with the α-NeuNAc(2-6)gal/galNAc residues (Table I ).
Representative examples of the major complex-type glycans present at the N -glycosylation sites Asn 28 and Asn 63 of glycodelin-A and glycodelin-S. Characteristic epitopes are marked by broken line. Adapted with permission from authors Dell et al. (1995) and Morris et al. (1996) and the American Society for Biochemistry and Molecular Biology.
Sperm-binding glycodelin isoforms
| Isoform . | Origin . | Typical carbohydrates . | Reference . |
|---|---|---|---|
| GdA | Endometrium, amniotic fluid, pregnancy serum | Sialylated and/or fucosylated lacNAc or lacdiNAc E-selectin ligands | Dell et al. , (1995) |
| Koistinen et al. , (2003) | |||
| Jeschke et al. , (2003) | |||
| GdF | Luteinized granulosa cells, follicular fluid, oviduct | Like GdA but more N -acetylglucosamine | Tse et al. , (2002) |
| Chiu et al. (2003a) | |||
| Yeung et al. (2006) | |||
| GdS | Seminal vesicles, seminal plasma | Fucosylated Lewis-x and Lewis-y, high mannose | Julkunen et al. (1984) |
| Koistinen et al. (1977) | |||
| Morris et al. (1996) | |||
| Chiu et al. (2005) | |||
| GdC | Cumulus cells | α-man, galNAc, β-gal, α-L-fuc | Chiu et al. (2006) |
| Isoform . | Origin . | Typical carbohydrates . | Reference . |
|---|---|---|---|
| GdA | Endometrium, amniotic fluid, pregnancy serum | Sialylated and/or fucosylated lacNAc or lacdiNAc E-selectin ligands | Dell et al. , (1995) |
| Koistinen et al. , (2003) | |||
| Jeschke et al. , (2003) | |||
| GdF | Luteinized granulosa cells, follicular fluid, oviduct | Like GdA but more N -acetylglucosamine | Tse et al. , (2002) |
| Chiu et al. (2003a) | |||
| Yeung et al. (2006) | |||
| GdS | Seminal vesicles, seminal plasma | Fucosylated Lewis-x and Lewis-y, high mannose | Julkunen et al. (1984) |
| Koistinen et al. (1977) | |||
| Morris et al. (1996) | |||
| Chiu et al. (2005) | |||
| GdC | Cumulus cells | α-man, galNAc, β-gal, α-L-fuc | Chiu et al. (2006) |
Gd, glycodelin.
Sperm-binding glycodelin isoforms
| Isoform . | Origin . | Typical carbohydrates . | Reference . |
|---|---|---|---|
| GdA | Endometrium, amniotic fluid, pregnancy serum | Sialylated and/or fucosylated lacNAc or lacdiNAc E-selectin ligands | Dell et al. , (1995) |
| Koistinen et al. , (2003) | |||
| Jeschke et al. , (2003) | |||
| GdF | Luteinized granulosa cells, follicular fluid, oviduct | Like GdA but more N -acetylglucosamine | Tse et al. , (2002) |
| Chiu et al. (2003a) | |||
| Yeung et al. (2006) | |||
| GdS | Seminal vesicles, seminal plasma | Fucosylated Lewis-x and Lewis-y, high mannose | Julkunen et al. (1984) |
| Koistinen et al. (1977) | |||
| Morris et al. (1996) | |||
| Chiu et al. (2005) | |||
| GdC | Cumulus cells | α-man, galNAc, β-gal, α-L-fuc | Chiu et al. (2006) |
| Isoform . | Origin . | Typical carbohydrates . | Reference . |
|---|---|---|---|
| GdA | Endometrium, amniotic fluid, pregnancy serum | Sialylated and/or fucosylated lacNAc or lacdiNAc E-selectin ligands | Dell et al. , (1995) |
| Koistinen et al. , (2003) | |||
| Jeschke et al. , (2003) | |||
| GdF | Luteinized granulosa cells, follicular fluid, oviduct | Like GdA but more N -acetylglucosamine | Tse et al. , (2002) |
| Chiu et al. (2003a) | |||
| Yeung et al. (2006) | |||
| GdS | Seminal vesicles, seminal plasma | Fucosylated Lewis-x and Lewis-y, high mannose | Julkunen et al. (1984) |
| Koistinen et al. (1977) | |||
| Morris et al. (1996) | |||
| Chiu et al. (2005) | |||
| GdC | Cumulus cells | α-man, galNAc, β-gal, α-L-fuc | Chiu et al. (2006) |
Gd, glycodelin.
Glycodelin produced by recombinant technologies may be either non-glycosylated or glycosylated, depending on the cells used for synthesis. For instance, recombinant glycodelin from Chinese hamster ovary cells is glycosylated and it reacts immunologically in the same way as glycodelin-A, yet their glycosylation patterns are different. By contrast, glycodelin produced in the human embryonic kidney 293 cells fulfils both immunological and glycosylation based criteria of glycodelin-A ( Van den Nieuwenhof et al. , 2000 ). Therefore, in the absence of specific information on the type of glycosylation, term glycodelin without any isoform designation is recommended.
Cellular origin and secretion in reproductive tissues
Glycodelin-A is synthesized in glandular and luminal surface epithelium of progesterone- or progestagen-exposed endometrium ( Julkunen et al. , 1986a ; Mandelin et al. , 1997 , 2001 ) (Table I ). During the estrogen dominated fertile window of a normal ovulatory cycle, absence of glycodelin-A synthesis in the endometrium is significant, because the glycoprotein has anti-fertilization activity (discussed later). In a normal ovulatory cycle, glycodelin expression peaks in the secretory endometrium 8–10 days after ovulation, whereas in maternal serum and amniotic fluid, the levels are highest at 10 and 16 weeks of pregnancy, respectively ( Julkunen et al. , 1985 ). Glycodelin-A is secreted mainly into endometrial/decidual gland lumen, from there to uterine fluid or amniotic fluid, and less to serum. Results of a study employing mutagenesis of the asparagines at the N -glycosylation sites (Asn 28 and Asn 63) suggest that glycosylation at Asn 28 plays a key role in the extracellular secretion of glycodelin, as mutation of Asn 28 brings about a significant decrease in the amount of secreted protein. Loss of both glycosylation sites dramatically reduces the secretion ( Jayachandran et al. , 2004 ). This study shows that glycosylation is essential for glycodelin secretion.
Glycodelin-S is one of the major secretory proteins in the seminal plasma, produced in seminal vesicle glands ( Petrunin et al. , 1980 ; Bohn et al. , 1982 ; Julkunen et al. , 1984 ; Koistinen et al. , 1997 ). No clinically meaningful association has been found between the glycodelin concentration in seminal plasma and sperm pathology ( Julkunen et al. , 1984 ), or the success of in vitro fertilization (IVF) ( Koistinen et al. , 2000 ).
Glycodelin-F is synthesized in luteinized granulosa cells of the ovary and it is present in follicular fluid (Table I ). Glycodelin has also been found in the oviduct, synthesized by its epithelial cells ( Julkunen et al. , 1986b , 1990 ; Laird et al. , 1995 ). Part of oviductal glycodelin is similar to glycodelin-F ( Yeung et al. , 2006 ). Cumulus oophorus cells contain glycodelin immunoreactivity but not glycodelin mRNA, indicating uptake rather than synthesis ( Tse et al. , 2002 ). The cumulus cell isoform is immunologically similar but it differs from the other three isoforms by glycosylation and charge ( Chiu et al. , 2006 ).
Outside the pelvic organs, glycodelin has been found in haematopoietic cells of the bone marrow ( Kämäräinen et al. , 1994 ; Morrow et al. , 1994 ), normal breast ( Kämäräinen et al. , 1999 ), glandular tissues in the lung and eccrine sweat glands ( Kämäräinen et al. , 1997 ). The type of glycosylation of glycodelin from these tissues remains to be characterized.
Biological behaviour and actions in the reproductive system
The difference in glycosylation between glycodelin and β-lactoglobulin is reflected in their different biological actions. Unlike β-lactoglobulin and many other lipocalins, glycodelin-A has not been found to bind retinol, retinoic acid or other lipocalin ligands ( Koistinen et al. , 1999 ). Glycodelin inhibits E-selectin mediated cell adhesion ( Jeschke et al. , 2003 ). This propensity is compatible with an observation that oligosaccharides with fucosylated lacdiNAc antennae present in glycodelin-A have been shown to block selectin-mediated adhesions, and a biantennary N-linked oligosaccharide bearing GalNAcβ1-4(Fucα1-3)GlcNAc antennae is an inhibitor of E-selectin-mediated adhesion ( Grinnell et al. , 1994 ). Glycodelin has also been shown to take part in cell differentiation, and it may be involved in tissue modelling ( Kämäräinen et al. , 1997 ; Koistinen et al. , 2005 ; Uchida et al. , 2005 ).
Binding of glycodelin isoforms on spermatozoa
All the four isoforms, glycodelin-A, -S, -F and -C, bind on the human sperm head (Tables II and III ). Glycodelin-F has two binding sites. One of them is shared with glycodelin-A, and glycodelin-A can displace maximally 70% of labelled glycodelin-F bound on the spermatozoa ( Chiu et al. , 2003b ). Immunocytochemical staining localizes glycodelin-F binding sites to the acrosomal region of the human spermatozoa. Studies on neoglycoproteins have shown that the binding of glycodelin-A to spermatozoa involves mannose, fucose and possibly E-selectin ligands, whereas that of glycodelin-F involves mannose, fucose and N -acetylglucosamine, but not the selectin ligands ( Chiu et al. , 2004 ).
Binding of glycodelin-A isoforms on different cell types
| Glycodelin-A . | Spermatozoa . | Monocyte . | T cell . | ||
|---|---|---|---|---|---|
| High affinity . | Low affinity . | CD14 + cell from PBMC . | U937 cell line . | ||
| KD (nM) | N/A | 21 ± 2 | 6.7 ± 1.1 | 48 ± 21 | N/A |
| Binding sites /cell | N/A | 1 884 260 ± 66 220 | 9 510–35 820 | N/A | N/A |
| Possible receptor | N/A | FUT5 | N/A | 250 kDa protein | CD45 |
| Action | Inhibits spermatozoa–zona pellucida binding | N/A | Inhibits chemotaxis | Inhibits proliferation Induces apoptosis | |
| Protects spermatozoa against lymphocyte attack | |||||
| Glycodelin-A . | Spermatozoa . | Monocyte . | T cell . | ||
|---|---|---|---|---|---|
| High affinity . | Low affinity . | CD14 + cell from PBMC . | U937 cell line . | ||
| KD (nM) | N/A | 21 ± 2 | 6.7 ± 1.1 | 48 ± 21 | N/A |
| Binding sites /cell | N/A | 1 884 260 ± 66 220 | 9 510–35 820 | N/A | N/A |
| Possible receptor | N/A | FUT5 | N/A | 250 kDa protein | CD45 |
| Action | Inhibits spermatozoa–zona pellucida binding | N/A | Inhibits chemotaxis | Inhibits proliferation Induces apoptosis | |
| Protects spermatozoa against lymphocyte attack | |||||
Kinetic data have been extracted from Chiu et al. (2003b) , Miller et al. (1998) , Vigne et al. (2001) and Rachmilewitz et al. (2003) . Data are expressed as mean and standard error of the mean (SEM). N/A, not available; KD , equilibrium dissociation constant, FUT5, sperm fucosyltransferase-5; PBMC, peripheral blood mononuclear cells.
Binding of glycodelin-A isoforms on different cell types
| Glycodelin-A . | Spermatozoa . | Monocyte . | T cell . | ||
|---|---|---|---|---|---|
| High affinity . | Low affinity . | CD14 + cell from PBMC . | U937 cell line . | ||
| KD (nM) | N/A | 21 ± 2 | 6.7 ± 1.1 | 48 ± 21 | N/A |
| Binding sites /cell | N/A | 1 884 260 ± 66 220 | 9 510–35 820 | N/A | N/A |
| Possible receptor | N/A | FUT5 | N/A | 250 kDa protein | CD45 |
| Action | Inhibits spermatozoa–zona pellucida binding | N/A | Inhibits chemotaxis | Inhibits proliferation Induces apoptosis | |
| Protects spermatozoa against lymphocyte attack | |||||
| Glycodelin-A . | Spermatozoa . | Monocyte . | T cell . | ||
|---|---|---|---|---|---|
| High affinity . | Low affinity . | CD14 + cell from PBMC . | U937 cell line . | ||
| KD (nM) | N/A | 21 ± 2 | 6.7 ± 1.1 | 48 ± 21 | N/A |
| Binding sites /cell | N/A | 1 884 260 ± 66 220 | 9 510–35 820 | N/A | N/A |
| Possible receptor | N/A | FUT5 | N/A | 250 kDa protein | CD45 |
| Action | Inhibits spermatozoa–zona pellucida binding | N/A | Inhibits chemotaxis | Inhibits proliferation Induces apoptosis | |
| Protects spermatozoa against lymphocyte attack | |||||
Kinetic data have been extracted from Chiu et al. (2003b) , Miller et al. (1998) , Vigne et al. (2001) and Rachmilewitz et al. (2003) . Data are expressed as mean and standard error of the mean (SEM). N/A, not available; KD , equilibrium dissociation constant, FUT5, sperm fucosyltransferase-5; PBMC, peripheral blood mononuclear cells.
Binding of glycodelin-F, -S and -C on spermatozoa
| . | Spermatozoa . | |
|---|---|---|
| . | High affinity . | Low affinity . |
| Glycodelin-F | ||
| KD (nM) | 3.9 ± 0.1 | 25 ± 2 |
| Binding sites/cell | 818 720 ± 18 060 | 2 028 740 ± 36 120 |
| Possible receptor | N/A | FUT5 |
| Action | Inhibits spermatozoa–zona pellucida binding | |
| Prevents premature acrosome reaction | ||
| Glycodelin-S | ||
| KD (nM) | 104 ± 20 | 1413 ± 316 |
| Binding sites/cell | 2 408 000 ± 301 000 | 5 117 000 ± 903 000 |
| Possible receptor | N/A | N/A |
| Action | Maintains spermatozoa in an uncapacitated state | |
| Glycodelin-C | ||
| KD (nM) | Positive co-operativity among binding sites | |
| Binding sites/cell | N/A | |
| Possible receptor | N/A | |
| Action | Stimulates spermatozoa–zona pellucida binding | |
| Displaces sperm-bound glycodelin-A/F | ||
| . | Spermatozoa . | |
|---|---|---|
| . | High affinity . | Low affinity . |
| Glycodelin-F | ||
| KD (nM) | 3.9 ± 0.1 | 25 ± 2 |
| Binding sites/cell | 818 720 ± 18 060 | 2 028 740 ± 36 120 |
| Possible receptor | N/A | FUT5 |
| Action | Inhibits spermatozoa–zona pellucida binding | |
| Prevents premature acrosome reaction | ||
| Glycodelin-S | ||
| KD (nM) | 104 ± 20 | 1413 ± 316 |
| Binding sites/cell | 2 408 000 ± 301 000 | 5 117 000 ± 903 000 |
| Possible receptor | N/A | N/A |
| Action | Maintains spermatozoa in an uncapacitated state | |
| Glycodelin-C | ||
| KD (nM) | Positive co-operativity among binding sites | |
| Binding sites/cell | N/A | |
| Possible receptor | N/A | |
| Action | Stimulates spermatozoa–zona pellucida binding | |
| Displaces sperm-bound glycodelin-A/F | ||
Kinetic data have been extracted from Chiu et al. (2003b , 2005 , 2006 ). Data were expressed as mean and (SEM). Information on monocyte or T cell effects of glycodelin-F, -S and -C is not available.
Binding of glycodelin-F, -S and -C on spermatozoa
| . | Spermatozoa . | |
|---|---|---|
| . | High affinity . | Low affinity . |
| Glycodelin-F | ||
| KD (nM) | 3.9 ± 0.1 | 25 ± 2 |
| Binding sites/cell | 818 720 ± 18 060 | 2 028 740 ± 36 120 |
| Possible receptor | N/A | FUT5 |
| Action | Inhibits spermatozoa–zona pellucida binding | |
| Prevents premature acrosome reaction | ||
| Glycodelin-S | ||
| KD (nM) | 104 ± 20 | 1413 ± 316 |
| Binding sites/cell | 2 408 000 ± 301 000 | 5 117 000 ± 903 000 |
| Possible receptor | N/A | N/A |
| Action | Maintains spermatozoa in an uncapacitated state | |
| Glycodelin-C | ||
| KD (nM) | Positive co-operativity among binding sites | |
| Binding sites/cell | N/A | |
| Possible receptor | N/A | |
| Action | Stimulates spermatozoa–zona pellucida binding | |
| Displaces sperm-bound glycodelin-A/F | ||
| . | Spermatozoa . | |
|---|---|---|
| . | High affinity . | Low affinity . |
| Glycodelin-F | ||
| KD (nM) | 3.9 ± 0.1 | 25 ± 2 |
| Binding sites/cell | 818 720 ± 18 060 | 2 028 740 ± 36 120 |
| Possible receptor | N/A | FUT5 |
| Action | Inhibits spermatozoa–zona pellucida binding | |
| Prevents premature acrosome reaction | ||
| Glycodelin-S | ||
| KD (nM) | 104 ± 20 | 1413 ± 316 |
| Binding sites/cell | 2 408 000 ± 301 000 | 5 117 000 ± 903 000 |
| Possible receptor | N/A | N/A |
| Action | Maintains spermatozoa in an uncapacitated state | |
| Glycodelin-C | ||
| KD (nM) | Positive co-operativity among binding sites | |
| Binding sites/cell | N/A | |
| Possible receptor | N/A | |
| Action | Stimulates spermatozoa–zona pellucida binding | |
| Displaces sperm-bound glycodelin-A/F | ||
Kinetic data have been extracted from Chiu et al. (2003b , 2005 , 2006 ). Data were expressed as mean and (SEM). Information on monocyte or T cell effects of glycodelin-F, -S and -C is not available.
Fucosyltransferases (FUT) constitute a family of glycosyltransferases that incorporate fucosyl residues into glycolipid or glycoprotein glycans, providing one of the possible termination steps of glycoconjugate biosynthesis of the sialyl Lewis x or sialyl Lewis a determinant that plays a role in cell–cell interaction ( Borsig et al. , 1996 ). It is believed that the function of glycosyltransferases on the cell surface is confined to their carbohydrate binding ability rather than glycosyltransferase function because of a lack of sugar nucleotide donors ( Colley, 1997 ). Using chemical cross-linking and anti-glycodelin antibody immunoprecipitation, the glycodelin-receptor complex was isolated and analysed by mass spectrometry and Western blot analysis using specific anti-FUT5 antibodies ( Chiu et al. , 2007 ). The results suggested that FUT5 serves as the receptor of glycodelin-A on human spermatozoa (Table IV ). Differential extraction of the surface labelled sperm proteins and immunofluorescence staining suggested that sperm FUT5 is an externally oriented integral membrane protein in the acrosomal region of the human spermatozoa. Subsequently, Chiu et al. (2007) succeeded in purification of biologically active FUT5 from human spermatozoa and, in co-immunoprecipitation experiments, they confirmed that the interaction between glycodelin-A and sperm FUT5 or recombinant FUT5 is highly specific. According to binding kinetic analyses, the KD of sperm FUT5 binding to solubilized zona pellucida is 43 pmol ml −1 . The ability of sperm FUT5 to bind to both glycodelin-A and -F, and to the zona pellucida, suggests that human sperm FUT5 is a receptor of glycodelin-A (and -F) and zona pellucida glycoproteins ( Chiu et al. , 2007 ). The likely mechanism by which these glycodelin isoforms inhibit spermatozoa–zona pellucida binding is by blocking the binding of sperm FUT5 to the zona pellucida.
Evidence that fucosyltransferase-5 (FUT5) is a glycodelin receptor on spermatozoa ( Chiu et al. , 2007 )
| In co-immunoprecipitation, solubilized zona pellucida reduces the binding of glycodelin-A to sperm FUT5 |
| Substrate of FUT5 specifically blocks the binding of glycodelin-A to spermatozoa in competition binding assay |
| Anti-FUT5 antibody and FUT5 acceptors inhibit spermatozoa-zona pellucida binding in hemizona binding assay |
| Indirect immunofluorescence staining localizes FUT5 to the acrosome region of human spermatozoa, a region that binds glycodelin-A and zona pellucida ( Chiu et al. , 2003a ) |
| Fluorophore-labelled sperm FUT5 binds strongly to intact and solubilized human zona pellucida |
| In co-immunoprecipitation, solubilized zona pellucida reduces the binding of glycodelin-A to sperm FUT5 |
| Substrate of FUT5 specifically blocks the binding of glycodelin-A to spermatozoa in competition binding assay |
| Anti-FUT5 antibody and FUT5 acceptors inhibit spermatozoa-zona pellucida binding in hemizona binding assay |
| Indirect immunofluorescence staining localizes FUT5 to the acrosome region of human spermatozoa, a region that binds glycodelin-A and zona pellucida ( Chiu et al. , 2003a ) |
| Fluorophore-labelled sperm FUT5 binds strongly to intact and solubilized human zona pellucida |
Evidence that fucosyltransferase-5 (FUT5) is a glycodelin receptor on spermatozoa ( Chiu et al. , 2007 )
| In co-immunoprecipitation, solubilized zona pellucida reduces the binding of glycodelin-A to sperm FUT5 |
| Substrate of FUT5 specifically blocks the binding of glycodelin-A to spermatozoa in competition binding assay |
| Anti-FUT5 antibody and FUT5 acceptors inhibit spermatozoa-zona pellucida binding in hemizona binding assay |
| Indirect immunofluorescence staining localizes FUT5 to the acrosome region of human spermatozoa, a region that binds glycodelin-A and zona pellucida ( Chiu et al. , 2003a ) |
| Fluorophore-labelled sperm FUT5 binds strongly to intact and solubilized human zona pellucida |
| In co-immunoprecipitation, solubilized zona pellucida reduces the binding of glycodelin-A to sperm FUT5 |
| Substrate of FUT5 specifically blocks the binding of glycodelin-A to spermatozoa in competition binding assay |
| Anti-FUT5 antibody and FUT5 acceptors inhibit spermatozoa-zona pellucida binding in hemizona binding assay |
| Indirect immunofluorescence staining localizes FUT5 to the acrosome region of human spermatozoa, a region that binds glycodelin-A and zona pellucida ( Chiu et al. , 2003a ) |
| Fluorophore-labelled sperm FUT5 binds strongly to intact and solubilized human zona pellucida |
Like glycodelin-A, glycodelin-S binds on the human sperm head via two binding sites that are saturable and reversible ( Chiu et al. , 2005 ). The binding sites of glycodelin-S are different because glycodelin-A and -F cannot displace glycodelin-S from its binding sites. Although the binding of glycodelin-S is specific, its affinity is low. The low affinity binding sites are more abundant than the high affinity binding sites. On the basis of indirect immunofluorescence staining the sperm-bound glycodelin-S covers the sperm head completely and this immunoreactivity is removed when spermatozoa migrate through cervical mucus surrogates. The low binding affinity of the carbohydrate-based interactions is likely to allow detachment of glycodelin-S from spermatozoa.
In follicular fluid, glycodelin-F is the main sperm–oocyte binding inhibitory isoform ( Chiu et al. , 2003a ), but follicular fluid also contains small amounts of glycodelin-A (P.C.N. Chiu and W.S.B. Yeung, unpublished observation). Glycodelin-C has been converted from glycodelin-A and -F by the cumulus cells ( Chiu et al. , 2006 ). Its protein core is identical with that of the other glycodelin isoforms, but glycodelin-C has a smaller molecular size, a higher isoelectric point and different lectin binding properties compared with the other isoforms (Table I ).
Capacitation
This is defined as a series of transformations that spermatozoa normally undergo during their migration through the female genital tract to reach and bind to the zona pellucida. During capacitation, extensive changes take place in all sperm compartments. Ion fluxes induce biochemical modifications, membrane lipids and proteins are reorganized, and complex signal transduction mechanisms are initiated ( de Lamirande et al. , 1997 ). At high physiological concentrations (>900 pmol ml −1 ) glycodelin-S significantly suppresses bovine serum albumin-induced capacitation of human spermatozoa, suggesting that glycodelin-S may contribute to an uncapacitated state of human spermatozoa in seminal plasma and prevent premature capacitation ( Chiu et al. , 2005 ). Deglycosylated glycodelin-S has no similar inhibitory effect, demonstrating the importance of glycosylation in this process. Interestingly, another study employing recombinant glycodelin has shown that whereas glycosylated glycodelin inhibits human sperm capacitation, non-glycosylated glycodelin stimulates it ( Dutta et al. , 2001 ). Compared with the binding kinetics of the other glycodelin isoforms, glycodelin-S binds to and detaches from spermatozoa at a faster rate than the other two isoforms do. The fast kinetics is obviously important for its biological action to take place because spermatozoa will be in contact with seminal plasma during a short time after ejaculation only. The weak binding affinity of glycodelin-S may also explain why labelled glycodelin immunoreactivity can be demonstrated on spermatozoa only when the cells are treated with high physiological concentration of glycodelin-S ( Chiu et al. , 2003b , 2005 ). It also explains the readiness of removal of bound glycodelin-S from spermatozoa during migration through the cervical mucus. Interestingly, glycodelin immunoreactivity has been found in the cervical mucus ( Pockley et al. , 1989 ), but its origin remains to be determined.
There is cholesterol efflux from human spermatozoa during bovine serum albumin and cyclodextrin induced capacitation of human spermatozoa. Glycodelin-S significantly reduces this efflux induced by either of the stimulators, and it exerts this effect upstream of protein kinase activation in the adenylyl cyclase/protein kinase A/tyrosine kinase signalling pathway. Again, these findings demonstrate the importance of carbohydrate moieties of glycodelin-S for its biological action, particularly because the said processes are activated upon removal of glycodelin-S from the spermatozoa. In vivo , the dissociation probably takes place during the passage of spermatozoa through the cervix, as suggested by in vitro experiments employing a cervical fluid surrogate ( Chiu et al. , 2005 ). In view of these observations glycodelin-S appears to play a role in maintaining an uncapacitated state in the human spermatozoa before their passage through the cervix.
Inhibition of sperm binding to the zona pellucida
Glycodelin-A was the first endogenous human glycoprotein that was found to potently and dose-dependently inhibit the binding of spermatozoa to the zona pellucida ( Oehninger et al. , 1995 ). This observation came from studies employing a hemizona assay (HZA) that was originally developed to predict the fertilizing potential of spermatozoa in the human, without inadvertent fertilization in vitro ( Burkman et al. , 1988 ). The HZA uses the bisected matching halves of a human zona pellucida, providing an internal control on zona to zona variability. One of the two halves can be exposed to a test substance, whereas the other serves as a control. In the case of glycodelin, the inhibition of sperm–oocyte binding was observed in HZA after a short pre-incubation time of glycodelin and the spermatozoa. This propensity of uterine glycodelin-A was glycosylation dependent, because differently glycosylated glycodelin-S had no similar activity ( Morris et al. , 1996 ). The result indicates that uterine glycodelin-A has anti-fertilizing activity (Table II ). This overtly surprising finding does not contradict with the current knowledge of the sequence of events in human reproduction, because glycodelin secretion is cyclically absent from endometrium during the fertile midcycle when the spermatozoa migrate through the uterine cavity to fertilize an oocyte in the fallopian tube.
Acrosome reaction
Initial sperm–oocyte binding in the mammals involves recognition of glycosylated proteins of the zona pellucida by glycosylated proteins on sperm surfaces ( Benoff, 1997 ). Once the binding of spermatozoa to the zona pellucida is engaged, acrosome reaction is induced via a G protein-mediated event ( Ward and Kopf, 1993 ). Glycodelin-F participates in the regulation of the acrosome reaction in an interesting fashion. The isoform is secreted from luteinized ovarian granulosa cells into pre-ovulatory follicular fluid ( Tse et al. , 2002 ) and transferred with the cumulus oophorus complex into the oviduct at ovulation. Glycodelin-F binds on the acrosome region of the sperm head, thereby inhibiting progesterone induced acrosome reaction. Glycodelin-A does not have this property. Glycodelin-F has even stronger an inhibitory activity on sperm-zona binding than glycodelin-A does ( Chiu et al. , 2003a ), and this capacity is lost upon deglycosylation ( Chiu et al. , 2003b ).
Role of the cumulus oophorus
At ovulation the oocyte and its associated cumulus cell mass containing follicular fluid are released and transported to the oviduct. The cumulus cells that surround the oocyte secrete hormones ( Shutt and Lopata, 1981 ), and they have been suggested to be involved in nutrition, selecting spermatozoa with normal morphology, induction of the acrosome reaction and support of oocyte nuclear maturation, fertilization and the subsequent embryo development ( Carrell et al. , 1993 ; Yanagimachi, 1994 ; Wongsrikeao et al. , 2005 ). Failure of cumulus oophorus formation results in problems in fertilization. For fertilization of the oocyte, the spermatozoa have to migrate through the cumulus cell mass.
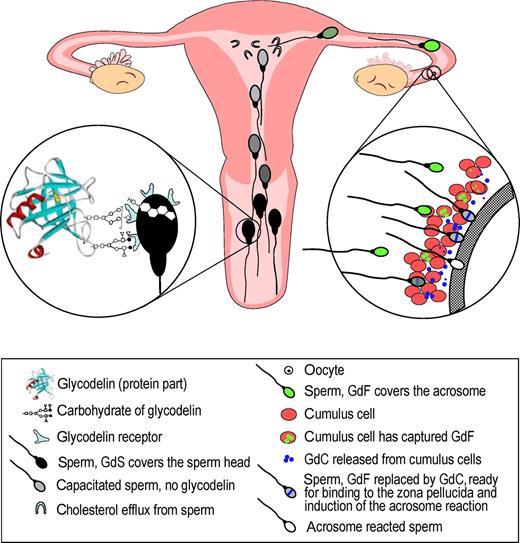
An interesting set of observations led to the discovery of the role of glycodelin-F in the fertilization process. Human follicular fluid was found to inhibit human sperm–zona pellucida binding ( Yao et al. , 1996 ), and the cumulus cells reduced this effect ( Hong et al. , 2003 ). To clarify the factors behind these observations, collaborative research between Hong Kong and Helsinki identified glycodelin as an important effector molecule in this phenomenon. As the sperm cells migrated through the cumulus oophorus matrix, both the glycodelin-A and glycodelin-F dependent inhibitory activities on gamete interaction were reduced (Figure 2 ). The uptake of these glycodelin isoforms by the cumulus cells was found to be unique among proteins of the lipocalin family. In addition to the uptake of glycodelin, the cumulus cells also partially deglycosylated glycodelin-F, yielding an interesting glycodelin isoform, glycodelin-C, that had opposite effects, i.e. it stimulated sperm–zona pellucida binding ( Chiu et al. , 2006 ). These observations may explain why removal of the cumulus cells before IVF does not improve the results, rather to the contrary ( Magier et al. , 1990 ). The results suggest that the biological role of glycodelin-F may be related to the prevention of premature progesterone-induced acrosome reaction before the spermatozoa have penetrated through the cumulus oophorus matrix on their way to bind to the zona pellucida. But there still are open questions. The stoichiometry between the cumulus cells and the spermatozoa that pass through this layer has not been determined, and it is not known if all the spermatozoa arriving at this site are bound to glycodelin-F in vivo . Furthermore, it is not known if all the spermatozoa that have migrated through the cumulus cell layer are free of glycodelin-F as they reach the zona pellucida, or how many of them carry the stimulatory glycodelin-C. It seems that the cumulus penetration of spermatozoa modifies them in two ways. The first is removal of the inhibitory effects of glycodelin-A and -F on sperm–oocyte binding and the acrosome reaction and the second is stimulation by glycodelin-C of the zona pellucida binding capacity of the spermatozoa ( Chiu et al. , 2006 ). Even if the cumulus cells did not remove or modify the inhibitory isoforms from all the spermatozoa, the results strongly suggest that the fertilizing spermatozoon is likely to be free of the unmodified, inhibitory isoforms. Whether the fertilizing spermatozoon requires stimulatory glycodelin-C bound on its head remains to be studied.
Many roles of glycodelin isoforms in sperm function Spermatozoa undergo several changes that have to be regulated in a timely manner before fertilization. Various glycoforms of glycodelin are involved in the regulation of these changes. After ejaculation the spermatozoa are covered by glycodelin-S (GdS) from seminal plasma, resulting in inhibition of premature capacitation and the capacitation-related cholesterol efflux from the sperm cells ( Chiu et al. , 2005 ). During migration in the Fallopian tube the spermatozoa will bind glycodelin-F (GdF) that inhibits sperm–oocyte binding and premature acrosome reaction ( Chiu et al. , 2003a ). Glycodelin is captured from the spermatozoa by the cumulus cells that convert it to glycodelin-C (GdC). This glycoform enhances sperm–oocyte binding, and inhibition of the acrosome reaction is removed. The fertilizing spermatozoon is likely to be free of inhibitory forms of glycodelin. All the above activities of glycodelin are dependent on its specific glycosylation patterns.
The immune system
Glycodelin is a rare human glycoprotein because it manifests both immunosuppressive and anti-fertilization activities, suggesting convergence of human immune and gamete recognition systems ( Clark et al. , 1996b ).
The first identification of immunosuppressive activity came from the observations that purified glycodelin and decidual tissue extract inhibit thymidine uptake in mixed lymphocyte culture and glycodelin antibodies neutralized the antiproliferative effects of decidual tissue extract ( Bolton et al. , 1987 ; Pockely and Bolton, 1989 , 1990 ). A receptor for glycodelin has been found on the human monocytes ( Miller et al. , 1998 ). Glycodelin also binds to pregnancy zone protein and α2-macroglobulin that may enhance its immunosuppressive activity ( Skornicka et al. , 2004 ).
T cells
Glycodelin inhibits T cell proliferation ( Rachmilewitz et al. , 1999 ), and it renders T cells less sensitive to stimulation ( Rachmilewitz et al. , 2001 ) (Table V ). The inhibitory activity of glycodelin is mainly directed to the Th-1 type cytokine response by selectively inhibiting the expression of a chemokine receptor (CXCR3) associated with the Th1 subtype and preventing repression of the transcriptional factor GATA-3, an event that is essential for differentiation along the Th1 lineage ( Mishan-Eisenberg et al. , 2004 ). Glycodelin appears to negatively regulate T cell activation by diminishing their responses in the contact site at the time of T cell receptor triggering ( Rachmilewitz et al. , 2002 ). This activity appears to be mediated by CD45, the tyrosine phosphatase receptor ( Rachmilewitz et al. , 2003 ). As glycodelin binds to the CD45 receptor on T cells, it may act as a calcium-dependent lectin to bind other T cell surface glycoproteins to mediate its immunoregulatory activities ( Ish-Shalom et al. , 2006 ). Importantly, glycodelin binding to T cells can be competitively inhibited with oligosaccharides, showing that glycodelin binds to T cell surfaces in a carbohydrate-dependent manner. Glycodelin has been suggested to act through its distinct receptors that are decorated by carbohydrates and expressed in different cells, wherein one of its surface molecular targets, CD45, mediates its T cell inhibitory activity ( Yaniv et al. , 2003 ).
Immunosuppressive activity of glycodelin
| Type of immune cell . | Effect by glycodelin . | Reference . |
|---|---|---|
| T cells | Inhibits T cell proliferation | Rachmilewitz et al. , (1999) |
| Renders T cells less sensitive to stimulation | Rachmilewitz et al. , (2001) | |
| Diminishes T cell responses in the contact site at the time of T cell receptor triggering | Rachmilewitz et al. , (2002) | |
| Inhibition mediated by CD45, the tyrosine phosphatase receptor | Rachmilewitz et al. , (2003) | |
| Inhibits expression of a chemokine receptor CXCR3 | Mishan-Eisenberg et al. , (2004) | |
| B cells | Inhibits B cell proliferation, IgM secretion and the surface expression of MHC class II | Yaniv et al. , (2003) |
| Inhibits B cell receptor mediated activation of human B cells | Yaniv et al. , (2003) | |
| NK cells | Inhibits cytotoxic activity of NK cells from peripheral blood | Okamoto et al. , (1991) |
| Increased expression in uterine NK cells suggesting local activity | Koopman et al. , (2003) |
| Type of immune cell . | Effect by glycodelin . | Reference . |
|---|---|---|
| T cells | Inhibits T cell proliferation | Rachmilewitz et al. , (1999) |
| Renders T cells less sensitive to stimulation | Rachmilewitz et al. , (2001) | |
| Diminishes T cell responses in the contact site at the time of T cell receptor triggering | Rachmilewitz et al. , (2002) | |
| Inhibition mediated by CD45, the tyrosine phosphatase receptor | Rachmilewitz et al. , (2003) | |
| Inhibits expression of a chemokine receptor CXCR3 | Mishan-Eisenberg et al. , (2004) | |
| B cells | Inhibits B cell proliferation, IgM secretion and the surface expression of MHC class II | Yaniv et al. , (2003) |
| Inhibits B cell receptor mediated activation of human B cells | Yaniv et al. , (2003) | |
| NK cells | Inhibits cytotoxic activity of NK cells from peripheral blood | Okamoto et al. , (1991) |
| Increased expression in uterine NK cells suggesting local activity | Koopman et al. , (2003) |
Immunosuppressive activity of glycodelin
| Type of immune cell . | Effect by glycodelin . | Reference . |
|---|---|---|
| T cells | Inhibits T cell proliferation | Rachmilewitz et al. , (1999) |
| Renders T cells less sensitive to stimulation | Rachmilewitz et al. , (2001) | |
| Diminishes T cell responses in the contact site at the time of T cell receptor triggering | Rachmilewitz et al. , (2002) | |
| Inhibition mediated by CD45, the tyrosine phosphatase receptor | Rachmilewitz et al. , (2003) | |
| Inhibits expression of a chemokine receptor CXCR3 | Mishan-Eisenberg et al. , (2004) | |
| B cells | Inhibits B cell proliferation, IgM secretion and the surface expression of MHC class II | Yaniv et al. , (2003) |
| Inhibits B cell receptor mediated activation of human B cells | Yaniv et al. , (2003) | |
| NK cells | Inhibits cytotoxic activity of NK cells from peripheral blood | Okamoto et al. , (1991) |
| Increased expression in uterine NK cells suggesting local activity | Koopman et al. , (2003) |
| Type of immune cell . | Effect by glycodelin . | Reference . |
|---|---|---|
| T cells | Inhibits T cell proliferation | Rachmilewitz et al. , (1999) |
| Renders T cells less sensitive to stimulation | Rachmilewitz et al. , (2001) | |
| Diminishes T cell responses in the contact site at the time of T cell receptor triggering | Rachmilewitz et al. , (2002) | |
| Inhibition mediated by CD45, the tyrosine phosphatase receptor | Rachmilewitz et al. , (2003) | |
| Inhibits expression of a chemokine receptor CXCR3 | Mishan-Eisenberg et al. , (2004) | |
| B cells | Inhibits B cell proliferation, IgM secretion and the surface expression of MHC class II | Yaniv et al. , (2003) |
| Inhibits B cell receptor mediated activation of human B cells | Yaniv et al. , (2003) | |
| NK cells | Inhibits cytotoxic activity of NK cells from peripheral blood | Okamoto et al. , (1991) |
| Increased expression in uterine NK cells suggesting local activity | Koopman et al. , (2003) |
B cells
Glycodelin also has an effect on the B cells (Table V ). A human B cell inhibitory receptor, CD22, binds to the sialylated lacNAc sequences ( Powell and Varki, 1994 ). It has been suggested that the oligosaccharides bearing sialylated lacNAc or lacdiNAc antennae present in glycodelin-A may manifest immunosuppressive effects by specifically blocking the adhesive and activation related events mediated by CD22 ( Dell et al. , 1995 ). Preliminary evidence indicates that glycodelin inhibits B cell receptor mediated activation of human B cells ( Yaniv et al. , 2003 ). Here, glycodelin inhibits B cell proliferation and the up-regulation of IgM and major histocompatibility complex (MHC), but not CD69 and CD86, regardless of the extent of B cell receptor triggering. These findings suggest that glycodelin affects some but not all B cell responses. The B cell inhibition by glycodelin is different from the T cell inhibition in that the extent of glycodelin-mediated inhibition does not correlate to the level of B cell receptor triggering, suggesting that glycodelin interferes with late events of B cell receptor signalling ( Yaniv et al. , 2003 ). Interestingly, CD22 binds to CD45, the leukocyte specific receptor linked phosphotyrosine phosphatase involved in T cell activation ( Stamenkovic et al. , 1991 ). Taken together, these studies suggest that glycodelin is a soluble regulatory factor capable of interacting with both T and B cells in a carbohydrate-dependent manner to affect both cellular and humoral immune responses ( Yaniv et al. , 2003 ).
Innate immunity
Besides its actions on humoral and cellular immunity, glycodelin inhibits cytotoxicity of peripheral blood natural killer (NK) cells ( Okamoto et al. , 1991 ) (Table V ). These cells are of specific interest because they need no prior exposure to react with foreign antigens, such as bacteria, viruses or an embryo, a semi-allograft. It is believed that uterine NK cells are derived from a subset of the NK cells in peripheral blood (reviewed in Dosiou and Giudice, 2005). There is selective, increased expression of glycodelin in the NK cells isolated from pregnancy decidua ( Koopman et al. , 2003 ). However, direct evidence that glycodelin inhibits cytotoxicity of uterine NK cells is not available yet, and the role of glycosylation in this process remains to be clarified.
Immunoprotection of spermatozoa
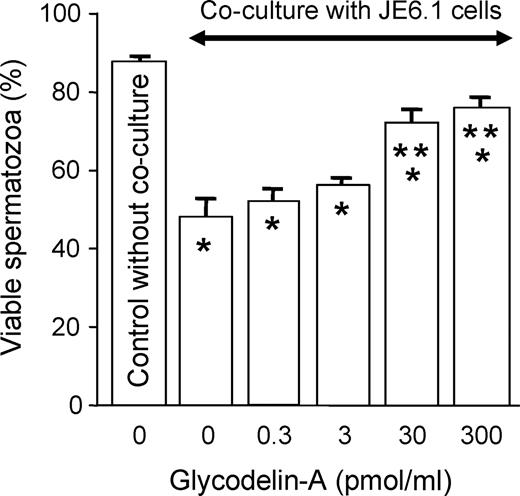
The expression of glycoconjugates by normal cells may protect them from immune responses, especially in those cases in which the MHC recognition is minimal or absent ( Clark et al. , 1997 ). In spite of the frequent exposure to antigens in spermatozoa and in seminal plasma, women are rarely immunized against spermatozoa. Should it happen, the question remains whether the various glycodelin glycoforms that bind on the human spermatozoa would protect them against immune responses in the female body. The immunosuppressive properties of the Lewis x/y epitopes present in glycodelin-S may contribute to the low immunogenicity of sperm in women ( Morris et al. , 1996 ). Recent findings (R. Tsang and W.S.B. Yeung, unpublished results) indicate that glycodelin-A treatment maintains viability of human spermatozoa in co-culture with a human lymphocyte cell line (Figure 3 ), and glycodelin bound spermatozoa are less likely to activate the lymphocytes. Obviously, glycosylation plays an important part here because, in the absence of glycosylation, glycodelin does not bind on the spermatozoa ( Chiu et al. , 2003b ), and deglycosylated glycodelin does not have the same effect. But, there are other mechanisms, e.g. attenuation of immune responses against the gametes or the blastocyst may be manifested by binding of selectin or ‘selectin-like’ receptors on the spermatozoa to the oligosaccharide ligands on immune effector cells ( Clark et al. , 1996b ).
Co-culture of human spermatozoa with JE6.1 lymphocyte cell line in the absence or presence of different concentrations of glycodelin-A. * P < 0.05, significant difference when compared with control without co-culture (column 1).* P < 0.05, significant difference when compared with control without glycodelin-A pretreatment (column 2).
Other aspects
Oligosaccharides with at least one fucosylated lacdiNAc antenna, such as present in glycodelin-A (Figure 1 ), have been shown to be 15–20-fold more potent inhibitors of selectin-mediated adhesions than either sialyl- or sulpho-Lewis x/a type oligosaccharides ( Grinnell et al. , 1994 ). The presence of such oligosaccharides in glycodelin suggests indirectly that glycodelin may manifest some of its immunosuppressive effects by blocking selectin-dependent adhesions ( Clark et al. , 1996a ). More recently, direct evidence has been provided that glycodelin inhibits E-selectin-mediated cell adhesion ( Jeschke et al. , 2003 ).
Apoptosis
Glycodelin-A but not glycodelin-S induces apoptosis in T cells, but not in monocytes ( Mukhopadhyay et al. , 2001 ). This function appears to be dependent on the presence of sialic acid residues ( Mukhopadhyay et al. , 2004 ). The immunosuppressive activity of glycodelin is related to its apoptogenic activity ( Jayachandran et al. , 2004 ). Studies employing mutagenesis of the asparagines at the N -glycosylation sites (Asn 28 and Asn 63) to glutamine show that the apoptogenic activity of glycodelin resides mainly in the protein backbone. The apoptogenic activity is present in glycodelin-A, whereas no similar activity is present in glycodelin-S and recombinant glycodelin products expressed in Pichia pastoris , or in Chinese hamster ovary cells ( Karande et al. , 2005 ). Recombinant glycodelin expressed in the SF21 insect cell line also yields an apoptotically active glycodelin, whereas the same gene expressed in the Tni insect cell line produces apoptotically inactive glycodelin ( Jayachandran et al. , 2006 ). These results suggest that, although the apoptogenic activity of glycodelin resides mainly in the protein backbone, certain glycans may modulate this activity either by masking or unmasking the functional region in the glycodelin molecule.
Micro-organisms
Another emerging relationship is that the same unusual carbohydrate sequences present in immunosuppressive glycodelin are also expressed on intravascular helminthic parasites ( Srivatsan et al. , 1992 ), Helicobacter pylori ( Aspinall and Monteiro, 1996 ) and HIV infected T lymphocytes (Kawashinagi et al. , 1994). This similarity suggests that mimicry or acquisition of the glycans used in this system by the pathogens may enable them to subvert or misdirect the host immune response to their own advantage ( Clark et al. , 1997 ).
Clinical aspects
Fertilization, implantation and placentation
In clinical practice, many ways have been tried to improve the fertilizing capacity of spermatozoa, from mere washing to specific substances and media. The classical Percoll™ treatment removes glycodelin-S from the sperm head. There is no systematic study addressing dislodging of glycodelin by the various media used for this purpose. Nevertheless, the present knowledge indicates that, outside their appropriate timing, binding of any of the three inhibitory glycodelin isoforms (-A, -F and -S) on the sperm head should have negative effects on the fertilizing capacity of spermatozoa. In respect of fertilization, it is significant that the binding of glycodelin-S is loose and reversible.
Due to ethical constraints, experimental studies on human implantation have not been feasible, and the importance of glycodelin in human implantation rests on its established immunosuppressive activities and abundance at the fetomaternal interface. In a normal fertile cycle, implantation takes place 8 days after the luteinizing hormone surge. At that time, the endometrium contains a full array of immune cells. According to global gene profiling studies, glycodelin expression is significantly increased during the window of implantation ( Kao et al. , 2002 ), and this change is translated into increased glycodelin synthesis and secretion at this site ( Julkunen et al. , 1986a ; reviewed in Seppälä et al. , 2002 ). Given its inhibitory effect on the peripheral blood NK cells ( Okamoto et al. , 1991 ) and the likelihood that uterine NK cells derive from peripheral blood NK cells (Dosiou and Giudice, 2005), an immunoprotective role has been suggested for glycodelin-A during implantation and placentation ( Okamoto et al. , 1991 ; Clark et al. , 1996a ).
Failure in reproductive performance may manifest itself at the level of implantation due to undeveloped endometrial microenvironment. This may result in failure to implant or insufficient support of early pregnancy after implantation. Relevant clinical studies on glycodelin in such conditions are listed in Table VI .
Clinical relevance of glycodelin research
| Clinical condition . | Typical change in glycodelin . | Reference . |
|---|---|---|
| Unexplained infertility | ||
| Retarded endometrial differentiation | Reduced expression in endometrial biopsy specimens and uterine flushing, | Li et al. , (1993a) |
| Reduced endometrial receptivity | Klentzeris et al. , (1994) | |
| Mackenna et al. , (1993) | ||
| Recurrent spontaneous abortion | Reduced serum level in mid-luteal phase | Tulppala et al. , (1995) |
| Reduced concentration in uterine flushing | Dalton et al. , (1998) | |
| Early pregnancy loss in PCOS | Reduced serum level in first trimester pregnancy | Jakubowicz et al. , (2004) |
| Endometrial and placental dysfunction | ||
| Outcome of IVF | ||
| Higher pregnancy rate | Reduced pretreatment serum level in mid-luteal phase | Westergaard et al. , (2004) |
| Greater increment in serum level from oocyte retrieval to embryo transfer | Liu et al. , (2006) | |
| Cancer, tumour tissue | ||
| Endometrial cancer | Expression related to reduced cell growth and differentiation | Koistinen et al. , (2005) |
| Ovarian serous adenocarcinoma | Expression related to better survival | Mandelin et al. , (2003) |
| Clinical condition . | Typical change in glycodelin . | Reference . |
|---|---|---|
| Unexplained infertility | ||
| Retarded endometrial differentiation | Reduced expression in endometrial biopsy specimens and uterine flushing, | Li et al. , (1993a) |
| Reduced endometrial receptivity | Klentzeris et al. , (1994) | |
| Mackenna et al. , (1993) | ||
| Recurrent spontaneous abortion | Reduced serum level in mid-luteal phase | Tulppala et al. , (1995) |
| Reduced concentration in uterine flushing | Dalton et al. , (1998) | |
| Early pregnancy loss in PCOS | Reduced serum level in first trimester pregnancy | Jakubowicz et al. , (2004) |
| Endometrial and placental dysfunction | ||
| Outcome of IVF | ||
| Higher pregnancy rate | Reduced pretreatment serum level in mid-luteal phase | Westergaard et al. , (2004) |
| Greater increment in serum level from oocyte retrieval to embryo transfer | Liu et al. , (2006) | |
| Cancer, tumour tissue | ||
| Endometrial cancer | Expression related to reduced cell growth and differentiation | Koistinen et al. , (2005) |
| Ovarian serous adenocarcinoma | Expression related to better survival | Mandelin et al. , (2003) |
Clinical relevance of glycodelin research
| Clinical condition . | Typical change in glycodelin . | Reference . |
|---|---|---|
| Unexplained infertility | ||
| Retarded endometrial differentiation | Reduced expression in endometrial biopsy specimens and uterine flushing, | Li et al. , (1993a) |
| Reduced endometrial receptivity | Klentzeris et al. , (1994) | |
| Mackenna et al. , (1993) | ||
| Recurrent spontaneous abortion | Reduced serum level in mid-luteal phase | Tulppala et al. , (1995) |
| Reduced concentration in uterine flushing | Dalton et al. , (1998) | |
| Early pregnancy loss in PCOS | Reduced serum level in first trimester pregnancy | Jakubowicz et al. , (2004) |
| Endometrial and placental dysfunction | ||
| Outcome of IVF | ||
| Higher pregnancy rate | Reduced pretreatment serum level in mid-luteal phase | Westergaard et al. , (2004) |
| Greater increment in serum level from oocyte retrieval to embryo transfer | Liu et al. , (2006) | |
| Cancer, tumour tissue | ||
| Endometrial cancer | Expression related to reduced cell growth and differentiation | Koistinen et al. , (2005) |
| Ovarian serous adenocarcinoma | Expression related to better survival | Mandelin et al. , (2003) |
| Clinical condition . | Typical change in glycodelin . | Reference . |
|---|---|---|
| Unexplained infertility | ||
| Retarded endometrial differentiation | Reduced expression in endometrial biopsy specimens and uterine flushing, | Li et al. , (1993a) |
| Reduced endometrial receptivity | Klentzeris et al. , (1994) | |
| Mackenna et al. , (1993) | ||
| Recurrent spontaneous abortion | Reduced serum level in mid-luteal phase | Tulppala et al. , (1995) |
| Reduced concentration in uterine flushing | Dalton et al. , (1998) | |
| Early pregnancy loss in PCOS | Reduced serum level in first trimester pregnancy | Jakubowicz et al. , (2004) |
| Endometrial and placental dysfunction | ||
| Outcome of IVF | ||
| Higher pregnancy rate | Reduced pretreatment serum level in mid-luteal phase | Westergaard et al. , (2004) |
| Greater increment in serum level from oocyte retrieval to embryo transfer | Liu et al. , (2006) | |
| Cancer, tumour tissue | ||
| Endometrial cancer | Expression related to reduced cell growth and differentiation | Koistinen et al. , (2005) |
| Ovarian serous adenocarcinoma | Expression related to better survival | Mandelin et al. , (2003) |
The value of histologic dating of the timed endometrial biopsy for clinical assessment of the fertility status has been questioned, because an equal incidence of morphologic delay may occur in fertile and infertile women ( Coutifaris et al. , 2004 ; Krazer, 2004 ). Where a delay is observed, it is usually caught up within 2 or 3 days ( Damario et al. , 2001 ). Histologically retarded endometrium shows reduced glycodelin immunostaining, indicating functional failure that may adversely affect uterine receptivity during implantation and placentation ( Klentzeris et al. , 1994 ).
Pinopodes are protrusions on the endometrial surface that have been suggested to be ultrastructural markers of endometrial receptivity ( Nikas et al. , 1995 ). Glycodelin emerges in the endometrial glands at the time when the pinopodes appear, with an inverse correlation with the progesterone receptor B. Glycodelin has been detected on the pinopodes ( Stavreus-Evers et al. , 2006 ), but its expression is not limited to these structures. Taken together, these observations clearly show the presence of immunosuppressive glycodelin-A in the receptive phase endometrium where it is likely to contribute to the fetomaternal defence mechanisms by interacting with the immune cells that are replete at the same site.
Ovarian failure, unexplained infertility and recurrent spontaneous abortion
In women with premature ovarian failure who conceived after ovum donation and embryo transfer with exogenous steroid support, the serum glycodelin levels in early pregnancy were lower than in the women with normal ovarian function. This suggests that factors under the control of the maternal ovary are involved in glycodelin production by the endometrium ( Critchley et al. , 1992 ). Besides progesterone that was replaced, relaxin appears to be another corpus luteum hormone that stimulates glycodelin secretion ( Stewart et al. , 1997 ; Tseng et al. , 1999 ). Subnormal secretion or absence of ovarian relaxin secretion may have contributed to the observed difference in women with premature ovarian failure.
Subnormal peri-implantation phase glycodelin levels have been reported in the uterine fluid of non-pregnant patients with unexplained infertility ( Mackenna et al. , 1993 ) and also in women with a history of recurrent spontaneous abortion ( Dalton et al. , 1998 ; Salim et al. , 2006 ). In the latter condition, also the circulating glycodelin concentration may be reduced at the mid-luteal phase ( Tulppala et al. , 1995 ). Although the glycodelin concentration of uterine secretions correlates well with endometrial morphology during the menstrual cycle ( Li et al. , 1993b ), particularly during the peri-implantation period ( Li et al. , 1993a ), the question remains whether this measurement would be clinically any more useful than immunohistochemical detection of glycodelin in endometrial biopsies, or the routine histologic dating. An advantage of determining glycodelin secretion from uterine flushings instead of endometrial biopsy is that uterine flushings should give a more comprehensive picture of uterine glycodelin secretion because, in biopsies, glycodelin expression varies from one endometrial site to another ( Li et al. , 1991 ). Therefore, in spite of its invasive nature, the measurement of glycodelin from uterine flushings has clinical potential, should a standardized methodology become widely applicable.
Polycystic ovary syndrome
This condition is frequently associated with increased secretion of luteinizing hormone, androgen and insulin, resulting in problems with ovulation, implantation and early pregnancy loss. In ovulatory cycles of the women with polycystic ovary syndrome (PCOS), glycodelin serum level increases during treatment with metformin, an insulin-reducing agent ( Jakubowicz et al. , 2001 ). During pregnancy, reduced glycodelin serum concentrations in the first trimester are associated with early pregnancy loss, suggesting failure in placentation ( Jakubowicz et al. , 2004 ). Interestingly, insulin has no acute glycodelin reducing effect ( Seppälä et al. , 2005 ), whereas its long-term effects on glycodelin secretion remain to be studied. Furthermore, it remains to be proven if the low glycodelin serum level plays any part in the pathogenesis of PCOS-related early pregnancy loss due to reduced fetomaternal defence mechanisms at the placentation site.
In vitro fertilization
Given the steep rise of serum glycodelin level from implantation onwards ( Julkunen et al. , 1985 ), prediction of the outcome of IVF by serum glycodelin levels has been thoroughly explored. Rather disappointedly, in many studies, the subnormal glycodelin serum levels at the implantation phase have not predicted fertile or infertile cycles in any consistent way (reviewed in Seppälä et al. , 2002 ). More recent reports point to potential clinical utility under specific circumstances. For instance, there is a significant correlation between low serum glycodelin levels on day 21 of the pretreatment cycle and a higher pregnancy rate following IVF/intracytoplasmic sperm injection in normogonadotrophic women subjected to the long protocol of pituitary down-regulation and gonadotrophin stimulation ( Westergaard et al. , 2004 ). In the subsequent treatment cycle, the glycodelin serum levels were significantly higher in conception than in non-conception cycles. The authors suggest that measuring the mid-luteal serum glycodelin level in the pretreatment cycle may offer a clinical test to decide whether infertility treatment should be initiated in that cycle or not. Perhaps, the low serum glycodelin level identified a specific group of women whose infertility was related to endometrial dysfunction, and this condition was corrected during ovarian stimulation for IVF, contributing to the high success rate.
Another approach is also of interest ( Liu et al. , 2006 ). In individual IVF cycles, comparison between serum glycodelin levels taken on the day of oocyte retrieval and on the day of embryo transfer gave information on success in terms of achieving a pregnancy. Although no overall difference in serum glycodelin was found on the day of oocyte retrieval or embryo transfer between the non-pregnant and pregnant groups, both the ratio and the difference of serum glycodelin levels on the days of embryo transfer and oocyte retrieval were higher in the pregnant group than in the non-pregnant group. This approach is of interest because it addresses intra-individual rather than inter-individual differences and, therefore, is free of the problem of wide individual variation in the glycodelin levels. The results are compatible with the fetoembryonic defence system hypothesis ( Clark et al. , 1996a ).
Both studies suggest clinical potential of the glycodelin test in predicting a short-term IVF success under specific situations, but in different ways. The results are not mutually controversial because the first study addressed low pretreatment levels and the second study measured the difference from oocyte retrieval to embryo transfer in the hormonally stimulated treatment cycles.
Another viewpoint in IVF comes from the observed apoptogenic activity of glycodelin, i.e. whether the glycodelin isoforms taken up (and possibly internalized) by the cumulus cells can exert apoptogenic activity before fertilization. The question is of interest because apoptosis has been used to estimate ovarian reserve in women undergoing IVF ( Seifer et al. , 1996 ). Here, the gametes and embryos derived from the cumulus complexes with no or minor apoptosis were found to have an increased chance of giving rise to optimum blastocysts ( Corn et al. , 2005 ).
Contraception
Contraceptive methods employing progestogens have an effect on endometrial glycodelin secretion. This has been shown in levonorgestrel-releasing intrauterine contraceptive system ( Mandelin et al. , 1997 ), subdermal implants ( Mandelin et al. , 2001 ) and levonorgestrel only containing pills taken for emergency contraception before the LH surge ( Durand et al. , 2005 ). In view of the anti-fertilizing effects of glycodelin-A, it would seem that induction of glycodelin synthesis before fertilization could contribute to the contraceptive effect of the above methods. However, the significance of this effect remains to be determined, because evidence of the magnitude of the effect in vivo is not available and the oocyte/cumulus cell complex can remove glycodelin from the spermatozoa and modify its activity before fertilization (see Role of the cumulus oophorus).
Cancer
Endometrial cancer
When endometrial adenocarcinoma cells (Ishikawa cells) were co-cultured with normal endometrial stromal cells in the presence of progesterone, proliferation was reduced concomitantly with induced glycodelin expression ( Arnold et al. , 2002 ). At the same time, the adenocarcinoma cells underwent conversion to a normal phenotype. These findings raised a question of whether glycodelin was a cause or a consequence of the reduction of malignant characteristics. Recent evidence indicates that glycodelin may be primary to the observed change. Experiments employing transfection of glycodelin cDNA into glycodelin-negative endometrial adenocarcinoma cells brought about induction of glycodelin expression at the same time as they showed increased differentiation and reduced tumour cell growth. A significant concomitant observation was reduced expression of the Bcl-XL gene, an anti-apoptotic survival gene involved in tumour cell growth and chemoresistance ( Koistinen et al. , 2005 ). The glycosylation profile of glycodelin induced by transfection in endometrial adenocarcinoma cells has not been determined, but the results are compatible with glycodelin's apoptogenic isoforms (see Apoptosis). Given that progesterone and progesterone antagonists stimulate glycodelin gene expression, the results may encourage a reappraisal of glycodelin synthesis-stimulating pathways in supporting chemotherapy of malignant endometrial tumours, notably those expressing the progesterone receptor. However, the results obtained with the glycodelin femtopeptide antibody are contradictory to the above results, as they show increased ‘glycodelin’ immunoreactivity in advanced malignant tumours (Horowwitz et al. , 2001). Obviously the glycodelin-unrelated specificity of the polyclonal anti-peptide antibody may contribute to the different results (see Nomenclature, antibodies and immunodetection).
Ovarian serous carcinoma
Ovarian cancer consists of many subtypes, serous carcinoma being the most common of them. Clinical stage and histological grade are the gold standards in the selection of clinical management, and many clinical and prognostic markers have been explored. Studies employing anti-glycodelin antibodies show that glycodelin expression in tumour cells is more frequent in well differentiated than in poorly differentiated carcinomas, and glycodelin expression is more frequent in early stage compared with advanced stage tumours ( Mandelin et al. , 2003 ). Importantly, in grade I/stage III patients, the 5-year overall survival of the patients with glycodelin expressing tumours is significantly higher than in those patients whose tumours did not contain glycodelin. The study shows that glycodelin expression in ovarian serous carcinoma is a favourable prognostic sign. These results are at variance with those employing the glycodelin femtopeptide antibody ( Horowitz et al. , 2001 ; Song et al. , 2001 ), as the femtopeptide antibody shows more intense staining in advanced tumours compared with local tumours or normal tissues, and it shows strong immunostaining in tumour blood vessels that are negative with the use of the anti-glycodelin antibody ( Mandelin et al. , 2003 ). Again, these differences are likely due to different specificities of the antibodies used in these studies.
Concluding remarks
It is now firmly established that glycodelin interacts by its unique carbohydrates with the cell surface of many cell types, particularly the gametes and the immune cells. In the gametes, most biological actions of glycodelin are inhibitory, such as the inhibition of sperm capacitation (glycodelin-S), sperm–oocyte binding (glycodelin-A and -F) and the acrosome reaction (glycodelin-F). All these activities involve binding of the specific glycodelin glycoform on the sperm head. Recent studies have uncovered an important role for the cumulus oophorus cells. These cells can take up glycodelin-A and -F and modify their glycans in such a way that the resulting glycodelin-C has stimulatory effects on the sperm–zona pellucida binding. The structure and biological role of the cumulus cell-modified glycodelin is now unfolding. Evidence for the involvement of glycodelin oligosaccharides in the cell signalling processes of the cellular, humoral and innate immune responses and apoptosis is also accumulating.
Finally, clinical research points to a number of areas in which significant changes take place in glycodelin secretion, providing functional information. As the removal of specific N -glycosylation sites by mutagenesis clearly shows that glycosylation is requisite for glycodelin secretion ( Jayachandran et al. , 2004 ), glycodelin secretion may be affected by either inappropriate glycosylation machinery or by another type of clinical dysfunction. So far, inappropriate glycosylation patterns of glycodelin have been addressed in one clinical study only, i.e. in respect of the fertilization potential of spermatozoa in vitro ( Koistinen et al. , 2000 ). The paucity of such studies is not surprising because aberrant glycosylation of glycodelin cannot be identified by any available routine test. Nevertheless, research on glycodelin glycosylation has led the way to a better understanding of the biology and physiology of reproduction, immunology and even cancer. Today, detection of inappropriate glycoprotein glycosylation remains a challenge for clinical research and practice alike, an glycodelin provides a well-characterized example of this complex issue for future studies.
Abbreviations
- Asn
asparagine
- Gly
glycine
- Glu
glutamic acid
- Leu
leucine
- Lys
lysine
- Phe
phenylalanine
- Pro
proline
- Thr
threonine
- Val
valine.
Acknowledgements
The authors wish to thank Mrs Annikki Löfhjelm for skillful assistance. Original studies of this review have been supported by grants from the University of Helsinki, the Academy of Finland, Helsinki University Central Hospital Research Funds, Finnish Cancer Foundation and the Research Grant Council, Hong Kong. Original studies by the authors of this review have been approved by the respective Institutional Review Boards/Ethical Committees at the Department of Obstetrics and Gynaecology, Helsinki University Central Hospital, Helsinki, Finland and the Department of Obstetrics and Gynaecology, University of Hong Kong, Queen Mary Hospital, Hong Kong, China.