-
PDF
- Split View
-
Views
-
Cite
Cite
Attilio Di Spiezio Sardo, Ivan Mazzon, Silvia Bramante, Stefano Bettocchi, Giuseppe Bifulco, Maurizio Guida, Carmine Nappi, Hysteroscopic myomectomy: a comprehensive review of surgical techniques, Human Reproduction Update, Volume 14, Issue 2, March/April 2008, Pages 101–119, https://doi.org/10.1093/humupd/dmm041
Close - Share Icon Share
Abstract
Hysteroscopic myomectomy currently represents the standard minimally invasive surgical procedure for treating submucous fibroids, with abnormal uterine bleeding and reproductive issues being the most common indications. While hysteroscopic myomectomy has been shown to be safe and effective in the control of menstrual disorders, its effects on infertility remain unclear. The review provides a comprehensive survey of all hysteroscopic techniques used to treat fibroids found completely within the uterine cavity (G0) and those with intramural development (G1 and G2). MEDLINE and EMBASE searches identified published papers from 1970. The choice of the technique mostly depends on the intramural extension of the fibroid, as well as on personal experience and available equipment. ‘Resectoscopic slicing’ still represents the ‘gold standard’ technique for treating fibroids G0, even if several other effective techniques including ablation by neodymium-yttrium-aluminum-garnet laser, morcellation and office myomectomy have been proposed. On the other hand, the present review clearly indicates that there is still no single technique proven to be unequivocally superior for treating fibroids G1 and G2. Most techniques aim at the transformation of an intramural fibroid into a totally intracavitary lesion, thus avoiding a deep cut into the myometrium. At present, the ‘cold loop’ technique seems to represent the best option as it allows a safe and complete removal of such fibroids in just one surgical procedure, while respecting the surrounding healthy myometrium.
Introduction
Uterine fibroids (also known as myomas or leiomyomas) are the most common benign solid tumours found in the female genital tract. They occur in ∼20–25% of women of reproductive age (Fernandez et al., 2001; Valle and Baggish, 2007) causing 3–5% of gynaecology consultations (Vidal, 1998).
Uterine leiomyomas arise from the muscular part of the uterus. As they grow, they usually migrate to a place of lower resistance: towards the abdominal cavity, thus becoming subserous masses or following the path of the intrauterine cavity thus becoming submucous fibroids (5–10% of uterine fibroids) (Ubaldi et al., 1995).
Localization of uterine fibroids seems to be an important factor in determining frequency and severity of symptomatology. Indeed, submucous fibroids may induce severe clinical symptoms such as excessive bleeding, usually during menses, colicky dysmenorrhoea (as the uterus tries, by means of contractions, to expel fibroids), and are thought to predispose patients to reproductive failure (Fernandez et al., 2001; Litta et al., 2003; Takeda et al., 2004; Indman, 2006; Sutton, 2006; Valle and Buggish, 2007). Furthermore, submucous fibroids are associated with chronic endometritis, they may have a greater risk for malignant change (Valle and Buggish, 2007) and are source of pre-term delivery, abnormal presentation, post-partum haemorrhage and puerperal infections (Bernard et al., 2000).
Most submucous fibroids occur at the corporeal sites of the uterine cavity. Some are fundal, others are anteriorly, posteriorly or laterally situated. Small fibroids may also arise from the cornual regions, thus interfering with the utero–tubal junction lumen. A few are located at the cervical canal (Valle and Buggish, 2007).
Hysterectomy and laparotomic excision have long been considered the two standard routes of surgical treatment for symptomatic submucous fibroids (Garcia and Tureck, 1984; Smith and Uhlir, 1990; Verkauf, 1992, Sudik et al., 1996; Glasser, 1997; Haney, 2000; Munoz et al., 2003; Campo et al., 2005).
In particular, hysterectomy has been routinely proposed to those patients in whom the desire to procreate had been satisfied, while the abdominal myomectomy has represented the only possible solution in young patients desiring a pregnancy.
However, the conservative approach requires the opening of the uterine cavity, which may be one of the factors responsible for altering the likelihood of subsequent conception (Berkeley et al., 1983). Furthermore, such an approach may compromise any future parturition as it requires caesarean section; in addition, it may lead to the development of pelvic post-operative adhesions which may further reduce rather than enhance fertility (Buttram and Reiter, 1981; Starks, 1988; Ubaldi et al., 1995).
The development of hysteroscopic techniques
The development of endoscopy has made these fibroids accessible and resectable from the inner surface of uterus (Walker and Stewart, 2005; Bradley, 2005).
At the beginnings of endoscopic surgery, the lesions were removed by somewhat rugged methods (e.g. ovum forceps were used to twist pedunculated fibroids off their pedicles and scissors were inserted outside the hysteroscopic sheath to cut the fibroid pedicle).
The first reported hysteroscopy myomectomy was performed in 1976, when Neuwirth and Amin resected a fibroid using an urologic resectoscope, monopolar current and 32% dextran 70 as distension medium.
In 1987, Hallez (1995) reported on the development of a gynaecologic resectoscope, changing the urologic instrument into a continuous-flow device with a 0° optic; cutting current was used but with 1.5% glycine as the distension medium.
During the last 20 years, thanks to advances in instruments and the refining of techniques, hysteroscopic myomectomy has acquired the status of ‘surgical technique’ and, at present it represents the standard minimally invasive surgical procedure for treating fibroids entirely or mostly located within the uterine cavity (Vercellini et al., 1999; Takeda et al., 2004).
Indications for hysteroscopic myomectomy
Abnormal uterine bleeding (AUB) represents the most common indication for hysteroscopic myomectomy in most publications reviewed with a percentage ranging from 60 to 84.1% (Neuwirth and Amin, 1976; Brooks et al., 1989; Derman et al., 1991; Indmann, 1993; Wamsteker et al., 1993; Donnez et al., 1994; Wortman and Dagget, 1995; Hallez, 1995; Phillips et al., 1995; Glasser, 1997; Emanuel et al., 1999; Hart et al., 1999; Vercellini et al., 1999; Munoz et al., 2003; Loffer, 2005; Campo et al., 2005; Marziani et al., 2005; Polena et al., 2007). Indeed, submucous fibroids have been advocated more than subserous and intramural ones as a cause of AUB, presumably due to distortion of the cavity and to an increase in the bleeding surface of the endometrium (Maher, 2003).
Although most women affected with fibroids are fertile, available evidence suggests that fibroids may interfere with fertility, with submucousal fibroids being reported to exert the most detrimental effects on pregnancy rates (Pritts, 2001; Donnez and Jadoul, 2002; Benecke et al., 2005; Somigliana et al., 2007).
Even though this association is not supported by a clear biological rationale, several hypotheses have been suggested to explain how submucous fibroids may cause infertility or repeated abortions. However, at present, none of these hypotheses is definitive (Somigliana et al., 2007).
Fibroids might interfere with sperm migration, ovum transport or embryo implantation; these effects might be mediated by alteration of uterine cavity contour causing mechanical pressure or by the occurrence of abnormal uterine contractility (Buttram and Reiter, 1981; Richards et al., 1998; Bettocchi et al., 2002; Farrugia et al., 2002; Oliveira et al., 2004). Fibroids may also be associated with implantation failure or gestation termination due to focal endometrial vascular disturbances, endometrial inflammation, secretion of vasoactive substances or an enhanced endometrial androgen environment (Buttram and Reiter, 1981; Cicinelli et al., 1995; Richards et al., 1998; Ng and Ho, 2002).
Reproductive problems represent the second leading indication for intervention, though the lack of randomized studies does not allow any definitive conclusion to be drawn regarding the improvement of spontaneous fertility after hysteroscopic myomectomy (Donnez and Jadoul, 2002; Somigliana et al., 2007; Stamatellos and Bontis, 2007).
Less frequent reported indications include dysmenorrhea (Hallez, 1995), aspecific pelvic pain (Munoz et al., 2003) and asymptomatic submucous fibroid in a woman candidate to start hormone replacement therapy (Hallez, 1995).
With the present review, we offer gynaecologists with special interest in endoscopy information regarding the instrumentation required to perform hysteroscopic myomectomy and the diagnostic tools and medications commonly used for an appropriate pre-surgical evaluation. Furthermore, it provides a comprehensive survey of all techniques used to treat fibroids completely within the uterine cavity as well as those with intramural development. Finally, the effects on menstrual pattern and infertility and the operative and long- term complications have been reviewed.
Materials and Methods
This review includes medical papers published in the English language on hysteroscopic myomectomy since 1970 and identified through a MEDLINE and EMBASE search using combinations of medical subject heading terms: hysteroscopy, myomectomy, pharmacological agents, gynaecological surgery, surgical technique, fibroid, fibroid, loop, laparoscopy and resectoscope. All pertinent articles were retrieved and reports were then selected through systematic review of all references. In addition, books and monographs of different languages on hysteroscopy and gynaecological surgery were consulted.
Pre-surgical evaluation of submucous fibroids
As hysteroscopic myomectomy may be sometimes a highly complex procedure, its real feasibility must be thoroughly evaluated preoperatively in order to minimize the incidence of incomplete resection and the complications that might occur during procedure.
The most widespread investigative techniques for pre-surgical evaluation are office hysteroscopy, transvaginal ultrasound scanning (TVS) and sonohysterography (SHG) (Fedele et al., 1991; Fukuda et al., 1993; Dodson, 1994; Cicinelli et al., 1995; Corson, 1995; Tulandi, 1996; Laifer-Narin, 1999; Perez-Medina et al., 2000; Cheng and Lin, 2002; Clark et al., 2002; Leone et al., 2003, 2007; Trew, 2004; Lasmar et al., 2005; Murakami et al., 2005; Salim et al., 2005; Vilos and Abu-Rafea, 2005; Sutton, 2006; Alborzi et al., 2007).
Besides giving us the certainty of the presence of the submucous fibroid, office hysteroscopy also enables the assessment of the intracavity component of the mass, its localization, its relationship with the uterine structures, the characteristics of the endometrium as well as the presence of possible associated intracavitary pathologies. Furthermore, it provides subjective assessment of fibroid size and indirect information regarding the depth of myometrial extension (Fedele et al., 1991; Corson, 1995; Emanuel and Wamsteker, 1997; Emanuel et al., 1997, 1999; Wieser et al., 2001; Clark et al., 2002; Murakami et al., 2005; Lasmar et al., 2005; Sutton, 2006).
However, even if a protruding dome of fibroid is identified at outpatient hysteroscopy, there is the possibility that it could sink into the myometrium (‘sinking fibroid’) during a hysteroresectoscopic procedure because of an increase in intrauterine pressure caused by the distension medium (Lin et al., 2000; Murakami et al., 2005).
TVS is not as useful as hysteroscopy in assessing the degree of intracavitary development of the fibroid. However, it is irreplaceable in the preoperative assessment as it provides two elements which would be otherwise unobtainable: the ‘myometrial free margin’ (thickness of the outer myometrial layer of the fibroid) as well as the presence of any other possibly associated pathology. For a submucous fibroid to be approached hysteroscopically, the ‘myometrial free margin’ should be at least 1 cm thick or in more expert hands at least a few millimetres thick. Scanning evidence of other associated pathologies (multiple fibroid, adnexial pathologies) may indicate the need for a different surgical approach. Furthermore, ultrasound scanning allows to evaluate the real size of the nodule (Lasmar et al., 2005; Murakami et al., 2005; Sutton, 2006).
SHG has been demonstrated to be superior to TVS in terms of diagnostic accuracy; furthermore, it allows to identify the exact location of the fibroid as well as the portion protruding into the cavity (Fukuda et al., 1993: Cicinelli et al., 1995; Farquhar et al., 2003; Leone et al., 2003, 2007; Salim et al., 2005; Botsis et al., 2006; Alborzi et al., 2007). Although many authors report that SHG could reduce the number of diagnostic hysteroscopies for pre-surgical evaluation (Turner et al., 1995; Bronz et al., 1997; Saidi et al., 1997; Williams and Marshburn, 1998; Bonnamy et al., 2002; Leone et al., 2003, 2007), this technique is limited by the inability or difficulty in obtaining tissue diagnosis (Botsis et al., 2006).
In case of a large uterus, with multiple fibroids, or if ultrasound scanning is technically difficult (i.e. obese patients), magnetic resonance imaging (MRI) can provide valuable information, being also helpful in differentiating between fibroids and adenomyosis (Hricak et al., 1986; Takeda et al., 2004; Lasmar et al., 2005; Murakami et al., 2005; Indman, 2006). Costs have prohibited its general use in clinical practice (Valle and Buggish, 2007).
Recently, Takeda et al. (2004) have proposed the use of ‘virtual hysteroscopy’ for preoperative evaluation of submucosal fibroids. Virtual endoscopy is a non-invasive technology used to display the image of the cavity inside the organ by processing the images acquired by a multislice helical computed tomography (CT) scanner using 3D computer graphics (3DCG) software as if one is observing the organ by real endoscopy.
Hysteroscopic classification of submucous fibroids
As the intramural extension of submucous fibroids may considerably vary, thus influencing the chance of achieving complete resection, a classification of different types of submucous fibroids was shown to be indispensable since the beginning of resectoscopic surgery for weighting the limits of surgical technique.
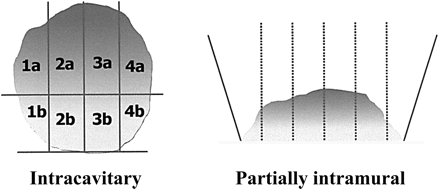
The classification developed by Wamsteker et al. (1993) and adopted by the European Society for Gynaecological Endoscopy (ESGE), which considers only the degree of myometrial penetration of the submucous fibroid, is currently worldwide used. According to this classification, a fibroid G0 is completely within the uterine cavity and appears only jointed to the cavity wall by a thin pedicle; a fibroid G1 has its larger part (>50%) in the uterine cavity; and a fibroid G2 has its larger part (>50%) in the myometrium (Wamsteker et al., 1993; Salim et al., 2005).
Lasmar et al. (2005) recently proposed a new preoperative classification of submucous fibroids which considers not only the degree of penetration of the fibroid into the myometrium, but also other parameters including the extension of the base of fibroid with respect to the wall of the uterus, the size of the nodule (cm) and the topography of the uterine cavity. A score ranging from 0 to 2 is given for each parameter and the patients are then allocated into one of the three groups of the classification on the basis of the total score (Table I). The authors found a higher correlation of this new scoring system with completeness of the myomectomy, time spent in surgery and fluid deficit, than scoring only based on the percentage of myometrial penetration (Lasmar et al., 2005).
Lasmar’s pre-surgical classification of submucous myomas
| Points . | Penetration . | Size, cm . | Basea . | Third . | Lateral wall (+1) . | . |
|---|---|---|---|---|---|---|
| 0 | 0 | ≤2 | ≤1/3 | Lower | ||
| 1 | ≤50% | >2–5 | >1/3 to 2/3 | Middle | ||
| 2 | >50% | >5 | >2/3 | Upper | ||
| Score | + | + | + | + | = |
| Points . | Penetration . | Size, cm . | Basea . | Third . | Lateral wall (+1) . | . |
|---|---|---|---|---|---|---|
| 0 | 0 | ≤2 | ≤1/3 | Lower | ||
| 1 | ≤50% | >2–5 | >1/3 to 2/3 | Middle | ||
| 2 | >50% | >5 | >2/3 | Upper | ||
| Score | + | + | + | + | = |
aIt refers to the extension of the base of the nodule with respect to the uterine wall on which the myoma is located. Score 0–4 (Group I): low complexity hysteroscopic myomectomy. Score 5–6 (Group II): complex hysteroscopic myomectomy, consider preparing with GnRH analogue and/or two stage surgery. Score 7–9 (Group III): recommend an alternative non-hysteroscopic technique.
Lasmar’s pre-surgical classification of submucous myomas
| Points . | Penetration . | Size, cm . | Basea . | Third . | Lateral wall (+1) . | . |
|---|---|---|---|---|---|---|
| 0 | 0 | ≤2 | ≤1/3 | Lower | ||
| 1 | ≤50% | >2–5 | >1/3 to 2/3 | Middle | ||
| 2 | >50% | >5 | >2/3 | Upper | ||
| Score | + | + | + | + | = |
| Points . | Penetration . | Size, cm . | Basea . | Third . | Lateral wall (+1) . | . |
|---|---|---|---|---|---|---|
| 0 | 0 | ≤2 | ≤1/3 | Lower | ||
| 1 | ≤50% | >2–5 | >1/3 to 2/3 | Middle | ||
| 2 | >50% | >5 | >2/3 | Upper | ||
| Score | + | + | + | + | = |
aIt refers to the extension of the base of the nodule with respect to the uterine wall on which the myoma is located. Score 0–4 (Group I): low complexity hysteroscopic myomectomy. Score 5–6 (Group II): complex hysteroscopic myomectomy, consider preparing with GnRH analogue and/or two stage surgery. Score 7–9 (Group III): recommend an alternative non-hysteroscopic technique.
Preoperative hormonal treatment
Whether treatment with GnRH agonist before myomectomy offers any significant advantage is still a matter of debate (Lethaby et al., 2001, 2002). However, a recent review by Gutmann and Corson (2005) reports that ‘the most clinically relevant indication for preoperative GnRH agonist use appears to be in patients with submucous fibroids’.
Benefits claimed include the following:
resolution of preoperative anaemia: these drugs create a state of amenorrhea, thus enabling patients suffering from menorrhagia to build up their blood counts and reducing the need for transfusion (Donnez et al., 1989, 1992, 1993; Isaacson, 2003)
reduction of endometrial thickness as well as the size and vascularization of fibroids (Donnez et al., 1989, 1992, 1993; Mencaglia and Tantini, 1993). This results in an improved operator’s visibility by limiting blood loss; furthermore, it leads to a reduced fluid absorption (through a reduction of uterine blood flow) (Parazzini et al., 1998) and a reduced length and difficulty of surgery.
possibility of surgical scheduling. Indeed, as patients do not necessarily need to be operated in the early proliferative phase, preoperative treatment also has a practical benefit in that it allows surgery to be performed at any time (Parazzini et al., 1998)
Universally accepted guidelines on the indications and duration of pretreatment with GnRH agonist (administered either as a long-acting monthly intramuscular injection or with daily dosing) are lacking in the international literature.
Hallez (1995, 1996) does not recommend any preoperative treatment; Hart et al. (1999) does not believe analogue use to be a risk factor for submucous fibroid reintervention; Donnez et al. (1995) claims that fibroids up to 2 cm do not require any preparation, those ranging from 2 to 4 cm have to be treated for 3 weeks with a progestogen or danazol, while GnRH agonist should be reserved only for those fibroids >4 cm. Other authors consider a large size as a contraindication for GnRH agonist therapy as severe haemorrhage after the administration of these drugs has been described (Indman, 1993).
We agree with those authors who consider these drugs particularly indicated for those fibroids with a diameter of >3 cm and/or with intramural portion as well as for patients suffering from secondary anaemia (Romer, 1998; Tulandi and al-Took, 1999; Romer et al., 2000; Valle and Buggish, 2007). A 6–8 weeks administration of GnRH agonist preoperatively is sufficient to shrink the fibroid by 30–50% (Donnez et al., 1992; Perino et al., 1993; Mencaglia and Tantini, 1993), for patients presenting with anaemia and a large submucous fibroid, such therapy can be prolonged up to 2–4 months to correct anaemia (in combination with iron supplement therapy) as well as to shrink the intrauterine lesion (Stamatellos and Bontis, 2007).
Evidence supporting the use of these drugs before hysteroscopic myomectomy only comes out from a few small [n = 20, Donnez et al. (1989); n = 25, Mencaglia and Tantini (1993); n = 58, Perino et al. (1993)] prospective studies. In the only one randomized study, Perino et al. (1993) have compared the post-operative outcomes following resectoscopic myomectomy for submucous fibroids <3 cm, showing a decreased volume of distension fluid, surgical time and bleeding in those patients (n = 33) preoperatively treated with GnRH agonist in comparison with controls (n = 25).
Conversely, it is well known that the preoperative treatment with these drugs is associated with some disadvantages including: (i) high costs; (ii) side effects (i.e. hot flushes, spotting); (iii) increased recurrence rate (these drugs may render small fibroids less visible) (Fedele et al., 1990) and (iv) increased risk of uterine perforation (due to a reduced myometrial thickness) (Bradley, 2002) and an increased risk of the ‘sinking’ phenomenon (due to a decreased elasticity of myometrial tissue caused by estrogen deficiency) (Lin et al., 2000).
Furthermore, a recent retrospective study by Campo et al. (2005) suggests that preoperative treatment with GnRH agonist does not seem to offer any advantage in terms of short- and long-term outcomes. In particular, those patients treated with GnRH agonist had significantly longer surgical times, compared with untreated patients, which has been ascribed by the authors to an increased cervical resistance to dilatation. However, such data needs to be confirmed by larger randomized studies.
Instrumentation
The operating hysteroscope (resectoscope) is the instrument that allows the performance of a submucous myomectomy under direct and constant visual control. It includes a straight-forward telescope (0°) or a slightly fore-oblique 12–30-° telescope with an outer diameter of 3–4 mm; an internal and an external sheaths of 24–27 Fr outer diameter (Table II) that provide a constant inflow and outflow of distension fluid for generating a continuous and efficient lavage system of the uterine cavity.
Main characteristics of the widely-used resectoscopes
| Manufacturer . | Diameter . | Telescope . | Electrosurgical system . |
|---|---|---|---|
| Storz | 26 Fr | 0°, 12°, 30° | Monopolar Bipolar |
| Circon ACMI | 25 Fr | 12°, 30° | Monopolar |
| Wolf | 27 Fr | 30° | Monopolar Bipolar |
| Gynecare | 27 Fr | 12°, 30° | Bipolar |
| Olympus | 26 Fr | 0°, 12° | Monopolar Bipolar |
| Manufacturer . | Diameter . | Telescope . | Electrosurgical system . |
|---|---|---|---|
| Storz | 26 Fr | 0°, 12°, 30° | Monopolar Bipolar |
| Circon ACMI | 25 Fr | 12°, 30° | Monopolar |
| Wolf | 27 Fr | 30° | Monopolar Bipolar |
| Gynecare | 27 Fr | 12°, 30° | Bipolar |
| Olympus | 26 Fr | 0°, 12° | Monopolar Bipolar |
Main characteristics of the widely-used resectoscopes
| Manufacturer . | Diameter . | Telescope . | Electrosurgical system . |
|---|---|---|---|
| Storz | 26 Fr | 0°, 12°, 30° | Monopolar Bipolar |
| Circon ACMI | 25 Fr | 12°, 30° | Monopolar |
| Wolf | 27 Fr | 30° | Monopolar Bipolar |
| Gynecare | 27 Fr | 12°, 30° | Bipolar |
| Olympus | 26 Fr | 0°, 12° | Monopolar Bipolar |
| Manufacturer . | Diameter . | Telescope . | Electrosurgical system . |
|---|---|---|---|
| Storz | 26 Fr | 0°, 12°, 30° | Monopolar Bipolar |
| Circon ACMI | 25 Fr | 12°, 30° | Monopolar |
| Wolf | 27 Fr | 30° | Monopolar Bipolar |
| Gynecare | 27 Fr | 12°, 30° | Bipolar |
| Olympus | 26 Fr | 0°, 12° | Monopolar Bipolar |
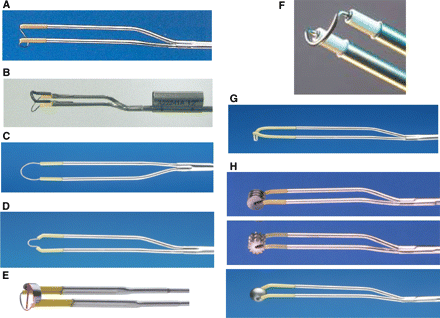
The operating hysteroscope contains a working element wherein electrosurgical (thermal loops and vaporizing electrodes) (Fig. 1) and mechanical instruments (cold loops) (Fig. 2) for the traditional resectoscopic surgery or laser fibres or a new Intra Uterine Morcellator (IUM) (Fig. 3) device can be attached.
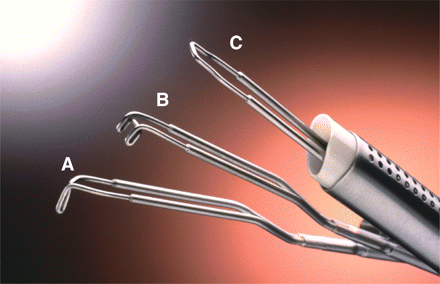
Conventionally-used thermal loops for resectoscopic myomectomy
(A) 24 Fr 30° U-shaped cutting loop for monopolar 26 Fr resectoscope (Karl Storz GmbH Co., Tuttlingen, Germany): it has a maximum cutting depth of 4 mm and represents the most frequently used loop; (B) 5 mm equatorial loop for monopolar 26 Fr resectoscope (Karl Storz GmbH Co); (C) 45° loop electrode with a short-arm safeguard (Olympus Medical System GmbH); (B) and (C) are used to treat submucous fibroids arising from uterine fundus; (D) 3 mm equatorial loop for monopolar 26 Fr resectoscope (Karl Storz GmbH Co): it is used to resect submucous fibroids located near the interstitial portions of the Fallopian tubes; (E) 8 mm 90° U-shaped cutting loop for bipolar 26 Fr resectoscope (Karl Storz GmbH Co): the direct current return via the electrode (arrow) prevents a current flow via the sheath; (F) Magnified view of 2.5 mm cutting loop for bipolar 27 Fr resectoscope (Gynecare; Ethicon Inc., Somerville, NJ); (G) Collin’s Electrode (Karl Storz GmbH Co): it is a cutting knife electrode, conventionally used to perform hysteroscopic metroplasty; however it can also be employed to perform hysteroscopic myomectomy (i.e. enucleation in toto); (H) Vaporizing electrodes (Karl Storz GmbH Co): they can have a cylindrical or spherical shape or a multidentate surface; this design works like an array of electrodes which, whether provided with pure cutting energy of high power, can vaporize tissue quite quickly and effectively without generating the ‘chips’ created by loop resection
Mazzon’s mechanical loops (Karl Storz GmbH Co) used for ‘cold loop’ myomectomy
A) Pointed loop (Knife-shaped): used to hook and lacerate the connective bridges which join the fibroid and the adjacent myometrium; (B) Rake loop (rake shaped with teeth): nearly completely replaced by pointed loop; (C) Cutting loop (rectangular): used to identify the cleavage plane between the fibroid and myometrium
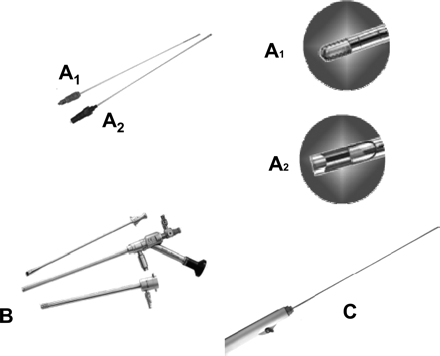
Intrauterine morcellator (IUM) (prototype: Smith & Nephew Endoscopy, Andover, MA)
It consists of a set of two metal, hollow, rigid, disposable tubes (A) that fit into each other and then are inserted into the working channel of a 9 mm operating hysteroscope (B). The inner tube rotates within the outer tube, driven mechanically by an electrically powered control unit and controlled by a foot pedal that activates rotation and regulates the direction of rotation of the inner tube. The control unit is connected to a handheld motor drive unit in which the IUM is inserted. Both tubes have a window-opening at the end with cutting edges. The rotary morcellator (A1) is recommended for polypectomy, while the reciprocating one (A2) for myomectomy. By means of a vacuum source (C) connected to the inner tube, the tissue is sucked into the window-opening and cut and ‘shaved’ as the inner tube is rotated. The removed tissue is discharged through the device and is available for pathology analysis
The application of resectoscopic surgery has been made possible by using the electric current. The electrosurgical system can be monopolar or bipolar: in the monopolar one, from the extremity of the resectoscope (active electrode) the flow of current, in order to close the circuit, must reach the plate (passive electrode). The use of monopolar electrodes requires non-conducting distending solution (sorbitol 5% or glycine 1.5%). The use of a bipolar set of instruments, in which both electrodes are introduced into the thermal loop, would be much safer. In this way the current will only have to pass through the tissue with which the thermal loop comes into contact, thus minimizing the danger deriving from the random passage through the corporeal structures. An intrauterine bipolar diathermy allows the use of an electrolitic uterine distension medium (normal saline). The passage of the electrical energy from the thermal loop to the tissues determines the cutting or coagulation action of the resectoscope.
There are various types of thermal loops with different shapes and sizes (Fig. 1A–F).
The diameters of thermal loops for bipolar resectoscopes are usually smaller than loops for a monopolar instrument with the same outer diameter, thus increasing the time required for resection (Indman, 2006). The bipolar loop operates in a similar way to a monopolar electrode; however, as tissue contact is not necessary for activation, the electrodes do not ‘stick’ in the tissue while cutting (Stamatellos and Bontis, 2007).
The cold loops are structurally more robust than the others as they are used in a mechanical way without electrical energy to carry out the enucleation of the intramural component of the fibroid (Fig. 2).
The resectoscope can also fit bipolar and monopolar vaporizing electrodes (Fig. 1H); The power required to vaporize tissue is 150–300 W of pure cut current delivered by any electrosurgical generator (Brooks, 1995; Vilos and Abu-Rafea, 2005). The vaporizing electrodes are also useful mechanical tools to be used to dissect the fibroid from its bed without electrosurgical activation.
The transhysteroscopic approach for the treatment of submucous fibroid includes also the use of lasers and of a new instrument called IUM.
Although argon, krypton and neodymium-yttrium-aluminum-garnet (Nd:yAG) lasers have all been successfully used, only the latter has found widespread application in hysteroscopic surgery (Ubaldi, 1995), being very popular in the late 1980s and early 1990s. Two techniques ‘touch’ and ‘non-touch’ may be used in hysteroscopic Nd:yAG laser surgery (Goldrath et al., 1981; Loffer, 1987). In the former, the laser fibre is in contact with the surface to be treated, whereas in the latter it is separated from it by a few millimetres. In hysteroscopic myomectomy both techniques are used (Ubaldi, 1995).
The 4–5 mm IUM (prototype: Smith & Nephew Endoscopy, Andover, MA) represents a new cutting device inserted into a straight working channel of a continuous flow 9 mm rigid operating hysteroscope (Fig. 3).
Hysteroscopic techniques
The choice of the technique for the hysteroscopic removal of submucous fibroids mostly depends on its type and location within the endometrial cavity. Furthermore, personal experience and the available equipment might then favour a particular technique rather then the other ones.
Fibroids completely within the uterine cavity (G0)
The operator has the possibility to choose among several alternative procedures: all of them are usually performed in the operating theatre under general anaesthesia, except office myomectomy.
Resectoscopic excision by slicing
The classical resectoscopic excision of intracavitary fibroid is carried out with the technique of slicing. It consists of repeated and progressive passages of the cutting loop, carried out with the standard technique (loop carried beyond the neoformation, with cutting only taking place during the backward or return movement of the loop). Excision usually begins from the top of the fibroid, progressing in a uniform way towards the base, also in the case of a pedunculated fibroid (Neuwirth and Amin, 1976; Mazzon and Sbiroli, 1997; Isaacson, 2003; Indman, 2006).
During the resection of the fibroid, particularly when there are voluminous neoformations or small cavities, the fragments sectioned and then accumulated into the cavity may interfere with a clear vision. Thus they must be removed from the uterine cavity by taking out the resectoscope after grasping the loose tissue elements with the loop electrode. Although the removal of tissue under visual control with the resectoscope is the most effective way, it requires a large number of steps which are tiring in the long run (Mazzon and Sbiroli, 1997; Emanuel and Wamsteker, 2005). Recently, a resectoscope with automatic chip aspiration (Resection Master by Gallinat, Richard Wolf GmbH, Knittlingen, Germany) has been developed. Thanks to an extremely effective pump with integrated pulse aspiration, the chips are aspirated immediately after they are produced and removed from the uterine cavity without the uterine water distension being impaired (Gallinat, 2005).
When dealing with the base of the fibroid, care must be taken to limit the surgical traumatism only to the area of the implant, thus avoiding the damage of the surrounding structures. As soon as the excision of the fibroid is finished, the base of the implant must be cleaned out until smooth and regular; the operation should be considered finished when the fasciculate structure of the myometrium is visualized.
It is well ascertained that intracavitary fibroids can be easily removed in a one-step procedure with fibroid size representing the main limiting factor. The operation may also be carried out by operating surgeons with average resectoscopic experience (Mazzon and Sbiroli, 1997; Isaacson, 2003; Indman, 2006).
Cutting of the base of the fibroid and its extraction
Ideally, when approaching a peduncolated fibroid, the basis of the pedicle might be cut by resectoscopic loop (Murakami et al., 2005) or Nd:yAG laser with the ‘no-touch’ technique (Valle and Baggish, 2007) or vaporizing electrode (Glasser, 1997). The resected node is then usually extracted with forceps. The fibroid can be grabbed blindly with a Corson forceps (Thomas Medical, Inc., Indianapolis, IN) or under direct visualization with an Isaacson optical tenaculum (Karl Storz Endoscopy, Culver City, CA) (Isaacson, 2003). Some other reports suggest that the resected fibroid node should be left in place until the remnant fibroid is excreted spontaneously during the first menstruation after surgery (Donnez et al., 1990; Isaacson, 2003). This is an attractive procedure but limited by frequent side effects including continuous colicky pain and intrauterine infection (Darwish, 2003).
Ablation by Nd:yAG laser
For fibroids 2 cm or less in diameter the Nd:yAG laser fibre may be used to ablate the fibroid. The technique first coagulates the surface vessels with the defocused laser fibre. Then the fibre is dragged repetitiously over the fibroid until it is flattened (touch technique). The disadvantages with this method are the time expended to reduce the fibroid and the lack of a tissue specimen for pathologic evaluation (Donnez et al., 1990; Gallinat et al., 1994; Smets et al., 1996; Valle and Baggish, 2007). Furthermore, laser equipment at present tends to be very expensive which significantly reduces its widespread use.
Vaporization of fibroid
The vaporization of fibroid is performed using spherical or cylindrical electrodes (Fig. 1H); the electrode is dragged along the surface of the fibroid until the nodule is reduced to a size compatible with removal by the means of Corson forceps or Isaacson optical tenaculum. The depth of vaporization depends on duration of contact, resistance (debris on the electrode) and wattage of the generator. It is important to move the electrode slowly across tissue, applying current only when moving in the direction towards the operator. Prolonged pressure in one spot exposed to this high current could result in uterine perforation (Glasser, 1997).
Fibroid vaporization has been reported to be significantly faster than traditional resectoscopic surgery (no fibroid chips to be removed) with an estimated blood loss <100 ml and a discrepancy between inflow and outflow volumes ranging 0–200 ml (Brooks, 1995; Vilos and Abu-Rafea, 2005).
The main disadvantage of vaporizing electrodes is the lack of tissue sample for pathology. While uterine sarcomas are vary rare, unfortunately they are not homogeneous. Therefore, a simple sample prior to vaporization does not rule out the disease. Consequently, it is mandatory that no fibroid be vaporized in its entirety but that substantial portions been retrieved for microscopic examination (Brooks, 1995; Glasser, 1997; Isaacson, 2003).
Another disadvantage is related to the use of high power which produces numerous gas bubbles which enter the vascular system. Providentially, these bubbles dissipate rapidly in the blood; as long as the rate of formation does not exceed the rate of dissipation, there are no significant clinical sequelae. A constant monitoring of patient’s end-tidal CO2 together with a close cooperation between surgeon and anaesthesiologist is needed to avoid serious complications (Isaacson, 2003).
Morcellation by IUM
Contrary to some other alternative techniques that use heat, coagulation or vaporization, the morcellation by IUM represents a new alternative technique which preserves tissue for histological examination.
Recently, Emanuel and Wamsteker (2005) have conducted a retrospective comparison between this technique and conventional resectoscopy.
They have shown that morcellation by IUM was effective for the treatment of fibroid G0 and G1 and faster than conventional resectoscopy. Indeed, the aspiration of tissue fragments through the instrument allowed the surgeons to save much time. However, further data are needed to evaluate long-term follow-up and to demonstrate whether this new technique might result in fewer fluid-related complications (physiological saline solution is used for distension and irrigation) and a shorter learning curve.
On the other hand, it should be underlined that this new technique cannot be used for the treatment of submucous fibroids with >50% intramural extension (G2).
Office hysteroscopic myomectomy
The development of smaller diameter hysteroscopes (<5 mm) with working channels and continuous flow systems has made it possible to treat several uterine pathologies in outpatient regimen without cervical dilatation and consequently without analgesia and/or local anaesthesia.
This new philosophy (‘see and treat hysteroscopy’) has reduced the distinction between a diagnostic and an operative procedure, introducing the concept of a single procedure in which the operative part was perfectly integrated in the diagnostic work-up (Bettocchi et al., 2003).
Mechanical operative instruments (scissors, biopsy cup, grasping and corkscrew) have long been the only way to apply the see and treat procedure in an outpatient setting (Bettocchi et al., 2004). The advent of bipolar technology, with introduction of electrosurgical systems dedicated to hysteroscopy and several types of 5 Fr electrodes, has increased the number of pathologies treated by office operative hysteroscopy, including fibroids <1.5–2 cm.
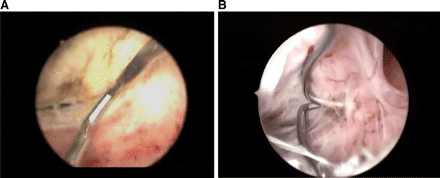
Endocavitary fibroids (G0) are first divided into two half-spheres and then each of these is sliced from the free edge to the base into two/three fragments (Fig. 4). These fragments must be large enough to be pulled out through the uterine cavity using 5 Fr. grasping forceps (Bettocchi et al., 2002).
Slicing technique to treat totally intracavitary and partially intramural submucous fibroid in office setting with 5Fr bipolar electrodes
‘a’ refers to the first half-sphere and ‘b’ to the second. Modified from Bettochi et al. (2002)
Few studies have investigated the effectiveness of this new approach (Farrugia and McMillan, 2000; Bettocchi et al., 2002; Clark et al., 2002) and are characterized by potential methodological weaknesses including the lack of a control group of women (Farrugia and McMillan, 2000; Bettocchi et al., 2002; Clark et al., 2002) and the relatively short-term follow-up (Farrugia and McMillan, 2000; Bettocchi et al., 2002; Clark et al., 2002). Larger prospective comparative studies are needed to better evaluate this promising approach in terms of symptom response and cost saving.
Fibroids with intramural development (G1-G2)
It is advisable to use expert operating surgeons for hysteroscopic resection of fibroids with intramural extension as it is technically difficult with a slow learning curve and it is associated with an higher risk of complications (Emanuel et al., 1999). The intramural extension of submucous fibroids influences the chance of achieving complete resection in one surgical session.
Conventionally, fibroids G1 and G2 should not exceed 5–6 and 4–5 cm, respectively, to be removed hysteroscopically, even if reports of removal of larger fibroids are available in the English literature (Neuwirth, 1983; Fried and Hulka, 1987; Hamou, 1993; Donnez et al., 1995; Phillips et al., 1995).
Several techniques have been proposed for the treatment of such submucous fibroids, most of them sharing the objective of producing an intracavitary protrusion of the intramural component. The advantages and limits of the most widely-used techniques are shown in Table III.
Advantages and limits of the most widespread techniques for hysterosocpic treatment of myomas G1-G2
| Author (year) . | Technique . | Advantages . | Limits . |
|---|---|---|---|
| Loffer et al. (1990) | Two-step myomectomy: the procedure can be performed only by means of traditional resectoscopic surgery (Loffer et al., 1990) or by Nd:yAG laser (Donnez et al., 1990). | - Safeness (possibility to operate at an intracavitary level) | - Two separate interventions |
| Donnez et al. (1990) | - High costs (GnRH agonist therapy, Nd:yAG laser, two surgeries) | ||
| - Only myomas with a reduced intramural development or of small dimensions can be treated with this technique. Indeed, in the case of myomas with a volumetrically significant intramural component, the part which remains after the first surgical operation may be excessively big: such a component, when migrating to the uterine cavity, will meet with resistance to its progression caused by the controlateral myometrial wall. As a result, during the second operation we will find a myoma which still has a significant intramural component, which will remain in the thickness of the wall at the end of the new excision only of the intracavitary part: it will therefore be necessary to carry out more surgical operations. However this limit might be solved by GnRH agonist therapy. | |||
| - ‘Sinking’ phenomenon (due to GnRH agonist therapy) | |||
| - Increased recurrence rate (due to GnRH agonist therapy) | |||
| Mazzon (1995) | ‘Cold loop’ myomectomy | - Theoretically one intervention | - Availability of cold loops |
| - Safeness (although the surgical action goes deeply into the uterine wall during enucleation with a cold loop, it always follows a reference plane (the surface of the intramural pole of the myoma) and constantly maintains the loop action under direct visual control; this means a smaller possibility of complications (perforation, bleeding) | - Training and high experience | ||
| - Respect of the surrounding healthly myometrium avoiding any needless cutting of the muscular fibres adjacent to the surgical area and reducing the thermal damage deriving from the loop of the resectoscope. This avoids any negative effect on the likelihood of subsequent conception and the uterine wall resistance | |||
| - Suitable also for large myomas | |||
| - Suitable also in case of myometrial free margin <1 cm | |||
| Lasmar et al. (2002) | ‘Enucleation in toto’ | - Theoretical one intervention | - The success of the procedure is higher for myomas with a intramural development >50% |
| Litta et al. (2003) | - Safeness (possibility to operate at an intracavitary level) | - The expulsion force of the myometrium is inversely correlated with the diameter of uterine cavity | |
| - Theoretical respect of the surrounding health myometrium | |||
| Hamou (1993) | Hydromassage | - Theoretical one intervention | - Need of electronically controlled irrigation and suction device |
| - Safeness (possibility to operate at an intracavitary level) | - The contractile reaction of the myometrium to such manoeuvres is neither predictable nor standardizable | ||
| - Theoretical respect of the surrounding health myometrium | - The migration of the intramural component of the myoma into the cavity is maximum for myomas with a reduced intramural development or of small dimensions | ||
| Bettocchi et al. (2002) | Office hysterosocpic myomectomy | - Office procedure | - Availability of 5Fr bipolar electrodes and generator |
| - Reduced anaesthetic risks | - Myoma G0-G1<1.5 cm | ||
| - Reduced costs | - Limited by patient’s compliance | ||
| - Theoretical one intervention | - Training and high experience | ||
| Indaman (2004) | Pharmacological-aided techniques (PGF-2α or carboprost) | - Theoretical one intervention | - The contractile reaction of the myometrium to such drugs is neither predictable nor standardizable |
| Murakami et al. (2003, 2006) | - Safeness (possibility to operate at an intracavitary level) - Theoretical respect of the surrounding health myometrium | - Extreme contraction of the uterus can interfere with visualization and one’s ability to manipulate the resectosocpe in the small cavity | |
| - Need of laparoscopic monitoring (Murakami’s technique) | |||
| - Side effects drug-related (nausea, vomiting, diarrhea, fever, bronchospasm in patients with bronchial asthma) |
| Author (year) . | Technique . | Advantages . | Limits . |
|---|---|---|---|
| Loffer et al. (1990) | Two-step myomectomy: the procedure can be performed only by means of traditional resectoscopic surgery (Loffer et al., 1990) or by Nd:yAG laser (Donnez et al., 1990). | - Safeness (possibility to operate at an intracavitary level) | - Two separate interventions |
| Donnez et al. (1990) | - High costs (GnRH agonist therapy, Nd:yAG laser, two surgeries) | ||
| - Only myomas with a reduced intramural development or of small dimensions can be treated with this technique. Indeed, in the case of myomas with a volumetrically significant intramural component, the part which remains after the first surgical operation may be excessively big: such a component, when migrating to the uterine cavity, will meet with resistance to its progression caused by the controlateral myometrial wall. As a result, during the second operation we will find a myoma which still has a significant intramural component, which will remain in the thickness of the wall at the end of the new excision only of the intracavitary part: it will therefore be necessary to carry out more surgical operations. However this limit might be solved by GnRH agonist therapy. | |||
| - ‘Sinking’ phenomenon (due to GnRH agonist therapy) | |||
| - Increased recurrence rate (due to GnRH agonist therapy) | |||
| Mazzon (1995) | ‘Cold loop’ myomectomy | - Theoretically one intervention | - Availability of cold loops |
| - Safeness (although the surgical action goes deeply into the uterine wall during enucleation with a cold loop, it always follows a reference plane (the surface of the intramural pole of the myoma) and constantly maintains the loop action under direct visual control; this means a smaller possibility of complications (perforation, bleeding) | - Training and high experience | ||
| - Respect of the surrounding healthly myometrium avoiding any needless cutting of the muscular fibres adjacent to the surgical area and reducing the thermal damage deriving from the loop of the resectoscope. This avoids any negative effect on the likelihood of subsequent conception and the uterine wall resistance | |||
| - Suitable also for large myomas | |||
| - Suitable also in case of myometrial free margin <1 cm | |||
| Lasmar et al. (2002) | ‘Enucleation in toto’ | - Theoretical one intervention | - The success of the procedure is higher for myomas with a intramural development >50% |
| Litta et al. (2003) | - Safeness (possibility to operate at an intracavitary level) | - The expulsion force of the myometrium is inversely correlated with the diameter of uterine cavity | |
| - Theoretical respect of the surrounding health myometrium | |||
| Hamou (1993) | Hydromassage | - Theoretical one intervention | - Need of electronically controlled irrigation and suction device |
| - Safeness (possibility to operate at an intracavitary level) | - The contractile reaction of the myometrium to such manoeuvres is neither predictable nor standardizable | ||
| - Theoretical respect of the surrounding health myometrium | - The migration of the intramural component of the myoma into the cavity is maximum for myomas with a reduced intramural development or of small dimensions | ||
| Bettocchi et al. (2002) | Office hysterosocpic myomectomy | - Office procedure | - Availability of 5Fr bipolar electrodes and generator |
| - Reduced anaesthetic risks | - Myoma G0-G1<1.5 cm | ||
| - Reduced costs | - Limited by patient’s compliance | ||
| - Theoretical one intervention | - Training and high experience | ||
| Indaman (2004) | Pharmacological-aided techniques (PGF-2α or carboprost) | - Theoretical one intervention | - The contractile reaction of the myometrium to such drugs is neither predictable nor standardizable |
| Murakami et al. (2003, 2006) | - Safeness (possibility to operate at an intracavitary level) - Theoretical respect of the surrounding health myometrium | - Extreme contraction of the uterus can interfere with visualization and one’s ability to manipulate the resectosocpe in the small cavity | |
| - Need of laparoscopic monitoring (Murakami’s technique) | |||
| - Side effects drug-related (nausea, vomiting, diarrhea, fever, bronchospasm in patients with bronchial asthma) |
Advantages and limits of the most widespread techniques for hysterosocpic treatment of myomas G1-G2
| Author (year) . | Technique . | Advantages . | Limits . |
|---|---|---|---|
| Loffer et al. (1990) | Two-step myomectomy: the procedure can be performed only by means of traditional resectoscopic surgery (Loffer et al., 1990) or by Nd:yAG laser (Donnez et al., 1990). | - Safeness (possibility to operate at an intracavitary level) | - Two separate interventions |
| Donnez et al. (1990) | - High costs (GnRH agonist therapy, Nd:yAG laser, two surgeries) | ||
| - Only myomas with a reduced intramural development or of small dimensions can be treated with this technique. Indeed, in the case of myomas with a volumetrically significant intramural component, the part which remains after the first surgical operation may be excessively big: such a component, when migrating to the uterine cavity, will meet with resistance to its progression caused by the controlateral myometrial wall. As a result, during the second operation we will find a myoma which still has a significant intramural component, which will remain in the thickness of the wall at the end of the new excision only of the intracavitary part: it will therefore be necessary to carry out more surgical operations. However this limit might be solved by GnRH agonist therapy. | |||
| - ‘Sinking’ phenomenon (due to GnRH agonist therapy) | |||
| - Increased recurrence rate (due to GnRH agonist therapy) | |||
| Mazzon (1995) | ‘Cold loop’ myomectomy | - Theoretically one intervention | - Availability of cold loops |
| - Safeness (although the surgical action goes deeply into the uterine wall during enucleation with a cold loop, it always follows a reference plane (the surface of the intramural pole of the myoma) and constantly maintains the loop action under direct visual control; this means a smaller possibility of complications (perforation, bleeding) | - Training and high experience | ||
| - Respect of the surrounding healthly myometrium avoiding any needless cutting of the muscular fibres adjacent to the surgical area and reducing the thermal damage deriving from the loop of the resectoscope. This avoids any negative effect on the likelihood of subsequent conception and the uterine wall resistance | |||
| - Suitable also for large myomas | |||
| - Suitable also in case of myometrial free margin <1 cm | |||
| Lasmar et al. (2002) | ‘Enucleation in toto’ | - Theoretical one intervention | - The success of the procedure is higher for myomas with a intramural development >50% |
| Litta et al. (2003) | - Safeness (possibility to operate at an intracavitary level) | - The expulsion force of the myometrium is inversely correlated with the diameter of uterine cavity | |
| - Theoretical respect of the surrounding health myometrium | |||
| Hamou (1993) | Hydromassage | - Theoretical one intervention | - Need of electronically controlled irrigation and suction device |
| - Safeness (possibility to operate at an intracavitary level) | - The contractile reaction of the myometrium to such manoeuvres is neither predictable nor standardizable | ||
| - Theoretical respect of the surrounding health myometrium | - The migration of the intramural component of the myoma into the cavity is maximum for myomas with a reduced intramural development or of small dimensions | ||
| Bettocchi et al. (2002) | Office hysterosocpic myomectomy | - Office procedure | - Availability of 5Fr bipolar electrodes and generator |
| - Reduced anaesthetic risks | - Myoma G0-G1<1.5 cm | ||
| - Reduced costs | - Limited by patient’s compliance | ||
| - Theoretical one intervention | - Training and high experience | ||
| Indaman (2004) | Pharmacological-aided techniques (PGF-2α or carboprost) | - Theoretical one intervention | - The contractile reaction of the myometrium to such drugs is neither predictable nor standardizable |
| Murakami et al. (2003, 2006) | - Safeness (possibility to operate at an intracavitary level) - Theoretical respect of the surrounding health myometrium | - Extreme contraction of the uterus can interfere with visualization and one’s ability to manipulate the resectosocpe in the small cavity | |
| - Need of laparoscopic monitoring (Murakami’s technique) | |||
| - Side effects drug-related (nausea, vomiting, diarrhea, fever, bronchospasm in patients with bronchial asthma) |
| Author (year) . | Technique . | Advantages . | Limits . |
|---|---|---|---|
| Loffer et al. (1990) | Two-step myomectomy: the procedure can be performed only by means of traditional resectoscopic surgery (Loffer et al., 1990) or by Nd:yAG laser (Donnez et al., 1990). | - Safeness (possibility to operate at an intracavitary level) | - Two separate interventions |
| Donnez et al. (1990) | - High costs (GnRH agonist therapy, Nd:yAG laser, two surgeries) | ||
| - Only myomas with a reduced intramural development or of small dimensions can be treated with this technique. Indeed, in the case of myomas with a volumetrically significant intramural component, the part which remains after the first surgical operation may be excessively big: such a component, when migrating to the uterine cavity, will meet with resistance to its progression caused by the controlateral myometrial wall. As a result, during the second operation we will find a myoma which still has a significant intramural component, which will remain in the thickness of the wall at the end of the new excision only of the intracavitary part: it will therefore be necessary to carry out more surgical operations. However this limit might be solved by GnRH agonist therapy. | |||
| - ‘Sinking’ phenomenon (due to GnRH agonist therapy) | |||
| - Increased recurrence rate (due to GnRH agonist therapy) | |||
| Mazzon (1995) | ‘Cold loop’ myomectomy | - Theoretically one intervention | - Availability of cold loops |
| - Safeness (although the surgical action goes deeply into the uterine wall during enucleation with a cold loop, it always follows a reference plane (the surface of the intramural pole of the myoma) and constantly maintains the loop action under direct visual control; this means a smaller possibility of complications (perforation, bleeding) | - Training and high experience | ||
| - Respect of the surrounding healthly myometrium avoiding any needless cutting of the muscular fibres adjacent to the surgical area and reducing the thermal damage deriving from the loop of the resectoscope. This avoids any negative effect on the likelihood of subsequent conception and the uterine wall resistance | |||
| - Suitable also for large myomas | |||
| - Suitable also in case of myometrial free margin <1 cm | |||
| Lasmar et al. (2002) | ‘Enucleation in toto’ | - Theoretical one intervention | - The success of the procedure is higher for myomas with a intramural development >50% |
| Litta et al. (2003) | - Safeness (possibility to operate at an intracavitary level) | - The expulsion force of the myometrium is inversely correlated with the diameter of uterine cavity | |
| - Theoretical respect of the surrounding health myometrium | |||
| Hamou (1993) | Hydromassage | - Theoretical one intervention | - Need of electronically controlled irrigation and suction device |
| - Safeness (possibility to operate at an intracavitary level) | - The contractile reaction of the myometrium to such manoeuvres is neither predictable nor standardizable | ||
| - Theoretical respect of the surrounding health myometrium | - The migration of the intramural component of the myoma into the cavity is maximum for myomas with a reduced intramural development or of small dimensions | ||
| Bettocchi et al. (2002) | Office hysterosocpic myomectomy | - Office procedure | - Availability of 5Fr bipolar electrodes and generator |
| - Reduced anaesthetic risks | - Myoma G0-G1<1.5 cm | ||
| - Reduced costs | - Limited by patient’s compliance | ||
| - Theoretical one intervention | - Training and high experience | ||
| Indaman (2004) | Pharmacological-aided techniques (PGF-2α or carboprost) | - Theoretical one intervention | - The contractile reaction of the myometrium to such drugs is neither predictable nor standardizable |
| Murakami et al. (2003, 2006) | - Safeness (possibility to operate at an intracavitary level) - Theoretical respect of the surrounding health myometrium | - Extreme contraction of the uterus can interfere with visualization and one’s ability to manipulate the resectosocpe in the small cavity | |
| - Need of laparoscopic monitoring (Murakami’s technique) | |||
| - Side effects drug-related (nausea, vomiting, diarrhea, fever, bronchospasm in patients with bronchial asthma) |
Excision only of the intracavitary component
In the past, several authors have proposed a progressive resectoscopic excision of only the intracavitary component of those fibroids with extensive intramural involvement (Neuwirth, 1978). Indeed, it was believed that the endometrium would recolonize the surgically operated area and that the residual intraparietal component of the fibroid would remain in the thickness of the wall, behaving like an intramural fibroid (usually asymptomatic). However, the constant intracavitary expulsion and the subsequent volumetric increase of the residual intramural component of the fibroid lead to the persistence of the initial symptomatology. That explains the clinical uselessness of such a treatment and its consequent fall into disuse.
Complete excision of fibroid by a two-step procedure
The observation of the rapid migration of the residual intramural component of the fibroid towards the uterine cavity (Loffer, 1990), with the parallel increase of myometrial thickness during hysteroscopic myomectomy (Yang and Lin, 2001), is the basis of this treatment, which represents the logical evolution of the earlier treatment which involved the excision of the only intracavitary component of the fibroid.
The technique described by Donnez et al. (1990) represents an effective mixture of hormonal treatment and hysteroscopic laser surgery. After 8 weeks of preoperative GnRH agonist therapy, a partial myomectomy of the intracavitary portion of the fibroid is carried out. The Nd:yAG laser fibre is then directed, as perpendicularly as possible, at the remaining (intramural) fibroid portion with the aim to reduce its size by decreasing its vascularity (transhysteroscopic myolysis). After another 8 weeks course of GnRH agonist therapy, a second hysteroscopic myomectomy is performed to remove the remnant intramural portion of the fibroid protruded in the uterine cavity as a consequence of uterine shrinkage. This technique has been successfully reported in 12 patients by Donnez in his original paper (Donnez et al., 1990), with a restoration of normal menstrual flow in all of them. In his largest series of fibroids with the biggest portion located into the uterine wall (n = 78), the author reports a success rate of 95% with only four patients requiring a third-look laser hysteroscopy to completely remove the fibroid (Donnez et al., 1995).
At present, most surgeons prefer to remove a fibroid through a two-steps procedure, by means of traditional resectoscopic surgery, as originally hypothesized by Loffer (1990). The technique consists of the following steps:
First surgical operation: excision only of the intracavitary portion of the fibroid, by means of the usual progressive resectoscopic excision. A hysteroscopic reassessment is carried out 20–30 days after the operation or after the first menstruation to verify that the intracavitary migration of the residual intramural component of the fibroid has taken place: once this has been verified, the second operation can be done.
Second surgical operation: complete excision, by means of slicing, of the residual component of the fibroid, which has now become intracavitary.
Optionally, first and second surgical operation can be preceded by GnRH agonist therapy.
Complete excision of fibroid by a one-step procedure
Excision of intramural component by slicing
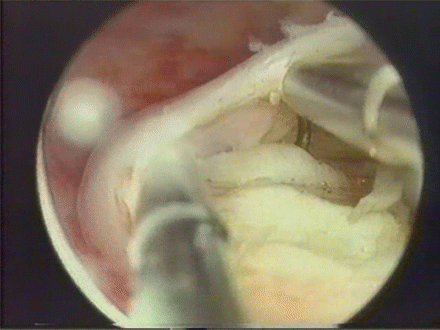
With this technique, after the usual progressive excision of the intracavitary portion of the fibroid, the operation continues with the slicing of the neoformation, included into the thickness of the uterine wall, until the operation is completed (Fig. 5). The main limit of this technique is the use of electrosurgery during the removal of the intramural component of the fibroid with the inevitable damage of the surrounding healthy myometrium (either directly during the cutting or indirectly because of thermal damage) and the increased risk of operative complications (such as perforation, bleeding, intravasation).
Excision of intramural component by slicing: the electrosurgery is used to slice the neoformation, included into the thickness of the uterine wall (Image kindly donated by I. Ardovino)
‘Cold loop’ myomectomy
This technique, developed by Mazzon (1995), is characterized by a sequence of three different operating steps:
Excision of the intracavitary component of the fibroid: this is carried out with the usual technique of slicing. It consists of repeated and progressive passages of the monopolar angled cutting loop, carried out with the standard technique. This action must stop at the level of the plane of the endometrial surface, so that the identification of the passage between the fibroid and the adjacent myometrial tissue is not impaired (cleavage plane).
Enucleation of the intramural component of the fibroid: once the cleavage plane is identified the usual cutting loop of the resectoscope is substituted by a suitable blunt dissection cold loop. Usually the rectangular loop is used first. This loop, once inserted into the plane between the fibroid and myometrium, is used in a mechanical way along the surface of the fibroid (usually clearly recognizable by its smooth, white and compact surface), thus bringing about its progressive blunt dissection from the myometrial wall (Fig. 6A). Then the single tooth loop is used to hook and lacerate the slender connective bridges which join the fibroid and the adjacent myometrium (Fig. 6B). During the entire phase of enucleation, electric energy must not be used in the thickness of the wall, and the loop must be used ‘cold’ or in a mechanical way.
Excision of the intramural component: at the end of the enucleation phase, the intramural part of the fibroid is totally dislocated inside the uterine cavity. At this point it can be treated as a neoformation with a total intracavitary development and therefore it can be completely and safety excised by means of the usual progressive excision using an angled cutting loop.
At present, studies evaluating of the effects of this technique are still lacking in the international literature. However, this technique is largely widespread through the Europe and since 1992, Mazzon himself has carried out >2000 hysteroscopies using this technique reporting good functional and anatomical results and a low complication rate (<2%). The fibroid was completely removed in one-step procedure in nearly 80% of cases (unpublished data).
(A) The rectangular loop is inserted into the plane between the fibroid and myometrium to progressively dissect it from the myometrial wall
(B) Connective bridges which join the fibroid and the adjacent myometrium are hooked by the single tooth cold loop (Images by I. Mazzon)
‘Enucleation in toto’
Litta’s technique: an elliptic incision of the endometrial mucosa that covers the fibroid is performed with a 90° Collins electrode (Fig. 1G), at the level of its reflection on the uterine wall until the cleavage zone of the fibroid is reached. Connecting bridges between fibroid and surrounding myocytes are slowly resected. The effect of such action is that the fibroid protrudes into the cavity, thus facilitating its removal by traditional slicing. The fibroid is pushed into the uterine cavity, enabling the surgeon to work safely and completely resect the intramural component with an angled cutting loop. This technique has been successfully reported in 41 out of 44 women with submucous fibroids G2 ranging from 2 to 4 cm (means diameter 3.2 cm) and myometrial free margin >4 mm at ultrasound (Litta et al., 2003).
Lasmar’s technique: the Collins electrode is used in shape of a ‘L’, to dissect the endometrium around the fibroid until getting to it. At this time, the direct mobilization of the fibroid is started in all directions, doing the coagulation only of the vessels that are bleeding. When the fibroid is in the cavity it is possible to remove it with grasping forceps (small fibroids) or to slice it in several pieces using the Collins electrode. This technique has been successfully reported in 98 cases (50 out of 98: direct mobilization plus grasping; 46 out of 98: direct mobilization plus slicing) (Lasmar and Barrozo, 2002).
Technique of ‘hydromassage’
Starting form the observation that the intramural portion of a submucous fibroid squeezes out of its base after contractions of the uterus during the removal of tissue chips (Loffer, 1990), Hamou (1993) proposed a ‘fibroid massage’ through rapid changes of intrauterine pressure using an electronically controlled irrigation and suction device (Endomat; Karl Storz GmbH Co., Tuttlingen, Germany). Indeed, interrupting and restarting the supply of distension liquid several times, myometrial contraction is stimulated, obtaining the maximum possible migration of the intramural component of the fibroid into the cavity. At present, series evaluating the effects of this technique are lacking in international literature.
‘Manual massage’ technique
At the beginning of 1990s, Hallez (1995) introduced a single-stage technique in which, after a partial myomectomy of the protruding dome of the fibroid, uterine contractions were induced by finger massage of the uterus (similar to obstetric manoeuvres as Crede’s one), thus expelling the residual intramural fibroid into the uterine cavity and making it accessibile for a safe hysteroscopic resection. Hallez (1995) reports good anatomical and functional results after resection with such technique of 222 submuocus fibroids with intramural development.
‘Two-resectoscope technique’
Lin et al. (2000) proposed a one-procedure hysteroscopic myomectomy by using two resectoscopes. A 7-mm resectoscope is first used to cut the capsule of the fibroid next to muscular layer of the uterus. This prevents the fibroid from sinking in the muscular layer as the procedure progresses. The fibroid is then dissected from the muscular layer. Then, after the fibroid has been dissected from the muscular layer, a standard resectoscope with a 9-mm external outer sheath is used to shave the body of the fibroid. A Lin fibroid grasper (Atom Medical Co., Tokyo, Japan) may be used to pull the fibroid further into the intrauterine cavity. The procedure is continuously monitorized by ultrasonography. The index technique has been successfully reported in only two infertile women presenting with menorrhagia.
Office hysteroscopic myomectomy
Fibroids <1.5–2 cm, with a minimal intramural component, can be removed in outpatient setting using smaller diameter hysteroscopes and 5Fr mechanical and bipolar instruments. In these cases, to avoid any myometrial stimulation or damage of the surrounding healthy myometrium, the fibroid is first gently separated from the capsule using mechanical instruments (grasping, forceps or scissor) as described for ‘cold loop’ resectoscopic myomectomy. Once the intramural section becomes intracavitary then it is sliced with the bipolar electrode (Fig. 5B) (Bettocchi et al., 2002).
Pharmacological-aided techniques
Several drugs may stimulate uterine contractions pushing the intramural part of the fibroid into the cavity, thus anticipating what generally happens spontaneously during the weeks following the operation. Murakami and colleagues (2003, 2006) proposed a transabdominal injection of prostaglandin F (PGF)-2α under laparoscopic monitoring, while Indman (2004) reported the successful use of intracervical carboprost, a methyl analogue of PGF-2α, in 8 out of 10 cases in which the drug was administered to facilitate resection of fibroids that otherwise could not be resected completely.
Global endometrial ablation
New instrumentation and the off-label use of some global (non-hysteroscopic) ablation techniques allow some selected patients with submucous fibroids, who have completed their family planning, to be treated only by endometrial ablation (Hickey and Farquhar, 2003; Loffer, 2006).
Conventional endometrial ablation techniques cannot be used when the uterine cavity is remarkably enlarged (>12 cm) and distorted as result of submucous or intramural myomas. Indeed, in such situation it is hard for most devices with a rigid intrauterine probe to access all areas of the endometrium.
Hydrothermal ablation system circulates free heated saline under hysteroscopic visualization and thus very readily adapts to an irregular cavity. It has already been demonstrated to be safe and effective in treating women with menorrhagia and submucous fibroids up to 4 cm in diameter (Glasser and Zimmerman, 2003).
Microwave endometrial ablation (MEA) is based on a generator supplying microwave energy to a 8 mm hand-held reusable probe. This results in a reliable 5–6 mm depth of endomyometrial penetration. Recently, a thinner (4 mm) curved microwave probe has been developed, in order to accomplish the complete coverage of the uterine cavity, even in case of enlarged (12–16 cm in length) and distorted cavity as a result of large submucous fibroids (Kanaoka et al., 2003, 2005). Available data about the outcome of MEA in patients with menorrhagia caused by submucous myomas are few but encouraging. The improvement of menorrhagia is accompanied with a significant shrinkage of myoma related to a necrotic degeneration recognizable by MRI 1–2 months after the procedure (Kanaoka et al., 2003, 2005).
Effects of hysteroscopic myomectomy on menstrual pattern and infertility
No meta-analysis of the association of submucous fibroids and AUB has been performed; however, most studies have shown that hysteroscopic myomectomy is safe and effective in the control of menstrual disorders with a success rate ranging from 70 to 99%. Usually the success rate declines as the follow-up period increases; this could be to due to a number of factors including the incomplete removal of fibroid (which could in time become larger and cause bleeding) as well as the occurrence of other dysfunctional factors as a cause of menorrhagia (Mazzon and Sbiroli, 1997).
The hysteroscopic technique (Table IV) adopted does not seem to significantly affect the success rate.
Bleeding control after hysteroscopic myomectomy (1976–2007)
| Author . | Cases (n) . | Main indications (%) . | Technique (n) . | Follow-up yearsa . | Bleeding control (%) . |
|---|---|---|---|---|---|
| Neuwirth and Amin (1976) | 5 | 80 AUB | Resectoscopic excision of myoma by slicing | 5 | 100 |
| 20 Infertility | |||||
| Brooks et al. (1989) | 62 | 79 AUB | Resectoscopic excision of myoma by slicing (57) | >3 months | 74 |
| 21 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (5) | ||||
| Derman et al. (1991) | 156 | 90.4 AUB | Resectoscopic excision of myoma by slicing (94) | 4 (1–16) | 83.9 |
| 9.6 Infertility | Endometrial ablation with or without resectoscopic myomectomy (62) | 77.5 | |||
| Indmann (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | (1–5) | 94 |
| Wamsteker et al. (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | 1.7 (0.8–2.5) | 94.1 |
| Donnez et al. (1994) | 366 | 100 AUB | Resectoscopic excision of myoma by slicing and Nd:yAG laser (two-step procedure) | 2 | 89 |
| Wortman et al. (1995) | 75 | 100 AUB | Resectoscopic excision of myoma by slicing with endometrial ablation | (1–5) | 84 |
| Brooks (1995) | 12 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization | (0.5–1) | 100 |
| Hallez (1995) | 284 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–8.8) | 76.3 |
| 11 Infertility | |||||
| Phillips et al. (1995) | 208 | 100 AUB | Resectoscopic excision of myoma by slicing (120) | (0.5–6) | 84.1 |
| Resectoscopic excision of myoma by slicing with endometrial ablation (88) | 88.5 | ||||
| Glasser (1997) | 35 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization (6) | 2 | 97 |
| Hysteroscopic myomectomy plus endometrial ablation by electrosurgical vaporization (29) | |||||
| Vercellini et al. (1999) | 101 | 71 AUB | Resectoscopic excision of myoma by slicing | 3.4 ± 1.9b | 70 |
| 29 AUB+infertility | |||||
| Hart et al. (1999) | 122 | 93 AUB | Resectoscopic excision of myoma by slicing | 2.3 (1–7.6) | 81.9 |
| 7 Infertility | |||||
| Emanuel et al. (1999) | 266 | 100 AUB | Resectoscopic excision of myoma by slicing | 3.8 (0.1–8.6) | 84.5 |
| Munoz et al. (2003) | 96 | 84 AUB | Resectoscopic excision of myoma by slicing | 2.8 (1–7) | 88.5 |
| 12 Infertility | |||||
| Loeffer (2005) | 177 | 91 AUB | Resectoscopic excision of myoma by slicing (104) | (1–15) | 80.8 |
| 9 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (73) | 95.9 | |||
| Campo et al. (2005) | 80 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–2) | 69.5 |
| 17 Infertility | |||||
| Marziani et al. (2005) | 107 | 78 AUB | Resectoscopic excision of myoma by slicing | (2–5) | 80.9 |
| 23 Infertility | |||||
| Polena et al. (2007) | 235 | 84.7% AUB | Resectoscopic excision of myoma by slicing (with endometrial ablation in 37% of patients) | 3.3 (1.5–5.5) | 94.4 |
| 6.8% Infertility |
| Author . | Cases (n) . | Main indications (%) . | Technique (n) . | Follow-up yearsa . | Bleeding control (%) . |
|---|---|---|---|---|---|
| Neuwirth and Amin (1976) | 5 | 80 AUB | Resectoscopic excision of myoma by slicing | 5 | 100 |
| 20 Infertility | |||||
| Brooks et al. (1989) | 62 | 79 AUB | Resectoscopic excision of myoma by slicing (57) | >3 months | 74 |
| 21 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (5) | ||||
| Derman et al. (1991) | 156 | 90.4 AUB | Resectoscopic excision of myoma by slicing (94) | 4 (1–16) | 83.9 |
| 9.6 Infertility | Endometrial ablation with or without resectoscopic myomectomy (62) | 77.5 | |||
| Indmann (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | (1–5) | 94 |
| Wamsteker et al. (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | 1.7 (0.8–2.5) | 94.1 |
| Donnez et al. (1994) | 366 | 100 AUB | Resectoscopic excision of myoma by slicing and Nd:yAG laser (two-step procedure) | 2 | 89 |
| Wortman et al. (1995) | 75 | 100 AUB | Resectoscopic excision of myoma by slicing with endometrial ablation | (1–5) | 84 |
| Brooks (1995) | 12 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization | (0.5–1) | 100 |
| Hallez (1995) | 284 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–8.8) | 76.3 |
| 11 Infertility | |||||
| Phillips et al. (1995) | 208 | 100 AUB | Resectoscopic excision of myoma by slicing (120) | (0.5–6) | 84.1 |
| Resectoscopic excision of myoma by slicing with endometrial ablation (88) | 88.5 | ||||
| Glasser (1997) | 35 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization (6) | 2 | 97 |
| Hysteroscopic myomectomy plus endometrial ablation by electrosurgical vaporization (29) | |||||
| Vercellini et al. (1999) | 101 | 71 AUB | Resectoscopic excision of myoma by slicing | 3.4 ± 1.9b | 70 |
| 29 AUB+infertility | |||||
| Hart et al. (1999) | 122 | 93 AUB | Resectoscopic excision of myoma by slicing | 2.3 (1–7.6) | 81.9 |
| 7 Infertility | |||||
| Emanuel et al. (1999) | 266 | 100 AUB | Resectoscopic excision of myoma by slicing | 3.8 (0.1–8.6) | 84.5 |
| Munoz et al. (2003) | 96 | 84 AUB | Resectoscopic excision of myoma by slicing | 2.8 (1–7) | 88.5 |
| 12 Infertility | |||||
| Loeffer (2005) | 177 | 91 AUB | Resectoscopic excision of myoma by slicing (104) | (1–15) | 80.8 |
| 9 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (73) | 95.9 | |||
| Campo et al. (2005) | 80 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–2) | 69.5 |
| 17 Infertility | |||||
| Marziani et al. (2005) | 107 | 78 AUB | Resectoscopic excision of myoma by slicing | (2–5) | 80.9 |
| 23 Infertility | |||||
| Polena et al. (2007) | 235 | 84.7% AUB | Resectoscopic excision of myoma by slicing (with endometrial ablation in 37% of patients) | 3.3 (1.5–5.5) | 94.4 |
| 6.8% Infertility |
AUB includes menorrhagia, metrorrhagia, post-menopausal bleeding, menorrhagia or metrorrhagia on hormonal replacement therapy. aData are expressed as median (range) if not otherwise stated; bmean ± SD.
Bleeding control after hysteroscopic myomectomy (1976–2007)
| Author . | Cases (n) . | Main indications (%) . | Technique (n) . | Follow-up yearsa . | Bleeding control (%) . |
|---|---|---|---|---|---|
| Neuwirth and Amin (1976) | 5 | 80 AUB | Resectoscopic excision of myoma by slicing | 5 | 100 |
| 20 Infertility | |||||
| Brooks et al. (1989) | 62 | 79 AUB | Resectoscopic excision of myoma by slicing (57) | >3 months | 74 |
| 21 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (5) | ||||
| Derman et al. (1991) | 156 | 90.4 AUB | Resectoscopic excision of myoma by slicing (94) | 4 (1–16) | 83.9 |
| 9.6 Infertility | Endometrial ablation with or without resectoscopic myomectomy (62) | 77.5 | |||
| Indmann (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | (1–5) | 94 |
| Wamsteker et al. (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | 1.7 (0.8–2.5) | 94.1 |
| Donnez et al. (1994) | 366 | 100 AUB | Resectoscopic excision of myoma by slicing and Nd:yAG laser (two-step procedure) | 2 | 89 |
| Wortman et al. (1995) | 75 | 100 AUB | Resectoscopic excision of myoma by slicing with endometrial ablation | (1–5) | 84 |
| Brooks (1995) | 12 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization | (0.5–1) | 100 |
| Hallez (1995) | 284 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–8.8) | 76.3 |
| 11 Infertility | |||||
| Phillips et al. (1995) | 208 | 100 AUB | Resectoscopic excision of myoma by slicing (120) | (0.5–6) | 84.1 |
| Resectoscopic excision of myoma by slicing with endometrial ablation (88) | 88.5 | ||||
| Glasser (1997) | 35 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization (6) | 2 | 97 |
| Hysteroscopic myomectomy plus endometrial ablation by electrosurgical vaporization (29) | |||||
| Vercellini et al. (1999) | 101 | 71 AUB | Resectoscopic excision of myoma by slicing | 3.4 ± 1.9b | 70 |
| 29 AUB+infertility | |||||
| Hart et al. (1999) | 122 | 93 AUB | Resectoscopic excision of myoma by slicing | 2.3 (1–7.6) | 81.9 |
| 7 Infertility | |||||
| Emanuel et al. (1999) | 266 | 100 AUB | Resectoscopic excision of myoma by slicing | 3.8 (0.1–8.6) | 84.5 |
| Munoz et al. (2003) | 96 | 84 AUB | Resectoscopic excision of myoma by slicing | 2.8 (1–7) | 88.5 |
| 12 Infertility | |||||
| Loeffer (2005) | 177 | 91 AUB | Resectoscopic excision of myoma by slicing (104) | (1–15) | 80.8 |
| 9 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (73) | 95.9 | |||
| Campo et al. (2005) | 80 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–2) | 69.5 |
| 17 Infertility | |||||
| Marziani et al. (2005) | 107 | 78 AUB | Resectoscopic excision of myoma by slicing | (2–5) | 80.9 |
| 23 Infertility | |||||
| Polena et al. (2007) | 235 | 84.7% AUB | Resectoscopic excision of myoma by slicing (with endometrial ablation in 37% of patients) | 3.3 (1.5–5.5) | 94.4 |
| 6.8% Infertility |
| Author . | Cases (n) . | Main indications (%) . | Technique (n) . | Follow-up yearsa . | Bleeding control (%) . |
|---|---|---|---|---|---|
| Neuwirth and Amin (1976) | 5 | 80 AUB | Resectoscopic excision of myoma by slicing | 5 | 100 |
| 20 Infertility | |||||
| Brooks et al. (1989) | 62 | 79 AUB | Resectoscopic excision of myoma by slicing (57) | >3 months | 74 |
| 21 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (5) | ||||
| Derman et al. (1991) | 156 | 90.4 AUB | Resectoscopic excision of myoma by slicing (94) | 4 (1–16) | 83.9 |
| 9.6 Infertility | Endometrial ablation with or without resectoscopic myomectomy (62) | 77.5 | |||
| Indmann (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | (1–5) | 94 |
| Wamsteker et al. (1993) | 51 | 100 AUB | Resectoscopic excision of myoma by slicing | 1.7 (0.8–2.5) | 94.1 |
| Donnez et al. (1994) | 366 | 100 AUB | Resectoscopic excision of myoma by slicing and Nd:yAG laser (two-step procedure) | 2 | 89 |
| Wortman et al. (1995) | 75 | 100 AUB | Resectoscopic excision of myoma by slicing with endometrial ablation | (1–5) | 84 |
| Brooks (1995) | 12 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization | (0.5–1) | 100 |
| Hallez (1995) | 284 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–8.8) | 76.3 |
| 11 Infertility | |||||
| Phillips et al. (1995) | 208 | 100 AUB | Resectoscopic excision of myoma by slicing (120) | (0.5–6) | 84.1 |
| Resectoscopic excision of myoma by slicing with endometrial ablation (88) | 88.5 | ||||
| Glasser (1997) | 35 | 100 AUB | Hysteroscopic myomectomy by electrosurgical vaporization (6) | 2 | 97 |
| Hysteroscopic myomectomy plus endometrial ablation by electrosurgical vaporization (29) | |||||
| Vercellini et al. (1999) | 101 | 71 AUB | Resectoscopic excision of myoma by slicing | 3.4 ± 1.9b | 70 |
| 29 AUB+infertility | |||||
| Hart et al. (1999) | 122 | 93 AUB | Resectoscopic excision of myoma by slicing | 2.3 (1–7.6) | 81.9 |
| 7 Infertility | |||||
| Emanuel et al. (1999) | 266 | 100 AUB | Resectoscopic excision of myoma by slicing | 3.8 (0.1–8.6) | 84.5 |
| Munoz et al. (2003) | 96 | 84 AUB | Resectoscopic excision of myoma by slicing | 2.8 (1–7) | 88.5 |
| 12 Infertility | |||||
| Loeffer (2005) | 177 | 91 AUB | Resectoscopic excision of myoma by slicing (104) | (1–15) | 80.8 |
| 9 AUB+infertility | Resectoscopic excision of myoma by slicing with endometrial ablation (73) | 95.9 | |||
| Campo et al. (2005) | 80 | 79 AUB | Resectoscopic excision of myoma by slicing | (0.5–2) | 69.5 |
| 17 Infertility | |||||
| Marziani et al. (2005) | 107 | 78 AUB | Resectoscopic excision of myoma by slicing | (2–5) | 80.9 |
| 23 Infertility | |||||
| Polena et al. (2007) | 235 | 84.7% AUB | Resectoscopic excision of myoma by slicing (with endometrial ablation in 37% of patients) | 3.3 (1.5–5.5) | 94.4 |
| 6.8% Infertility |
AUB includes menorrhagia, metrorrhagia, post-menopausal bleeding, menorrhagia or metrorrhagia on hormonal replacement therapy. aData are expressed as median (range) if not otherwise stated; bmean ± SD.
The failure of hysteroscopic treatment seems to be related to the growth of fibroids in other sites, the association of fibroids with adenomyosis and the incomplete treatment of partially intramural submucous fibroids (Donnez et al., 1995). The complete resection of the intramural part of the fibroid is certainly advisable to improve the control of menorrhagia, with lower chance of subsequent recurrence (Wamsteker et al., 1993; Parent et al., 1994; Van Dongen et al., 2006).
Furthermore, uterine size (Emanuel et al., 1999; Hart et al., 1999), fibroid size (Hart et al., 1999) and the number of fibroids (Emanuel et al., 1999) found at hysteroscopy seem to have an independent prognostic value for recurrence of AUB.
In women who have completed their family planning, good long-term results in controlling bleeding have been achieved by concomitant hysteroscopic endometrial ablation which leads to an amenorrhoic status in up to 95.5% of patients (Brooks et al., 1989; Derman et al., 1991; Phillips et al., 1995; Glasser, 1997; Loffer, 2000, 2005; Polena et al., 2007).
The effects of hysteroscopic myomectomy on the reproductive outcome in infertile women have been investigated by several authors (Hunt and Wallah, 1974; Donnez et al., 1990; Valle, 1990; Corson and Brooks, 1991; Goldenberg et al., 1995; Preutthipan and Theppisai, 1998; Giatras et al., 1999; Varasteh et al., 1999; Vercellini et al., 1999; Bernard et al., 2000; Fernandez et al., 2001; Pritts, 2001; Donnez and Jadoul, 2002; Munoz et al., 2003; Shokeir, 2005; Stamatellos et al., 2006; Somigliana et al., 2007), but unfortunately the evidence thus far is not of the highest quality. Reported post-surgical pregnancy rate varies from 16.7 to 76.9% with a mean of 45%. This large variation may be mainly related to difficulty in controlling for multiple infertility factors, to sample size and follow-up discrepancies and to differences in patients’ (i.e. age, primary or secondary infertility) and fibroid characteristics (i.e. number, size, intramural portion and presence of concomitant intramural fibroids) (Cheong and Ledger, 2007; Somigliana et al., 2007).
Uterine fibroids as an isolated cause of infertility are very uncommon. Two papers (Buttram and Reiter, 1981; Verkauf, 1992), generally cited as sources of epidemiological data about this issue, reported that uterine fibroids as an isolated cause of infertility range from 1 to 2.4%. However, these studies do not provide a reliable estimate of the real impact of fibroids on infertility as routine diagnostic evaluations performed were not listed and they precede the advent and widespread use of new instrumentations (i.e. TVS and endoscopic procedures) (Somigliana et al., 2007).
Several studies (Ubaldi et al., 1995; Bernard et al., 2000; Fernandez et al., 2001) have shown that post-operative reproductive outcome is adversely affected by the presence of additional infertility factors. Particularly, Fernandez et al. (2001) reported in their retrospective series that the pregnancy rate was 41.6% when the fibroid was the only apparent cause, compared with 26.3% with the presence of one factor and 6.3% with two or more additional factors.
In another retrospective study (Bernard et al., 2000) a statistically significant difference in delivery rate was found between patients with one fibroid and those with a number of fibroids equal or superior to two. Furthermore, the authors report that patients without an intramural fibroid associated had a significantly greater delivery rate and a significantly shorter delay of conception than those found in patients with associated intramural fibroids. These differences cannot be due to uterine cavity abnormalities as the associated intramural fibroids did not cause any distortion of the uterine cavity as assessed by an office hysteroscopy performed four weeks after resection. These results reinforce the hypothesis that other mechanisms associated with fibroids may contribute to infertility.
In a recent prospective study, Stamatellos et al. (2006) has reported increased fertility rates after hysteroscopic myomectomy of type 0 and type 1 fibroids in previously infertile women. Interestingly, in patients with type 2 fibroids fertility rate did not increase, in contrast with patients with type 2 fibroids who received expectant management (control group).
Fernandez et al. (2001) described higher pregnancy rates after the removal of larger fibroids, although the difference was not statistically significant. Indeed, the pregnancy rate after the removal of fibroids >5 cm in size was 57%, whereas it was 23% for fibroids <5 cm.
Finally, assessing the real impact of hysteroscopic myomectomy on subsequent fertility seems to be highly hampered by the fact that most studies addressing this issue were retrospective (Donnez et al., 1990; Goldenberg et al., 1995; Giatras et al., 1999; Varasteh et al., 1999; Vercellini et al., 1999; Bernard et al., 2000; Fernandez et al., 2001; Munoz et al., 2003) and did not have a control group (Donnez et al., 1990; Valle, 1990; Hallez, 1995; Goldenberg et al., 1995; Vercellini et al., 1999; Giatras et al., 1999; Bernard et al., 2000; Fernandez et al., 2001; Munoz et al., 2003; Shokeir, 2005).
Only one study (Varasteh et al., 1999) had a control group of infertile women and the authors showed that the removal of submucosal fibroids >2 cm carried a significant benefit in terms of pregnancy and live birth rates. However, the limited sample size (control group: n = 19; myomectomy group: n = 36) reduces the strength of these results.
Only a randomized controlled study would provide a definitive assessment of the advantages of such technique on reproductive outcomes and would be useful to confirm that fibroids are not purely coincidental with infertility (Donnez and Jadoul, 2002, Somigliana et al., 2007); such a study should be performed comparing pregnancy rates between infertile women with submucous fibroids resected or left in situ (Pritts, 2001). However, such a study would be hard to justify because such lesions often cause menorrhagia, they require histologic diagnosis and they are likely to contribute to infertility (Varasteh et al., 1999).
Also, technical factors such as the surgeon’s skill and experience as well as the applied techniques surely play an important role (Stamatellos et al., 2006). Thus, as similar studies are lacking in international literature, it might be interesting to evaluate whether the choice of a myometrial sparing technique would further increase the reproductive outcomes.
The advent of assisted reproductive techniques and in particular of IVF has offered a useful tool to elucidate the impact of fibroids on embryo implantation. However, there is no definite consensus on whether fibroids affect the outcome and should be removed prior to any attempt. Their location, followed by size, is considered the main factor determining this impact (Gianaroli et al., 2005; Kolankaya and Arici, 2006).
Four meta-analysis (Pritts, 2001; Donnez and Jadoul, 2002; Benecke et al., 2005; Somigliana et al., 2007) have aimed to assess the impact of fibroids on IVF cycles.
Pritts (2001) reported significantly lower pregnancy (RR 0.32), implantation (RR 0.28), and delivery (RR 0.75) rates in patients with submucosal fibroids and abnormal uterine cavities in comparison with infertile controls without fibroids. Results from Donnez and Jadoul (2002) confirm a negative impact only of submucous fibroids on embryo implantation.
Conversely, Benecke et al. (2005) reported a negative impact also of intramural fibroids. Recently, Somigliana et al. (2007) performed an updated meta-analysis reporting significantly lower pregnancy and delivery rates for patients with submucosal fibroids [odds ratio (OR): 0.3; OR: 0.3) and intramural fibroids (OR: 0.8; OR: 0.7).
The impact of hysteroscopic myomectomy on IVF outcome has been less extensively investigated. Among the three studies evaluating the impact of previous myomectomy on IVF cycles (Seoud et al., 1992; Narayan et al., 1994; Surrey et al., 2005), only two (Narayan et al., 1994; Surrey et al., 2005) include patients operated for submucousal fibroids.
Meta-analysis of these two studies reports that hysteroscopic myomectomy does not appear to negatively affect the chance of pregnancy in IVF cycles (Somigliana et al., 2007). However, positive answers could be doubted, as they were based on two retrospective studies only, both of them characterized by a limited number of patients.
Operative and long-term complications
Hysteroscopic myomectomy is one of the most advanced operative hysteroscopic procedures as it is associated, particularly for complex cases, with a significantly higher rate of complications than other hysteroscopic procedures (Jansen et al., 2000; Propst et al., 2000; Aydeniz et al., 2002; Murakami et al., 2005). Reported data show a rate of complication ranging from 0.3 to 28%, fluid overload and uterine perforation being the most frequent complications occurring during surgery (Loffer, 1990; Valle, 1990; Corson and Brooks, 1991; Derman et al., 1991; Serden and Brooks, 1991; Pace, 1993; Wamsteker et al., 1993; Hallez, 1995; Jansen et al., 2000; Propst et al., 2000; Agostini et al., 2002; Darwish, 2003). Other intraoperative complications include bleeding, cervical trauma and air embolism, while late complications include post-operative intrauterine adhesion (IUA) (Wamstecker et al., 1993; Hallez, 1995; Giatras et al., 1999; Taskin et al., 2000; Acunzo et al., 2003; Guida et al., 2004; Nappi et al., 2007) and uterine rupture during pregnancy (Derman et al., 1991; Yaron et al., 1994; Darwish, 2003; Valle and Buggish, 2007).
Uterine perforation
Uterine perforation may occur during cervical dilatation, hysteroscope insertion and intramyometrial tissue resection. In particular, the risk of perforation increases in case of fibroids with intramural component whereas an aggressive uterine fibroid resection into the myometrium is carried out (Mazzon, 1995; Murakami et al., 2005). In case of uterine perforation, the procedure should be terminated immediately and, if there is a mechanical perforation in which bowel damage is not suspected, the patient can be observed and discharged if stable (Indman, 2006). If a perforation occurs secondarily to an activated electrode then it should be assumed that there is a bowel injury until proven otherwise, and laparoscopy must be done without delay (Indman, 2006).
Intravasation and electrolyte imbalance
The most dangerous complication during hysteroscopic myomectomy is an excessive intravasation of the fluid used to distend and irrigate the uterine cavity. Severe fluid overload can cause pulmonary edema, hyponatremia, heart failure, cerebral edema and even death (Emanuel et al., 1997). Fluid absorption occurs through the open veins of the fibroid and possibly through transperitoneal absorption from retrograde flow through the Fallopian tubes. Guidelines indicate that fluid intravasation of 750 ml during surgery requires planned termination of the operation (Loffer, 2000) and that the intervention must be immediately stopped when balance exceeds of 1000 ml (Munoz et al., 2003) or, according to other authors, 1500–2000 ml (West and Robinson, 1989; Baumann et al., 1990; Istre et al., 1994). The risk factors for intravasation during hysteroscopic myomectomy are not completely elucidated because no studies have tested their independent contribution or relation to fluid loss. The main factor seems to be the intramural extension of the fibroid (Emanuel et al., 1997); indeed, in cases of fibroids with deep intramural extension, intravasation will increase mainly because of damage to larger-sized vessels (Emanuel et al., 1997). Other reported factors possibly associated with a higher risk of intravasation include the length of the operation (Corson et al., 1994, Emmanuel et al., 1997), the size of the fibroid (Maher and Hill, 1990) and the total inflow volume (Corson et al., 1994).
Management of this risk relies on close monitoring of the fluid balance and interruption of the procedure before excessive fluid absorption occurs. The difference between the amount of inflow and outflow fluid (including also fluid leaking from the vagina) could be assessed by dedicated operating room personnel or by modern electronic balances and pumps (i.e. Hydromat Gyn and Equimat, Karl Storz GmbH Co., Tuttlingen, Germany; HysteroBalance/HysteroFlow, Olympus Medical System GmbH, Hamburg, Germany).
The use of normal saline combined with bipolar energy reduces the risk of hyponatremia (eliminating the problem of the accumulation of the free water), but an excessive intravasation (>1500 ml) still remains a risk and might cause cardiac overload (Murakami et al., 2005).
Post-operative IUA
The incidence of post-operative IUAs represents the major long-term complication of hysterosocpic myomectomy ranging from 1 to 13% (Wamstecker et al., 1993; Hallez, 1995; Giatras et al., 1999). To minimize the risk of post-operative IUA, it is necessary to avoid forced cervical manipulation, and trauma of healthy endometrium and myometrium surrounding the fibroid; it is also advisable to reduce the usage of electrosurgery especially during the removal of fibroids with extensive intramural involvement (Mazzon, 1995) and multiple fibroids on opposing endometrial surfaces (Indman, 2006). An early second-look hysteroscopy after any hysteroscopic surgery is another effective preventive and therapeutic strategy (Wheeler and Taskin, 1993).
Several pharmacologic (conjugated estrogen, levonorgestrel-releasing intrauterine device) and barrier agents, including foley catheter, hyaluronic acid gel and hyaluronic acid and carboxymethylcellulos (Seprafilm) have been used to reduce IUA development. A recent review has clearly indicated that there is no single modality proven to be unequivocally effective at preventing post-operative adhesion formation for hysteroscopic surgery (Nappi et al., 2007).
Uterine rupture during pregnancy
Uterine rupture may occur in a subsequent pregnancy after surgery invading the myometrium, perforation during entry or during surgery. Therefore when any of the above events occurs, it is important that the surgeon explains to the patient about the risk of uterine rupture in a subsequent pregnancy and to document this discussion clearly in the medical records (Valle and Buggish, 2007).
A recent review of the complications after hysterosocpic myoemctomy only reports two cases of uterine rupture following such surgery (Derman et al., 1991; Yaron et al., 1994).
According to some authors the interval between uterine operation infringing on the myometrium and attempts for pregnancy should not be less than one year from the date of uterine surgery (Valle and Buggish, 2007). Although some surgeons believe that caesarean section should be preferred whenever you are dealing with fibroids with intramural development (Keltz et al., 1998; Cravello et al., 2004), currently there is lack of strong evidence to suggest this mode of delivery to reduce the risk of uterine rupture.
Conclusions
Hysteroscopic myomectomy currently represents one of the greatest advances in the field of hysteroscopic surgery. Ideally, it should result in the complete removal of the fibroid (reducing the chance of recurrence and re-growth) (Wamsteker et al., 1993; Parent et al., 1994; Van Dongen et al., 2006) without traumatizing the normal surrounding uterine tissue.
It is well ascertained that fibroids developing completely within the uterine cavity can be easily removed in a single procedure with fibroid size representing the main limiting factor (Mazzon and Sbiroli, 1997; Isaacson, 2003; Indman, 2006).
Resectoscopic slicing still represents the standard widely-used technique for treating such fibroids (Neuwirth, 1995; Mazzon and Sbiroli, 1997; Isaacson, 2003; Gallinat, 2005; Indman, 2006) though several other techniques have been introduced. It is less expensive than laser treatment and quick to perform when you have adequate training. Recently, an innovative and effective device called IUM has been proposed and it may become in the near future a valid alternative to the traditional transcervical resectoscopic myomectomy (Emanuel and Wamsteker, 2005).
Furthermore, the development of smaller diameter scopes with working channels and continuous flow systems together with the establishment of bipolar technology, have made possible the outpatient treatment of small (<1.5–2 cm) totally intracavitary fibroids as well as those with minimal intramural development thus avoiding both cervical dilatation and any anaesthesia or analgesia (Bettocchi et al., 2002; Clark et al., 2002).
On the other hand, the resection of fibroids with intramural extension is advisable only for expert surgeons as it is technically difficult and has a higher risk of complications than other hysteroscopic procedures (Propst et al., 2000). The frequency of complications and the chance of achieving the complete resection of the lesion at one surgical time, may widely vary depending on the extension of intramural component and the operative technique (Loffer, 1990; Valle, 1990; Derman et al., 1991; Corson and Brooks, 1991; Serden and Brooks, 1991; Pace, 1993; Wamsteker et al., 1993; Hallez, 1995; Jansen et al., 2000; Propst et al., 2000; Agostini et al., 2002; Darwish, 2003; Van Dongen et al., 2006). Furthermore, while differences in equipment do not seem to have significant effects on surgery for fibroids G0, advanced ‘state of the art’ equipment is necessary for carrying out safe hysteroscopic myomectomy for fibroids with intramural extension (Murakami et al., 2005).
Several techniques have been developed to completely remove such fibroids, all of those aiming at the transformation of an intramural fibroid into a totally intracavitary lesion, thus avoiding a deep cut into the myometrium.
While G1 fibroids may be often completely removed in one step, as the uterus contracts and tends to expel the intramural component into the cavity during surgery, the removal of G2 fibroids may be much more problematic.
In such cases, despite of recent evidence indicating that an incomplete removal does not always necessitate subsequent surgery, patients should always be advised regarding the possibility of another surgical step (Van Dongen et al., 2006).
The present review clearly indicates that there is still no single technique proven to be unequivocally superior to the others for treating fibroids with intramural development (G1-G2). The two-step technique seems to be very effective and safe; however, the extended GnRH agonist treatment and repeated hysteroscopies can cause greater distress in patients. One-step myomectomy remains more desirable and the ‘cold loop’ myomectomy seems to represent the best option as it allows a safe and complete removal of such fibroids in just one surgical procedure, while respecting the surrounding healthy myometrium. This might reduce the risk of operative (i.e. bleeding, perforation) and long-term complication (i.e. IUA and uterine rupture).
Other techniques including ‘enucleation in toto‘, ‘hydromassage’ and pharmacologic aided-techniques may be helpful to induce the shrinkage of the intramural portion in the uterine cavity, but the contractile reaction of the myometrium is neither predictable nor standardizable, and this represents an uncertain variable.