-
PDF
- Split View
-
Views
-
Cite
Cite
Emmy van den Boogaard, Rosa Vissenberg, Jolande A. Land, Madelon van Wely, Joris A.M. van der Post, Mariette Goddijn, Peter H. Bisschop, Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review, Human Reproduction Update, Volume 17, Issue 5, September-October 2011, Pages 605–619, https://doi.org/10.1093/humupd/dmr024
Close - Share Icon Share
Abstract
Thyroid dysfunction and thyroid autoimmunity are prevalent among women of reproductive age and are associated with adverse pregnancy outcomes. Preconception or early pregnancy screening for thyroid dysfunction has been proposed but is not widely accepted. We conducted a systematic review of the literature on the clinical significance of thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy.
Relevant studies were identified by searching Medline, EMBASE and the Cochrane Controlled Trials Register.
From a total of 14 208 primary selected titles, 43 articles were included for the systematic review and 38 were appropriate for meta-analyses. No articles about hyperthyroidism were selected. Subclinical hypothyroidism in early pregnancy, compared with normal thyroid function, was associated with the occurrence of pre-eclampsia [odds ratio (OR) 1.7, 95% confidence interval (CI) 1.1–2.6] and an increased risk of perinatal mortality (OR 2.7, 95% CI 1.6–4.7). In the meta-analyses, the presence of thyroid antibodies was associated with an increased risk of unexplained subfertility (OR 1.5, 95% CI 1.1–2.0), miscarriage (OR 3.73, 95% CI 1.8–7.6), recurrent miscarriage (OR 2.3, 95% CI 1.5–3.5), preterm birth (OR 1.9, 95% CI 1.1–3.5) and maternal post-partum thyroiditis (OR 11.5, 95% CI 5.6–24) when compared with the absence of thyroid antibodies.
Pregnant women with subclinical hypothyroidism or thyroid antibodies have an increased risk of complications, especially pre-eclampsia, perinatal mortality and (recurrent) miscarriage. Future research, within the setting of clinical trials, should focus on the potential health gain of identification, and effect of treatment, of thyroid disease on pregnancy outcome.
Introduction
Thyroid dysfunction and autoimmunity are not uncommon among women of reproductive age. The prevalence of thyroid dysfunction during pregnancy is estimated to be 2–3% and is mainly caused by chronic autoimmune thyroiditis. Thyroid auto-antibodies are found in 5–15% of women of reproductive age, but are not necessarily accompanied by thyroid dysfunction. Nevertheless, both thyroid dysfunction and thyroid autoimmunity have independently been associated with adverse pregnancy outcomes during all trimesters of pregnancy (Abalovich et al., 2007a).
In the general population, miscarriage occurs in ∼15% of all clinically recognized pregnancies and recurrent miscarriage in 1–3% of all couples trying to conceive (Regan and Rai, 2000). Complications later in pregnancy that have been associated with thyroid disorders are pre-eclampsia (incidence 5–10%), preterm delivery (incidence 10–15%) and placental abruption (incidence ∼1%) (Cunningham and Lindheimer, 1992; Ananth et al., 2006).
In order to achieve an optimal pregnancy outcome, namely a healthy full-term live birth, all circumstances should be optimal in early pregnancy. Adequate functioning of the maternal thyroid is especially important during the first trimester, when development of the fetal brain starts and the fetus does not yet produce its own thyroid hormones. The exact prevalence of thyroid dysfunction and thyroid autoimmunity among pregnant women as well as the clinical consequences is still unclear: the same applies to the treatment possibilities and their effects on pregnancy outcome.
Guidelines on treatment of hypo- and hyperthyroidism in non-pregnant women and men are generally well defined (Baskin et al., 2002; Gharib et al., 2004) but only a few guidelines are specifically related to obstetric care (Endocrine Society, 2007). Endocrinologists agree upon the need for hormone replacement therapy in pregnant women with subclinical hypothyroidism, even in case of only marginally increased thyroid-stimulating hormone (TSH) levels (Poppe and Glinoer, 2003; Casey et al., 2005). Therapy has also been recommended in euthyroid women with circulating antibodies against thyroperoxidase (TPO-Ab) and/or thyroglobulin (Tg-Ab) (Negro et al., 2006).
General screening for thyroid dysfunction either preconception or in (early) pregnancy has been proposed but is not widely accepted (American College of Obstetricians and Gynecologists, ACOG, 2002; Abalovich et al., 2007a). It remains to be established whether screening and subsequent treatment will improve clinical outcome and which risk factors contribute to the complications resulting from thyroid abnormalities. The potential benefit of any screening strategy critically depends on the relative contribution of thyroid dysfunction to adverse pregnancy outcomes and on the impact of treatment.
Studies on treatment interventions in patients with thyroid disorders can only be justified if an association between the thyroid condition and obstetric outcome has been demonstrated. Therefore, in order to gain insight into the clinical significance of thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy, we conducted a systematic review and meta-analyses of the literature.
Methods
Relevant studies were identified by searching Medline, EMBASE and the Cochrane Controlled Trials Register, published until May 2010. Date limit for inclusion was based upon the availability of reliable free thyroxine (fT4) assays, which excluded articles published before 1975 (Ball et al., 1989). Search criteria used were related to thyroid function, thyroid autoimmunity and pregnancy outcome. Specifically the following search terms were used: thyroid*, hyperthyr*, hypothyr*, tpo*, tsh, thyrotropin receptor antibod*, thyroid stimulating immunoglobulin*, thyrotropin-binding inhibit*, thyroxine, thyrotropin, thyroid microsomal antibodies, fertility, infertility, abortion*, miscarriag*, pregnan*, obstetric*, gestation* preterm deliver*, premature deliver*, intrauterine growth retardation*, fetal growth restriction*, intrauterine growth restriction* and child development*. Mesh terms used were: thyroid gland, thyroid diseases immunoglobulins, thyroid-stimulating, thyrotropin, thyroxine, fertility, infertility, pregnancy pregnancy outcome, pregnancy complications, fetal growth retardation and child development. There were no language limitations for the initial search. Randomised Controlled Trials (RCTs), cohort studies and case–control studies were included. Data on the effect of T4 replacement therapy were excluded.
Titles and subsequently abstracts of the articles were screened by two reviewers independently (E.v.d.B., R.V.). Included articles for full text screening were compared during a consensus meeting. In case of disagreement, a third reviewer (M.G. or P.B.) was consulted for the decision on inclusion or exclusion for full-text evaluation. Articles that did not contribute to the answer of our research questions after full text evaluation were excluded. Only articles that described at least 10 patients were eligible. Hypothyroidism was defined as low free T4 and TSH concentrations (Braverman and Utiger, 2005) and subclinical hypothyroidism as a high TSH and normal free T4 (Surks et al., 2004). Hyperthyroidism was defined as low TSH with high free T4 or normal free T4 in case of subclinical hyperthyroidism (Canaris et al., 2000). Articles that did not report concentrations of TSH and/or free T4, and articles on thyroid antibodies in non-euthyroid populations were excluded. After consensus the remaining articles were included for critical appraisal and assessed by two reviewers independently (E.v.d.B., R.V.). Articles were judged on scientific quality according to the CONSORT and STROBE statement (von Elm et al., 2007; Schulz et al., 2010). Levels of evidence were attributed according to the Oxford Centre for Evidence-Based Medicine (Oxford Centre for Evidence-based Medicine, 2009). Articles in foreign languages were translated and included if eligible, except for articles in Chinese, Japanese, Russian and Bulgarian.
In case of adequate clinical and statistical homogeneity, summarized odds ratios (ORs) were calculated using random effect models. Software of Review Manager 5 was used to perform the meta-analyses (available from Cochrane). Meta-analysis on thyroid autoimmunity was performed on the presence of antibodies, i.e. TPO-Ab and/or Tg-Ab. In studies that reported both TPO-Ab and Tg-Ab, TPO-Ab was used for meta-analysis, since this is the most commonly and most frequently tested type of antibody. When applicable, i.e. enough data were reported, a subgroup meta-analysis on TPO-Ab and Tg-Ab was performed separately. This was carried out to approximate clinical practice more precisely and to achieve applicability of the results in all clinical settings.
Results
Figure 1 shows the selection process after the search: 435 articles were selected for critical appraisal, all dealing with fertility, pregnancy outcome and/or the post-natal period. Of the 43 included articles in this systematic review, 4 reported on hypothyroidism (Haddow et al., 1999; Klein et al., 2001; Rao et al., 2008; Negro et al., 2010), 5 on subclinical hypothyroidism (Allan et al., 2000; Casey et al., 2007; Abalovich et al., 2007b; Cleary-Goldman et al., 2008; Li et al., 2009) and 36 on thyroid antibodies (Fung et al., 1988; Feldt-Rasmussen et al., 1990; Stagnaro-Green et al., 1990; Lejeune et al., 1993; Pratt et al., 1993; Singh et al., 1995; Geva et al., 1996; Roberts et al., 1996; Bussen and Steck, 1997; Iijima et al., 1997; Kim et al., 1998; Kutteh et al., 1999a, b; Mavragani et al., 1999; Muller et al., 1999; Dendrinos et al., 2000; Mecacci et al., 2000; Rushworth et al., 2000; Sakaihara et al., 2000; Poppe et al., 2002; Sieiro et al., 2004; Stagnaro-Green et al., 2005; Ghafoor et al., 2006; Negro et al., 2006, 2007a, b; Shoenfeld et al., 2006; Abalovich et al., 2007b; Mamede da et al., 2007; Bellver et al., 2008; Iravani et al., 2008; Kilic et al., 2008; Montaner et al., 2008; Benhadi et al., 2009; Li et al., 2009; Sezer et al., 2009). Articles on subclinical hypothyroidism and antibodies were, in case of the same outcome measures, included in the meta-analysis. Patients in the included studies were pregnant women or non-pregnant women with unexplained subfertility or recurrent miscarriage. Definitions of (unexplained) subfertility and recurrent miscarriage used in the included articles are described in Table I. Controls were all women, either euthyroid or without the adverse pregnancy outcome.
Characteristics and quality features of the 43 studies included in the systematic review of clinical impact of thyroid disorders before conception and in the first trimester of pregnancy.
| First author . | Year . | Study type . | Participants . | Hormone levels . | Patients . | Controls . | Outcome measure(s) . | Quality features . |
|---|---|---|---|---|---|---|---|---|
| Fung et al. | 1988 | Cohort | 901 pregnant women | Reference range TSH, T4, T3 from control group | 100 women with Tg-Ab/microsomal Ab | 120 women without Ab detectable, euthyroid | PPTD | Matching: yes |
| Tg-Ab and microsomal Ab: positive >+2 SD in control group | Detectable, euthyroid | |||||||
| Feldt-Rasmussen et al. | 1990 | Cohort | 736 healthy euthyroid pregnant women | TSH (0.3–5 mU/l) | 36 women with TPO-Ab and/or Tg-Ab in first trimester | 20 women without TPO-Ab and/or Tg-Ab in first trimester | PPTD (transient or persistent thyroid dysfunction within 1 year after delivery, thyreotoxicosis or hypothyroidism) | Matching: no |
| T4 (56–129 nmol/l) | ||||||||
| T3 (1.6–2.8 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (>100 U/ml) | ||||||||
| Stagnaro-Green et al. | 1990 | Cohort | 552 pregnant euthyroid women | Thyrotropin (TSH) (0.2–5 U/l) | 100 women positive for TPO-Ab and/or Tg-Ab | 392 negative for TPO-Ab and/or Tg-Ab | MC (in first or second trimester) | Matching: no |
| T4 (58–161 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (<0.20 arbitrary units by ELISA) | ||||||||
| Lejeune et al. | 1993 | Prospective cohort | 363 pregnant women, euthyroid, <14 weeks gestational age | TSH not defined | 23 women positive for TPO-Ab and/or Tg-Ab | 340 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| TPO-Ab (>150 U/ml) | ||||||||
| Tg-Ab (>100 U/ml) | ||||||||
| Pratt et al. | 1993 | Prospective cohort | 42 non-pregnant euthyroid women with a history of RM | TSH (0.35–7.0 μIU/ml) | 13 women positive for TPO-Ab and/or Tg-Ab | 29 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| fT4 (0.9–2.1 ng/dl) | ||||||||
| TPO-Ab, Tg-Ab (>5 U/ml) | ||||||||
| Singh et al. | 1995 | Cohort | 487 infertile patients conceiving after ART (artificial reproductive techniques) (IVF) | TSH not defined | 106 women positive for TPO-Ab and/or Tg-Ab, euthyroid | 381 women negative for TPO-Ab and/or Tg-Ab, euthyroid | MC (not defined) | Matching: no |
| TPO-Ab and Tg-Ab (sample antibody index 0–3.8) | ||||||||
| Geva et al. | 1996 | Prospective cohort | 78 patients with mechanical (tubal obstruction) or unexplained infertility in IVF program | Tg-Ab (>1:400) | 16 women positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 55 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | Pregnancy after IVF, MC after IVF | Matching: no |
| Antimicrosomal Ab (>1:1600) | ||||||||
| Roberts et al. | 1996 | Case–control | 33 pregnant women | TSH (0–5 mU/l) | 11 pregnant women with RM (≥3 MC) | 11 healthy women in the first trimester of an ongoing pregnancy | TPO-Ab, Tg-Ab | Matching: no |
| T4 (55–144 nmol/l) | ||||||||
| TPO-Ab (0–1 U/ml) | 11 pregnant women with 1 MC | |||||||
| Tg-Ab (0–8 U/ml) | ||||||||
| Bussen and Steck | 1997 | Case–control | 56 non-pregnant women of reproductive age, euthyroid | TPO-Ab (>100 IU/ml) | 28 non-pregnant women with RM (≥3 MC) | 28 multigravidae without previous MC or endocrine dysfunction | TPO-Ab, Tg-Ab (combined) | Matching: no |
| Tg-Ab (>100 IU/ml) | ||||||||
| Iijima et al. | 1997 | Cohort | 1179 healthy euthyroid pregnant women with singleton gestations | Tg-Ab, antimicrosomal Ab (titer: >1:100) | 125 antimicrosomal Ab positive, 32 Tg-Ab positive | 951 women negative for antimicorsomal Ab or Tg-Ab | MC (pregnancy loss after existence of gestational sac or fetus), PTD (<37 weeks), stillbirth, PIH (>140/90 mmHg), birthweight, malformations, SGA (<1.2 SD), LGA (>1.5 SD) | Matching: no |
| Kim et al. | 1998 | Cohort | 79 euthyroid women with tubal factor or unexplained infertility who underwent IVF | TPO-Ab and Tg-Ab (>100 U/ml) | 28 euthyroid positive for TPO-Ab and/or Tg-Ab | 51 euthyroid without TPO-Ab and/or Tg | MC | Matching: no |
| Haddow et al. | 1999 | Cohort | 25 216 pregnant women | Thyrotropin (>99.7‰ of the mean values of all women or between 98–99.6‰) | 47 pregnant women >99.7‰ | 124 matched pregnant women with normal values | Neuropsychological development tests in their children | Matching: yes |
| 15 women between 98 and 99.6‰ of the mean value of all women | ||||||||
| Kutteh et al. | 1999a | Case–control/cohort | 1073 Non-pregnant euthyroid healthy women and women undergoing IVF | TSH (0.45–4.5 μIU/ml) | 873 infertile women undergoing ART | 200 healthy reproductive-aged parous controls | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (>40 IU/ml) | Pregnancy rate, delivery rate | |||||||
| Tg-Ab (>67 IU/ml) | 143 TPO/Tg-Ab positive women undergoing ART | 143 TPO/Tg-Ab negative women undergoing ART | Matching: yes | |||||
| Kutteh et al. | 1999b | Case–control | 1588 women of reproductive age | TSH 0.45–4.5 5 μIU/ml | 700 women with RM (≥2 MC)) | 200 healthy females | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (0–65 IU/ml) and Tg-Ab (0–120 IU/ml) | 688 women with a history of infertility who were undergoing ART (described above) | |||||||
| Mavragani et al. | 1999 | Case–control | 80 women Ro/SSA positive or with autoimmune disorder Ro/SSA negative | TPO-Ab (>60 IU/ml) | 40 anti Ro-SSA positive women | 40 age-matched women with an autoimmune disorder age-matched anti Ro/SSA negative | TPO-Ab, Tg-Ab | Matching: yes |
| Tg-Ab (>50 IU/ml) | ||||||||
| Muller et al. | 1999 | Cohort | 173 Non-pregnant women eligible for IVF | TSH (0.2–4.5 μIU/ml) | 25 women TPO-Ab positive, euthyroid | 148 women TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| TPO-Ab (>80 U/ml) | Outcome of pregnancy after IVF | |||||||
| Allan et al. | 2000 | Cohort | 9403 pregnant women at gestational age of 15–18 weeks | TSH (<6 mU/l) | 9194 pregnant women with normal TSH | 172 pregnancies in women with increased TSH | PA, PIH, CS, fetal death, PND | Matching: yes |
| Dendrinos et al. | 2000 | Case–control | 45 non-pregnant women, at least 6 months after last pregnancy | TSH (0.5–4.6 µIU/ml) | 30 RM patients (≥3 consecutive losses) | 15 healthy parous controls | TPO-Ab, Tg-Ab | Matching: yes |
| TPO/Tg-Ab(<2 IU/ml) | ||||||||
| Mecacci et al. | 2000 | Case–control | 138 non-pregnant women with RM, PND or PE | TSH (0.2–4.0 μU/l) | 29 RM patients (≥2 losses <12 weeks, unexplained) | 69 healthy non-pregnant women | TPO-Ab and/or Tg-Ab | Matching: yes |
| fT4 (7.8–18.4 pg/ml) | ||||||||
| TPO-Ab (>10 IU/ml) | ||||||||
| Tg-Ab (>50 IU/ml) | ||||||||
| Rushworth et al. | 2000 | Cohort | 870 non-pregnant women with RM (≥3 consecutive losses) | TSH (0.5–5.0 mIU/l) | 24 women, euthyroid positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 81 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | MC (first trimester) | Matching: no |
| Tg-Ab (titer >1:100) antimicrosomal Ab (titer 1:400) | ||||||||
| Sakaihara et al. | 2000 | Cohort | 4022 pregnant women, euthyroid | TSH (0.2–6.0 mU/l), | 131 women positive for Tg-Ab and/or antimicrosomal Ab | 1030 women negative for Tg-Ab and/or antimicrosomal Ab | PPTD (hyperthyroidism, hypothyroidism 1 and 3 months post-partum) | Matching: no |
| fT4 (7.7–29.0 pmol/l) | ||||||||
| Tg-Ab, antimicrosomal Ab (100-fold dilutions) | ||||||||
| Klein et al. | 2001 | Case–control | Offspring of 164 mothers who were tested for thyroid function during pregnancy | TSH at 17 weeks of gestation | 8-year-old offspring of 20 untreated hypothyroid mothers (TSH 88–99.85th ‰) and 20 (TSH >99.85th ‰) | 8-year-old offspring of 124 control mothers | IQ | Matching: yes |
| (TSH <98th ‰) | ||||||||
| Poppe et al. | 2002 | Case–control | 538 non-pregnant women | TSH (0.27–4.2 mIU/l) | 438 infertility patients, 197 female (endometriosis, tubal disease and ovarian dysfunction), 168 male factor, 73 idiopathic) | 100 parous controls | TPO-Ab | Matching: yes |
| fT 4(9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Sieiro et al. | 2004 | Cohort | 534 pregnant women | TSH (0.4–3.8 mU/l) | 29 TPO-Ab positive women, euthyroid | 505 TPO-Ab negative women, euthyroid | MC (spontaneous ending of pregnancy before 20 weeks) | Matching: no |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (0–40 U/l) | ||||||||
| Stagnaro-Green et al. | 2005 | Case–control | 953 women who had delivered | TSH (0.35–2.99 mIU/l) | 124 women with preterm delivery | 124 women who delivered at term | TPO-Ab, Tg-Ab | Matching: yes |
| TPO-Ab, Tg-Ab (sensitivity assay 0.3 U/ml) | ||||||||
| Ghafoor et al. | 2006 | Prospective Cohort | 1500 euthyroid pregnant women | TPO-Ab (>100 U/ml) | 168 TPO-Ab positive women | 1332 TPO-Ab negative women | MC, prematurity | Matching: yes |
| Negro et al. | 2006 | Case–control | 1074 Pregnant women, euthyroid | TSH (0.27–4.2 mIU/l) | 58 patients TPO-Ab positive | 869 patients TPO-Ab negative | MC, PIH, PE, PTD, PA | Matching: yes |
| fT4 (9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kIU/l) | ||||||||
| Shoenfeld et al. | 2006 | Case–control | 269 patients with autoimmune disease and/or reproductive failure (recurrent pregnancy loss, infertility) | TPO-Ab, Tg-Ab (>2 SD than the mean level in control group) | 109 RM ((≥3 MC in first and second trimester) | 120 healthy females, euthyroid | TPO-Ab, Tg-Ab | Matching: yes |
| Abalovich et al. | 2007b | Case–control | 399 women of reproductive age | TSH (0.5–5 mIU/l) | 244 women consulting on infertility (>1 year, 94% known causes) | 155 healthy women with confirmed fertility | TPO-Ab, subclinical hypothyroidism | Matching: no |
| T4 (4.5–12 μg/dl) | ||||||||
| TPO-Ab (>35 IU/ml) | ||||||||
| Casey et al. | 2007 | Cohort | 17 298 singleton pregnant women | TSH (0.08–3.0 mU/l) | 598 with subclinical hypothyroidism (normal TSH, fT4 <0.86 ng/dl) | 16 011 normal TSH, fT4 euthyroid | PIH, PE, GDM, PA, PTD (36 weeks or less), CS, fetal malformation, low Apgar scores (<3 after 5 min), admission NICU, RDS, PND, birthweight | Matching: yes |
| fT4 (lower limit 0.86 ng/dl) | ||||||||
| Mamede da et al. | 2007 | Cohort | 98 pregnant women | TSH (0.4–3.8 μm/l), | 10 TPO-Ab positive women, euthyroid | 88 TPO-Ab negative women, euthyroid | PPTD (hypo/hyperthyroidism) | Matching: yes |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (>40 U/l) | ||||||||
| Negro et al. | 2007a | Cohort | 423 women undergoing IVF | TSH (0.27–4.2 mU/l) | 49 TPO-Ab positive, euthyroid | 374 TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| fT4 (12–33.5 pmol/l) | Outcome of pregnancy after IVF | |||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Negro et al. | 2007b | RCT | 2143 euthyroid pregnant women | TSH (0.27–4.2 mIU/l) | 84 euthyroid pregnant women TPO-Ab positive | 85 euthyroid pregnant women TPO-Ab negative | PPTD (hyperthyroidism, hypothyroidism) permanent hypothyroidism (12 months post-partum), MC | Randomization: computer generated |
| fT4 (9.3–18.0 ng/l, 12–33.5 pmol/l) | ||||||||
| TPO-Ab (0–100 kIU/l) | Concealed: yes | |||||||
| Blinding: yes | ||||||||
| ITT: yes | ||||||||
| Bellver et al. | 2008 | Case–control | 119 women undergoing ART | TSH (0.25–5 μUI/ml) | 30 RM patients | 32 Oocyte donors | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (0.73–2.2 ng/dl) | 26 Implantation failure (IF) | 31 UI | ||||||
| TPO-Ab (>25 IU/ml) | 26 IF+31 Unexplained infertility (UI) (57 subfertile couples) | 32 oocyte | ||||||
| Tg-Ab (>100 IU/ml) | ||||||||
| Cleary-Goldman et al. | 2008 | Cohort | 10 990 women with singleton pregnancies | TSH and T4 (between 2.5 and 97.5th ‰) | 240 subclinical hypothyroidism (TSH >97.5th and fT4 between 2.5 and 97.5th ‰) | 10 518 euthyroid state (TSH and T4 between 2.5th and 97.5th ‰) | MC (<24 weeks), PIH (>140/90 mmHg), PE, GDM, placenta previa, PA, preterm onset on labor (<37weeks), PPROM (<37weeks), PTD (<37 weeks), LBW (<2500 gr), macrosomia (>4000 gr), PND | Matching: yes |
| TPO-Ab (>35 IU/ml) | ||||||||
| Tg-Ab (>40 IU/ml) | ||||||||
| Iravani et al. | 2008 | Case–control | 910 euthyroid, non-pregnant women | TSH (0.4–4.4 mIU/l) | 641 women with RM (≥3) | 269 non-pregnant healthy euthyroid controls, age matched | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (4.5–10.9 μg/dl) | ||||||||
| TPO-Ab (>40 IU/ml) | ||||||||
| Tg-Ab (>125 IU/ml) | ||||||||
| Kilic et al. | 2008 | Prospective cohort | 69 (54 eligible) patients with unexplained infertility undergoing IVF | TSH (0.005–100.0 μgIU/Ml) | 23 TPO-Ab or Tg-Ab positive patients, euthyroid | 31 TPO-Ab or Tg-Ab negative patients, euthyroid | IVF outcome | Matching: yes |
| Ft4 (0.023–7.77 ng/dl) | ||||||||
| TPO-Ab (>34 IU/ml) | ||||||||
| Tg-Ab (>115 IU/ml) | ||||||||
| Montaner et al. | 2008 | Cohort | 619 pregnant women without former DM | TPO-Ab (>12 IU/ml) | 62 TPO-Ab positive, euthyroid | 557 TPO-Ab negative, euthyroid | GDM | Matching: yes |
| Rao et al. | 2008 | Case–control | 333 non-pregnant women | TSH (0.3–5.0 μIU/ml) | 163 RM patients | 170 health controls, age matched | Hypothyroidism | Matching: yes |
| T4 (5.0–12.5 µg/dl) | ||||||||
| Benhadi et al. | 2009 | Cohort | 2497 Women with singleton pregnancy without overt hypo-hyperthyroidism | TSH (0.34–5.60 mU/l) | 146 TPO-Ab positive | 2351 TPO-Ab negative | MC (<22 weeks), fetal death (22 weeks-delivery) or neonatal death (0–7 days after delivery) | Matching: yes |
| fT4 (7.5–21.2 pmol/l) | ||||||||
| TPO-Ab (0–80 kU/l) | ||||||||
| Sezer et al. | 2009 | Cohort | 128 euthyroid healthy pregnant women with 1 MC | TSH (0.3–4.5 mIU/l) | 28 TPO-Ab or TG-Ab positive | 100 TPO-Ab or Tg-Ab negative | MC | Matching: yes |
| fT4 (10–22 pmol/l) | ||||||||
| TPO-Ab (<34 IU/ml) | ||||||||
| Tg-Ab (<115 IU/ml) | ||||||||
| Li et al. | 2009 | Cohort | 1268 healthy pregnant women without overt thyroid disease | TSH (0.12–4.21 mIU/l) | 18 women with subclinical hypothyroidism | 36 euthyroid controls TPO-Ab negative | CS, mean intelligence scores | Matching: yes |
| fT4 (11.9–24.6 pmol/l) | ||||||||
| TPO-Ab (0–50 IU/ml) | 34 TPO-Ab positive euthyroid | 68 euthyroid controls TPO-Ab negative | ||||||
| Negro et al. | 2010 | RCT | 4562 pregnant women | TSH (>2,5 mIU/l) | 34 hypothyroid from the case finding low risk for thyroid disease group (not universal screening group) | 1769 euthyroid patients with or without Ab | MC, PIH, PE, GDM, PA, CS, RD NICU admission, LBW (<2500 gr), PTD (<37 weeks), Low Apgar Score (<3 after 5 min), PND | Randomization: |
| TPO-Ab (>100 kIU/l) | Computer generated | |||||||
| Concealed: yes | ||||||||
| Blinding: yes | ||||||||
| ITT: no |
| First author . | Year . | Study type . | Participants . | Hormone levels . | Patients . | Controls . | Outcome measure(s) . | Quality features . |
|---|---|---|---|---|---|---|---|---|
| Fung et al. | 1988 | Cohort | 901 pregnant women | Reference range TSH, T4, T3 from control group | 100 women with Tg-Ab/microsomal Ab | 120 women without Ab detectable, euthyroid | PPTD | Matching: yes |
| Tg-Ab and microsomal Ab: positive >+2 SD in control group | Detectable, euthyroid | |||||||
| Feldt-Rasmussen et al. | 1990 | Cohort | 736 healthy euthyroid pregnant women | TSH (0.3–5 mU/l) | 36 women with TPO-Ab and/or Tg-Ab in first trimester | 20 women without TPO-Ab and/or Tg-Ab in first trimester | PPTD (transient or persistent thyroid dysfunction within 1 year after delivery, thyreotoxicosis or hypothyroidism) | Matching: no |
| T4 (56–129 nmol/l) | ||||||||
| T3 (1.6–2.8 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (>100 U/ml) | ||||||||
| Stagnaro-Green et al. | 1990 | Cohort | 552 pregnant euthyroid women | Thyrotropin (TSH) (0.2–5 U/l) | 100 women positive for TPO-Ab and/or Tg-Ab | 392 negative for TPO-Ab and/or Tg-Ab | MC (in first or second trimester) | Matching: no |
| T4 (58–161 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (<0.20 arbitrary units by ELISA) | ||||||||
| Lejeune et al. | 1993 | Prospective cohort | 363 pregnant women, euthyroid, <14 weeks gestational age | TSH not defined | 23 women positive for TPO-Ab and/or Tg-Ab | 340 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| TPO-Ab (>150 U/ml) | ||||||||
| Tg-Ab (>100 U/ml) | ||||||||
| Pratt et al. | 1993 | Prospective cohort | 42 non-pregnant euthyroid women with a history of RM | TSH (0.35–7.0 μIU/ml) | 13 women positive for TPO-Ab and/or Tg-Ab | 29 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| fT4 (0.9–2.1 ng/dl) | ||||||||
| TPO-Ab, Tg-Ab (>5 U/ml) | ||||||||
| Singh et al. | 1995 | Cohort | 487 infertile patients conceiving after ART (artificial reproductive techniques) (IVF) | TSH not defined | 106 women positive for TPO-Ab and/or Tg-Ab, euthyroid | 381 women negative for TPO-Ab and/or Tg-Ab, euthyroid | MC (not defined) | Matching: no |
| TPO-Ab and Tg-Ab (sample antibody index 0–3.8) | ||||||||
| Geva et al. | 1996 | Prospective cohort | 78 patients with mechanical (tubal obstruction) or unexplained infertility in IVF program | Tg-Ab (>1:400) | 16 women positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 55 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | Pregnancy after IVF, MC after IVF | Matching: no |
| Antimicrosomal Ab (>1:1600) | ||||||||
| Roberts et al. | 1996 | Case–control | 33 pregnant women | TSH (0–5 mU/l) | 11 pregnant women with RM (≥3 MC) | 11 healthy women in the first trimester of an ongoing pregnancy | TPO-Ab, Tg-Ab | Matching: no |
| T4 (55–144 nmol/l) | ||||||||
| TPO-Ab (0–1 U/ml) | 11 pregnant women with 1 MC | |||||||
| Tg-Ab (0–8 U/ml) | ||||||||
| Bussen and Steck | 1997 | Case–control | 56 non-pregnant women of reproductive age, euthyroid | TPO-Ab (>100 IU/ml) | 28 non-pregnant women with RM (≥3 MC) | 28 multigravidae without previous MC or endocrine dysfunction | TPO-Ab, Tg-Ab (combined) | Matching: no |
| Tg-Ab (>100 IU/ml) | ||||||||
| Iijima et al. | 1997 | Cohort | 1179 healthy euthyroid pregnant women with singleton gestations | Tg-Ab, antimicrosomal Ab (titer: >1:100) | 125 antimicrosomal Ab positive, 32 Tg-Ab positive | 951 women negative for antimicorsomal Ab or Tg-Ab | MC (pregnancy loss after existence of gestational sac or fetus), PTD (<37 weeks), stillbirth, PIH (>140/90 mmHg), birthweight, malformations, SGA (<1.2 SD), LGA (>1.5 SD) | Matching: no |
| Kim et al. | 1998 | Cohort | 79 euthyroid women with tubal factor or unexplained infertility who underwent IVF | TPO-Ab and Tg-Ab (>100 U/ml) | 28 euthyroid positive for TPO-Ab and/or Tg-Ab | 51 euthyroid without TPO-Ab and/or Tg | MC | Matching: no |
| Haddow et al. | 1999 | Cohort | 25 216 pregnant women | Thyrotropin (>99.7‰ of the mean values of all women or between 98–99.6‰) | 47 pregnant women >99.7‰ | 124 matched pregnant women with normal values | Neuropsychological development tests in their children | Matching: yes |
| 15 women between 98 and 99.6‰ of the mean value of all women | ||||||||
| Kutteh et al. | 1999a | Case–control/cohort | 1073 Non-pregnant euthyroid healthy women and women undergoing IVF | TSH (0.45–4.5 μIU/ml) | 873 infertile women undergoing ART | 200 healthy reproductive-aged parous controls | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (>40 IU/ml) | Pregnancy rate, delivery rate | |||||||
| Tg-Ab (>67 IU/ml) | 143 TPO/Tg-Ab positive women undergoing ART | 143 TPO/Tg-Ab negative women undergoing ART | Matching: yes | |||||
| Kutteh et al. | 1999b | Case–control | 1588 women of reproductive age | TSH 0.45–4.5 5 μIU/ml | 700 women with RM (≥2 MC)) | 200 healthy females | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (0–65 IU/ml) and Tg-Ab (0–120 IU/ml) | 688 women with a history of infertility who were undergoing ART (described above) | |||||||
| Mavragani et al. | 1999 | Case–control | 80 women Ro/SSA positive or with autoimmune disorder Ro/SSA negative | TPO-Ab (>60 IU/ml) | 40 anti Ro-SSA positive women | 40 age-matched women with an autoimmune disorder age-matched anti Ro/SSA negative | TPO-Ab, Tg-Ab | Matching: yes |
| Tg-Ab (>50 IU/ml) | ||||||||
| Muller et al. | 1999 | Cohort | 173 Non-pregnant women eligible for IVF | TSH (0.2–4.5 μIU/ml) | 25 women TPO-Ab positive, euthyroid | 148 women TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| TPO-Ab (>80 U/ml) | Outcome of pregnancy after IVF | |||||||
| Allan et al. | 2000 | Cohort | 9403 pregnant women at gestational age of 15–18 weeks | TSH (<6 mU/l) | 9194 pregnant women with normal TSH | 172 pregnancies in women with increased TSH | PA, PIH, CS, fetal death, PND | Matching: yes |
| Dendrinos et al. | 2000 | Case–control | 45 non-pregnant women, at least 6 months after last pregnancy | TSH (0.5–4.6 µIU/ml) | 30 RM patients (≥3 consecutive losses) | 15 healthy parous controls | TPO-Ab, Tg-Ab | Matching: yes |
| TPO/Tg-Ab(<2 IU/ml) | ||||||||
| Mecacci et al. | 2000 | Case–control | 138 non-pregnant women with RM, PND or PE | TSH (0.2–4.0 μU/l) | 29 RM patients (≥2 losses <12 weeks, unexplained) | 69 healthy non-pregnant women | TPO-Ab and/or Tg-Ab | Matching: yes |
| fT4 (7.8–18.4 pg/ml) | ||||||||
| TPO-Ab (>10 IU/ml) | ||||||||
| Tg-Ab (>50 IU/ml) | ||||||||
| Rushworth et al. | 2000 | Cohort | 870 non-pregnant women with RM (≥3 consecutive losses) | TSH (0.5–5.0 mIU/l) | 24 women, euthyroid positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 81 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | MC (first trimester) | Matching: no |
| Tg-Ab (titer >1:100) antimicrosomal Ab (titer 1:400) | ||||||||
| Sakaihara et al. | 2000 | Cohort | 4022 pregnant women, euthyroid | TSH (0.2–6.0 mU/l), | 131 women positive for Tg-Ab and/or antimicrosomal Ab | 1030 women negative for Tg-Ab and/or antimicrosomal Ab | PPTD (hyperthyroidism, hypothyroidism 1 and 3 months post-partum) | Matching: no |
| fT4 (7.7–29.0 pmol/l) | ||||||||
| Tg-Ab, antimicrosomal Ab (100-fold dilutions) | ||||||||
| Klein et al. | 2001 | Case–control | Offspring of 164 mothers who were tested for thyroid function during pregnancy | TSH at 17 weeks of gestation | 8-year-old offspring of 20 untreated hypothyroid mothers (TSH 88–99.85th ‰) and 20 (TSH >99.85th ‰) | 8-year-old offspring of 124 control mothers | IQ | Matching: yes |
| (TSH <98th ‰) | ||||||||
| Poppe et al. | 2002 | Case–control | 538 non-pregnant women | TSH (0.27–4.2 mIU/l) | 438 infertility patients, 197 female (endometriosis, tubal disease and ovarian dysfunction), 168 male factor, 73 idiopathic) | 100 parous controls | TPO-Ab | Matching: yes |
| fT 4(9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Sieiro et al. | 2004 | Cohort | 534 pregnant women | TSH (0.4–3.8 mU/l) | 29 TPO-Ab positive women, euthyroid | 505 TPO-Ab negative women, euthyroid | MC (spontaneous ending of pregnancy before 20 weeks) | Matching: no |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (0–40 U/l) | ||||||||
| Stagnaro-Green et al. | 2005 | Case–control | 953 women who had delivered | TSH (0.35–2.99 mIU/l) | 124 women with preterm delivery | 124 women who delivered at term | TPO-Ab, Tg-Ab | Matching: yes |
| TPO-Ab, Tg-Ab (sensitivity assay 0.3 U/ml) | ||||||||
| Ghafoor et al. | 2006 | Prospective Cohort | 1500 euthyroid pregnant women | TPO-Ab (>100 U/ml) | 168 TPO-Ab positive women | 1332 TPO-Ab negative women | MC, prematurity | Matching: yes |
| Negro et al. | 2006 | Case–control | 1074 Pregnant women, euthyroid | TSH (0.27–4.2 mIU/l) | 58 patients TPO-Ab positive | 869 patients TPO-Ab negative | MC, PIH, PE, PTD, PA | Matching: yes |
| fT4 (9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kIU/l) | ||||||||
| Shoenfeld et al. | 2006 | Case–control | 269 patients with autoimmune disease and/or reproductive failure (recurrent pregnancy loss, infertility) | TPO-Ab, Tg-Ab (>2 SD than the mean level in control group) | 109 RM ((≥3 MC in first and second trimester) | 120 healthy females, euthyroid | TPO-Ab, Tg-Ab | Matching: yes |
| Abalovich et al. | 2007b | Case–control | 399 women of reproductive age | TSH (0.5–5 mIU/l) | 244 women consulting on infertility (>1 year, 94% known causes) | 155 healthy women with confirmed fertility | TPO-Ab, subclinical hypothyroidism | Matching: no |
| T4 (4.5–12 μg/dl) | ||||||||
| TPO-Ab (>35 IU/ml) | ||||||||
| Casey et al. | 2007 | Cohort | 17 298 singleton pregnant women | TSH (0.08–3.0 mU/l) | 598 with subclinical hypothyroidism (normal TSH, fT4 <0.86 ng/dl) | 16 011 normal TSH, fT4 euthyroid | PIH, PE, GDM, PA, PTD (36 weeks or less), CS, fetal malformation, low Apgar scores (<3 after 5 min), admission NICU, RDS, PND, birthweight | Matching: yes |
| fT4 (lower limit 0.86 ng/dl) | ||||||||
| Mamede da et al. | 2007 | Cohort | 98 pregnant women | TSH (0.4–3.8 μm/l), | 10 TPO-Ab positive women, euthyroid | 88 TPO-Ab negative women, euthyroid | PPTD (hypo/hyperthyroidism) | Matching: yes |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (>40 U/l) | ||||||||
| Negro et al. | 2007a | Cohort | 423 women undergoing IVF | TSH (0.27–4.2 mU/l) | 49 TPO-Ab positive, euthyroid | 374 TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| fT4 (12–33.5 pmol/l) | Outcome of pregnancy after IVF | |||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Negro et al. | 2007b | RCT | 2143 euthyroid pregnant women | TSH (0.27–4.2 mIU/l) | 84 euthyroid pregnant women TPO-Ab positive | 85 euthyroid pregnant women TPO-Ab negative | PPTD (hyperthyroidism, hypothyroidism) permanent hypothyroidism (12 months post-partum), MC | Randomization: computer generated |
| fT4 (9.3–18.0 ng/l, 12–33.5 pmol/l) | ||||||||
| TPO-Ab (0–100 kIU/l) | Concealed: yes | |||||||
| Blinding: yes | ||||||||
| ITT: yes | ||||||||
| Bellver et al. | 2008 | Case–control | 119 women undergoing ART | TSH (0.25–5 μUI/ml) | 30 RM patients | 32 Oocyte donors | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (0.73–2.2 ng/dl) | 26 Implantation failure (IF) | 31 UI | ||||||
| TPO-Ab (>25 IU/ml) | 26 IF+31 Unexplained infertility (UI) (57 subfertile couples) | 32 oocyte | ||||||
| Tg-Ab (>100 IU/ml) | ||||||||
| Cleary-Goldman et al. | 2008 | Cohort | 10 990 women with singleton pregnancies | TSH and T4 (between 2.5 and 97.5th ‰) | 240 subclinical hypothyroidism (TSH >97.5th and fT4 between 2.5 and 97.5th ‰) | 10 518 euthyroid state (TSH and T4 between 2.5th and 97.5th ‰) | MC (<24 weeks), PIH (>140/90 mmHg), PE, GDM, placenta previa, PA, preterm onset on labor (<37weeks), PPROM (<37weeks), PTD (<37 weeks), LBW (<2500 gr), macrosomia (>4000 gr), PND | Matching: yes |
| TPO-Ab (>35 IU/ml) | ||||||||
| Tg-Ab (>40 IU/ml) | ||||||||
| Iravani et al. | 2008 | Case–control | 910 euthyroid, non-pregnant women | TSH (0.4–4.4 mIU/l) | 641 women with RM (≥3) | 269 non-pregnant healthy euthyroid controls, age matched | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (4.5–10.9 μg/dl) | ||||||||
| TPO-Ab (>40 IU/ml) | ||||||||
| Tg-Ab (>125 IU/ml) | ||||||||
| Kilic et al. | 2008 | Prospective cohort | 69 (54 eligible) patients with unexplained infertility undergoing IVF | TSH (0.005–100.0 μgIU/Ml) | 23 TPO-Ab or Tg-Ab positive patients, euthyroid | 31 TPO-Ab or Tg-Ab negative patients, euthyroid | IVF outcome | Matching: yes |
| Ft4 (0.023–7.77 ng/dl) | ||||||||
| TPO-Ab (>34 IU/ml) | ||||||||
| Tg-Ab (>115 IU/ml) | ||||||||
| Montaner et al. | 2008 | Cohort | 619 pregnant women without former DM | TPO-Ab (>12 IU/ml) | 62 TPO-Ab positive, euthyroid | 557 TPO-Ab negative, euthyroid | GDM | Matching: yes |
| Rao et al. | 2008 | Case–control | 333 non-pregnant women | TSH (0.3–5.0 μIU/ml) | 163 RM patients | 170 health controls, age matched | Hypothyroidism | Matching: yes |
| T4 (5.0–12.5 µg/dl) | ||||||||
| Benhadi et al. | 2009 | Cohort | 2497 Women with singleton pregnancy without overt hypo-hyperthyroidism | TSH (0.34–5.60 mU/l) | 146 TPO-Ab positive | 2351 TPO-Ab negative | MC (<22 weeks), fetal death (22 weeks-delivery) or neonatal death (0–7 days after delivery) | Matching: yes |
| fT4 (7.5–21.2 pmol/l) | ||||||||
| TPO-Ab (0–80 kU/l) | ||||||||
| Sezer et al. | 2009 | Cohort | 128 euthyroid healthy pregnant women with 1 MC | TSH (0.3–4.5 mIU/l) | 28 TPO-Ab or TG-Ab positive | 100 TPO-Ab or Tg-Ab negative | MC | Matching: yes |
| fT4 (10–22 pmol/l) | ||||||||
| TPO-Ab (<34 IU/ml) | ||||||||
| Tg-Ab (<115 IU/ml) | ||||||||
| Li et al. | 2009 | Cohort | 1268 healthy pregnant women without overt thyroid disease | TSH (0.12–4.21 mIU/l) | 18 women with subclinical hypothyroidism | 36 euthyroid controls TPO-Ab negative | CS, mean intelligence scores | Matching: yes |
| fT4 (11.9–24.6 pmol/l) | ||||||||
| TPO-Ab (0–50 IU/ml) | 34 TPO-Ab positive euthyroid | 68 euthyroid controls TPO-Ab negative | ||||||
| Negro et al. | 2010 | RCT | 4562 pregnant women | TSH (>2,5 mIU/l) | 34 hypothyroid from the case finding low risk for thyroid disease group (not universal screening group) | 1769 euthyroid patients with or without Ab | MC, PIH, PE, GDM, PA, CS, RD NICU admission, LBW (<2500 gr), PTD (<37 weeks), Low Apgar Score (<3 after 5 min), PND | Randomization: |
| TPO-Ab (>100 kIU/l) | Computer generated | |||||||
| Concealed: yes | ||||||||
| Blinding: yes | ||||||||
| ITT: no |
Ab, antibody; ART, artificial reproductive techniques; CS, cesarean section; GDM, gestational diabetes mellitus; IF, infertility; LGA, large for gestational age; MC, miscarriage; NICU, neonatal intensive care unit; PA, placental abruption; PE, pre-eclampsia; PIH, pregnancy induced hypertension; PND, perinatal death; PPTD, post-partum thyroid disease; PTD, preterm delivery; RDS, respiratory distress syndrome; RM, recurrent miscarriage; SGA, small for gestational age; ITT, intention to treat.
Notes:
All studies have an adequate sample size (n> 10).
Two RCTs were included (Negro et al., 2007b, 2010) All other studies were level II studies: cohort and case–control studies.
Microsomal antibodies are the previous nomenclature for TPO antibodies.
Characteristics and quality features of the 43 studies included in the systematic review of clinical impact of thyroid disorders before conception and in the first trimester of pregnancy.
| First author . | Year . | Study type . | Participants . | Hormone levels . | Patients . | Controls . | Outcome measure(s) . | Quality features . |
|---|---|---|---|---|---|---|---|---|
| Fung et al. | 1988 | Cohort | 901 pregnant women | Reference range TSH, T4, T3 from control group | 100 women with Tg-Ab/microsomal Ab | 120 women without Ab detectable, euthyroid | PPTD | Matching: yes |
| Tg-Ab and microsomal Ab: positive >+2 SD in control group | Detectable, euthyroid | |||||||
| Feldt-Rasmussen et al. | 1990 | Cohort | 736 healthy euthyroid pregnant women | TSH (0.3–5 mU/l) | 36 women with TPO-Ab and/or Tg-Ab in first trimester | 20 women without TPO-Ab and/or Tg-Ab in first trimester | PPTD (transient or persistent thyroid dysfunction within 1 year after delivery, thyreotoxicosis or hypothyroidism) | Matching: no |
| T4 (56–129 nmol/l) | ||||||||
| T3 (1.6–2.8 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (>100 U/ml) | ||||||||
| Stagnaro-Green et al. | 1990 | Cohort | 552 pregnant euthyroid women | Thyrotropin (TSH) (0.2–5 U/l) | 100 women positive for TPO-Ab and/or Tg-Ab | 392 negative for TPO-Ab and/or Tg-Ab | MC (in first or second trimester) | Matching: no |
| T4 (58–161 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (<0.20 arbitrary units by ELISA) | ||||||||
| Lejeune et al. | 1993 | Prospective cohort | 363 pregnant women, euthyroid, <14 weeks gestational age | TSH not defined | 23 women positive for TPO-Ab and/or Tg-Ab | 340 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| TPO-Ab (>150 U/ml) | ||||||||
| Tg-Ab (>100 U/ml) | ||||||||
| Pratt et al. | 1993 | Prospective cohort | 42 non-pregnant euthyroid women with a history of RM | TSH (0.35–7.0 μIU/ml) | 13 women positive for TPO-Ab and/or Tg-Ab | 29 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| fT4 (0.9–2.1 ng/dl) | ||||||||
| TPO-Ab, Tg-Ab (>5 U/ml) | ||||||||
| Singh et al. | 1995 | Cohort | 487 infertile patients conceiving after ART (artificial reproductive techniques) (IVF) | TSH not defined | 106 women positive for TPO-Ab and/or Tg-Ab, euthyroid | 381 women negative for TPO-Ab and/or Tg-Ab, euthyroid | MC (not defined) | Matching: no |
| TPO-Ab and Tg-Ab (sample antibody index 0–3.8) | ||||||||
| Geva et al. | 1996 | Prospective cohort | 78 patients with mechanical (tubal obstruction) or unexplained infertility in IVF program | Tg-Ab (>1:400) | 16 women positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 55 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | Pregnancy after IVF, MC after IVF | Matching: no |
| Antimicrosomal Ab (>1:1600) | ||||||||
| Roberts et al. | 1996 | Case–control | 33 pregnant women | TSH (0–5 mU/l) | 11 pregnant women with RM (≥3 MC) | 11 healthy women in the first trimester of an ongoing pregnancy | TPO-Ab, Tg-Ab | Matching: no |
| T4 (55–144 nmol/l) | ||||||||
| TPO-Ab (0–1 U/ml) | 11 pregnant women with 1 MC | |||||||
| Tg-Ab (0–8 U/ml) | ||||||||
| Bussen and Steck | 1997 | Case–control | 56 non-pregnant women of reproductive age, euthyroid | TPO-Ab (>100 IU/ml) | 28 non-pregnant women with RM (≥3 MC) | 28 multigravidae without previous MC or endocrine dysfunction | TPO-Ab, Tg-Ab (combined) | Matching: no |
| Tg-Ab (>100 IU/ml) | ||||||||
| Iijima et al. | 1997 | Cohort | 1179 healthy euthyroid pregnant women with singleton gestations | Tg-Ab, antimicrosomal Ab (titer: >1:100) | 125 antimicrosomal Ab positive, 32 Tg-Ab positive | 951 women negative for antimicorsomal Ab or Tg-Ab | MC (pregnancy loss after existence of gestational sac or fetus), PTD (<37 weeks), stillbirth, PIH (>140/90 mmHg), birthweight, malformations, SGA (<1.2 SD), LGA (>1.5 SD) | Matching: no |
| Kim et al. | 1998 | Cohort | 79 euthyroid women with tubal factor or unexplained infertility who underwent IVF | TPO-Ab and Tg-Ab (>100 U/ml) | 28 euthyroid positive for TPO-Ab and/or Tg-Ab | 51 euthyroid without TPO-Ab and/or Tg | MC | Matching: no |
| Haddow et al. | 1999 | Cohort | 25 216 pregnant women | Thyrotropin (>99.7‰ of the mean values of all women or between 98–99.6‰) | 47 pregnant women >99.7‰ | 124 matched pregnant women with normal values | Neuropsychological development tests in their children | Matching: yes |
| 15 women between 98 and 99.6‰ of the mean value of all women | ||||||||
| Kutteh et al. | 1999a | Case–control/cohort | 1073 Non-pregnant euthyroid healthy women and women undergoing IVF | TSH (0.45–4.5 μIU/ml) | 873 infertile women undergoing ART | 200 healthy reproductive-aged parous controls | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (>40 IU/ml) | Pregnancy rate, delivery rate | |||||||
| Tg-Ab (>67 IU/ml) | 143 TPO/Tg-Ab positive women undergoing ART | 143 TPO/Tg-Ab negative women undergoing ART | Matching: yes | |||||
| Kutteh et al. | 1999b | Case–control | 1588 women of reproductive age | TSH 0.45–4.5 5 μIU/ml | 700 women with RM (≥2 MC)) | 200 healthy females | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (0–65 IU/ml) and Tg-Ab (0–120 IU/ml) | 688 women with a history of infertility who were undergoing ART (described above) | |||||||
| Mavragani et al. | 1999 | Case–control | 80 women Ro/SSA positive or with autoimmune disorder Ro/SSA negative | TPO-Ab (>60 IU/ml) | 40 anti Ro-SSA positive women | 40 age-matched women with an autoimmune disorder age-matched anti Ro/SSA negative | TPO-Ab, Tg-Ab | Matching: yes |
| Tg-Ab (>50 IU/ml) | ||||||||
| Muller et al. | 1999 | Cohort | 173 Non-pregnant women eligible for IVF | TSH (0.2–4.5 μIU/ml) | 25 women TPO-Ab positive, euthyroid | 148 women TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| TPO-Ab (>80 U/ml) | Outcome of pregnancy after IVF | |||||||
| Allan et al. | 2000 | Cohort | 9403 pregnant women at gestational age of 15–18 weeks | TSH (<6 mU/l) | 9194 pregnant women with normal TSH | 172 pregnancies in women with increased TSH | PA, PIH, CS, fetal death, PND | Matching: yes |
| Dendrinos et al. | 2000 | Case–control | 45 non-pregnant women, at least 6 months after last pregnancy | TSH (0.5–4.6 µIU/ml) | 30 RM patients (≥3 consecutive losses) | 15 healthy parous controls | TPO-Ab, Tg-Ab | Matching: yes |
| TPO/Tg-Ab(<2 IU/ml) | ||||||||
| Mecacci et al. | 2000 | Case–control | 138 non-pregnant women with RM, PND or PE | TSH (0.2–4.0 μU/l) | 29 RM patients (≥2 losses <12 weeks, unexplained) | 69 healthy non-pregnant women | TPO-Ab and/or Tg-Ab | Matching: yes |
| fT4 (7.8–18.4 pg/ml) | ||||||||
| TPO-Ab (>10 IU/ml) | ||||||||
| Tg-Ab (>50 IU/ml) | ||||||||
| Rushworth et al. | 2000 | Cohort | 870 non-pregnant women with RM (≥3 consecutive losses) | TSH (0.5–5.0 mIU/l) | 24 women, euthyroid positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 81 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | MC (first trimester) | Matching: no |
| Tg-Ab (titer >1:100) antimicrosomal Ab (titer 1:400) | ||||||||
| Sakaihara et al. | 2000 | Cohort | 4022 pregnant women, euthyroid | TSH (0.2–6.0 mU/l), | 131 women positive for Tg-Ab and/or antimicrosomal Ab | 1030 women negative for Tg-Ab and/or antimicrosomal Ab | PPTD (hyperthyroidism, hypothyroidism 1 and 3 months post-partum) | Matching: no |
| fT4 (7.7–29.0 pmol/l) | ||||||||
| Tg-Ab, antimicrosomal Ab (100-fold dilutions) | ||||||||
| Klein et al. | 2001 | Case–control | Offspring of 164 mothers who were tested for thyroid function during pregnancy | TSH at 17 weeks of gestation | 8-year-old offspring of 20 untreated hypothyroid mothers (TSH 88–99.85th ‰) and 20 (TSH >99.85th ‰) | 8-year-old offspring of 124 control mothers | IQ | Matching: yes |
| (TSH <98th ‰) | ||||||||
| Poppe et al. | 2002 | Case–control | 538 non-pregnant women | TSH (0.27–4.2 mIU/l) | 438 infertility patients, 197 female (endometriosis, tubal disease and ovarian dysfunction), 168 male factor, 73 idiopathic) | 100 parous controls | TPO-Ab | Matching: yes |
| fT 4(9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Sieiro et al. | 2004 | Cohort | 534 pregnant women | TSH (0.4–3.8 mU/l) | 29 TPO-Ab positive women, euthyroid | 505 TPO-Ab negative women, euthyroid | MC (spontaneous ending of pregnancy before 20 weeks) | Matching: no |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (0–40 U/l) | ||||||||
| Stagnaro-Green et al. | 2005 | Case–control | 953 women who had delivered | TSH (0.35–2.99 mIU/l) | 124 women with preterm delivery | 124 women who delivered at term | TPO-Ab, Tg-Ab | Matching: yes |
| TPO-Ab, Tg-Ab (sensitivity assay 0.3 U/ml) | ||||||||
| Ghafoor et al. | 2006 | Prospective Cohort | 1500 euthyroid pregnant women | TPO-Ab (>100 U/ml) | 168 TPO-Ab positive women | 1332 TPO-Ab negative women | MC, prematurity | Matching: yes |
| Negro et al. | 2006 | Case–control | 1074 Pregnant women, euthyroid | TSH (0.27–4.2 mIU/l) | 58 patients TPO-Ab positive | 869 patients TPO-Ab negative | MC, PIH, PE, PTD, PA | Matching: yes |
| fT4 (9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kIU/l) | ||||||||
| Shoenfeld et al. | 2006 | Case–control | 269 patients with autoimmune disease and/or reproductive failure (recurrent pregnancy loss, infertility) | TPO-Ab, Tg-Ab (>2 SD than the mean level in control group) | 109 RM ((≥3 MC in first and second trimester) | 120 healthy females, euthyroid | TPO-Ab, Tg-Ab | Matching: yes |
| Abalovich et al. | 2007b | Case–control | 399 women of reproductive age | TSH (0.5–5 mIU/l) | 244 women consulting on infertility (>1 year, 94% known causes) | 155 healthy women with confirmed fertility | TPO-Ab, subclinical hypothyroidism | Matching: no |
| T4 (4.5–12 μg/dl) | ||||||||
| TPO-Ab (>35 IU/ml) | ||||||||
| Casey et al. | 2007 | Cohort | 17 298 singleton pregnant women | TSH (0.08–3.0 mU/l) | 598 with subclinical hypothyroidism (normal TSH, fT4 <0.86 ng/dl) | 16 011 normal TSH, fT4 euthyroid | PIH, PE, GDM, PA, PTD (36 weeks or less), CS, fetal malformation, low Apgar scores (<3 after 5 min), admission NICU, RDS, PND, birthweight | Matching: yes |
| fT4 (lower limit 0.86 ng/dl) | ||||||||
| Mamede da et al. | 2007 | Cohort | 98 pregnant women | TSH (0.4–3.8 μm/l), | 10 TPO-Ab positive women, euthyroid | 88 TPO-Ab negative women, euthyroid | PPTD (hypo/hyperthyroidism) | Matching: yes |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (>40 U/l) | ||||||||
| Negro et al. | 2007a | Cohort | 423 women undergoing IVF | TSH (0.27–4.2 mU/l) | 49 TPO-Ab positive, euthyroid | 374 TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| fT4 (12–33.5 pmol/l) | Outcome of pregnancy after IVF | |||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Negro et al. | 2007b | RCT | 2143 euthyroid pregnant women | TSH (0.27–4.2 mIU/l) | 84 euthyroid pregnant women TPO-Ab positive | 85 euthyroid pregnant women TPO-Ab negative | PPTD (hyperthyroidism, hypothyroidism) permanent hypothyroidism (12 months post-partum), MC | Randomization: computer generated |
| fT4 (9.3–18.0 ng/l, 12–33.5 pmol/l) | ||||||||
| TPO-Ab (0–100 kIU/l) | Concealed: yes | |||||||
| Blinding: yes | ||||||||
| ITT: yes | ||||||||
| Bellver et al. | 2008 | Case–control | 119 women undergoing ART | TSH (0.25–5 μUI/ml) | 30 RM patients | 32 Oocyte donors | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (0.73–2.2 ng/dl) | 26 Implantation failure (IF) | 31 UI | ||||||
| TPO-Ab (>25 IU/ml) | 26 IF+31 Unexplained infertility (UI) (57 subfertile couples) | 32 oocyte | ||||||
| Tg-Ab (>100 IU/ml) | ||||||||
| Cleary-Goldman et al. | 2008 | Cohort | 10 990 women with singleton pregnancies | TSH and T4 (between 2.5 and 97.5th ‰) | 240 subclinical hypothyroidism (TSH >97.5th and fT4 between 2.5 and 97.5th ‰) | 10 518 euthyroid state (TSH and T4 between 2.5th and 97.5th ‰) | MC (<24 weeks), PIH (>140/90 mmHg), PE, GDM, placenta previa, PA, preterm onset on labor (<37weeks), PPROM (<37weeks), PTD (<37 weeks), LBW (<2500 gr), macrosomia (>4000 gr), PND | Matching: yes |
| TPO-Ab (>35 IU/ml) | ||||||||
| Tg-Ab (>40 IU/ml) | ||||||||
| Iravani et al. | 2008 | Case–control | 910 euthyroid, non-pregnant women | TSH (0.4–4.4 mIU/l) | 641 women with RM (≥3) | 269 non-pregnant healthy euthyroid controls, age matched | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (4.5–10.9 μg/dl) | ||||||||
| TPO-Ab (>40 IU/ml) | ||||||||
| Tg-Ab (>125 IU/ml) | ||||||||
| Kilic et al. | 2008 | Prospective cohort | 69 (54 eligible) patients with unexplained infertility undergoing IVF | TSH (0.005–100.0 μgIU/Ml) | 23 TPO-Ab or Tg-Ab positive patients, euthyroid | 31 TPO-Ab or Tg-Ab negative patients, euthyroid | IVF outcome | Matching: yes |
| Ft4 (0.023–7.77 ng/dl) | ||||||||
| TPO-Ab (>34 IU/ml) | ||||||||
| Tg-Ab (>115 IU/ml) | ||||||||
| Montaner et al. | 2008 | Cohort | 619 pregnant women without former DM | TPO-Ab (>12 IU/ml) | 62 TPO-Ab positive, euthyroid | 557 TPO-Ab negative, euthyroid | GDM | Matching: yes |
| Rao et al. | 2008 | Case–control | 333 non-pregnant women | TSH (0.3–5.0 μIU/ml) | 163 RM patients | 170 health controls, age matched | Hypothyroidism | Matching: yes |
| T4 (5.0–12.5 µg/dl) | ||||||||
| Benhadi et al. | 2009 | Cohort | 2497 Women with singleton pregnancy without overt hypo-hyperthyroidism | TSH (0.34–5.60 mU/l) | 146 TPO-Ab positive | 2351 TPO-Ab negative | MC (<22 weeks), fetal death (22 weeks-delivery) or neonatal death (0–7 days after delivery) | Matching: yes |
| fT4 (7.5–21.2 pmol/l) | ||||||||
| TPO-Ab (0–80 kU/l) | ||||||||
| Sezer et al. | 2009 | Cohort | 128 euthyroid healthy pregnant women with 1 MC | TSH (0.3–4.5 mIU/l) | 28 TPO-Ab or TG-Ab positive | 100 TPO-Ab or Tg-Ab negative | MC | Matching: yes |
| fT4 (10–22 pmol/l) | ||||||||
| TPO-Ab (<34 IU/ml) | ||||||||
| Tg-Ab (<115 IU/ml) | ||||||||
| Li et al. | 2009 | Cohort | 1268 healthy pregnant women without overt thyroid disease | TSH (0.12–4.21 mIU/l) | 18 women with subclinical hypothyroidism | 36 euthyroid controls TPO-Ab negative | CS, mean intelligence scores | Matching: yes |
| fT4 (11.9–24.6 pmol/l) | ||||||||
| TPO-Ab (0–50 IU/ml) | 34 TPO-Ab positive euthyroid | 68 euthyroid controls TPO-Ab negative | ||||||
| Negro et al. | 2010 | RCT | 4562 pregnant women | TSH (>2,5 mIU/l) | 34 hypothyroid from the case finding low risk for thyroid disease group (not universal screening group) | 1769 euthyroid patients with or without Ab | MC, PIH, PE, GDM, PA, CS, RD NICU admission, LBW (<2500 gr), PTD (<37 weeks), Low Apgar Score (<3 after 5 min), PND | Randomization: |
| TPO-Ab (>100 kIU/l) | Computer generated | |||||||
| Concealed: yes | ||||||||
| Blinding: yes | ||||||||
| ITT: no |
| First author . | Year . | Study type . | Participants . | Hormone levels . | Patients . | Controls . | Outcome measure(s) . | Quality features . |
|---|---|---|---|---|---|---|---|---|
| Fung et al. | 1988 | Cohort | 901 pregnant women | Reference range TSH, T4, T3 from control group | 100 women with Tg-Ab/microsomal Ab | 120 women without Ab detectable, euthyroid | PPTD | Matching: yes |
| Tg-Ab and microsomal Ab: positive >+2 SD in control group | Detectable, euthyroid | |||||||
| Feldt-Rasmussen et al. | 1990 | Cohort | 736 healthy euthyroid pregnant women | TSH (0.3–5 mU/l) | 36 women with TPO-Ab and/or Tg-Ab in first trimester | 20 women without TPO-Ab and/or Tg-Ab in first trimester | PPTD (transient or persistent thyroid dysfunction within 1 year after delivery, thyreotoxicosis or hypothyroidism) | Matching: no |
| T4 (56–129 nmol/l) | ||||||||
| T3 (1.6–2.8 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (>100 U/ml) | ||||||||
| Stagnaro-Green et al. | 1990 | Cohort | 552 pregnant euthyroid women | Thyrotropin (TSH) (0.2–5 U/l) | 100 women positive for TPO-Ab and/or Tg-Ab | 392 negative for TPO-Ab and/or Tg-Ab | MC (in first or second trimester) | Matching: no |
| T4 (58–161 nmol/l) | ||||||||
| TPO-Ab and/or Tg-Ab (<0.20 arbitrary units by ELISA) | ||||||||
| Lejeune et al. | 1993 | Prospective cohort | 363 pregnant women, euthyroid, <14 weeks gestational age | TSH not defined | 23 women positive for TPO-Ab and/or Tg-Ab | 340 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| TPO-Ab (>150 U/ml) | ||||||||
| Tg-Ab (>100 U/ml) | ||||||||
| Pratt et al. | 1993 | Prospective cohort | 42 non-pregnant euthyroid women with a history of RM | TSH (0.35–7.0 μIU/ml) | 13 women positive for TPO-Ab and/or Tg-Ab | 29 women negative for TPO-Ab and/or Tg-Ab | MC in the next pregnancy | Matching: yes |
| fT4 (0.9–2.1 ng/dl) | ||||||||
| TPO-Ab, Tg-Ab (>5 U/ml) | ||||||||
| Singh et al. | 1995 | Cohort | 487 infertile patients conceiving after ART (artificial reproductive techniques) (IVF) | TSH not defined | 106 women positive for TPO-Ab and/or Tg-Ab, euthyroid | 381 women negative for TPO-Ab and/or Tg-Ab, euthyroid | MC (not defined) | Matching: no |
| TPO-Ab and Tg-Ab (sample antibody index 0–3.8) | ||||||||
| Geva et al. | 1996 | Prospective cohort | 78 patients with mechanical (tubal obstruction) or unexplained infertility in IVF program | Tg-Ab (>1:400) | 16 women positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 55 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | Pregnancy after IVF, MC after IVF | Matching: no |
| Antimicrosomal Ab (>1:1600) | ||||||||
| Roberts et al. | 1996 | Case–control | 33 pregnant women | TSH (0–5 mU/l) | 11 pregnant women with RM (≥3 MC) | 11 healthy women in the first trimester of an ongoing pregnancy | TPO-Ab, Tg-Ab | Matching: no |
| T4 (55–144 nmol/l) | ||||||||
| TPO-Ab (0–1 U/ml) | 11 pregnant women with 1 MC | |||||||
| Tg-Ab (0–8 U/ml) | ||||||||
| Bussen and Steck | 1997 | Case–control | 56 non-pregnant women of reproductive age, euthyroid | TPO-Ab (>100 IU/ml) | 28 non-pregnant women with RM (≥3 MC) | 28 multigravidae without previous MC or endocrine dysfunction | TPO-Ab, Tg-Ab (combined) | Matching: no |
| Tg-Ab (>100 IU/ml) | ||||||||
| Iijima et al. | 1997 | Cohort | 1179 healthy euthyroid pregnant women with singleton gestations | Tg-Ab, antimicrosomal Ab (titer: >1:100) | 125 antimicrosomal Ab positive, 32 Tg-Ab positive | 951 women negative for antimicorsomal Ab or Tg-Ab | MC (pregnancy loss after existence of gestational sac or fetus), PTD (<37 weeks), stillbirth, PIH (>140/90 mmHg), birthweight, malformations, SGA (<1.2 SD), LGA (>1.5 SD) | Matching: no |
| Kim et al. | 1998 | Cohort | 79 euthyroid women with tubal factor or unexplained infertility who underwent IVF | TPO-Ab and Tg-Ab (>100 U/ml) | 28 euthyroid positive for TPO-Ab and/or Tg-Ab | 51 euthyroid without TPO-Ab and/or Tg | MC | Matching: no |
| Haddow et al. | 1999 | Cohort | 25 216 pregnant women | Thyrotropin (>99.7‰ of the mean values of all women or between 98–99.6‰) | 47 pregnant women >99.7‰ | 124 matched pregnant women with normal values | Neuropsychological development tests in their children | Matching: yes |
| 15 women between 98 and 99.6‰ of the mean value of all women | ||||||||
| Kutteh et al. | 1999a | Case–control/cohort | 1073 Non-pregnant euthyroid healthy women and women undergoing IVF | TSH (0.45–4.5 μIU/ml) | 873 infertile women undergoing ART | 200 healthy reproductive-aged parous controls | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (>40 IU/ml) | Pregnancy rate, delivery rate | |||||||
| Tg-Ab (>67 IU/ml) | 143 TPO/Tg-Ab positive women undergoing ART | 143 TPO/Tg-Ab negative women undergoing ART | Matching: yes | |||||
| Kutteh et al. | 1999b | Case–control | 1588 women of reproductive age | TSH 0.45–4.5 5 μIU/ml | 700 women with RM (≥2 MC)) | 200 healthy females | TPO-Ab, Tg-Ab | Matching: no |
| TPO-Ab (0–65 IU/ml) and Tg-Ab (0–120 IU/ml) | 688 women with a history of infertility who were undergoing ART (described above) | |||||||
| Mavragani et al. | 1999 | Case–control | 80 women Ro/SSA positive or with autoimmune disorder Ro/SSA negative | TPO-Ab (>60 IU/ml) | 40 anti Ro-SSA positive women | 40 age-matched women with an autoimmune disorder age-matched anti Ro/SSA negative | TPO-Ab, Tg-Ab | Matching: yes |
| Tg-Ab (>50 IU/ml) | ||||||||
| Muller et al. | 1999 | Cohort | 173 Non-pregnant women eligible for IVF | TSH (0.2–4.5 μIU/ml) | 25 women TPO-Ab positive, euthyroid | 148 women TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| TPO-Ab (>80 U/ml) | Outcome of pregnancy after IVF | |||||||
| Allan et al. | 2000 | Cohort | 9403 pregnant women at gestational age of 15–18 weeks | TSH (<6 mU/l) | 9194 pregnant women with normal TSH | 172 pregnancies in women with increased TSH | PA, PIH, CS, fetal death, PND | Matching: yes |
| Dendrinos et al. | 2000 | Case–control | 45 non-pregnant women, at least 6 months after last pregnancy | TSH (0.5–4.6 µIU/ml) | 30 RM patients (≥3 consecutive losses) | 15 healthy parous controls | TPO-Ab, Tg-Ab | Matching: yes |
| TPO/Tg-Ab(<2 IU/ml) | ||||||||
| Mecacci et al. | 2000 | Case–control | 138 non-pregnant women with RM, PND or PE | TSH (0.2–4.0 μU/l) | 29 RM patients (≥2 losses <12 weeks, unexplained) | 69 healthy non-pregnant women | TPO-Ab and/or Tg-Ab | Matching: yes |
| fT4 (7.8–18.4 pg/ml) | ||||||||
| TPO-Ab (>10 IU/ml) | ||||||||
| Tg-Ab (>50 IU/ml) | ||||||||
| Rushworth et al. | 2000 | Cohort | 870 non-pregnant women with RM (≥3 consecutive losses) | TSH (0.5–5.0 mIU/l) | 24 women, euthyroid positive for Tg-Ab and/or antimicrosomal Ab, euthyroid | 81 women negative for Tg-Ab and/or antimicrosomal Ab, euthyroid | MC (first trimester) | Matching: no |
| Tg-Ab (titer >1:100) antimicrosomal Ab (titer 1:400) | ||||||||
| Sakaihara et al. | 2000 | Cohort | 4022 pregnant women, euthyroid | TSH (0.2–6.0 mU/l), | 131 women positive for Tg-Ab and/or antimicrosomal Ab | 1030 women negative for Tg-Ab and/or antimicrosomal Ab | PPTD (hyperthyroidism, hypothyroidism 1 and 3 months post-partum) | Matching: no |
| fT4 (7.7–29.0 pmol/l) | ||||||||
| Tg-Ab, antimicrosomal Ab (100-fold dilutions) | ||||||||
| Klein et al. | 2001 | Case–control | Offspring of 164 mothers who were tested for thyroid function during pregnancy | TSH at 17 weeks of gestation | 8-year-old offspring of 20 untreated hypothyroid mothers (TSH 88–99.85th ‰) and 20 (TSH >99.85th ‰) | 8-year-old offspring of 124 control mothers | IQ | Matching: yes |
| (TSH <98th ‰) | ||||||||
| Poppe et al. | 2002 | Case–control | 538 non-pregnant women | TSH (0.27–4.2 mIU/l) | 438 infertility patients, 197 female (endometriosis, tubal disease and ovarian dysfunction), 168 male factor, 73 idiopathic) | 100 parous controls | TPO-Ab | Matching: yes |
| fT 4(9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Sieiro et al. | 2004 | Cohort | 534 pregnant women | TSH (0.4–3.8 mU/l) | 29 TPO-Ab positive women, euthyroid | 505 TPO-Ab negative women, euthyroid | MC (spontaneous ending of pregnancy before 20 weeks) | Matching: no |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (0–40 U/l) | ||||||||
| Stagnaro-Green et al. | 2005 | Case–control | 953 women who had delivered | TSH (0.35–2.99 mIU/l) | 124 women with preterm delivery | 124 women who delivered at term | TPO-Ab, Tg-Ab | Matching: yes |
| TPO-Ab, Tg-Ab (sensitivity assay 0.3 U/ml) | ||||||||
| Ghafoor et al. | 2006 | Prospective Cohort | 1500 euthyroid pregnant women | TPO-Ab (>100 U/ml) | 168 TPO-Ab positive women | 1332 TPO-Ab negative women | MC, prematurity | Matching: yes |
| Negro et al. | 2006 | Case–control | 1074 Pregnant women, euthyroid | TSH (0.27–4.2 mIU/l) | 58 patients TPO-Ab positive | 869 patients TPO-Ab negative | MC, PIH, PE, PTD, PA | Matching: yes |
| fT4 (9.3–18.0 ng/l) | ||||||||
| TPO-Ab (>100 kIU/l) | ||||||||
| Shoenfeld et al. | 2006 | Case–control | 269 patients with autoimmune disease and/or reproductive failure (recurrent pregnancy loss, infertility) | TPO-Ab, Tg-Ab (>2 SD than the mean level in control group) | 109 RM ((≥3 MC in first and second trimester) | 120 healthy females, euthyroid | TPO-Ab, Tg-Ab | Matching: yes |
| Abalovich et al. | 2007b | Case–control | 399 women of reproductive age | TSH (0.5–5 mIU/l) | 244 women consulting on infertility (>1 year, 94% known causes) | 155 healthy women with confirmed fertility | TPO-Ab, subclinical hypothyroidism | Matching: no |
| T4 (4.5–12 μg/dl) | ||||||||
| TPO-Ab (>35 IU/ml) | ||||||||
| Casey et al. | 2007 | Cohort | 17 298 singleton pregnant women | TSH (0.08–3.0 mU/l) | 598 with subclinical hypothyroidism (normal TSH, fT4 <0.86 ng/dl) | 16 011 normal TSH, fT4 euthyroid | PIH, PE, GDM, PA, PTD (36 weeks or less), CS, fetal malformation, low Apgar scores (<3 after 5 min), admission NICU, RDS, PND, birthweight | Matching: yes |
| fT4 (lower limit 0.86 ng/dl) | ||||||||
| Mamede da et al. | 2007 | Cohort | 98 pregnant women | TSH (0.4–3.8 μm/l), | 10 TPO-Ab positive women, euthyroid | 88 TPO-Ab negative women, euthyroid | PPTD (hypo/hyperthyroidism) | Matching: yes |
| fT4 (0.8–2.0 ng/dl) | ||||||||
| TPO-Ab (>40 U/l) | ||||||||
| Negro et al. | 2007a | Cohort | 423 women undergoing IVF | TSH (0.27–4.2 mU/l) | 49 TPO-Ab positive, euthyroid | 374 TPO-Ab negative, euthyroid | Pregnancy after IVF | Matching: yes |
| fT4 (12–33.5 pmol/l) | Outcome of pregnancy after IVF | |||||||
| TPO-Ab (>100 kU/l) | ||||||||
| Negro et al. | 2007b | RCT | 2143 euthyroid pregnant women | TSH (0.27–4.2 mIU/l) | 84 euthyroid pregnant women TPO-Ab positive | 85 euthyroid pregnant women TPO-Ab negative | PPTD (hyperthyroidism, hypothyroidism) permanent hypothyroidism (12 months post-partum), MC | Randomization: computer generated |
| fT4 (9.3–18.0 ng/l, 12–33.5 pmol/l) | ||||||||
| TPO-Ab (0–100 kIU/l) | Concealed: yes | |||||||
| Blinding: yes | ||||||||
| ITT: yes | ||||||||
| Bellver et al. | 2008 | Case–control | 119 women undergoing ART | TSH (0.25–5 μUI/ml) | 30 RM patients | 32 Oocyte donors | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (0.73–2.2 ng/dl) | 26 Implantation failure (IF) | 31 UI | ||||||
| TPO-Ab (>25 IU/ml) | 26 IF+31 Unexplained infertility (UI) (57 subfertile couples) | 32 oocyte | ||||||
| Tg-Ab (>100 IU/ml) | ||||||||
| Cleary-Goldman et al. | 2008 | Cohort | 10 990 women with singleton pregnancies | TSH and T4 (between 2.5 and 97.5th ‰) | 240 subclinical hypothyroidism (TSH >97.5th and fT4 between 2.5 and 97.5th ‰) | 10 518 euthyroid state (TSH and T4 between 2.5th and 97.5th ‰) | MC (<24 weeks), PIH (>140/90 mmHg), PE, GDM, placenta previa, PA, preterm onset on labor (<37weeks), PPROM (<37weeks), PTD (<37 weeks), LBW (<2500 gr), macrosomia (>4000 gr), PND | Matching: yes |
| TPO-Ab (>35 IU/ml) | ||||||||
| Tg-Ab (>40 IU/ml) | ||||||||
| Iravani et al. | 2008 | Case–control | 910 euthyroid, non-pregnant women | TSH (0.4–4.4 mIU/l) | 641 women with RM (≥3) | 269 non-pregnant healthy euthyroid controls, age matched | TPO-Ab, Tg-Ab | Matching: yes |
| fT4 (4.5–10.9 μg/dl) | ||||||||
| TPO-Ab (>40 IU/ml) | ||||||||
| Tg-Ab (>125 IU/ml) | ||||||||
| Kilic et al. | 2008 | Prospective cohort | 69 (54 eligible) patients with unexplained infertility undergoing IVF | TSH (0.005–100.0 μgIU/Ml) | 23 TPO-Ab or Tg-Ab positive patients, euthyroid | 31 TPO-Ab or Tg-Ab negative patients, euthyroid | IVF outcome | Matching: yes |
| Ft4 (0.023–7.77 ng/dl) | ||||||||
| TPO-Ab (>34 IU/ml) | ||||||||
| Tg-Ab (>115 IU/ml) | ||||||||
| Montaner et al. | 2008 | Cohort | 619 pregnant women without former DM | TPO-Ab (>12 IU/ml) | 62 TPO-Ab positive, euthyroid | 557 TPO-Ab negative, euthyroid | GDM | Matching: yes |
| Rao et al. | 2008 | Case–control | 333 non-pregnant women | TSH (0.3–5.0 μIU/ml) | 163 RM patients | 170 health controls, age matched | Hypothyroidism | Matching: yes |
| T4 (5.0–12.5 µg/dl) | ||||||||
| Benhadi et al. | 2009 | Cohort | 2497 Women with singleton pregnancy without overt hypo-hyperthyroidism | TSH (0.34–5.60 mU/l) | 146 TPO-Ab positive | 2351 TPO-Ab negative | MC (<22 weeks), fetal death (22 weeks-delivery) or neonatal death (0–7 days after delivery) | Matching: yes |
| fT4 (7.5–21.2 pmol/l) | ||||||||
| TPO-Ab (0–80 kU/l) | ||||||||
| Sezer et al. | 2009 | Cohort | 128 euthyroid healthy pregnant women with 1 MC | TSH (0.3–4.5 mIU/l) | 28 TPO-Ab or TG-Ab positive | 100 TPO-Ab or Tg-Ab negative | MC | Matching: yes |
| fT4 (10–22 pmol/l) | ||||||||
| TPO-Ab (<34 IU/ml) | ||||||||
| Tg-Ab (<115 IU/ml) | ||||||||
| Li et al. | 2009 | Cohort | 1268 healthy pregnant women without overt thyroid disease | TSH (0.12–4.21 mIU/l) | 18 women with subclinical hypothyroidism | 36 euthyroid controls TPO-Ab negative | CS, mean intelligence scores | Matching: yes |
| fT4 (11.9–24.6 pmol/l) | ||||||||
| TPO-Ab (0–50 IU/ml) | 34 TPO-Ab positive euthyroid | 68 euthyroid controls TPO-Ab negative | ||||||
| Negro et al. | 2010 | RCT | 4562 pregnant women | TSH (>2,5 mIU/l) | 34 hypothyroid from the case finding low risk for thyroid disease group (not universal screening group) | 1769 euthyroid patients with or without Ab | MC, PIH, PE, GDM, PA, CS, RD NICU admission, LBW (<2500 gr), PTD (<37 weeks), Low Apgar Score (<3 after 5 min), PND | Randomization: |
| TPO-Ab (>100 kIU/l) | Computer generated | |||||||
| Concealed: yes | ||||||||
| Blinding: yes | ||||||||
| ITT: no |
Ab, antibody; ART, artificial reproductive techniques; CS, cesarean section; GDM, gestational diabetes mellitus; IF, infertility; LGA, large for gestational age; MC, miscarriage; NICU, neonatal intensive care unit; PA, placental abruption; PE, pre-eclampsia; PIH, pregnancy induced hypertension; PND, perinatal death; PPTD, post-partum thyroid disease; PTD, preterm delivery; RDS, respiratory distress syndrome; RM, recurrent miscarriage; SGA, small for gestational age; ITT, intention to treat.
Notes:
All studies have an adequate sample size (n> 10).
Two RCTs were included (Negro et al., 2007b, 2010) All other studies were level II studies: cohort and case–control studies.
Microsomal antibodies are the previous nomenclature for TPO antibodies.
Quality of the studies
The characteristics of the included articles and quality assessment are reported in Table I. Two RCTs were included (Negro et al., 2007b, 2010). All other studies were evidence-level II studies, i.e. cohort and case–control studies.
The effect of thyroid dysfunction and autoimmunity on fertility
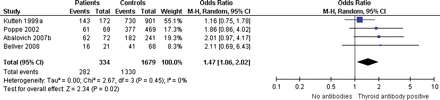
One study reported on the relation between subclinical hypothyroidism and unexplained subfertility in 40 women with subclinical hypothyroidism and 359 controls (Abalovich et al., 2007b). Subclinical hypothyroidism was associated with an increased risk of unexplained subfertility [one study, OR 4.0, 95% confidence interval (CI) 1.7–9.8]. Four studies reported on the relation between thyroid antibodies and unexplained subfertility and could be included in a meta-analysis (Fig. 2) (Kutteh et al., 1999a; Poppe et al., 2002; Abalovich et al., 2007b; Bellver et al., 2008). Summarized data included 334 patients with anti-thyroid antibodies and 1679 controls. In antibody-positive women subfertility occurred more frequently (four studies, OR 1.5, 95% CI 1.1–2.0).
Forest plot of Odds Ratio's and 95% Confidence Interval of pooled studies comparing euthyroid thyroid antibody positive patients with euthyroid antibody negative controls according to the risk of unexplained subfertility.
Seven studies reported on thyroid antibodies in relation to IVF outcome. A total of 1760 women undergoing IVF for different reasons could be included in the meta-analysis, 330 with thyroid antibodies and 1430 controls (Supplementary data, Fig. S1a) (Geva et al., 1996; Kim et al., 1998; Muller et al., 1999; Kutteh et al., 1999a; Negro et al., 2007a; Bellver et al., 2008; Kilic et al., 2008). No association was found between the presence of thyroid antibodies and the clinical pregnancy rates after IVF (seven studies, OR 0.67, 95% CI 0.36–1.4).
The effect of thyroid dysfunction and autoimmunity on early pregnancy
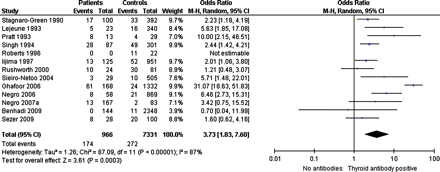
One study reported on the relation between untreated hypothyroidism (determined retrospectively using frozen serum) and miscarriages, showing an increased risk for miscarriage in women with untreated hypothyroidism compared with euthyroid controls (OR 5.78, 95% CI 2.4–14) (Negro et al., 2010). Another study, with 240 patients with subclinical hypothyroidism and 10 518 controls did not show any difference in miscarriage rate (OR 0.69, 95% CI 0.10–5.0) (Cleary-Goldman et al., 2008). Data from 13 studies were included to determine the risk for miscarriage rate in relation to thyroid antibodies (Fig. 3) (Stagnaro-Green et al., 1990; Lejeune et al., 1993; Pratt et al., 1993; Singh et al., 1995; Roberts et al., 1996; Iijima et al., 1997; Rushworth et al., 2000; Sieiro et al., 2004; Ghafoor et al., 2006; Negro et al., 2006, 2007a; Benhadi et al., 2009; Sezer et al., 2009). Data from 12 studies reporting on 966 thyroid antibody positive patients and 7331 controls without thyroid antibodies could be included in the meta-analysis and showed an increased risk of miscarriage in patients with thyroid antibodies (12 studies, OR 3.7, 95% CI 1.8–7.6). Five studies reported on pregnancy outcome after IVF (Supplementary data, Fig. S1b) (Geva et al., 1996; Kim et al., 1998; Muller et al., 1999; Kutteh et al., 1999a; Negro et al., 2007a). In contrast to spontaneous pregnancy, there was no evidence for an increased risk of miscarriage in IVF pregnancies in women with antibodies, compared with women without antibodies (five studies, OR 1.6, 95% CI 0.76–3.5).
Forest plot of Odds Ratio's and 95% Confidence Interval of pooled studies comparing euthyroid thyroid antibody positive patients with euthyroid antibody negative controls according to the risk of miscarriage.
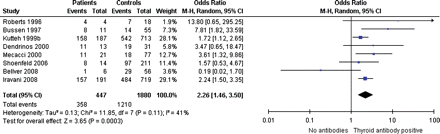
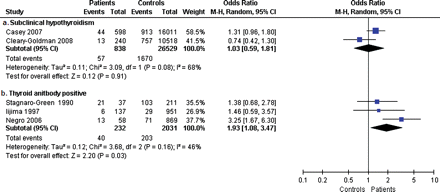
Thyroid function and recurrent miscarriage was studied in one study, with 8 hypothyroid patients and 325 euthyroid controls (Rao et al., 2008). There was no evidence for a difference in risk for recurrent miscarriage between the two groups (one study, OR 7.6, 95% CI 0.92–62). Antibodies in women with recurrent miscarriage were investigated in eight of the included studies, reporting on 460 patients with thyroid antibodies and 1923 antibody-negative controls (Fig. 4) (Roberts et al., 1996; Bussen and Steck, 1997; Kutteh et al., 1999b; Dendrinos et al., 2000; Mecacci et al., 2000; Shoenfeld et al., 2006; Bellver et al., 2008; Iravani et al., 2008). Patients with recurrent miscarriage more often had thyroid antibodies (eight studies, OR 2.3, 95% CI 1.5–3.5). One study could not be included in the meta-analysis, since only the OR was documented and not the exact number of patients in both groups (Mavragani et al., 1999): this study reported an OR for recurrent miscarriage in women with thyroid antibodies of 2.6, with an OR of 2.6 for TPO-Ab and 4.1 for Tg-Ab.
Forest plot of Odds Ratio's and 95% Confidence Interval of pooled studies comparing euthyroid thyroid antibody positive patients with euthyroid antibody negative controls according to the risk of recurrent miscarriage.
The effect of thyroid dysfunction and autoimmunity on late pregnancy complications
The relation between hypothyroidism and gestational diabetes mellitus (GDM) was addressed in one study, reporting no difference between patients and controls (one study, OR 2.3, 95% CI 0.67–7.5) (Negro et al., 2010). Meta-analysis of two studies on subclinical hypothyroidism and GDM resulted in a pooled OR of 1.4, 95% CI 0.64–2.8 (Supplementary data, Fig. S2) (Casey et al., 2007; Cleary-Goldman et al., 2008). The study on antibodies did not report any relationship with GDM (one study, OR 1.2, 95% CI 0.45–3.17) (Montaner et al., 2008).
Pregnancy-induced hypertension was investigated in six studies; one study on hypothyroidism, three studies on subclinical hypothyroidism and two studies on thyroid antibodies. The study among women with hypothyroidism showed no association with pregnancy-induced hypertension (one study, OR 1.8, 95% CI 0.54–6.0) (Negro et al., 2010). Meta-analysis did not show any association between subclinical hypothyroidism and pregnancy-induced hypertension (three studies, OR 1.00, 95% CI 0.79–1.29) (Supplementary data, Fig. S3a) (Allan et al., 2000; Casey et al., 2007; Cleary-Goldman et al., 2008). The pooled OR for thyroid antibodies versus no antibodies and pregnancy-induced hypertension was 1.2 (two studies, 95% CI 0.59–2.6), indicating no difference (Supplementary data, Fig. S3b) (Iijima et al., 1997; Negro et al., 2006).
Hypothyroidism and pre-eclampsia, reported in one study, showed no association (one study, OR 1.52, 95% CI 0.36–6.5) (Negro et al., 2010). Subclinical hypothyroidism compared with normal thyroid function in the studies included in the meta-analysis was significantly related to the occurrence of pre-eclampsia (two studies, OR 1.7, 95% CI 1.1–2.6) (Supplementary data, Fig. S4) (Casey et al., 2007; Cleary-Goldman et al., 2008). Data from the included study on antibodies and pre-eclampsia did not indicate any relation (one study, OR 1.4, 95% CI 0.42–4.8) (Negro et al., 2006).
In one study reporting on placenta praevia the risk in patients with subclinical hypothyroidism when compared with euthyroid patients appeared to be comparable (one study, OR 0.98, 95% CI 0.13–7.1) (Cleary-Goldman et al., 2008).
One study showed an increased risk for placental abruption in hypothyroid patients (one study, OR 10.7, 95% CI 1.2–94) (Negro et al., 2010). In a meta-analysis of two studies reporting on placental abruption, the pooled risk was not significantly increased in subclinical hypothyroid patients (two studies, OR 1.9, 95% CI 0.96–3.7) (Supplementary data, Fig. S5) (Casey et al., 2007; Cleary-Goldman et al., 2008). In 58 euthyroid patients with thyroid antibodies and 869 euthyroid controls without antibodies, no difference in incidence of placental abruption was described (one study, OR 3.8, 95% CI 0.42–35) (Negro et al., 2006).
The relationship between clinical hypothyroidism and preterm onset of labor was reported in one study, not showing a significant difference (one study, OR 2.6, 95% CI 0.91–7.7) (Negro et al., 2010). The study reporting on subclinical hypothyroidism also did not show any difference (one study, OR 0.99, 95% CI 0.57–1.7) (Cleary-Goldman et al., 2008). This latter study also looked at preterm premature rupture of membranes, for which no increased risk was observed (one study, OR 1.6, 95% CI 0.66–4.0). Six studies reported on preterm delivery before 37 weeks of gestational age. The study on hypothyroidism found the risk of preterm birth to be comparable in hypothyroid and in euthyroid patients (one study, OR 2.6, 95% CI 0.99–6.9) (Negro et al., 2010). The meta-analysis on subclinical hypothyroidism and preterm delivery, describing 838 patients and 26 529 controls, showed no difference between the two groups (two studies OR 1.0, 95% CI 0.59–1.8) (Fig. 5a) (Casey et al., 2007; Cleary-Goldman et al., 2008). Thyroid antibodies in the meta-analysis were associated with an increased risk of preterm delivery (three studies OR 1.9, 95% CI 1.1–3.5) (Fig. 5b) (Stagnaro-Green et al., 1990; Iijima et al., 1997; Negro et al., 2006).
Forest plot of Odds Ratio's and 95% Confidence Interval of pooled studies comparing (a) patients with subclinical hypothyroidism with eythyroid controls and (b) euthyroid thyroid antibody positive patients with euthyroid antibody negative controls according to the risk of preterm delivery <37 weeks gestation.
Cesarean delivery rate was not increased in patients with hypothyroidism (one study, OR 1.5, 95% CI 0.68–3.2) (Negro et al., 2010). The meta-analysis on 788 patients with subclinical hypothyroidism and 25 241 healthy euthyroid controls showed a comparable risk for cesarean section (three studies, OR 1.1, 95% CI 0.91–1.3) (Supplementary data, Fig. S6) (Allan et al., 2000; Casey et al., 2007; Li et al., 2009). Thyroid antibodies were not related to cesarean section (one study, OR 1.2, 95% CI 0.51–2.9) (Li et al., 2009).
The effect of thyroid dysfunction and autoimmunity on neonatal outcome
Perinatal mortality was reported in one study, and it was not significantly different in hypothyroid and euthyroid patients (one study, OR 2.4, 95% CI 0.14–42) (Negro et al., 2010). Meta-analysis on three studies, reporting on 1010 subclinical hypothyroid patients and 35 723 euthyroid controls, revealed an increased risk of perinatal mortality in subclinical hypothyroid patients (three studies, OR 2.7, 95% CI 1.6–4.7) (Supplementary data, Fig. S7) (Allan et al., 2000; Casey et al., 2007; Cleary-Goldman et al., 2008). The presence of thyroid antibodies did not increase the risk of perinatal mortality but was reported in only one study (one study, OR 0.49, 95% CI 0.03–8.6) (Benhadi et al., 2009).
Low birthweight defined as a weight of <2500 g at term and high birthweight defined as a weight of >4000 g were reported in three studies (Casey et al., 2007; Cleary-Goldman et al., 2008; Negro et al., 2010). No evidence was found for a relationship between hypothyroidism and low or high birthweight (one study, OR 2.6, 95% CI 0.90–7.6 and OR 2.4, 95% CI 0.81–6.8, respectively) (Negro et al., 2010). In a meta-analysis of 838 patients and 26 259 controls, subclinical hypothyroidism appeared not to be associated with low or high birthweight (two studies, pooled OR 0.93, CI 0.46–1.9 and OR 0.63, CI 0.37–1.1, respectively) (Supplementary data, Fig. S8a and b) (Casey et al., 2007; Cleary-Goldman et al., 2008).
The neonatal outcome was significantly worse in hypothyroid patients than in euthyroid patients as was the risk of admission to the Neonatal Intensive Care Unit (NICU) (one study, OR 4.7, 95% CI 1.9–12) (Negro et al., 2010). This risk was also increased in subclinical hypothyroid patients (one study, OR 1.8, 95% CI 1.2–1.8) (Casey et al., 2007). There was no evidence for an increase in respiratory distress syndrome (RDS) in children born to hypothyroid patients (one study, OR 2.4, 95% CI 0.31–18) (Negro et al., 2010). The same was reported for subclinical hypothyroidism, addressed in one study (one study, OR 1.7, 95% CI 0.98–2.8) (Casey et al., 2007). The risk of an Apgar score <3 after 5 min was comparable in hypothyroid and euthyroid patients (one study, OR 4.8, 95% CI 0.61–39) (Negro et al., 2010). The study on subclinical hypothyroidism and low Apgar score, reporting on 598 patients and 16 011 controls, indicated an increased risk for low Apgar score in patients (one study, OR 2.2, CI 1.1–4.3) (Casey et al., 2007).
Congenital malformations were addressed in two studies, reporting no increased risk in children of patients with subclinical hypothyroidism (one study, OR 0.89, 95% CI 0.39–2.0), nor in children of patients with thyroid autoimmunity (1 study, OR 0.54, 95% CI 0.13–2.3) (Iijima et al., 1997; Casey et al., 2007).
Three studies reported on intelligence score in the offspring of mothers with thyroid dysfunction or autoimmunity (Haddow et al., 1999; Klein et al., 2001; Li et al., 2009). A meta-analysis could not be performed, since outcome measures were reported as intelligence and development scores (continuous variables) and definitions differed between the studies. The study on children of 62 hypothyroid—sometimes treated—women compared with 1245 control children showed an association of hypothyroidism with lower scores on attention and word discrimination (P = 0.01 and P = 0.04, respectively) but no difference in intelligence score (Haddow et al., 1999). The study on subclinical hypothyroidism and TPO-Ab in association with intelligence and motor scores showed decreased intelligence and motor scores in children of women with subclinical hypothyroidism (one study, OR 16, 95% CI 4.7–52 and OR 9.2, 95% CI 2.9–29, respectively, in multivariable analyses) (Li et al., 2009). TPO-Ab were also associated with lower scores on intellectual and motor development (one study, OR 6.7, 95% CI 2.3–19 and OR 8.3, 95% CI 3.3–21, respectively, in multivariable analyses) (Li et al., 2009). The third study showed an inverse correlation between severity of maternal hypothyroidism and intelligence score in the offspring (Klein et al., 2001). TSH >99.85th percentile was associated with lower intelligence scores in the offspring (>1 SD below control mean) compared with women with TSH in the normal range (one study, OR 4.7, 95% CI 1.5–14 in multilevel analyses).
The effect of thyroid autoimmunity on post-natal maternal complications
A relation between thyroid autoimmunity and post-partum thyroid disease in the mother was reported in five studies, which were all included in the meta-analysis (Fung et al., 1988; Feldt-Rasmussen et al., 1990; Sakaihara et al., 2000; Mamede da et al., 2007; Negro et al., 2007b). The meta-analysis, including 305 antibody-positive euthyroid patients and 1342 healthy controls, showed an increased risk of post-partum maternal thyroid disease (five studies, OR 12, 95% CI 5.6–24) (Supplementary data, Fig. S9).
Subgroup analyses of thyroid antibodies
The relationship between the presence of thyroid antibodies and adverse pregnancy outcomes was not different for TPO-Ab compared with Tg-Ab, with the exception of unexplained subfertility. The presence of TPO-Ab was related to unexplained subfertility, while this relationship could not be found for Tg-Ab (four studies, OR 1.5, 95% CI 1.1–2.1 for TPO-Ab, OR 1.1, 95% CI 0.68–1.7 for Tg-Ab) (Supplementary data, Fig. S10) (Kutteh et al., 1999a; Poppe et al., 2002; Abalovich et al., 2007b; Bellver et al., 2008). This difference is most likely explained by the fact that Tg-Ab is present less often than TPO-Ab in cases of autoimmune hypothyroidism and is thus a less sensitive marker for detecting of thyroid autoimmunity.
Discussion
The results of this review provide clear evidence for a relationship between the presence of thyroid antibodies or subclinical hypothyroidism on several pregnancy outcome parameters. Subclinical hypothyroidism, compared with normal thyroid function, was associated with the occurrence of pre-eclampsia and showed an increased risk of perinatal mortality. Meta-analyses on the presence of thyroid antibodies showed an increased risk of unexplained subfertility, miscarriage, recurrent miscarriage, preterm birth and post-partum thyroid disease. In contrast to spontaneous pregnancy, miscarriage after IVF was not associated with the presence of thyroid antibodies.
In the current review, by performing meta-analyses we have found associations that have been unclear or underreported so far. Subclinical hypothyroidism in early pregnancy, compared with normal thyroid function, is associated with the occurrence of pre-eclampsia (OR 1.7, 95% CI 1.1–2.6). We also showed a significantly increased risk of perinatal mortality in women with subclinical hypothyroidism in early pregnancy (OR 2.6, 95% CI 1.6–4.7), a relationship which needs attention, especially in respect of therapeutic options. If, for example, thyroxin supplementation early in pregnancy can reduce perinatal mortality, an important clinical health gain may be achieved. A causal relationship cannot be found between subclinical hypothyroidism and a higher incidence of RDS but the increase in mortality may be related to the increased risk of low Apgar scores and NICU admission in the offspring of these patients. Reasons for mortality are not systematically described in the included studies. Our findings emphasize the importance of normal thyroid function in early pregnancy and even before pregnancy. This review is the first to show the association between thyroid antibodies and unexplained subfertility (OR 1.5, 95% CI 1.1–2.0), while individual studies had only demonstrated a trend so far. This review showed an association between the presence of thyroid antibodies and recurrent miscarriage (OR 2.3, 95% CI 1.5–3.5). Not all individual studies reported showed this association but the meta-analysis was conclusive on this point, showing the additional value of pooled studies compared with individual studies.
Several hypotheses exist on the causality between thyroid autoimmunity and obstetric complications. The first hypothesis is that the autoimmunity increases the risk for hypothyroidism, owing to the chronic lymphocytic thyroiditis that is associated with the presence of TPO-Ab. The thyroid then may fail to respond adequately to the increased demand for thyroid hormone during pregnancy. The second hypothesis is that thyroid antibodies can be considered an expression of autoimmunity in general and adverse obstetric outcome may be caused by other underlying autoimmune diseases e.g. anticardiolipin antibodies. The third hypothesis assumes that age is more important than the presence of antibodies, since the amount of antibodies increases with aging (Sinclair, 2006) and age in itself is a risk factor for obstetric complications (Dulitzki et al., 1998).The third hypothesis seems the least plausible hypothesis for a number of reasons. The majority of the studies included in this review used age-matched control-groups as a reference to their patients. After exclusion of studies not using age-matched control groups, patients with thyroid antibodies still had an increased risk of miscarriage compared with euthyroid patients without antibodies (OR 5.4, 95% CI 1,8–16; Supplementary data, Fig. S10).
Some limitations of this systematic review should be considered. As mentioned, the included articles used different cutoff levels for TSH, T4 and antibodies, and different inclusion criteria for the patients. This should be considered when using the results for clinical application. For instance, antibody positivity was based on the threshold reported in the individual studies and is shown for each study in Table I. TPO-Ab thresholds vary substantially among the studies, but most studies used a more or less generally accepted cutoff value between 50 and 100 kU/l for TPO-Ab. Nevertheless, some degree of population heterogeneity cannot be excluded. Since we used random effect models to perform the meta-analyses of pooled data in case of heterogeneity, and since the majority of data showed a very similar trend, we consider the results to be generally applicable. Individual patient data meta-analysis regarding subclinical hypothyroidism and the different antibodies could be considered in order to calculate a more specific pooled OR for some outcomes and to address the issue of different reference values (Broeze et al., 2009). For some thyroid abnormalities, a limited number of studies on associations with obstetric outcomes were available.
This systematic review does not provide information on the treatment outcome of thyroid dysfunction, as it was not the aim of our study. Nevertheless, the findings in this review are a logical first step prior to any study on the effect of treatment in early pregnancy. Several studies have been performed on the treatment options in thyroid dysfunction and thyroid autoimmunity. The Cochrane review on treatment of (sub)clinical hypothyroidism in pregnancy was limited to women with a single miscarriage and only included three trials studying different treatment options and a meta-analysis could not be performed (Reid et al., 2010). The study on the treatment with levothyroxin in TPO-Ab positive women showed a reduction in preterm birth and a non-significant trend towards reduction in miscarriages (Negro et al., 2006). A reduction in pre-eclampsia was not seen after treatment with levothyroxin (Negro et al., 2006). This is not surprising, since our meta-analysis did not demonstrate an association between thyroid autoimmunity and pregnancy-induced hypertension, and the single article selected about pre-eclampsia did not show a significant relationship between thyroid autoimmunity and pre-eclampsia. In other words, if an association cannot be demonstrated, treatment options can never be expected to work and, even if causality is suspected, treatment remains to be proven. Not all relationships described in our diagnostic review were addressed in the Cochrane review. This remains an important topic for future research.
We conclude that patients with subclinical hypothyroidism are facing an increased risk of pre-eclampsia and the hitherto under-reported risk for perinatal mortality. The presence of thyroid antibodies in euthyroid patients is associated with unexplained subfertility (which was so far unknown), miscarriage, recurrent miscarriage, preterm birth <37 weeks and post-partum thyroid disease. Special attention in pregnant women at risk for, or diagnosed with, thyroid abnormalities and in non-pregnant patients with a history of recurrent miscarriage is desirable. Therapeutic options and thereby the viability of a standardized screening program remain to be established in the near future.
Authors' roles
J.A.L., J.A.M.P., M.G., and P.H.B. all contributed substantially to the conception and design of this review. E.v.d.B. and R.V. screened all titles, abstracts, articles and extracted data for meta-analyses. M.G. and P.H.B. were third reviewer in case consensus could not be reached directly. M.W. supervised the analysis and interpretation of data. E.v.d.B. drafted the article, all other authors critically revised multiple versions of the manuscript. All authors gave their final approval of the version to be published.
Conflict of interest
None declared.
Funding
No external funding was either sought or obtained for this study.
Acknowledgements
We thank H.C. Dyserinck, clinical librarian, for her help with the literature search.