-
PDF
- Split View
-
Views
-
Cite
Cite
A.M. Sanchez, P. Viganò, E. Somigliana, P. Panina-Bordignon, P. Vercellini, M. Candiani, The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary, Human Reproduction Update, Volume 20, Issue 2, March/April 2014, Pages 217–230, https://doi.org/10.1093/humupd/dmt053
Close - Share Icon Share
Abstract
Clinical data suggest that the presence of an ovarian endometrioma may cause per se damage to the surrounding otherwise healthy ovarian tissue. However, the basic research has so far done a limited job in trying to understand the potential detrimental effect of an endometrioma presence in the context of the ovarian physiology. We have reviewed the literature with the aim of characterizing the pathophysiology of the endometrioma focusing mostly on factors and mechanisms potentially affecting the surrounding, otherwise normal, ovarian tissue.
Comprehensive searches of PUBMED were conducted to identify human studies published from 1991 to 2013 in the English language on the cellular and molecular characterization of the various endometrioma components.
An endometrioma contains free iron, reactive oxygen species (ROS), proteolytic enzymes and inflammatory molecules in concentrations from tens to hundreds of times higher than those present in peripheral blood or in other types of benign cysts. The cyst fluid causes substantial changes in the endometriotic cells that it baths from gene expression modifications to genetic mutations The physical barrier between the cyst contents and the normal ovarian tissue is a thin wall composed of the ovarian cortex itself or fibroreactive tissue. ROS potentially permeating the surrounding tissues and proteolytic substances degrading the adjacent areas are likely to cause the substitution of normal ovarian cortical tissue with fibrous tissue in which the cortex-specific stroma is reduced. The fibrosis is associated with smooth muscle metaplasia and followed by follicular loss and intraovarian vascular injury. Follicular density in tissue surrounding the endometriotic cyst was consistently shown to be significantly lower than in healthy ovaries but this pathological change does not appear to be caused by the stretching of surrounding tissues owing to the presence of a cyst.
There is sufficient molecular, histological and morphological evidence, in part deriving from knowledge of the pathophysiology, to support a deleterious effect of the endometrioma on the adjacent ovarian cortical tissue, independent of the mere mechanical stretching owing to its size.
Introduction
Our knowledge of the pathophysiology of endometriomas (or ‘chocolate cysts’) is still limited as it is supported by controversial theories proposed for its pathogenesis. The term ‘chocolate cyst’ was applied to describe an ovarian cyst lined with endometrial tissue histologically and functionally similar to eutopic endometrium and in which the internal fluid is generally thought to arise from the accumulation of menstrual debris deriving from the shedding of the active implants inside the cyst (Sampson, 1921; Donnez et al, 2012). However, independently from its pathogenesis, clinical treatment of the ovarian endometriotic cyst is presently the focus of much interest and controversy due to the overwhelming evidence demonstrating that ovarian reserve is negatively affected following surgical excision of these cysts (Garcia-Velasco and Somigliana, 2009). The rate of spontaneous ovulation is lower in operated ovaries (Candiani, 2005) and serum levels of anti-Mullerian hormone decrease after surgery (Chang et al., 2010). In women selected for IVF, responsiveness to hyperstimulation, the number of developing follicles and the number of oocytes retrieved are reduced in the gonad where an endometrioma has been surgically removed (Loh et al., 1999; Somigliana et al., 2003; Ragni et al., 2005; Gupta et al., 2006; Duru et al., 2007). Upon removal of bilateral endometriomas, IVF outcome is significantly impaired (Somigliana et al., 2008) and a 2% rate of premature menopause following surgery has been reported (Busacca et al., 2006). There is also some evidence that the magnitude of damage in terms of negative impact on IVF parameters is related to the dimension of the excised cyst but data are conflicting (Tang et al., 2013).
The debate is now moving to the causes underlying the reported ovarian damage. Of relevance here is that there are important clinical data supporting the view that the damage may, in part, precede surgery. More specifically, only one-third of spontaneous ovulation occurs in the ovaries affected by an endometrioma (Benaglia et al., 2009). Insights from IVF procedures are equivocal (Almog et al., 2011; Benaglia et al., 2011) but we have recently demonstrated that in women with bilateral endometriomas undergoing an IVF cycle, responsiveness to ovarian hyperstimulation and number of oocytes retrieved were significantly reduced compared with age-matched unexposed control subjects (Benaglia et al., 2013).
Unfortunately, evidence from the basic science perspective is scanty in this context. Indeed, the basic research has so far been inadequate in elucidating the potential detrimental effect of an endometrioma presence in the context of the ovarian physiology. We deemed it important to further explore this topic from a more basic standpoint. Thus, we have reviewed the literature on the pathophysiology of the endometrioma with a specific focus on factors and mechanisms that may potentially affect negatively the surrounding ovarian environment, with the wider aim to provide support to physicians in their clinical strategy. This review represents a basis for further investigation on this surprisingly neglected topic.
Methods
We searched PUBMED for articles published in the English language between September 1991 and January 2013, including clinical and experimental studies, using the following MeSH search terms: ‘endometrioma’ OR ‘ovarian endometriotic cyst’ combined with ‘content’ OR ‘fluid’ OR ‘wall’ OR ‘capsule’ OR ‘follicle’ OR ‘gene expression’ OR ‘vasculature’ OR ‘mutation’ with restriction to the human species. Data were extracted independently by two investigators (A.M.S. and P.V.) who also performed an initial screening of the title and abstract of all articles to exclude citations deemed irrelevant to both observers. Reference lists of the selected articles and from other reviews were also evaluated.
Results
The database search identified 2549 articles. After detailed screening of the titles, we have excluded studies with a strict clinical aim. Then after screening the titles and abstracts, we selected 72 studies that have characterized the endometrioma and its surrounding tissue from histological, cellular and molecular standpoints with a particular focus on factors potentially affecting the normal ovarian tissue.
The molecular milieu inside the cyst
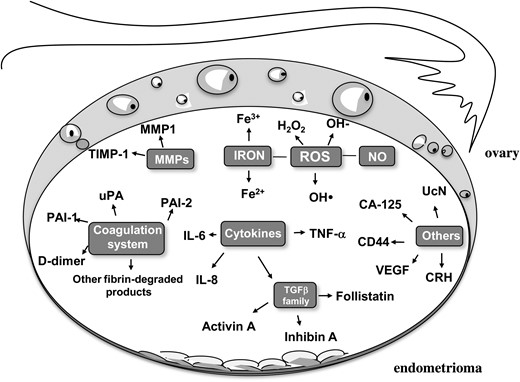
An endometrioma contains a chocolate colored fluid which is thought to contain non-resorbed blood derived from repeated hemorrhages of the endometriotic cells in the cyst during menstrual cycles. As a result of this assumption, molecular analysis of the fluid has been limited. An issue to be clarified is whether the content of an endometriotic cyst could be a potential source of toxicity for the surrounding healthy tissue (Fig. 1). The following paragraphs will consider the compounds inside the cyst with some potential to induce a deleterious effect on the surrounding healthy tissue. The specific mechanisms of toxicity should also be elucidated but data available so far in this regard are very limited. Some hypotheses will be proposed. However, this aspect of pathophysiology would represent a starting point for further investigations aimed at better unraveling this topic.
The ‘toxic’ network of endometrioma fluid. MMP1, Matrix metalloproteinase 1; TIMP-1, metalloproteinase tissue inhibitor 1; uPA, urokinase plasminogen activator; PAI-1, plasminogen activator inhibitor-1; PAI-2, plasminogen activator inhibitor-2; UcN, urocortin; CRH, corticotrophin-releasing hormone; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6; IL-8, interleukin 8; TGF-β, transforming growth factor beta: ROS, reactive oxygen species; H2O2, hydrogen peroxide.
Iron, reactive oxygen species and nitric oxide
Free iron in endometriotic cysts exceeds 100 nmol/l (100 μmol/g) and these levels are much higher compared with the normal serum levels (0.013–0.027 mmol/l) or the contents of non-endometriotic ovarian cysts (0.075 ± 0.081 nmol/l). The concentrations reported in endometriotic cysts are similar to those found in lutein cysts and hemorrhagic corpora lutea (Iizuka et al., 1998), further indicating that the nature of the fluid in endometriotic cysts would actually be old blood. Interestingly, the average concentration of free iron in endometriotic cysts is comparable with the iron concentration reported in hepatic carcinoma tissue, which is a sufficient amount of iron to induce a malignant transformation (Yamaguchi et al., 2008).
Iron present in heme, in iron-sulphur clusters or closely associated with proteins, plays a pivotal role in a variety of physiological cellular functions, such as oxygen transport, energy metabolism, electron transport and modulation of hydrogen peroxide levels. However, non-protein-bound ‘free’ or ‘catalytic’ iron, as the most abundant transition metal in the human body, could mediate the production of reactive oxygen species (ROS) via the Fenton reaction. When stored ferric iron (Fe3+) is reduced to ferrous iron (Fe2+), oxygen-free radicals are generated (Jomova and Valko, 2011).
Free radical species are unstable and highly reactive. They become stable by acquiring electrons from nucleic acids, lipids, proteins, carbohydrates or any nearby molecule, causing a cascade of chain reactions resulting in cellular damage. Free radical species include ROS and reactive nitrogen species (Agarwal et al., 2008).
ROS include superoxide anion, hydrogen peroxide and hydroxyl radicals. Oxidative stress results when the production of ROS exceeds the capacity of cellular antioxidant defences to remove these toxic agents. In fact, ROS are initially regulated by the action of antioxidants, which convert ROS into an inactive state (Van Langendonckt et al., 2002; Vercellini et al., 2011). Importantly, ROS are highly diffusible through different cellular compartments and can be freely diffusible through cell membrane (Yang et al., 2013). It has been reported that ROS are secreted in the interstitial fluids where their favorable role in focal sterilization of the extracellular milieu may be over-shadowed by their destructive influence on otherwise healthy tissue (Bryan et al., 2012).
Specific oxidative stress-related factors have been evaluated in the cyst fluid: (i) lactose dehydrogenase level (used as a marker of tissue damage) measured 7717 ± 4540 IU/l in endometriotic cyst compared with 64.5 ± 102.5 IU/l in other ovarian benign cysts; (ii) concentrations of lipid peroxide were significantly higher in endometriotic cysts (75.7 ± 73.4 nmol/ml) than in other types of cysts (2.9 ± 5.4 nmol/ml) and (iii) 8-hydroxydeoxyguanosine (8-OHdG, a oxidized nucleoside of DNA very frequently detected as a marker of DNA damage) was 0.58 ± 0.61 ng/ml in endometriotic cysts and 0.02 ± 0.05 ng/ml in other types of cyst (Yamaguchi et al., 2008). These high concentrations of ROS in the endometrioma support the idea that these cysts are exposed to greater oxidative stress than other types of cyst. Of further interest, when immortalized endometrial glandular cells and immortalized ovarian surface epithelial cells were treated in vitro with the contents of various cysts at 1:5 dilution, significantly higher ROS production was observed when cells were treated with the fluid of endometriotic cysts compared with either the contents of non-endometriotic cysts or with ferric nitrilotriacetate used as iron in the free form (Yamaguchi et al., 2008). Thus, the fluid in the endometriotic cyst is a strong inducer of oxidative stress in viable cells. ROS-induced oxidative stress alters cellular function by regulating gene expression and protein activity of pro-inflammatory cytokines, adhesion molecules, growth and angiogenic factors as well as by affecting the normal action of important signaling pathways, such as the mitogen-activated protein kinase (MAPK) pathways, the AP-1 transcription factor, the NF-kB pathway and hypoxia-inducible transcription factors (Michiels et al., 2002; Wu, 2006; Defrere et al., 2011).
In addition, reactive nitrogen species, in particular nitric oxide (NO), are present in endometrioma fluid in the 20–40 μM/l concentration range (Shawky et al., 2001), which is similar to the concentration detected in peripheral blood (45.0 ± 1.7 μM/l) (Manau et al., 2000). The analysis of NO has been, however, limited to a small number of patients and no comparison with other types of cysts has been performed. An excess of NO is also toxic since it can damage proteins, carbohydrates, nucleotides and lipids.
The plasminogen activation system
The plasminogen activation system is composed of a set of proteolytic enzymes that play an important role in the coagulation process and in the extracellular matrix (ECM) degradation process. A central step of the plasminogen activation system is the extracellular conversion of the ubiquitous inactive plasminogen to the broad-spectrum serine protease plasmin implicated in numerous pathophysiological processes requiring the remodeling of ECM and basal membranes (Rabbani and Mazar, 2001). The aberrant expression of components of this system in tissues has been associated with the acquisition of novel functional roles including those related to tissue invasion and fibrosis (Samarakoon et al., 2013). In particular, plasminogen activator inhibitor-1 (PAI-1) controls ECM degradation facilitating accumulation of matrix structural elements that promotes a fibrotic response with associated recruitment of inflammatory cells, macrophages and myofibroblasts (Samarakoon et al., 2013). The plasminogen activation system in the endometriotic cyst fluid and in other types of cysts, as well as in ovarian tumors, has been studied by Boss et al. (2002). They found significant differences in concentrations of urokinase plasminogen activator (uPA), PAI-1 and PAI-2 in samples from malignant, borderline and benign ovarian tumors. In agreement with previous findings (Casslen et al., 1991), higher concentrations of uPA have been found in malignant and borderline, compared with benign tumors. However, a novel observation of this study was related to the endometrioma that represents a special entity in this context. PAI-1 concentration in the endometrioma fluid was in the same range as found in malignant tumors [median (25–75th percentilies), 247 (158–601) μg/l and 246 (105–480) μg/l, respectively] but much higher than in benign cysts [37 (9.2–149) μg/l]. UPA and PAI-2 concentrations in endometrioma were >7- and 15-fold higher than those found in cyst fluid from malignant tumors [155 μg/l (99–212) and 199 μg/l (146–267) versus 21.7 μg/l (10.1–43.0) and 12.2 μg/l (4.9–25.0) in endometrioma and cancer, respectively] and >50-fold higher than those in benign cysts, supporting a particular degradative capacity of the endometrioma fluid (Boss et al., 2002).
The potential for damage by these large amounts of coagulation-related products is evident in case of cyst rupture. According to some case reports, the rupture of an ovarian endometriotic cyst can cause the rapid elevation in plasma D dimer level, a specific marker of degradation of cross-linked polymers and of active fibrinolysis (Kline et al., 2005). It is reasonable to consider that the leaked contents of an endometriotic cyst containing fibrin-degraded products (including D-dimer) and the prompt absorption of these products through the peritoneal surface led to an elevation of D-dimer levels in the blood (to >100 μg/ml). It has to be considered that, usually the plasma D-dimer level does not exceed 100 μg/ml (normal plasma levels 0.5 μg/ml), even in cases of acute leukemia or with a major embolism (Fujiwara et al., 2003). Moreover, the rupture of an endometrioma frequently induces elevation in body temperature, serum C reactive protein and white blood count, which are considered as a response to the local acute and devastating inflammatory reaction induced by the fluid of the cyst on the peritoneal tissues as they come into direct contact. These findings suggest that the fluid of the cyst contains biologically active molecules able to provoke a strong inflammatory reaction in normal tissue.
In line with this, the instillation of human endometrioma fluid in the peritoneal cavity of rabbits can cause adhesion formation (Smith et al., 2007), known to be initiated by an inflammatory reaction followed by an imbalance between tissue PA and PAI-1 resulting in decreased fibrinolytic activity and increased fibrin exudate (Somigliana et al., 2012).
Total matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-containing enzymes able to degrade ECM components such as collagen and proteoglycans and, as such, they are involved in both physiologic functions, such as tissue remodeling and menstruation, and in many diseases. An increase in the expression of MMP-1 (Kokorine et al., 1997) and MMP-9 (Chung et al., 2001) has been reported in ovarian endometriotic tissue. Mizumoto et al. (2002) measured total MMP-1 and tissue inhibitor of metalloproteinase-1 (TIMP-1) levels in endometrioma fluid of 20 patients. Levels of both MMP-1 and TIMP-1 varied with the menstrual cycle being higher during menstruation. However, total MMP-1 levels were dramatically higher (menstrual phase: 706 ± 417 ng/ml; proliferative phase 136 ± 60 ng/ml; secretory phase 61 ± 15 ng/ml) than those detected normally in plasma (1.3 ± 1.1 ng/ml) (Li et al., 2010) or serum (Thrailkill et al., 2009). TIMP-1 was detected at levels lower than that normally detected in serum or in the peritoneal fluid of women with endometriosis (Sharpe-Timms et al., 1998; Chegini et al., 2001). Though it is uncertain whether the detected MMP-1 maintains its activity in the fluid of endometrioma, this enzyme may play a role in the modeling of the surrounding connective tissue as MMPs normally do in the healthy endometrium.
Cytokines
Various cytokines with different regulatory effects on growth and differentiation and which are representative of an underlying inflammatory process have been found in endometriotic cyst fluid (Velasco et al., 2010). Levels of interleukin (IL)-8 were shown to be higher (3470.3 ± 312 pg/ml) compared with those in follicular cysts (49.2 ± 9.6 pg/ml) and serous cysts (833.3 ± 320 pg/ml) but similar to those found in ovarian carcinoma (Fasciani et al., 2001). Significantly higher concentrations of IL-8 in the endometrioma compared with other types of cysts (mostly serous) but slightly lower than those found in ovarian carcinoma were detected by Darai et al. (2003). According to these authors, tumor necrosis factor α and IL-6 levels were similar in endometriomas and other benign ovarian cysts. Conversely, Velasco et al. (2010) reported higher levels of IL-6 and IL-8 in the endometrioma cyst compared with other types of cysts. ILs have the ability to mediate ovarian leukocyte chemotaxis and to promote influx of granulocytes in and around the follicles in order to favor follicular rupture (Polec et al., 2009; Bryan et al., 2012). It is unknown whether the inflammatory component of the endometrioma may intensify these phenomena but a dysregulated production of pro-inflammatory cytokines is thought to adversely impact follicular function (Bedaiwy et al., 2007) and ovarian reserve (Opoien et al., 2013).
The activin system
Activins are members of the transforming growth factor-β (TGF-β) superfamily composed of disulfide linked dimers of β-subunits (βA and βB) that can also dimerize with an α-subunit to form inhibin A (αβA) and inhibin B (αβB). Consequently, three forms of activin have been described, activin A (βAβA), activin B (βBβB) and activin AB (βAβB) (de Kretser et al., 2012). Follistatin, a powerful activin-binding protein, is the major factor controlling the biological actions of activins (Nakamura et al., 1990). The activin/follistatin system acts primarily as a local growth regulatory system that controls proliferation, differentiation and apoptosis in many cell types.
Reis et al. (2001) found that inhibin A and activin A concentrations in the cystic fluid of ovarian endometriosis were slightly higher than in the peritoneal fluid and significantly higher than in serum. Inhibin A concentrations were 91 ± 10 pg/ml in the serum, 241 ± 47 pg/ml in the peritoneal fluid and 392 ± 96 pg/ml in the cystic fluid. Activin A levels were 0.38 ± 0.05 ng/ml in serum, 1.82 ± 0.39 ng/ml in the peritoneal fluid and 2.23 ± 1.31 ng/ml in the cystic fluid. The authors suggested these data strongly supported the local production and secretion of inhibin A and activin A in ovarian endometriotic cysts by the endometriotic cells lining the inside of the cyst but their biological effect in this context is not clear. A local modulation of endometriotic cell growth and differentiation has been suggested (Reis et al., 2001). Moreover, activin A has also been implicated in cell invasion in physiologic processes such as embryo implantation and in endometrial cancer. In both cytotrophoblasts and endometrial cells, activin A stimulation induces production of MMP-2 and MMP-9. More interestingly, activin A was shown to promote invasion of cultured endometrial cells in a layer of confluent peritoneal mesothelial cells grown in a Matrigel™ invasion assay with an effect of >2-fold versus control cells (Ferreira et al., 2008). A homeostatic balance inside the cyst involving activin A, inhibins and follistatin potentially affecting the invasiveness of the surrounding tissue might be suggested.
Soluble adhesion molecules
The CD44 proteoglycan is a multistructural (i.e. has different isoforms) and multifunctional adhesion molecule. As a cell surface molecule, it may be released by the cells; thus, soluble CD44 (sCD44) can be found in cell culture supernatants as well as in serum. The most frequently expressed form in lymphocytes is known as the standard form of CD44 (CD44s). CD44 was suggested to be involved in the metastatic process of both human malignancies and experimental animal tumors (Bendardaf et al., 2006). An involvement of CD44 in the progression of lymphoma has been shown by faster growth and dissemination after transfection of lymphoma cells with CD44. Darai et al. (1998) measured sCD44s concentrations in the endometrioma fluid which were significantly higher (1006 ± 679 ng/ml) than those found in cystadenomas (244 ± 251 ng/ml), borderline tumors (765 ± 218 ng/ml) and in ovarian carcinomas (623 ± 357 ng/ml). Similar results were subsequently reported by Igarashi et al. (2003) as they found 10-fold higher sCD44 levels in ovarian endometriotic cysts than in serous, mucinous, and dermoid ovarian cysts. In this study, sCD44 concentration in the endometriotic cyst was as high as that seen in ovarian cancer. The high sCD44 content in endometrioma represents a further crucial mechanism for the invasive potential of the cells forming this entity. Levels of soluble E-cadherin were also higher in endometriomas compared with cystadenomas but lower than that detected in borderline or malignant tumors (Darai et al., 1998). Changes in E-cadherin may affect the adhesive ability of the tissues resulting in a modification of the normal tissue architecture (Charalabopoulos et al., 2006).
CA-125
CA-125 is found inside the endometriotic cyst at concentrations much higher than in serum (>10.000 and <100 IU/ml, respectively) (Koninckx et al., 1992; Velasco et al., 2010). The reason for these higher concentrations inside the cyst is still unknown. However, CA-125 is generally considered an indicator of inflammation.
The wall of the cyst
The wall of the cyst has received limited attention in research. This is quite surprising considering that the nature of the wall is fundamental to clarifying some pathogenic aspects. To clarify a potential detrimental effect of the cyst on the surrounding ovarian tissue, the issue here is: ‘Is this wall an impermeable barrier or are the endometrioma contents able, at least in part, to leak out from the cyst?’ This review cannot provide an answer to this question owing to the limited evidence currently available. There are however some interesting observations than may derive from the knowledge of the pathophysiology of the cyst and which deserve particular attention in order to clarify this issue. Unlike non-endometriotic cysts (dermoids and serous or mucinous), in which a real anatomic capsule is present, the endometrioma is not surrounded by a capsule (Muzii et al., 2002). This makes this type of cyst an unusual entity from a morphological, structural and also surgical point of view. Therefore, the nature of this wall would require a more in-depth analysis than that dedicated so far.
Histology
The histologic nature of the endometrioma wall has greatly contributed to the debate on pathogenesis of the endometrioma. There are only a few studies that report in detail on the histologic features of the endometrioma wall.
Hughesdon (1957) has described the structure of ovaries with endometriomas in a series of 32 surgical specimens. In about 90% of the cases, the wall was identifiable as disorganized, stretched ovarian cortex and signs of metaplasia into smooth muscle cells were evident in this ovarian cortex (Hughesdon, 1957).
Martin and Berry (1990) have studied 41 chocolate cysts of which only 61% were histologically confirmed as endometrioma while the others were diagnosed as corpus lutea or corpus albicans. The authors described these cysts as surrounded by fibrosis and a rim of normal-appearing ovary with a generally flattened white internal lining with red or red and brown streaks scattered throughout the internal wall. Histologically, the brown areas were hemosiderin-laden stroma remnants.
According to Brosens et al. (1994), who have evaluated 59 hemorragic cysts, typical features of an endometrioma included the presence of adhesions between the frontal site of the ovary and the posterior leaf of the broad ligament or pelvic wall and, at the site of adhesions, the ovarian cortex was retracted or scarified. At histology, the internal wall presented red foci frequently characterized by an endometrial surface epithelium with or without stroma, white surface areas of ovarian cortex with or without follicles and pigmented areas represented by fibrous tissue with macrophages. Interestingly, according to these authors, in the later stages, the normal cortex is progressively replaced by pigmentation and by a fibroreactive tissue, which masks the original tissue (Brosens et al., 1994).
Donnez et al. (1996) have studied biopsies from 814 ovarian cysts. They found that the endometrioma wall often comprised flattened columnar epithelium with a stroma of endometrial type, often surrounded by fibroreactive tissue with haemosiderin-laden macrophages. Frequently, the internal lining of endometriotic tissue was absent and only granulation and fibrous tissue with macrophages could be detected. Signs of metaplastic changes, including the presence of ciliated cells, were observed in 62% of cases. Mesothelial inclusions in the form of invaginations of the celomic mesothelial cells in ovarian cortex were described with frequent aspects of continuum between the mesothelium and the endometriotic tissue (Donnez et al., 1996).
Fukunaga (2000) have described metaplastic changes into smooth muscle cells located in the rim of the cyst or in the endometrial stroma in 18.9% of 265 ovarian endometriosis cases. Other authors have subsequently described ovarian smooth muscle cell metaplasia in association with the endometrioma with a higher frequency (Mechsner et al., 2005; Odagiri et al., 2009). Two possible cell sources for these changes have been proposed: (i) endometrial stromal cells in endometriotic foci or (ii) ovarian stromal cells in the rim of endometriosis (Fukunaga, 2000). Metaplasia implies a change in the expression of tissue-specific genes and the response to signals that a cell would not normally receive (Quinlan et al., 2007). Tissue damage and inflammation predispose to the development of metaplasia (Quinlan et al., 2007; Burke and Tosh 2012). Typically, a change in cellular phenotype disrupts the normal tissue histoarchitecture. A physiological smooth muscle network is known to be present in the cortical stroma and periovulatory follicle and is thought to be involved in regulating the contractile activity associated with ovarian follicle rupture (Mechsner et al., 2005; Choi et al., 2011). An ovarian cortical environment in which the component of smooth muscle cells is dysregulated is likely to respond aberrantly to the finely tuned signals underlying these phenomena (Quinlan et al., 2007).
Hachisuga and Kawarabayashi (2002) have divided 73 cases of ovarian endometriomas into those in which the cyst was easily stripped from the ovarian tissue and those in which the cyst was hardly stripped, and the mean wall thickness was 2.1 ± 0.8 and 1.8 ± 0.4 mm, respectively. Histologically, the wall of the cysts that were easily stripped was characterized by the regular presence of ovarian stroma and of primordial follicles in 68.9% of the cases. No follicles or ovarian stroma were found in hardly stripped cysts (Hachisuga and Kawarabayashi, 2002).
Muzii et al. (2007) studied the wall of 70 cysts demonstrating a mean wall thickness of 1.4 ± 0.6 mm. Ovarian stroma, follicles or both were found in 81% of the cyst wall specimens. An endometrial lining covered the internal surface of the cyst wall in 60% of the entire area, with a range of 10–98%. This means that in some cysts, only 10% of the area was covered by endometrium (Muzii et al., 2007). In 40% of the cyst surface (with a range of 2–90%), no epithelium was identifiable, and the inner surface of the cyst was covered by fibrotic tissue. The maximal penetration of the endometriotic tissue was less than 1.5 mm, although in the portion of the cyst wall where the penetration of endometriosis was deeper, the total cyst wall thickness was also greater, even 2.4 ± 0.3 mm.
A layer of full thickness ovarian cortex with stroma and follicles definitively characterizes the wall of the endometrioma, although the frequency of ovarian cortex found varied according to the different studies and the various techniques employed: 83% by Hughesdon (1957), 15% by Brosens et al. (1994), 34% by Scurry et al. (2001) and 81% by Muzii et al. (2007).
Pathogenic clues
Various hypotheses have been proposed for the genesis of ovarian endometriotic cysts that are mostly based on the histological observations of the cyst wall and the cyst internal lining. A detailed explanation of the pathogenic theories at the basis of the endometrioma formation is beyond the scope of this paper but the various notions underlying them may add some interesting information on the unique aspects of the endometriotic cyst.
Metaplasia of the invaginated mesothelial inclusions
The presence of ovarian epithelial invaginations in continuum with ectopic endometrial tissue suggests that the mesothelium covering the ovary can invaginate into the ovarian cortex forming mesothelial inclusions and that the celomic metaplasia of these invaginated epithelial inclusions could be responsible for the formation of endometriomas with changes into typical endometrial glands and stroma (Donnez et al., 1996).
The mesothelium is implicated in the transport of fluid and particulate matter across the serosal cavities, in the synthesis of pro-inflammatory cytokines, growth factors and ECM proteins to aid in serosal repair, in leukocyte migration in response to inflammatory mediators and in the release of factors promoting the disposition and clearance of fibrins, such as plasminogen (Schor et al., 2000; Flessner, 2005; Fernandez-Borja et al., 2010). Based on its nature, this barrier between the fluid of the cyst and the normal ovarian tissue might be incompletely impermeable.
Superficial implants and invagination of the ovarian cortex
The presence of adhesions between the gonad and the peritoneum and the observation that active superficial endometriotic implants are mostly located at the site of cyst inversion would suggest that the ovary would adhere to the posterior leaf of the broad ligament because of the presence of an endometriotic ovarian implant deriving from retrograde menstruation creating an inflammatory milieu with the subsequent formation of adhesions (Brosens, 1998). Then, the cortex would invaginate and the typical tarry fluid would be formed by accumulation of menstrual debris from bleeding of the active implant invading the surrounding ovarian cortex. In this situation, the normal ovarian cortex eroded by the superficial implant shedding would be bathed by the endometrioma fluid.
Superficial implant and corpus luteum invasion
A variant of the previous theory assumes that a possible alternative source of the entrapped blood would be a corpus luteum that does not undergo resorption (Vercellini et al., 2009). More specifically, the ovary would adhere to the broad ligament because of the presence of superficial endometriotic implants with the subsequent formation of adhesions. Subsequently, the attached implants would guide ovulation to occur in this site since the mechanism of follicle dehiscence requires an inflammatory process. Endometriotic cells can then invade the newly formed hemorrhagic corpus luteum. A less marked erosion and damage of the normal ovarian cortex could be envisioned in this situation as the corpus luteum structure would protect the surrounding tissue. Indeed, in corpus luteum formation, fibronectin and collagen change dynamically to produce a firm tissue with a well-developed matrix (Irving-Rodgers et al., 2006).
The cellular elements lining the internal surface and inside the cyst
The endometriotic cells
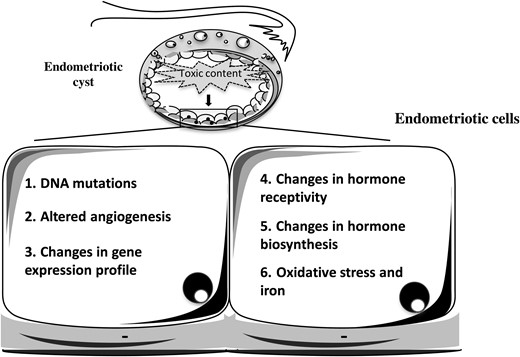
The endometriotic cells lining the inside of the cysts have received a great deal of attention over the years, mostly because of the need to compare the cellular and molecular elements in eutopic versus ectopic endometrium. The molecular analysis of these cells may shed some light on some of the potential deleterious effects of the endometrioma fluid on the surrounding cellular components (Fig. 2). Indeed, the cyst environment is thought to induce a high frequency of somatic mutations in the cells lining the inside of the cyst, potentially contributing to cancer development (Viganò et al., 2006; Yamaguchi et al., 2008,Wiegand et al., 2010; Mangili et al., 2012). The local factors in the cysts claimed to be responsible for the induction of genetic alterations in endometriotic cells lining the inside of ovarian endometriomas are as follows: (i) inflammatory factors and cytokines that, via NF-kB activation, have been found to promote angiogenesis, cell proliferation, inhibition of apoptosis and production of ROS that may, in turn, induce DNA damage and mutations; (ii) an altered steroid hormone balance or responsiveness as estrogens have been linked to the pathogenesis of gynecological cancers (Bulun et al., 2012); (iii) iron-associated oxidative stress, currently considered the most important trigger, being able to attack ‘fragile’ sites in the genome, including the susceptible p53 pathway (Vercellini et al., 2011; Munksgaard and Blaakaer, 2012; Shigetomi et al., 2012; Viganò et al., 2012). Endometriotic epithelial and stromal cells lining the internal surface of the ovarian cysts have indeed been shown by immunohistochemistry to express higher levels of 8-OHdG and acrolein-modified 20-deoxyadenosine (oxidized nucleosides of DNA) and of lipoperoxides (Kao et al., 2005; Kobayashi et al., 2012) (Fig. 2). 4-Hydroxy-2-nonenal-modified proteins and carboxymethyllysine, as markers of modified proteins, were increased mainly in the ectopic stromal cells of the cyst (Kobayashi et al., 2012). Most of these products were rare in the eutopic endometrium (Slater et al., 2005; Kobayashi et al., 2012). A massive iron staining was also observed in the stroma of the cyst mostly located in macrophages (Kobayashi et al., 2012). Ngo et al. (2009) have demonstrated higher endogenous oxidative stress with an increase in ROS production in endometriotic cells isolated from the ovary. Superoxide anion and hydrogen peroxide were increased by 39% and 2.5-fold, respectively, in endometriotic stromal cells compared with endometrial stromal cells from women without endometriosis. The detoxification pathways represented by superoxide dismutase, catalase and glutathione peroxidase activity as compensatory feedback mechanisms to control oxidative stress, remained insufficient to overcome the increased oxidative stress. In particular, the drop in catalase levels is frequently observed in tumor cells (Ngo et al., 2009).
Molecular alterations typical of the endometriotic cells lining the internal surface of the cyst.
Finally, there seem to be differences in the gene expression profile between eutopic and ectopic endometrium inside the cyst supporting the idea that the endometrioma internal milieu may modify gene expression in the surrounding cellular components. The identification of the genes aberrantly expressed in ectopic endometrium lining the inside of the cyst involves very numerous studies and their description is beyond the scope of this review (Cox et al., 2001; Arimoto et al., 2003; Wu et al., 2006; Eyster et al., 2007; Hever et al., 2007; Mettler et al., 2007; Borghese et al., 2008; Zafrakas et al., 2008; Di Carlo et al., 2009; Morelli et al., 2009; Khan et al., 2012; Lin et al., 2012; Chen et al., 2013). There are, however, some additional considerations. In general, characterization of the isolated endometriotic cells from ovarian cysts does not receive the necessary level of attention. Studies that normally isolate endometriotic cells from endometriomas to be used for mRNA extraction usually check their purity using a vimentin staining for stromal cells and a cytokeratin staining for epithelial cells. However, the ordinary fibrous stroma of the ovarian cortex and of the ovarian medulla and the fibroreactive tissue always stain with vimentin antibody (Santini et al., 1993). Thus, it is unclear how the ovarian stroma can be distinguished from the endometriotic stromal cells through a conventional tissue collection thus raising severe doubts on the isolation of a pure endometriotic cell component from the endometriotic cyst.
Similarly, the use of ectopic tissue taken or scraped from the internal lining of the cyst wall for mRNA extraction raises some doubts regarding possible contamination by the ovarian components or by fibroreactive tissue. Most of the studies failed to clearly describe the method used to collect pure ectopic tissue from the internal lining. Differences in pigmentation are not reliable (Brosens et al, 1994). No study has reported the exclusion of some cysts in the collection because of a limited amount of ectopic tissue, as expected from the histologic information. Therefore, although these studies may be useful to understand how, and to what extent, the endometrioma fluid may modify the gene expression profile of the cells it baths, information derived from these studies needs to be considered with caution.
The macrophages
Hemosiderin-laden macrophages are a frequent histologic finding of an endometriotic cyst. Understanding the nature of these macrophages is critical as macrophages can mediate opposite effects depending on their specialization. They may be phagocytic cells that eliminate invading pathogens and elicit specific anti-tumor adaptive immunity responses (Capobianco and Rovere-Querini, 2013). Such macrophages are referred to as classically activated/inflammatory macrophages or M1 cells. Macrophages can also undergo a distinct activation program, the ‘alternative activation’, M2 cells, as they tune inflammatory responses and adaptive immunity, exhibit pro-tumor activity and promote a wound healing-like tumor microenvironment. Data from animal models of endometriosis would suggest that macrophages in the endometriotic lesions are ‘alternatively activated’ and play a role in lesion development by promoting lesion growth supporting the typical glandular and stromal architecture and sustaining vessel integrity. Moreover, macrophages from patients with endometriosis express typical markers of ‘alternative activation’, especially high levels of scavenger receptors CD206 and CD163. CD206 belongs to the C-type lectin superfamily and contributes to the removal or inactivation of inflammatory signals. CD163 mediates endocytosis of haptoglobin-hemoglobin complexes with degradation of heme-iron components that can be recycled for erytropoiesis (Capobianco and Rovere-Querini, 2013). Endometriotic cysts actually contain dense infiltrates of CD163-positive macrophages (Canet et al., 2012). Iron present in high levels in the cyst may bind to haptoglobin released by red blood cells. The complex may be internalized by macrophages via the CD163 hemoglobin/haptoglobin scavenger receptor (Lousse et al., 2009). ‘Alternatively activated’ macrophages actually have the ability to effectively internalize and recycle the metal, as supported by the relative increase of macrophages overloaded with iron (Defrere et al., 2011).
It has been observed recently that macrophages within the endometriotic cysts are also positive for CDC42, an intracellular protein of the RHO GTPase family that regulates the actin cytoskeleton and is involved in cellular transformation (Canet et al., 2012). CDC42 is thought to be essential for phagocytic cup formation by macrophages. Finally, macrophages infiltrating endometriotic lesions are a major source of soluble factors, particularly members of the TGF-β family and may therefore be important regulators of many cell functions inside and around the cyst (Bacci et al., 2009; Defrere et al., 2011).
The environment around the endometriotic cyst
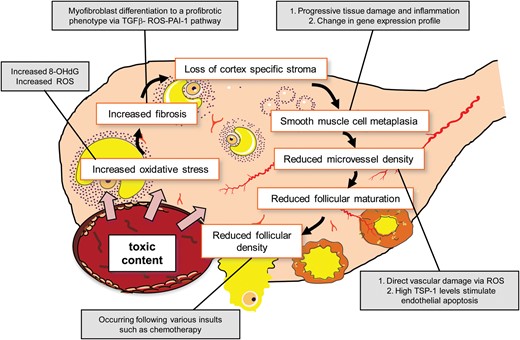
If we suppose that the presence of an endometrioma determines ovarian damage even before any eventual surgical intervention, the normal ovarian cortex surrounding the cyst should show cellular and molecular signs of such damage (Fig. 3). The following section of this review will deal with this aspect.
Unfavourable events occurring in the otherwise healthy ovarian tissue surrounding an endometrioma. 8-OHdG, 8-hydroxydeoxyguanosine; PAI-I, plasminogen activator inhibitor-I; ROS, reactive oxgyen species; TGF, transforming growth factor; TSP-1, thrombospondin-1.
ROS and the contribution to tissue fibrosis
The oxidative stress status in the normal ovarian cortex surrounding the endometrioma was studied by Matsuzaki and Schubert (2010). This study is very informative for the specific purpose of this review as it shows that the amount of oxidative stress affecting the normal ovarian cortex surrounding an endometrioma is greater than that in other types of cysts. Ovarian tissue from patients who underwent laparoscopic cystectomy for endometriotic cysts and other benign ovarian cysts (dermoid and serous) was immunostained for 8-OHdG (Matsuzaki and Schubert, 2010). The immunostaining for 8-OHdG was >10-fold greater in the ovarian cortex of patients with endometrioma compared with patients with other cysts. Explanations for this finding can be various; the presence of the cyst as an entity inducing a structural distortion of the ovarian architecture may initiate a local ovarian inflammatory reaction causing ROS production; alternatively, factors present in the cyst, for example iron, might diffuse into the surrounding tissue causing ROS generation; finally, several ROS are membrane permeable, making them excellent candidate paracrine molecules for the surrounding tissues (Bryan et al., 2012). It has been reported that ROS have the potential to cause catastrophic damage to healthy tissue if left unharnessed (Bryan et al., 2012).
It is very important to underline that ROS, together with the potent profibrotic PAI-1 and members of the TGF-β family are all involved in tissue fibrosis. The pathological manifestations of fibrosis are the expansion of mesenchymal elements, including myofibroblasts, excessive accumulation of ECM proteins and tissue contraction. Myofibroblasts, as the cells most responsible for the development of fibrosis, derive from activated resident fibroblasts or from fibroblasts deriving from epithelial-to-mesenchymal transition or endothelial-to-mesenchymal transition. They synthesize mostly collagen and fibronectin (Radisky et al., 2007; Barnes and Gorin, 2011; Ghosh and Vaughan, 2012). TGF-β1 and ROS are the principal factors responsible for myofibroblast differentiation to a profibrotic phenotype (Barnes and Gorin, 2011). TGF-β1 stimulates ROS production by various mechanisms that, in turn, engage downstream signaling pathways (small mother against decapentaplegic proteins, MAPK family) resulting in the expression of a subset of profibrotic genes among which PAI-1 is one of the best characterized. PAI-1, controlling the activities of plasmin and plasmin-dependent MMPs, regulates the extent and locale of collagen matrix remodeling (Samarakoon et al., 2013). The demonstration of increased oxidative stress affecting the normal ovarian cortex surrounding an endometrioma strongly supports the possibility that a ROS-induced fibrogenic response may be induced at this level.
Oxidative metabolism has also been reported to be involved in every stage of ovarian follicular development and oocyte maturation. Oxidative stress imbalance in the ovarian follicular fluid environment has a detrimental effect on oocyte and embryo development, and pregnancy outcome (Pasqualotto et al., 2004; Das et al., 2006; Tamura et al., 2008). Indeed, it induces oocyte apoptosis and necrosis in early follicles (Zhang et al., 2006). Thus, an alteration in the oxidative stress status in the normal cortex might, at least in part, explain the reduced rate of spontaneous ovulation in ovaries affected by an endometrioma and the reduced number of developing follicles and oocytes retrieved in IVF cycles in women with bilateral endometriomas (Benaglia et al., 2013).
Some studies have also demonstrated that granulosa cells from infertile patients with endometriosis exhibit more signs of oxidative stress when compared with those from unaffected patients (Seino et al., 2002; Karuputhula et al., 2013). Interestingly, a recent study observed that, in a cohort of women with endometriosis undergoing IVF procedures, more than one-third of the follicles contained ROS in concentrations ≥107 cps/400 μl, which was estimated as the upper limit beyond which formation of viable top/good embryos is not favorable. Indeed, the fertilization rate and the rate of embryo formation from oocytes retrieved from those follicles were very low (11.8 and 16.6%, respectively) (Jana et al., 2010). However, all these studies did not specifically focus on patients affected by endometriomas and the peritoneal environment may also have a role.
The follicular pattern
One of the first studies conducted with the aim of examining the ovarian follicle pattern surrounding different types of cysts was performed by Maneschi et al. in 1993. Specimens obtained from the area of maximum distension of the ovarian cortex overlying benign cysts were obtained from 48 patients. Cortical tissue surrounding dermoid cysts, cystadenomas and endometriomas, that is stretched and thinned by the growth of a benign tumor, showed morphological patterns similar to a normal ovarian cortex in, respectively, 92, 77 and 19% of the cases and with a regular vascular network in 84, 78 and 22% of the samples, respectively. Follicular maturation up to the antral stage was much less frequent in tissue surrounding endometriomas than in other types of cysts (Maneschi et al., 1993). The results of this study, unique for its far-sightedness, support the idea that endometriomas greatly affect the morphological and functional characteristics of normal ovarian tissue while excluding a role of stretching per se as a factor responsible for this damage.
Some studies have described the characteristics of the ovarian tissue inadvertently stripped during a laparoscopic intervention for cyst removal in relation to the adequacy and precision of the surgical procedure. Muzii et al. found that the ovarian tissue adjacent to the endometrioma wall differed morphologically from the normal ovarian tissue, as in most cases no follicles or scanty primordial follicles were present (Muzii et al., 2002). According to Dogan et al. (2011) there were no differences in mean cortical tissue thickness, or the number of primordial, primary or secondary follicles between endometriomas and non-endometriotic cysts. Functional follicle loss was, however, slightly but significantly higher in ovaries with an endometrioma. Conversely, Shi et al. (2011) reported that the number of ovarian cortical samples showing follicles and the mean number of follicles per sample were lower for endometriomas than for non-endometriotic cysts. In the context of the evaluation of endometrioma-mediated damage to the ovary, the results of these studies should be considered with caution as the samples analyzed were not taken randomly from the ovarian cortex but were part of the tissues removed with the cyst; therefore sampling biases should be considered.
The study of the quality of the cortex surrounding the endometrioma has recently received an increased interest mostly for the rapid diffusion of ovarian tissue cryopreservation techniques. In this context, Schubert et al. have evaluated the ovarian cortex surrounding dermoid, serous and endometriosis cysts for follicular density with the purpose of establishing whether it represents an appropriate parameter to evaluate the efficacy of some cryopreservation procedures. A 1–2 cm2 piece of ovarian cortex overlying the cyst was analyzed. Follicular densities in ovarian cortex surrounding dermoid cysts were higher than for endometriotic and serous cysts in both histological (median follicular density per mm3: 13.04, 0.31 and 0.89, respectively) and viability analyses (median number of viable follicles per mm2: 2.93, 0.05 and 0.71, respectively). Histologically, the tissue surrounding dermoid and serous cysts showed a normal morphological pattern while endometrioma-surrounded cortex showed an increased tissue fibrosis (Schubert et al., 2005).
Kitajima et al. have compared the follicular density and the histologic features in apparently normal ovarian cortical tissue from ovaries with small endometriomas and from the contralateral healthy ovaries. A piece of thick ovarian cortical tissue that looked normal at a macroscopic level was excised away from the site of endometrioma fenestration. The results of this study have demonstrated that: (i) follicular density was significantly lower in cortex from ovaries with endometriomas (6.3 ± 4.1/mm3) than without (25.1 ± 15.0/mm3); (ii) fibrosis was significantly more frequent in cortex from ovaries with endometriomas (80%) than without (27%) and (iii) the presence of fibrosis and concomitant loss of cortex-specific stroma were found in 55% of cortical samples from ovaries with endometriomas but it was never observed in samples from contralateral healthy ovaries. Interestingly, since evaluated endometriomas were <4 cm in size, it might be hypothesized that follicular loss occurs even at early stages of disease development. Thus, this study supports previous observations indicating that factors other than the simple mechanical tissue stretching might be responsible for reduced follicular density in the ovarian cortex surrounding endometriomas, as this phenomenon was independent of size (Kitajima et al., 2011).
The loss of cortex-specific stroma should be considered in the context of its potential harmful effect on folliculogenesis. The ovarian stroma not only provides a blood supply though its capillaries to the primordial follicles but also acts in a co-ordinated and synergistic way with other compartments to induce the transition from primordial to primary follicles (Motta et al., 2003; Skinner, 2005; Oktem and Urman, 2010). Ovarian cortical stromal cells are the source of theca cells (Motta et al., 2003; Skinner 2005; Oktem and Urman, 2010). Stroma-derived keratinocyte-growth factor, fibroblast growth factor-2, bone-morphogenic protein-4 have been implicated as positive regulators of primordial follicle growth (Oktem and Urman, 2010) and were also found in islands of stromal cells not associated with the follicles (Skinner, 2005).
The follicular density in ovarian biopsies obtained from the tissue surrounding endometriotic and non-endometriotic cysts (mostly dermoid) has been also evaluated (Kuroda et al., 2012). The authors demonstrated that follicular density in healthy ovarian tissue was lower in patients with an endometrioma compared with the non-endometriotic cyst group. This effect was more evident in patients <35 years of age (follicle density 29.2 ± 7.3/cm2 in tissue surrounding endometriomas and 66.5 ± 13.8/cm2 in tissue surrounding non-endometriotic cysts) compared with older patients (follicle density 7.5 ± 1.5/cm2 in tissue surrounding endometriomas and 9.6 ± 3.7/cm2 in tissue surrounding non-endometriotic cysts).
In summary, most authors agree that follicular density is lower in the ovarian cortex adjacent to the endometriotic cyst. This phenomenon is associated with tissue alterations, such as formation of fibrosis and vascular deficiency, and does not seem to be related to the mere mechanical stretching. Interestingly, these pathological changes, injury to blood vessels and focal fibrosis have also been observed in the cortex of ovaries exposed to chemotherapy (Meirow et al., 2007), and similarly no follicles are seen in the fibrotic zone. Thus, this pattern of damage to the entire organ may be common among different harmful processes that, in general, tend to diminish the ovarian reserve.
The intralesional and intraovarian vasculature
One hypothesis proposed for the potential intrinsic ovarian damage induced by the cyst implies ovarian vascular damage. To gain insight into this aspect we have reviewed papers addressing how the vascular system in the ovary is affected by an endometrioma.
The results from various studies are quite different depending on which part of the ovarian tissue is considered. Intralesional microvessel density (MVD) was assessed in two studies. In the study by Ceyhan et al. (2008) MVD was mainly evaluated by CD34 staining of vascular endothelial cells, and was found to correlate positively with vascular endothelial growth factor (VEGF) and cyclooxygenase-2 immunohistochemical expression in the cyst and negatively with cyst diameter. Huang et al. (2012) evaluated MVD in ovarian endometriotic lesions by using the blood vessel markers von Willebrand factor (vWF) and alpha-smooth muscle actin (α-SMA), which are useful markers for endothelial cells and pericytes, respectively. MVD, assessed by either anti-vWF or anti-α-SMA staining, was increased in ovarian endometriotic lesions compared with eutopic endometrium of control women (Huang et al., 2012).
Very different results were obtained when considering the specific vascular system of the ovary. Maneschi et al. (1993) reported that an adequate vascular network (>10 capillaries/low power field) was more frequently observed in cortical tissue surrounding teratomas (84%) and cytoadenomas (78%) than that surrounding endometriomas (22%).
Qiu et al. have assessed ovarian interstitial blood flow changes and correlated them with two indices of vascular injury, thrombospondin-1 (TSP-1) and MVD by CD34 staining, in patients with endometriotic and non-endometriotic ovarian cysts. The vascular network in the ovarian interstitium primarily involves a capillary network, which originates from the ovarian branches of uterine arteries and from ovarian arteries. This network plays a key role in maintaining ovarian structure and function (Qiu et al., 2012). TSP-1 is widely recognized as a negative angiogenic regulator, owing to its inhibitory effects on endothelial cell proliferation, and as an important modulator of blood perfusion (Zhao et al., 2008; Garside et al., 2010; Csanyi et al., 2012). The study by Qiu et al. (2012) confirmed previous findings indicating that interstitial blood flow around benign ovarian tumors was adequate but typically formed a strip shape. Conversely, an evident reduction of blood perfusion within ovarian interstitial arteries in patients with endometriomas was observed, supportive of blood vessel damage during the pathological process of endometrioma formation and development. In interstitial specimens, a higher expression of TSP-1 and a lower MVD could be observed in patients with endometrioma, suggesting again that blood vessels in the ovarian interstitium of ovaries affected by an endometrioma have been destroyed. Two possible mechanisms can be proposed to explain this vascular dysfunction: (i) the imbalance between ROS production and the ability of biological systems to detoxify the reactive intermediates leads to impaired vascular relaxation, enhanced endothelial transcytosis, up-regulation of proinflammatory molecules and alteration of endothelial cell fibrinolytic activity. Oxidation of biological molecules, resulting in structural and functional abnormalities of the cells of the vascular wall, is the best characterized process (Manea 2010). (ii) Alternatively, a mechanism mediated by TSP-1 may be envisioned. TSP-1 levels surrounding vascular smooth muscle cells rise sharply in response to injury. Via its ubiquitously expressed cell surface receptor CD47, TSP-1 activates adenylate cyclase and stimulates apoptosis. However, TSP-1 is also able to stimulate the production of ROS in vascular smooth muscle cells and induces a vascular dysfunction by promoting oxidative stress (Csanyi et al., 2012).
Conclusions and future research
The results of this review strongly suggest that the presence of an endometrioma results in damage to the affected ovary, independent of the mechanical stretching and of its size. The occurrence of this damage is supported by important morphological and functional effects and substantial differences compared with the normal tissue.
The cyst contains cellular damage-mediating factors, proteolytic enzymes and inflammatory molecules in concentrations from tens to hundreds of times higher than those present in serum or in other types of cysts. Factors such as iron and ROS, present at high concentrations inside the cyst, can be taken up by the cells in direct contact. Iron bound to transferrin or other proteins can bind with high affinity to receptors on the surface of cells and the complex undergoes endocytosis. Many ROS are membrane permeable (Yang et al., 2013). In line with these observations, high amounts of ROS and iron deposits have been found in endometriotic cells and macrophages lining the inside of the cyst. The cyst fluid causes dramatic modifications to the endometriotic cells that it baths, from changes in the expression of critical genes to severe genetic alterations potentially initiating tumorigenesis.
Thus, having established that the endometrioma fluid is highly deleterious for its surrounding cells, the issue remains whether the same toxic factors can also negatively affect the otherwise healthy ovarian tissue surrounding the cyst. The barrier between the cyst fluid and the normal ovarian tissue is represented by a wall ∼1 mm thick, composed of ovarian cortex itself or fibroreactive tissue. Based on the evidence presented herein, a series of events is likely to occur: (i) ROS permeate inside the surrounding cells or are formed in the otherwise healthy tissue as a response to the cyst presence, as shown by the finding that a marker of DNA damage is 10-fold higher in the tissue surrounding the endometrioma than other types of cyst (Matsuzaki and Schubert, 2010); (ii) ROS and TGF-β induce tissue fibrosis, also involving the actions of proteolytic enzymes (Radisky et al., 2007; Bryan et al., 2012; Samarakoon et al., 2013); (iii) an increased fibrosis causes a reduction of cortex-specific stromal cells. Stromal cells are essential for the structure of the follicle and act as mediators of nutrients and molecular signals (Skinner 2005; Oktem and Urman 2010; Kitajima et al., 2011); (iv) fibrosis formation in cortical tissue is also a common pathogenic feature of follicular loss (Meirow et al., 2007) as most authors agree that the follicular density in healthy tissue is at least 2-fold higher than in tissue surrounding the endometriotic cyst (Kitajima et al., 2011; Kuroda et al., 2012); (v) smooth muscle metaplasia also occurs, mostly in the rim of endometriotic cysts and probably disrupting the organization of the physiological smooth muscle network present in the cortical stroma and periovulatory follicle (Fukunaga, 2000); (vi) inhibition of ovarian angiogenesis and capillary loss are consequently mediated directly by increased ROS levels and indirectly by the cellular injury that in turn triggers over-expression of factors affecting the vascular system, such as TSP-1 (Csanyi et al., 2012). These signs of potential damage are illustrated in Fig. 3.
Surgical excision of an endometrioma has been shown to be detrimental to the ovarian reserve (Garcia-Velasco and Somigliana, 2009; Somigliana et al., 2011). This review does not help in clarifying which of the two events, the surgical removal of the cyst or the presence of the cyst itself, might be more catastrophic to the otherwise healthy tissue. There are however some data to support a deleterious effect of the cyst itself on the surrounding tissue. To further investigate this issue, the presence of molecular signs of gonadotoxic insults should be better evaluated in the ovarian cortical stroma surrounding endometriomas. Similarly, the characterization of molecular and cellular features of follicles developing adjacent to the cyst during IVF cycles is needed as data available in this context are scanty (Opoien et al., 2013). This review also does not clarify whether the ovarian damage mediated by the cyst is acute or progressive over time. Clinically this is a very important point, critical for directing the treatment towards one of the possible different approaches. Other critical matters are clarifying whether and how the medical therapy or specific strategies of the surgical therapy may limit or slow the damage caused by a cyst. Understanding and clarifying these issues represent tasks to be accomplished in the future.
Authors' roles
A.M.S and P.Vi. provided a substantial contribution to the review conception and drafted the article; E.S. analyzed the data and critically revised the manuscript; P.P.-B. contributed to the design and revised the manuscript; P.Ve. contributed to the interpretation of the data and critically revised the manuscript; M.C. contributed to the design of the review and critically revised the paper for important intellectual content. All authors approved the final version of the article.
Funding
A.M.S. is supported by a fellowship from Fondazione Giorgio Pardi, Milano.
Conflict of interest
The authors have no financial, personal or competing interests.
Acknowledgement
We thank Dr Meera Nanjundan for comments and revision of the manuscript.