-
PDF
- Split View
-
Views
-
Cite
Cite
Tyl H. Taylor, Susan A. Gitlin, Jennifer L. Patrick, Jack L. Crain, J. Michael Wilson, Darren K. Griffin, The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans, Human Reproduction Update, Volume 20, Issue 4, July/August 2014, Pages 571–581, https://doi.org/10.1093/humupd/dmu016
Close - Share Icon Share
Chromosomal mosaicism, the presence of two or more distinct cell lines, is prevalent throughout human pre- and post-implantation development and can lead to genetic abnormalities, miscarriages, stillbirths or live births. Due to the prevalence and significance of mosaicism in the human species, it is important to understand the origins, mechanisms and incidence of mosaicism throughout development.
Literature searches were conducted utilizing Pubmed, with emphasis on human pre- and post-implantation mosaicism.
Mosaicism persists in two separate forms: general and confined. General mosaicism is routine during human embryonic growth as detected by preimplantation genetic screening at either the cleavage or blastocyst stage, leading to mosaicism within both the placenta and fetus proper. Confined mosaicism has been reported in the brain, gonads and placenta, amongst other places. Mosaicism is derived from a variety of mechanisms including chromosome non-disjunction, anaphase lagging or endoreplication. Anaphase lagging has been implicated as the main process by which mosaicism arises in the preimplantation embryo. Furthermore, mosaicism can be caused by any one of numerous factors from paternal, maternal or exogenous factors such as culture media or possibly controlled ovarian hyperstimulation during in vitro fertilization (IVF). Mosaicism has been reported in as high as 70 and 90% of cleavage- and blastocyst-stage embryos derived from IVF, respectively.
The clinical consequences of mosaicism depend on which chromosome is involved, and when and where an error occurs. Mitotic rescue of a meiotic error or a very early mitotic error will typically lead to general mosaicism while a mitotic error at a specific cell lineage point typically leads to confined mosaicism. The clinical consequences of mosaicism are dependent on numerous aspects, with the consequences being unique for each event.
Introduction
Chromosomal mosaicism is defined as the presence of two or more chromosomally distinct cell lines within an individual. At its core, chromosomal mosaicism is the failure of chromosomes to properly segregate during mitosis, leading to the gain or loss of whole chromosomes, a phenomenon known as aneuploidy. Chromosomal mosaicism has been implicated in genetic diseases, miscarriages and preimplantation embryo wastage (Hassold and Hunt, 2001). Moreover, mosaicism has been shown in cancer (Lengauer et al., 1998) and in an increased incidence of trisomy 21 conceptions (Kovaleva, 2010), and has been associated with aging (Ly et al., 2000). Although prevalent, the exact threshold at which mosaicism switches from clinically irrelevant to relevant is unknown and differs depending on the stage and severity at onset. Due to the prevalence and significance of mosaicism in the human species, it is important to understand the origins, mechanisms and incidences of mosaicism throughout development. The purpose of this review is to explain the different types of mosaicism, the mechanisms from which mosaicism derives, the incidences of mosaicism during development, and the clinical consequences and relevance of chromosomal mosaicism.
General mosaicism
General mosaicism is the presence of a two or more cell lines throughout the entire organism. In order for the mosaic cell lineage to be present within the entire organism, the aneuploidy in question must derive from a mitotic event during the first days of embryonic development, prior to any cellular differentiation. At this stage, mosaicism has been found to range between 65 and 70% (Wells and Delhanty, 2000; Mertzanidou et al., 2013). However, simply because preimplantation embryos are mosaic does not mean that the abnormal cell line(s) will continue to propagate during development. Research has shown that euploid cells proliferate at a higher rate than aneuploidy cells (Ruangvutilert et al., 2000). Scott et al. (2012) describe live births from diagnosed aneuploid cleavage- and blastocyst-stage embryos, although at a significantly lower rate than from euploid embryos. This indicates that aneuploidy and mosaicism may play a limited role in development, and its influence during development may be dependent on the degree of mosaicism and what chromosome is involved.
Chromosomal mosaicism is common during preimplantation development. However, due to the low number of cells present during preimplantation development, any abnormality can have much more of an impact as development continues. Furthermore, mosaicism can become isolated during embryonic development, leading to mosaicism confined to a particular area.
Confined mosaicism
Confined mosaicism refers to chromosomal mosaicism that is only present in a particular area. For example, confined chromosomal mosaicism has been reported in the brain (Yurov et al., 2007), placenta (Kalousek and Dill, 1983) and gonads, amongst other places. A major aspect of research about confined mosaicism deals with the relationship between the placenta and the developing fetus.
Confined placental mosaicism (CPM) is defined as chromosomal differences between the fetus and placenta. CPM was first reported in the human placenta by Warburton et al. (1978). They discovered that roughly 10% of trisomic conceptions contained a mosaic cell line. CPM has been linked to intrauterine growth retardation (Kalousek, 1990), spontaneous abortions, intrauterine death, stillbirth (Benn, 1998) and abnormal placental function (Koplan et al., 1991). CPM is believed to occur in roughly 1–2% (Kalousek et al., 1992) of all placental tissue analysis.
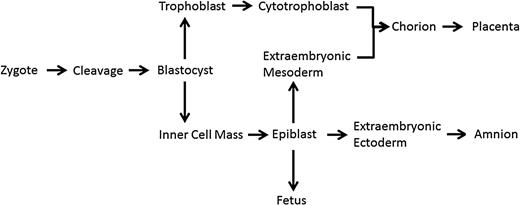
Cell lineage from zygote (1 cell) stage to the fetus, including extraembryonic materials of the extraembryonic mesoderm (EEM) and cytotrophoblast (adapted from Crane and Cheung, 1988; Delozier-Blanchet, 1991).
Regardless of whether mosaicism is present in the entire individual or is confined, the mechanisms by which mosaicism occurs are the same.
Methods
This article provides a review of the literature concerning chromosomal mosaicism in humans and its mechanisms, origin and incidence in both pre- and post-implantation development. Only Pubmed was used to identify relevant articles, utilizing the following terms: ‘mosaicism in IVF’, ‘mosaicism preimplantation development’, ‘mosaicism post-implantation development’, ‘human chromosomal mosaicism’, ‘chromosomal mosaicism and IVF’, ‘placental mosaicism’, ‘confined mosaicism’ and ‘general mosaicism’. Furthermore, relevant articles referenced within articles found in Pubmed were also reviewed based upon their relevance as determined by the authors.
Mechanisms
Proper chromosome segregation
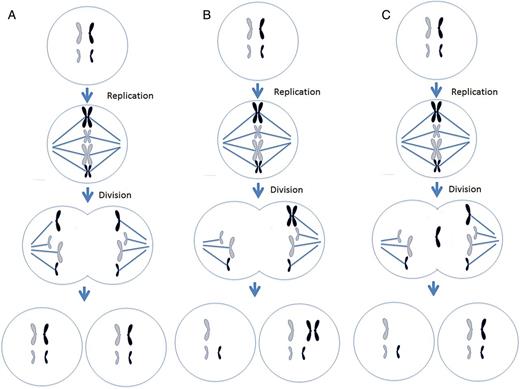
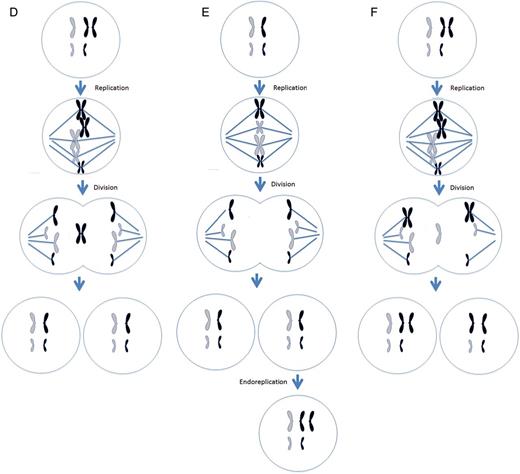
Different mechanisms leading to chromosome malsegregation in humans. For each figure, two different chromosomes are present, black chromosomes are paternal in origin and white are maternal in origin. (A) Proper segregation of chromosomes during mitosis. (B) A mitotic non-disjunction event in a paternal chromosome. (C) An anaphase lagging event involving a paternal chromosome. (D) A trisomy rescue event involving a paternal chromosome. (E) An endoreplication event involving a paternal chromosome. (F) A trisomy rescue event with uniparental disomy in the paternal chromosomes.
Centrosomes are organelles within the cells that define the poles of the spindle apparatus and begin the production of microtubules used to capture chromosomes. These microtubules randomly attach to the chromosomes on the centromere at a site called the kinetochore. The kinetochore complex on the centromere of the chromosomes is composed of multiple proteins that are involved in the capture and movement of chromosomes along the microtubules (Ma et al., 2003). As the chromosome division starts, microtubules (still connected to the kinetochores) begin to depolymerize, effectively pulling the chromosomes towards the spindle poles. Once the chromatids are on opposite poles, cytokinesis begins and the cell divides, creating two identical cells (Fig. 2A). In some mammals and invertebrates, research suggests that a mitotic spindle checkpoint exists to protect the cell from dividing its chromosomes unequally (Brunet et al., 2003; Wassmann et al., 2003; Homer et al., 2005). If a chromosome(s) is not attached to the microtubules, the cell will prevent itself from dividing until the chromosome(s) is captured by the microtubules. This mechanism assures proper segregation of the chromosomes. However, during the first mitotic divisions in human preimplantation development, this checkpoint seems non-existent, leading to chromosome malsegregation and subsequent aneuploidy and mosaicism (Braude et al., 1988).
There are three main mechanisms by which chromosomal mosaicism can occur leading to a gain and/or loss of chromosomes: non-disjunction, anaphase lagging and chromosome gain referred to as endoreplication. Endoreplication and anaphase lagging account for the majority of chromosomal errors during embryonic development, while non-disjunction occurs to a lesser extent.
Non-disjunction
Non-disjunction is the failure of sister chromatids to separate during mitosis. Instead of separating, the entire chromosome (two chromatids) is pulled to one cell, creating a cell with a monosomy and another cell with a trisomy (Fig. 2B). If non-disjunction occurs prior to cell differentiation, for example in a preimplantation embryo, a general mosaic is created. Non-disjunction can also lead to a confined mosaicism. For example, if the non-disjunction event occurs in the trophoblast, after differentiation, then only the placenta will contain the mosaic cell lines, while the embryo proper could be euploid.
The incidence of non-disjunction during preimplantation development is subject to debate, and depends greatly on the stage of development and chromosome involved. For example, non-disjunction has been shown to be the least prevalent mechanism associated with aneuploidy during meiosis I and II amongst the autosomes (Forman et al., 2013; Handyside et al., 2013), but it is established as the main mechanism for sex chromosome malsegregation during the first cleavage stage divisions (Bean et al., 2001, 2002). It is evident that chromosomes may be more or less susceptible to non-disjunction depending on stage of development.
Anaphase lagging
Anaphase lagging is the failure of a single chromatid to be incorporated into the nucleus resulting in a monosomy of that chromosome in one cell and a disomy in the corresponding chromosome in the other cell (Fig. 2C). Anaphase lagging occurs when the chromatid fails to attach to the spindle or when the chromatid attaches to the spindle but then fails to be incorporated in the nucleus. If this mechanism occurs prior to differentiation, then the organism will contain two distinct cell lines, thereby creating a general mosaic. If this event occurs after differentiation in the trophoblast, then the placenta will contain a normal and monosomic cell line, an example of CPM.
In a study utilizing discarded Day 5 embryos, Ioannou et al. (2012) demonstrated that monosomy can occur at a 7x greater rate than trisomy. This would implicate anaphase lagging as the main source of mosaicism in human preimplantation development. This observation is supported by Coonen et al. (2004) and Capalbo et al. (2013) who found anaphase lagging at rates of 5x and 3x that of non-disjunction, respectively.
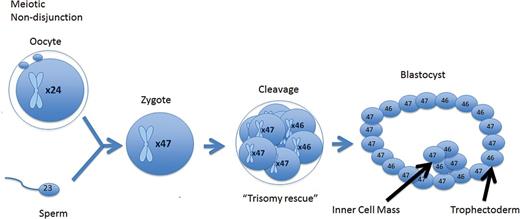
If a cell presents with a trisomy, then the process of anaphase lagging can ‘correct’ the trisomy and revert the reciprocal chromosome back to disomy, a process referred to as trisomic rescue (Fig. 2D). Although the frequency of this process is largely unknown, reports indicate that trisomy rescue of meiotic errors can occur during preimplantation development (Barbash-Hazan et al., 2009; Capalbo et al., 2013).
Endoreplication
Endoreplication is the replication of a chromosome without division. This results in a trisomic chromosome in one cell and a disomic chromosome in the other. Chromosome gain is believed to derive from two mechanisms, a cell cycle malfunction in which a chromosome is replicated without subsequent cytokinesis or when mitosis is initiated and shortly thereafter shutdown, resulting in a replicated chromosome. Regardless of the mechanism, the result is the same: the gain of a single chromosome (Fig. 2E). Another name for endoreplication is polyploidy. Polyploidy has been shown to exist in blood, gut, skin and brain (for review see Fox and Duronio, 2013).
Uniparental disomy
Non-disjunction, anaphase lagging and endoreplication lead to aneuploidy. However, there are mitotic events that lead to mosaicism, but still present a disomic cell line. Instead of chromosomes presenting in a gain or loss fashion, a phenomenon known as uniparental disomy (UPD) can occur. As UPD implies, there are two chromosomes present; however, instead of one maternal and paternal chromosome, there are two copies of either a maternal or paternal chromosomes. This may be the result of a trisomic rescue event after an error during meiosis (Fig. 2F). The most frequent chromosome that UPD occurs in is chromosome 15, where two paternal copies is referred to as Angelman syndrome or two maternal copies is known as Prader-Willi syndrome. Other chromosomes that can present with UPD are chromosomes 7, 11 and 16 (Kaluosek et al., 1992).
UPD and endoreplication occur to a lesser extent while anaphase lagging and non-disjunction seem to be responsible for a majority of whole chromosomal abnormalities within the human embryo (Daphnis et al., 2008). These mechanisms promote improper chromosome segregation, leading to multiple cell lines presenting with either chromosome gain and/or loss. Because of this, it is important to understand the origins of these mechanisms and what can lead to these errors.
Origins
Paternal origin
The centrosome is inherited from the sperm and is responsible for the first mitotic divisions within the human embryo (Palermo et al., 1994). The disruption of the sperm centrosome can produce mosaicism in the preimplantation embryo (Palermo et al., 1997). Furthermore, research has indicated that sperm aster formation could be delayed in infertile males when compared with fertile males (Terada et al., 2004; Yoshimoto-Kakoi et al., 2008). A delay in sperm aster formation could cause a delay in syngamy and subsequent cleavages and possibly induce aneuploidy. Thus, studies have shown that chromosomal aneuploidies are more prevalent in patients with severe male factor infertility (Gianaroli et al., 2000; Silber et al., 2003; Magli et al., 2009). If that is the case, then it is possible that mosaicism may also be more prevalent during preimplantation development in this group of patients.
Maternal origin
The increase in aneuploidy with maternal age is well documented (Munne et al., 1995, 2002a, b). Although mosaicism is a mitotically derived phenomenon, the proper segregation of chromosomes can be hindered by maternal processes. As previously stated, the centrosome is paternally inherited but the mitochondria and mRNA stores necessary for proper chromosome division originate from the oocyte. Indeed, research has indicated that mitochondrial function is affected by maternal age, possibly influencing chromosome segregation (Schon et al., 2000; Wilding et al., 2001).
The oocyte pool in a woman has been arrested at prophase since prenatal development. As the woman ages, so too does the length of exposure of the oocytes to reactive oxygen species and environmental factors that may have negative effects during embryological development. Furthermore, an increase in reproductive age correlates with a decrease in the cohesion molecule which is responsible for binding the sister chromatids together (Hodges et al., 2005; Duncan et al., 2012). If this cohesion is reduced in older women, this could cause an unequal separation of chromosomes leading to aneuploidy. Depending on when and where in development the error occurs, this could lead to mosaicism. Finally, some of the genes necessary for mitosis have been shown to be down-regulated in fibroblasts from older patients when compared with younger patients (Ly et al., 2000), although unfortunately this study did not give an indication of the actual ages. Interestingly, no correlation has been observed between the incidence of preimplantation chromosomal mosaicism and maternal age (Munne and Cohen, 1998; Munne et al., 2002a, b).
Abnormalities in spindles are also more prevalent in older women. For example, meiotic spindles from older women can have an abnormal shape and produce more chromosome misalignment compared with spindles of younger women, which appear to have normal configurations (Battaglia et al., 1996). Although the meiotic spindle does not directly influence mitotic chromosome segregation, it is possible that the presence of abnormal meiotic spindles could suggest that the process of chromosome segregation in older women is flawed from the onset, leading to mosaicism. An increase in mitotic spindle abnormalities of arrested Day 3 and 4 embryos compared with blastocysts has been reported (Chatzimeletiou et al., 2005). Furthermore, poor quality blastocysts have been shown to have more abnormal spindles when compared with good quality ones (Hashimoto et al., 2013). If abnormal spindles are present within a human embryo, then one would expect a higher incidence of aneuploidies, which may indicate a direct relationship between maternal age, spindle abnormalities and chromosomal mosaicism.
While there is a direct relationship between advanced maternal age and aneuploidy, no relationship between maternal age and mosaicism has been shown. In any case, aneuploidy has also been shown to be prominent in young, fertile, oocyte donors and women <35 years old (Baart et al., 2006; Munne et al., 2006; Fragouli et al. 2009; Ata et al., 2012). It is possible that other cells within the embryos tested in these studies could present with different cell lines, which would indicate that mosaicism, regardless of maternal age, may be a pathological phenomenon during human preimplantation development.
External influences
External factors also contribute to mosaicism. For example, the production of oocytes for in vitro fertilization (IVF) requires controlled ovarian hyperstimulation of ovaries by exogenous follicle-stimulating hormone. Hyperstimulation has been implicated in increased rates of cleavage stage aneuploidy. Munne et al. (1997) have demonstrated different mosaicism rates between IVF centers, implicating differences in stimulation protocols as a potential reason. However, other research has shown that even embryos derived from unstimulated ovaries produce similar rates of chromosomal aneuploidies (Verpoest et al., 2008).
Munne et al. (1997) have suggested that embryo culture conditions may also cause differences in mosaicism. Improper culture conditions can compromise embryo quality. Furthermore, 5% oxygen versus atmospheric oxygen levels has been found to improve embryo quality and decrease sex chromosome mosaicism when compared with culture in atmospheric oxygen levels (Bean et al., 2002; De Los Santos et al., 2013). Proper embryo culture is essential for embryo development and poorer quality embryos tend to have higher rates of chromosomal abnormalities (Munne et al., 2007). Thus, it is plausible that embryo culture may increase aneuploidy and subsequent mosaicism in the human preimplantation embryo (Beyer et al., 2009). However, in vitro-derived blastocysts have lower rates of aneuploidy when compared with cleavage-stage embryos (Fragouli et al., 2014). Embryos that develop to the blastocyst stage have progressed further than cleavage-stage embryos and so the culture media may have less of an effect on chromosome segregation as development progresses.
Incidence in preimplantation embryos
Cleavage embryos
Mosaicism occurs in ∼15–90% of all cleavage stage human embryos (Harper et al., 1995; Daphnis et al., 2005; Rubio et al., 2007). It is believed that if a majority of the cells are diploid, then these embryos could be deemed viable, even though they are general mosaics. Indeed, a majority of embryos do contain a combination of diploid and aneuploid cells, referred to as diploid-aneuploid mosaic embryos. In a systematic review looking at all the cells from each embryo, van Echten-Arends et al. (2011) found that 73% of 815 cleavage embryos examined were mosaic and 59% of all embryos were diploid-aneuploid mosaic. The authors noted that not all embryos were examined with the same technology, indicating that the mosaic rate may be higher when more chromosomes are analyzed. However, other research indicates similar rates of mosaicism at the cleavage stage (Wells and Delhanty, 2000; Vanneste et al., 2009; Mertzanidou et al., 2013). It seems that mosaicism and aneuploidies are routine during the first cleavage divisions in human preimplantation development.
Blastocysts
The blastocyst is composed of two distinct parts, the trophectoderm which will become the placenta and the inner cell mass which will become the fetus (Fig. 1). Thus, the blastocyst represents the first stage of cellular differentiation in human embryonic development whereby totipotent cells become pluripotent cells. Compared with cleavage-stage embryos, similar rates of mosaicism appear to exist in the human blastocyst. Liu et al. (2012) reported that 69% of abnormal blastocysts from women of advanced age are mosaic for both the inner cell mass and trophectoderm. However, research from younger women has demonstrated that 80% of blastocysts are euploid, with a majority of the abnormal blastocysts presenting with only one or two structural chromosome abnormalities. This suggested a lower level mosaicism at the blastocyst stage (Johnson et al., 2010). Furthermore, Fragouli et al. (2011) demonstrated that roughly one-third of all blastocysts are mosaic, while Northrop et al. (2010) found that only 16% (8/50) were mosaic. Regardless of the variability in results, mosaicism is still prevalent at the blastocyst stage.
Incidence postimplantation
CPM is detected via CVS samples typically at 10–12 weeks and is typically conducted on patients who are at an increased risk of genetic abnormalities; therefore, the true incidence of CPM in a general population is unknown. Research indicates that 1–2% of viable pregnancies present with a chromosomally abnormal placenta but a normal fetus (Ledbetter et al., 1992). The diagnosis of CPM is determined from a limited number of cells from one particular biopsy site, and it is also possible that the cells sampled could accidently be from the mother (a euploid individual), an artifact known as maternal contamination. When maternal contamination is controlled for, CPM exists in roughly 6% of all pregnancies (Griffin et al., 1997). CVS sampling takes place at 10–12 weeks, when the placenta is not yet fully mature, therefore CPM quantified at CVS sampling has been shown to be different from that in term placenta (Schuring-Blom et al., 1993). When term placentas have been reanalyzed after a 10-week diagnosis of CPM, trisomic cells have been found within both the cytotrophoblast and the EEM (Fig. 1; Schuring-Blom et al., 1993; Artan et al., 1995).
A majority of these abnormalities within CPM are autosomal trisomies (Lestou and Kalousek, 1998). Unfortunately, there is not always concordance between the EEM and cytotrophoblasts. For example, trisomies 2 and 17 are more prevalent in the EEM, while trisomies 3, 6, 9, 12, 13, 14, 16, 18, 20, 21 and 22 are more prevalent in the cytotrophoblasts. The trisomies that present equally between the two tissues are chromosomes 4, 5, 7, 8, 11 and 15 (Hahnemann and Vejerslev, 1997; Lebedev, 2011). These errors could be attributed to either meiotic errors or mitotic errors during development. If meiotic in origin, the errors would also be present throughout the embryo including the EEM unless trisomic rescue occurred prior to differentiation. Likewise, it is possible that a non-disjunction event or endoreplication occurred at the onset of cellular divergence to either the EEM or cytotrophoblasts creating a mosaic within those lines compared with other embryonic and extraembryonic tissue. Regardless of how the error occurred, it is evident that chromosomes are differently affected during development.
Clinical consequences
The clinical consequences of mosaicism are dependent on a variety of factors including when during development the error occurs and if the error can continue to propagate. Because the cleavage-stage embryo only contains 4–8 cells, the consequences of chromosomal mosaicism are much more pronounced during this stage then if mosaicism occurs when more cells are present. For example, it was previously mentioned that the rate of mosaicism at the cleavage stage varies greatly from 15 to 90%; however, the rate of mosaicism seen in prenatal diagnosis ranges from 1 to 2% (Ledbetter et al., 1992). This would indicate a selection mechanism against mosaicism in the later stages of development. Therefore, mosaicism during the preimplantation stage has a greater consequence than postimplantation mosaicism.
It is believed that the actual fetus only derives from three cells of the inner cell mass (Markert and Peters, 1978). Any cells not destined to become the fetus may not be a true representation of the fetus itself but rather of extraembryonic tissue (i.e. EEM, cytotrophoblasts, placenta, chorion, etc.). Studies have shown that abnormal cells can be forced away from the fetus lineage (James and West, 1994). Stetten et al. (2004) have found that 76% of abnormal CVS results have a normal amniocentesis result; however, they note that it is unknown if the fetus presents with low, undetectable levels of mosaicism. These tissues could be mosaic while the fetus itself is normal, at least phenotypically. In line with this, Staals et al. (2003) reported a mosaic female for trisomy 12 with normal development. Down syndrome patients with low levels of mosaicism tend to have less severe manifestations than typical Down syndrome patients, indicating that the clinical significance of mosaicism is directly associated with the ratio of abnormal to normal cells (Leon et al., 2010).
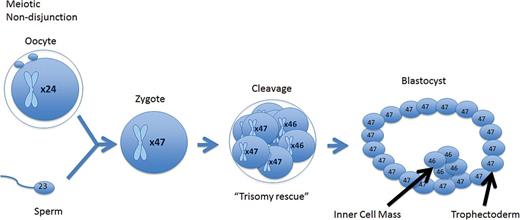
A meiotic error that is corrected at the cleavage stage resulting in a mosaic cleavage-stage embryo. When forming a blastocyst, the mosaic cell line is isolated to the trophectoderm, while the euploid cell line is isolated to the inner cell mass.
A meiotic error that is corrected at the cleavage stage resulting in a mosaic cleavage-stage embryo. When forming a blastocyst, the mosaic cell line does not become isolated and persists throughout the trophectoderm inner cell mass.
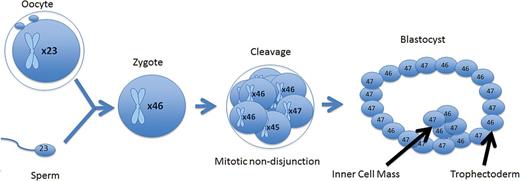
A mitotic error at the cleavage stage that resulted in a mosaic cleavage-stage embryo. When forming a blastocyst, the mosaic cell line does not become isolated and persists throughout the trophectoderm and inner cell mass.
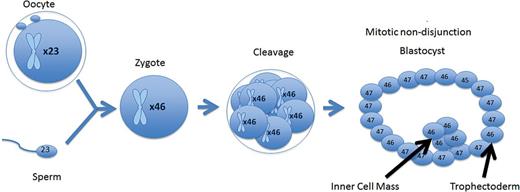
A mitotic error that occurred in the trophectoderm of the blastocyst. The blastocyst is a mosaic; however, the error is isolated to the trophectoderm, while the inner cell mass remains euploid.
The clinical consequences of confined mosaicism are dependent upon the location of the mosaicism. For example, germ line mosaicism has been found to be associated with an increase in trisomic oocytes (Delhanty, 2011). In one report, CPM of trisomy 16 was discovered to also have propagated in the oocytes of the corresponding fetus (Stavropoulos et al., 1998). Likewise, confined mosaicism for trisomy 21 was detected in the ovaries of eight fetuses that were phenotypically normal (Hulten et al., 2008, 2010). It is evident that the effects of the mosaicism depend greatly on the location of the mosaic cell line along with what chromosome is involved.
The clinical consequences of mosaicism are difficult to pinpoint and explain due to differences between individual chromosomes. For example, mosaicism of a particular chromosome may affect muscular development, while mosaicism of another chromosome may affect organ development. It is not only the particular chromosome that matters but the prevalence or sheer numbers of the particular mosaic cell line. If an individual has a few muscle cells that are mosaic and the remaining are normal, then the effects of those abnormal cells is masked by the number of normal cells. The opposite also holds true, if all but a few muscle cells are mosaic, then the normal cells will be masked by the mosaic cells.
As previously mentioned, certain trisomies are more prevalent in certain tissues. This would suggest that there is a selection against some chromosomal abnormalities at certain stages of fetal development. This could be due to different growth rates of abnormal and normal cells. Likewise, each chromosome acts differently under different conditions and at different times during prenatal development. For example, if trisomy 13, 18 and sex chromosomes are detected in CVS, they typically will also present themselves within the fetal lineage (Hahnemann and Vejerslev, 1997), while CPM presenting with trisomy 2, 3, 7 and 8 are typically not associated with any adverse events and normally lead to a chromosomally normal fetus (Kalousek et al., 1996; Hahnemann and Vejerslev, 1997; van Haelst et al., 2001; Sifakis et al., 2010). Taken together, the clinical relevance and consequences of mosaicism depends on a variety of factors.
Conclusion
The understanding that not all chromosomal mosaicism events are equal has profound consequences, particularly in the IVF community where embryos are tested for chromosome copy number during a procedure known as preimplantation genetic screening (PGS). During this procedure, the polar body, a single cell from a Day 3 cleavage-stage embryo, or multiple cells from the trophoblast (which becomes the placenta; Fig. 1) of a blastocyst is removed and chromosomal aneuploidy is determined through a variety of means. These results should reflect the chromosomal complement of the fetus; however, as described, mosaicism is both present and routine during preimplantation development, specifically at the cleavage and blastocyst stages. If aneuploidy is detected with PGS, regardless of what chromosome is involved, the embryo is deemed abnormal and discarded. Hence, the discarding of potentially viable, euploid embryos may be occurring due to mosaicism.
Mosaicism is a widespread and common occurrence in humans, prevalent in both pre- and postimplantation. The type of mosaicism and its clinical consequence is dependent upon a variety of aspects including where and when during development the aneuploidy and subsequent mosaicism is generated, and if the aneuploidy can propagate. Furthermore, each chromosome contains genes for different cellular processes. Therefore, the consequences of mosaicism are widespread and unique for each incident.
Authors' roles
T.H.T.: manuscript preparation, critique and critical discussion of manuscript. S.A.G., J.L.P, J.L.C., J.M.W. and D.K.G.: critique and critical discussion of manuscript.
Funding
No particular funding was used for this study.
Conflict of interest
The authors have no conflict of interest to declare.