-
PDF
- Split View
-
Views
-
Cite
Cite
Kun Woo Kim, Jae-Ik Lee, Ji Sung Kim, Young-Jin Lee, Won-Jun Choi, Han Jung, Kook-Yang Park, Chul-Hyun Park, Kuk-Hui Son, Risk factors for urinary retention following minor thoracic surgery, Interactive CardioVascular and Thoracic Surgery, Volume 20, Issue 4, April 2015, Pages 486–492, https://doi.org/10.1093/icvts/ivu445
Close - Share Icon Share
Abstract
Our goals were (i) to identify the incidence and risk factors of postoperative urinary retention in minor thoracic surgery patients and (ii) to develop a scoring system to predict postoperative urinary retention in these patients.
Two hundred and ninety-two consecutive patients who underwent thoracic surgery without a pre- or intraoperative indwelling urinary catheter under general anaesthesia were used to identify the risk factors of postoperative urinary retention (post-void residual urine >200 ml) and to develop the scoring system predicting the incidence of this complication. We investigated past history, type of operation, operation time, amount of administered intravenous fluids, medications used perioperatively as well as demographic data.
The incidence rate of postoperative urinary retention was 11.6% (34/292). Independent risk factors and their scores were the following: age above 40 years (P < 0.001; two points); male (P = 0.002; one point); diabetes mellitus (P = 0.002; one point) and lung resection (P < 0.001; two points). The cut-off value for a model predicting postoperative urinary retention was five points (C-index = 0.88; 95% confidence interval: 0.83–0.94), with 73% sensitivity and 90% specificity.
In minor thoracic surgery patients, special attention should be paid to detect postoperative urinary retention in those with the following characteristics: age over 40 years, male gender, history of diabetes mellitus and candidates for lung resection. The use of the developed scoring system may help in identifying those high-risk patients who need more aggressive management to prevent bladder overdistension and associated urinary complications.
INTRODUCTION
Postoperative urinary retention (POUR) is defined as an acute urinary retention following an operation, and is a frequently encountered postoperative complication [1–3]. The incidence rate of POUR has been reported with much variability to be from 5 to 70%. This wide range has been attributed to differences in patient populations, its multifactorial aetiology and the lack of uniform defining criteria [1]. The diagnosis of POUR has clinical implications such as delayed discharge, potential risk of systemic infection from urinary catheterization and possible long-term bladder dysfunction [4–6]. Although the relation between POUR and various surgical procedures such as vascular, gynaecological, colorectal, orthopaedic surgery has been reported [2, 3, 7–9], little is known about POUR following thoracic surgery.
With recent advances in the field of thoracic surgery, the awareness and identification of patients at risk of developing POUR assumes greater significance. Specifically, it can impact minor thoracic surgery including fast-track and minimally invasive surgical procedures, whose advantages can be mitigated by prolonged hospital stays and the eventual economic burden resulting from POUR. However, because there is currently no clear consensus for the detection and optimal management of POUR, a large number of thoracic surgery patients still suffer adverse outcomes related to POUR. Ideally to manage these patients, special attention to POUR is required along with the application of a commonly accepted system that can identify patients at high risk for the development of POUR.
The aims of this study were two-fold: (i) to identify the incidence and risk factors of POUR in minor thoracic surgery patients and (ii) to develop a scoring system to predict POUR in these patients.
MATERIALS AND METHODS
Strategy to avoid urological complications
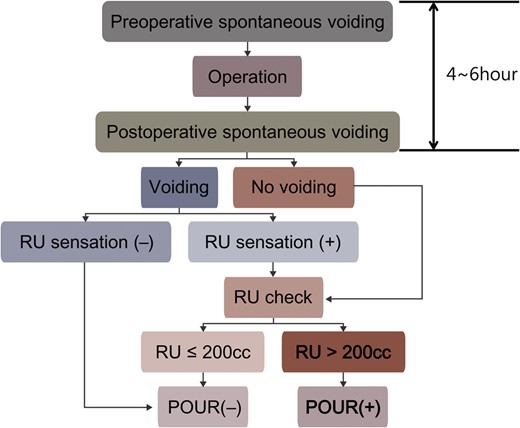
In 2010, we began to observe an increase in the occurrence of the adverse outcomes related to POUR in our thoracic surgery population. After consultation with one of our urology colleagues (Han Jung), we adopted a strict protocol for the prevention of these adverse outcomes (Fig. 1). According to the protocol, patients were routinely given intravenous fluid (10 ml/h) from the midnight before operation. On the day of operation, all patients were made to urinate immediately before they were brought into the operation room to minimize the interval between the last preoperative voiding and surgery. During the induction of general anaesthesia, indwelling urinary catheters were inserted only in the patients with operation time exceeding 3 h or who required meticulous monitoring of the intraoperative fluid status. In case of patients in whom an indwelling urinary catheter was not inserted intraoperatively, they were encouraged to ambulate early and urinate in a toilet whether they felt desire to micturate or not after the operation (within a period not exceeding 6 h from the time of preoperative voiding). If they were not able to void at all, in-and-out urinary catheterization was performed. Also in cases where the patient felt discomfort or residual urine (RU) sensation after voiding, RU was measured by catheterization. If the post-void RU volume was greater than 200 ml, the same assessments and procedures were repeated to prevent bladder overdistension at intervals of 4 h until the patients were able to void comfortably with the RU volume less than 200 ml, or after 4 failed attempts an indwelling urinary catheter was placed.
Protocol to reduce the adverse outcomes from POUR. RU: residual urine; POUR: postoperative urinary retention.
Definition of POUR
The definition of POUR remains unclear in the literature. In the present study, POUR was arbitrarily defined as an inability to void or a post-void RU volume greater than 200 ml, measured with in-and-out urinary catheterization, in the patients who felt discomfort or RU sensation after voiding 4–6 h after preoperative voiding (Fig. 1).
Patients
From June 2010 to April 2013, a total of 489 patients underwent elective thoracic surgery under general anaesthesia by a single surgeon at Gachon University Gil Hospital. Of these 489 patients, 196 patients in whom an indwelling urinary catheter was inserted before or during operation were excluded. Of the remaining 293 patients, we excluded 1 patient whose post-void RU was not able to be measured because of pre-existing anuria with continuous ambulatory peritoneal dialysis. Finally, 292 patients were enrolled in this retrospective study. No patients had epidural analgesia perioperatively for pain control, instead intravenous patient-controlled analgesia was used in 246 patients (84%, 246/292). Video-assisted thoracoscopic surgery (VATS) was performed in 223 patients (76%, 223/292), whereas 69 patients (24%, 69/292) underwent open procedures. Table 1 summarizes the details about the types of surgery. For risk analysis, the data were collected from medical records retrospectively (Table 2). Past medical histories and medications used perioperatively were investigated. Regarding fluid balance during anaesthesia, we adopted only the amount of fluid intake, because the amount of blood loss was negligible or not checked in most cases, and urine output was not able to be measured due to the absence of a urinary catheter. This study was approved by the Gachon University Gil Hospital Institutional Review Board.
Types of surgery
| Types of surgery . | n . | POUR, n (%) . |
|---|---|---|
| Total | 292 | 34 (11.6%) |
| Lung parenchyma | 178 | 32 (18.0%) |
| Wedge resection | 176 | 31 |
| Lobectomy | 2 | 1 |
| Pleura | 9 | 0 (0%) |
| Biopsy | 9 | 0 |
| Mediastinum | 40 | 2 (5%) |
| Mass excision | 29 | 1 |
| Lymph node biopsy | 11 | 1 |
| Oesophagus | 4 | 0 (0%) |
| Mass enucleation | 4 | 0 |
| Chest wall | 33 | 0 (0%) |
| Nuss operation | 12 | 0 |
| Mass excision | 12 | 0 |
| Rib resection | 8 | 0 |
| Wound repair | 1 | 0 |
| Autonomic nervous system | 28 | 0 (0%) |
| Sympathicotomy | 28 | 0 |
| Types of surgery . | n . | POUR, n (%) . |
|---|---|---|
| Total | 292 | 34 (11.6%) |
| Lung parenchyma | 178 | 32 (18.0%) |
| Wedge resection | 176 | 31 |
| Lobectomy | 2 | 1 |
| Pleura | 9 | 0 (0%) |
| Biopsy | 9 | 0 |
| Mediastinum | 40 | 2 (5%) |
| Mass excision | 29 | 1 |
| Lymph node biopsy | 11 | 1 |
| Oesophagus | 4 | 0 (0%) |
| Mass enucleation | 4 | 0 |
| Chest wall | 33 | 0 (0%) |
| Nuss operation | 12 | 0 |
| Mass excision | 12 | 0 |
| Rib resection | 8 | 0 |
| Wound repair | 1 | 0 |
| Autonomic nervous system | 28 | 0 (0%) |
| Sympathicotomy | 28 | 0 |
POUR: postoperative urinary retention.
Types of surgery
| Types of surgery . | n . | POUR, n (%) . |
|---|---|---|
| Total | 292 | 34 (11.6%) |
| Lung parenchyma | 178 | 32 (18.0%) |
| Wedge resection | 176 | 31 |
| Lobectomy | 2 | 1 |
| Pleura | 9 | 0 (0%) |
| Biopsy | 9 | 0 |
| Mediastinum | 40 | 2 (5%) |
| Mass excision | 29 | 1 |
| Lymph node biopsy | 11 | 1 |
| Oesophagus | 4 | 0 (0%) |
| Mass enucleation | 4 | 0 |
| Chest wall | 33 | 0 (0%) |
| Nuss operation | 12 | 0 |
| Mass excision | 12 | 0 |
| Rib resection | 8 | 0 |
| Wound repair | 1 | 0 |
| Autonomic nervous system | 28 | 0 (0%) |
| Sympathicotomy | 28 | 0 |
| Types of surgery . | n . | POUR, n (%) . |
|---|---|---|
| Total | 292 | 34 (11.6%) |
| Lung parenchyma | 178 | 32 (18.0%) |
| Wedge resection | 176 | 31 |
| Lobectomy | 2 | 1 |
| Pleura | 9 | 0 (0%) |
| Biopsy | 9 | 0 |
| Mediastinum | 40 | 2 (5%) |
| Mass excision | 29 | 1 |
| Lymph node biopsy | 11 | 1 |
| Oesophagus | 4 | 0 (0%) |
| Mass enucleation | 4 | 0 |
| Chest wall | 33 | 0 (0%) |
| Nuss operation | 12 | 0 |
| Mass excision | 12 | 0 |
| Rib resection | 8 | 0 |
| Wound repair | 1 | 0 |
| Autonomic nervous system | 28 | 0 (0%) |
| Sympathicotomy | 28 | 0 |
POUR: postoperative urinary retention.
Univariable analysis to determine the predictors of POUR
| Characteristics . | POUR (n = 34) . | No POUR (n = 258) . | P-value . |
|---|---|---|---|
| Patient | |||
| Age ≥40 | 30 (88%) | 107 (41%) | <0.001 |
| Male | 28 (82%) | 159 (62%) | 0.018 |
| Body mass index (kg/m2) | 23 ± 3 | 22 ± 4 | 0.118 |
| History of | |||
| Diabetes mellitus | 11 (32%) | 10 (4%) | <0.001 |
| Brain disease | 2 (6%) | 5 (2%) | 0.190 |
| Spinal cord disease | 2 (6%) | 10 (4%) | 0.418 |
| Renal disease | 1 (3%) | 7 (3%) | 0.637 |
| Previous pelvic operation | 5 (15%) | 14 (5%) | 0.055 |
| Benign prostatic hyperplasia | 2 (6%) | 3 (1%) | 0.105 |
| Laboratory finding | |||
| Preoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 6 (2%) | 0.020 |
| Postoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 7 (3%) | 0.040 |
| Preoperative creatinine >1.2 mg/dl | 1 (3%) | 3 (1%) | 0.392 |
| Postoperative creatinine >1.2 mg/dl | 2 (6%) | 3 (1%) | 0.127 |
| Urine analysis | |||
| Bacteriuria | 1 (3%) | 10 (4%) | 1.000 |
| Pyuria | 4 (12%) | 26 (10%) | 0.764 |
| Glycosuria | 6 (18%) | 8 (3%) | 0.002 |
| Ketonuria | 1 (3%) | 8 (3%) | 1.000 |
| Proteinuria | 2 (6%) | 13 (5%) | 0.689 |
| Haematuria | 2 (6%) | 15 (6%) | 1.000 |
| Anaesthesia | |||
| Fentanyl | 34 (100%) | 254 (98%) | 1.000 |
| Propofol | 34 (100%) | 252 (98%) | 1.000 |
| Zofran | 30 (88%) | 208 (81%) | 0.282 |
| Keromin | 14 (41%) | 125 (48%) | 0.425 |
| Ephedrine | 6 (18%) | 18 (7%) | 0.045 |
| Perdipine | 5 (15%) | 31 (12%) | 0.587 |
| Dexamethasone | 6 (18%) | 82 (32%) | 0.091 |
| Macperan | 13 (38%) | 102 (40%) | 0.884 |
| Atropine | 0 (0%) | 7 (3%) | 1.000 |
| Beta-blocker | 2 (6%) | 43 (17%) | 0.102 |
| Pentobarbital | 0 (0%) | 4 (2%) | 1.000 |
| Total fluid input (ml) | 331 ± 159 | 390 ± 206 | 0.107 |
| Anaesthesia time (min) | 118 ± 32 | 112 ± 39 | 0.344 |
| Operation | |||
| Operation time (min) | 75 ± 36 | 70 ± 39 | 0.430 |
| VATS | 23 (68%) | 200 (78%) | 0.203 |
| Lung resection | 32 (94%) | 146 (57%) | <0.001 |
| Chest tube | 34 (100%) | 199 (77%) | <0.001 |
| Analgesia | |||
| Opioid | 2 (6%) | 4 (2%) | 0.146 |
| NSAIDs | 23 (68%) | 120 (47%) | 0.020 |
| Patient-controlled analgesia | 33 (97%) | 213 (83%) | 0.029 |
| Characteristics . | POUR (n = 34) . | No POUR (n = 258) . | P-value . |
|---|---|---|---|
| Patient | |||
| Age ≥40 | 30 (88%) | 107 (41%) | <0.001 |
| Male | 28 (82%) | 159 (62%) | 0.018 |
| Body mass index (kg/m2) | 23 ± 3 | 22 ± 4 | 0.118 |
| History of | |||
| Diabetes mellitus | 11 (32%) | 10 (4%) | <0.001 |
| Brain disease | 2 (6%) | 5 (2%) | 0.190 |
| Spinal cord disease | 2 (6%) | 10 (4%) | 0.418 |
| Renal disease | 1 (3%) | 7 (3%) | 0.637 |
| Previous pelvic operation | 5 (15%) | 14 (5%) | 0.055 |
| Benign prostatic hyperplasia | 2 (6%) | 3 (1%) | 0.105 |
| Laboratory finding | |||
| Preoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 6 (2%) | 0.020 |
| Postoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 7 (3%) | 0.040 |
| Preoperative creatinine >1.2 mg/dl | 1 (3%) | 3 (1%) | 0.392 |
| Postoperative creatinine >1.2 mg/dl | 2 (6%) | 3 (1%) | 0.127 |
| Urine analysis | |||
| Bacteriuria | 1 (3%) | 10 (4%) | 1.000 |
| Pyuria | 4 (12%) | 26 (10%) | 0.764 |
| Glycosuria | 6 (18%) | 8 (3%) | 0.002 |
| Ketonuria | 1 (3%) | 8 (3%) | 1.000 |
| Proteinuria | 2 (6%) | 13 (5%) | 0.689 |
| Haematuria | 2 (6%) | 15 (6%) | 1.000 |
| Anaesthesia | |||
| Fentanyl | 34 (100%) | 254 (98%) | 1.000 |
| Propofol | 34 (100%) | 252 (98%) | 1.000 |
| Zofran | 30 (88%) | 208 (81%) | 0.282 |
| Keromin | 14 (41%) | 125 (48%) | 0.425 |
| Ephedrine | 6 (18%) | 18 (7%) | 0.045 |
| Perdipine | 5 (15%) | 31 (12%) | 0.587 |
| Dexamethasone | 6 (18%) | 82 (32%) | 0.091 |
| Macperan | 13 (38%) | 102 (40%) | 0.884 |
| Atropine | 0 (0%) | 7 (3%) | 1.000 |
| Beta-blocker | 2 (6%) | 43 (17%) | 0.102 |
| Pentobarbital | 0 (0%) | 4 (2%) | 1.000 |
| Total fluid input (ml) | 331 ± 159 | 390 ± 206 | 0.107 |
| Anaesthesia time (min) | 118 ± 32 | 112 ± 39 | 0.344 |
| Operation | |||
| Operation time (min) | 75 ± 36 | 70 ± 39 | 0.430 |
| VATS | 23 (68%) | 200 (78%) | 0.203 |
| Lung resection | 32 (94%) | 146 (57%) | <0.001 |
| Chest tube | 34 (100%) | 199 (77%) | <0.001 |
| Analgesia | |||
| Opioid | 2 (6%) | 4 (2%) | 0.146 |
| NSAIDs | 23 (68%) | 120 (47%) | 0.020 |
| Patient-controlled analgesia | 33 (97%) | 213 (83%) | 0.029 |
POUR: postoperative urinary retention; VATS: video-assisted thoracoscopic surgery; NSAIDs: non-steroidal anti-inflammatory drugs.
Univariable analysis to determine the predictors of POUR
| Characteristics . | POUR (n = 34) . | No POUR (n = 258) . | P-value . |
|---|---|---|---|
| Patient | |||
| Age ≥40 | 30 (88%) | 107 (41%) | <0.001 |
| Male | 28 (82%) | 159 (62%) | 0.018 |
| Body mass index (kg/m2) | 23 ± 3 | 22 ± 4 | 0.118 |
| History of | |||
| Diabetes mellitus | 11 (32%) | 10 (4%) | <0.001 |
| Brain disease | 2 (6%) | 5 (2%) | 0.190 |
| Spinal cord disease | 2 (6%) | 10 (4%) | 0.418 |
| Renal disease | 1 (3%) | 7 (3%) | 0.637 |
| Previous pelvic operation | 5 (15%) | 14 (5%) | 0.055 |
| Benign prostatic hyperplasia | 2 (6%) | 3 (1%) | 0.105 |
| Laboratory finding | |||
| Preoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 6 (2%) | 0.020 |
| Postoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 7 (3%) | 0.040 |
| Preoperative creatinine >1.2 mg/dl | 1 (3%) | 3 (1%) | 0.392 |
| Postoperative creatinine >1.2 mg/dl | 2 (6%) | 3 (1%) | 0.127 |
| Urine analysis | |||
| Bacteriuria | 1 (3%) | 10 (4%) | 1.000 |
| Pyuria | 4 (12%) | 26 (10%) | 0.764 |
| Glycosuria | 6 (18%) | 8 (3%) | 0.002 |
| Ketonuria | 1 (3%) | 8 (3%) | 1.000 |
| Proteinuria | 2 (6%) | 13 (5%) | 0.689 |
| Haematuria | 2 (6%) | 15 (6%) | 1.000 |
| Anaesthesia | |||
| Fentanyl | 34 (100%) | 254 (98%) | 1.000 |
| Propofol | 34 (100%) | 252 (98%) | 1.000 |
| Zofran | 30 (88%) | 208 (81%) | 0.282 |
| Keromin | 14 (41%) | 125 (48%) | 0.425 |
| Ephedrine | 6 (18%) | 18 (7%) | 0.045 |
| Perdipine | 5 (15%) | 31 (12%) | 0.587 |
| Dexamethasone | 6 (18%) | 82 (32%) | 0.091 |
| Macperan | 13 (38%) | 102 (40%) | 0.884 |
| Atropine | 0 (0%) | 7 (3%) | 1.000 |
| Beta-blocker | 2 (6%) | 43 (17%) | 0.102 |
| Pentobarbital | 0 (0%) | 4 (2%) | 1.000 |
| Total fluid input (ml) | 331 ± 159 | 390 ± 206 | 0.107 |
| Anaesthesia time (min) | 118 ± 32 | 112 ± 39 | 0.344 |
| Operation | |||
| Operation time (min) | 75 ± 36 | 70 ± 39 | 0.430 |
| VATS | 23 (68%) | 200 (78%) | 0.203 |
| Lung resection | 32 (94%) | 146 (57%) | <0.001 |
| Chest tube | 34 (100%) | 199 (77%) | <0.001 |
| Analgesia | |||
| Opioid | 2 (6%) | 4 (2%) | 0.146 |
| NSAIDs | 23 (68%) | 120 (47%) | 0.020 |
| Patient-controlled analgesia | 33 (97%) | 213 (83%) | 0.029 |
| Characteristics . | POUR (n = 34) . | No POUR (n = 258) . | P-value . |
|---|---|---|---|
| Patient | |||
| Age ≥40 | 30 (88%) | 107 (41%) | <0.001 |
| Male | 28 (82%) | 159 (62%) | 0.018 |
| Body mass index (kg/m2) | 23 ± 3 | 22 ± 4 | 0.118 |
| History of | |||
| Diabetes mellitus | 11 (32%) | 10 (4%) | <0.001 |
| Brain disease | 2 (6%) | 5 (2%) | 0.190 |
| Spinal cord disease | 2 (6%) | 10 (4%) | 0.418 |
| Renal disease | 1 (3%) | 7 (3%) | 0.637 |
| Previous pelvic operation | 5 (15%) | 14 (5%) | 0.055 |
| Benign prostatic hyperplasia | 2 (6%) | 3 (1%) | 0.105 |
| Laboratory finding | |||
| Preoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 6 (2%) | 0.020 |
| Postoperative blood urea nitrogen >22 mg/dl | 4 (12%) | 7 (3%) | 0.040 |
| Preoperative creatinine >1.2 mg/dl | 1 (3%) | 3 (1%) | 0.392 |
| Postoperative creatinine >1.2 mg/dl | 2 (6%) | 3 (1%) | 0.127 |
| Urine analysis | |||
| Bacteriuria | 1 (3%) | 10 (4%) | 1.000 |
| Pyuria | 4 (12%) | 26 (10%) | 0.764 |
| Glycosuria | 6 (18%) | 8 (3%) | 0.002 |
| Ketonuria | 1 (3%) | 8 (3%) | 1.000 |
| Proteinuria | 2 (6%) | 13 (5%) | 0.689 |
| Haematuria | 2 (6%) | 15 (6%) | 1.000 |
| Anaesthesia | |||
| Fentanyl | 34 (100%) | 254 (98%) | 1.000 |
| Propofol | 34 (100%) | 252 (98%) | 1.000 |
| Zofran | 30 (88%) | 208 (81%) | 0.282 |
| Keromin | 14 (41%) | 125 (48%) | 0.425 |
| Ephedrine | 6 (18%) | 18 (7%) | 0.045 |
| Perdipine | 5 (15%) | 31 (12%) | 0.587 |
| Dexamethasone | 6 (18%) | 82 (32%) | 0.091 |
| Macperan | 13 (38%) | 102 (40%) | 0.884 |
| Atropine | 0 (0%) | 7 (3%) | 1.000 |
| Beta-blocker | 2 (6%) | 43 (17%) | 0.102 |
| Pentobarbital | 0 (0%) | 4 (2%) | 1.000 |
| Total fluid input (ml) | 331 ± 159 | 390 ± 206 | 0.107 |
| Anaesthesia time (min) | 118 ± 32 | 112 ± 39 | 0.344 |
| Operation | |||
| Operation time (min) | 75 ± 36 | 70 ± 39 | 0.430 |
| VATS | 23 (68%) | 200 (78%) | 0.203 |
| Lung resection | 32 (94%) | 146 (57%) | <0.001 |
| Chest tube | 34 (100%) | 199 (77%) | <0.001 |
| Analgesia | |||
| Opioid | 2 (6%) | 4 (2%) | 0.146 |
| NSAIDs | 23 (68%) | 120 (47%) | 0.020 |
| Patient-controlled analgesia | 33 (97%) | 213 (83%) | 0.029 |
POUR: postoperative urinary retention; VATS: video-assisted thoracoscopic surgery; NSAIDs: non-steroidal anti-inflammatory drugs.
Statistical analysis
Statistical analysis was done using the standard statistics software (SAS, version 9.3; SAS Institute, Cary, NC, USA). The χ2 test and Fisher's exact test were used to compare categorical variables, and Student's t-test was used to compare the means of continuous variables. All the significant variables in univariable analyses were put into the multivariable model after considering co-linearity. To reduce the model, we performed variable selection using a stepwise method with the significance level of 0.1 for entering into the model and the significance level of 0.2 for staying in the model. Although the selected variables seemed clinically relevant, the model building method was repeated with 1000 bootstrap samples to select reliable variables. If the final stepwise model variables occur in a majority (>50%) of the bootstrap models, the original final stepwise regression model can be judged to be stable [10]. Only reliable (bootstrap frequency >50% in 1000 simulated samples) predictors were used to construct the final aggregate score to predict POUR. Internal validation of the model was assessed by bootstrapping. Optimism-corrected C-index was estimated using 200 bootstrap samples. Finally, the scoring system to predict POUR was developed by proportional weighting of the significant and reliable predictor estimates. A P-value of less than 0.05 was considered statistically significant.
RESULTS
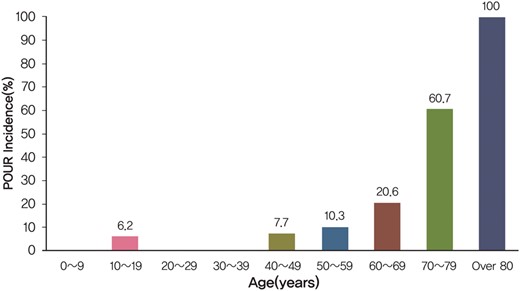
In our cohort, 34 patients (11.6%, 34/292) met our criteria for POUR. In most cases (94%, 32/34), it was detected after lung resection (Table 1). As shown in Fig. 2, the incidence of POUR tended to increase with increasing age and rose sharply in the patients who were 40 years or older. Therefore, the age of 40 years was used to categorize the age variable in the risk analysis when determining the predictors of POUR. Of 34 POUR patients, 5 patients eventually underwent indwelling urinary catheterization after 4 failed attempts using in-and-out urinary catheters, each for an average of 8 days (range 2–18 days). One of these patients was subsequently treated for a urinary tract infection. The mean hospital stay was longer for patients with POUR than for those without POUR, though it did not reach statistical significance (10 ± 5 vs 7 ± 4 days, P = 0.286).
Incidence of POUR according to the patients' age. POUR: postoperative urinary retention.
Predictors of POUR following minor thoracic surgery
Risk analysis was performed to determine the predictors of POUR following minor thoracic surgery. Univariable analysis revealed that old age, male gender, history of diabetes mellitus (DM), elevated preoperative and postoperative blood urea nitrogen, glycosuria in the preoperative urine analysis, lung resection, chest tube insertion and the use of patient controlled analgesia, non-steroidal anti-inflammatory drugs (NSAIDs) and ephedrine hydrochloride were significantly associated with POUR (Table 2). However, in multivariable analysis of those variables, age over 40 (odds ratio [OR], 12.424; 95% confidence interval [CI]: 3.899–39.583; P < 0.001), male gender (OR, 5.319; 95% CI: 1.844–15.341; P = 0.002), history of DM (OR, 7.026; 95% CI: 2.040–24.195; P = 0.002) and candidate for lung resection (OR, 17.333; 95% CI: 3.571–84.122; P < 0.001) were independent predictors of POUR. The full and reduced models were presented in Table 3.
Full and reduced multivariable models
| Variables . | Full model . | Reduced modela . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age ≥40 | 11.527 | 3.422–38.824 | 12.424 | 3.899–39.583 |
| Male | 5.585 | 1.867–16.709 | 5.319 | 1.844–15.341 |
| Diabetes mellitus | 8.263 | 2.287–29.850 | 7.026 | 2.040–24.195 |
| Lung resection | 14.004 | 2.540–77.200 | 17.333 | 3.571–54.122 |
| Preoperative elevated blood urea nitrogen | 0.977 | 0.893–1.068 | ||
| Chest tube | 1.087 | 0.001–infinite | ||
| Patient-controlled analgesia | 1.506 | 0.039–58.132 | ||
| Ephedrine | 1.752 | 0.467–6.573 | ||
| NSAIDs | 2.396 | 0.887–6.471 | ||
| Variables . | Full model . | Reduced modela . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age ≥40 | 11.527 | 3.422–38.824 | 12.424 | 3.899–39.583 |
| Male | 5.585 | 1.867–16.709 | 5.319 | 1.844–15.341 |
| Diabetes mellitus | 8.263 | 2.287–29.850 | 7.026 | 2.040–24.195 |
| Lung resection | 14.004 | 2.540–77.200 | 17.333 | 3.571–54.122 |
| Preoperative elevated blood urea nitrogen | 0.977 | 0.893–1.068 | ||
| Chest tube | 1.087 | 0.001–infinite | ||
| Patient-controlled analgesia | 1.506 | 0.039–58.132 | ||
| Ephedrine | 1.752 | 0.467–6.573 | ||
| NSAIDs | 2.396 | 0.887–6.471 | ||
NSAIDs: non-steroidal anti-inflammatory drugs.
aStepwise logistic regressing analyses using the bootstrap resampling technique were applied to reduce the model. Only reliable predictors (i.e. bootstrap frequency >50% in 1000 simulated samples) were used to construct a reduced model.
Full and reduced multivariable models
| Variables . | Full model . | Reduced modela . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age ≥40 | 11.527 | 3.422–38.824 | 12.424 | 3.899–39.583 |
| Male | 5.585 | 1.867–16.709 | 5.319 | 1.844–15.341 |
| Diabetes mellitus | 8.263 | 2.287–29.850 | 7.026 | 2.040–24.195 |
| Lung resection | 14.004 | 2.540–77.200 | 17.333 | 3.571–54.122 |
| Preoperative elevated blood urea nitrogen | 0.977 | 0.893–1.068 | ||
| Chest tube | 1.087 | 0.001–infinite | ||
| Patient-controlled analgesia | 1.506 | 0.039–58.132 | ||
| Ephedrine | 1.752 | 0.467–6.573 | ||
| NSAIDs | 2.396 | 0.887–6.471 | ||
| Variables . | Full model . | Reduced modela . | ||
|---|---|---|---|---|
| OR . | 95% CI . | OR . | 95% CI . | |
| Age ≥40 | 11.527 | 3.422–38.824 | 12.424 | 3.899–39.583 |
| Male | 5.585 | 1.867–16.709 | 5.319 | 1.844–15.341 |
| Diabetes mellitus | 8.263 | 2.287–29.850 | 7.026 | 2.040–24.195 |
| Lung resection | 14.004 | 2.540–77.200 | 17.333 | 3.571–54.122 |
| Preoperative elevated blood urea nitrogen | 0.977 | 0.893–1.068 | ||
| Chest tube | 1.087 | 0.001–infinite | ||
| Patient-controlled analgesia | 1.506 | 0.039–58.132 | ||
| Ephedrine | 1.752 | 0.467–6.573 | ||
| NSAIDs | 2.396 | 0.887–6.471 | ||
NSAIDs: non-steroidal anti-inflammatory drugs.
aStepwise logistic regressing analyses using the bootstrap resampling technique were applied to reduce the model. Only reliable predictors (i.e. bootstrap frequency >50% in 1000 simulated samples) were used to construct a reduced model.
Scoring system to predict the risk of POUR
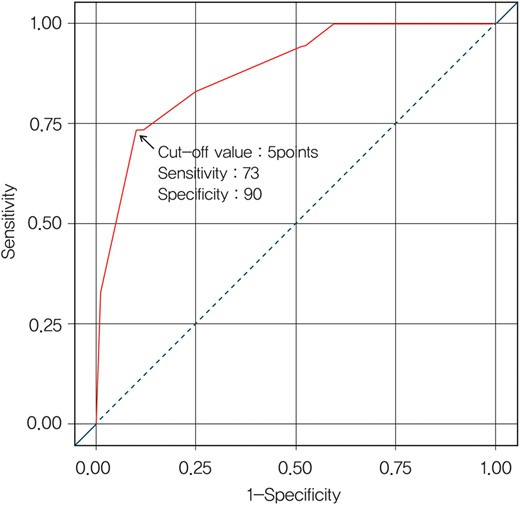
Based on the results of risk analysis, we developed a scoring system to predict the risk of POUR. Stepwise logistic regression identified the following significant and reliable predictors of POUR: age over age 40 (coefficient, 2.520; standard error [SE], 0.591; bootstrap frequency, 74%), male gender (coefficient, 1.671; SE, 0.540; bootstrap frequency, 59%), history of DM (coefficient, 1.950; SE, 0.631; bootstrap frequency, 77%) and candidate for lung resection (coefficient, 2.853; SE, 0.806; bootstrap frequency, 67%). A scoring system for POUR was established using weighting factors that were based on relative size of the regression coefficient of each variable. The points of individual factors were the following: male gender and history of DM, one point, and age over age 40 and candidates for lung resection, two points. To obtain a cumulative score, the individual points were summed for each factor to obtain a score from 0 to 6. Factors were then grouped into two risk classes according to their aggregate scores (cut-off value = five points with 73% sensitivity and 90% specificity), which were significantly associated with an incremental risk of POUR. A cut-off level was established using the point closest to the top-left part of the receiver operating characteristic (ROC) curve (Fig. 3). Patients in Class A (score ≤4) had a 3.7% risk of POUR, whereas patients in Class B (score ≥5) had a 49% risk of POUR (P < 0.001; Table 4). The apparent C-index was 0.88 and the optimism-corrected C-index was 0.87.
Scoring system and incidence of POUR by class of risk
| Risk class . | 292 patients . | ||
|---|---|---|---|
| POUR (+) . | POUR (−) . | Incidence of POUR (%) . | |
| Class A (score 0–4; low risk) | 9 | 232 | 3.7 |
| Class B (score 5, 6; high risk) | 25 | 26 | 49.0 |
| χ2, P-value | <0.001 | ||
| C-index | 0.88 (95% confidence limits 0.83 and 0.94) | ||
| Risk class . | 292 patients . | ||
|---|---|---|---|
| POUR (+) . | POUR (−) . | Incidence of POUR (%) . | |
| Class A (score 0–4; low risk) | 9 | 232 | 3.7 |
| Class B (score 5, 6; high risk) | 25 | 26 | 49.0 |
| χ2, P-value | <0.001 | ||
| C-index | 0.88 (95% confidence limits 0.83 and 0.94) | ||
POUR: postoperative urinary retention.
Scoring system and incidence of POUR by class of risk
| Risk class . | 292 patients . | ||
|---|---|---|---|
| POUR (+) . | POUR (−) . | Incidence of POUR (%) . | |
| Class A (score 0–4; low risk) | 9 | 232 | 3.7 |
| Class B (score 5, 6; high risk) | 25 | 26 | 49.0 |
| χ2, P-value | <0.001 | ||
| C-index | 0.88 (95% confidence limits 0.83 and 0.94) | ||
| Risk class . | 292 patients . | ||
|---|---|---|---|
| POUR (+) . | POUR (−) . | Incidence of POUR (%) . | |
| Class A (score 0–4; low risk) | 9 | 232 | 3.7 |
| Class B (score 5, 6; high risk) | 25 | 26 | 49.0 |
| χ2, P-value | <0.001 | ||
| C-index | 0.88 (95% confidence limits 0.83 and 0.94) | ||
POUR: postoperative urinary retention.
ROC curve according to the cumulative score; 73% sensitivity and 90% specificity at the cut-off value of five points and the area under the curve is 0.8825.
DISCUSSION
Currently, there are no or limited data on how many patients are diagnosed with POUR following thoracic surgery and which factors influence the development of POUR in these patients. Most previous studies evaluated POUR in heterogeneous groups that included patients who underwent various types of surgeries and anaesthesia. In those studies, the number of thoracic surgery patients was too small to determine the incidence and the risk factors of POUR [11–15]. Our cohort is large (n = 292) and composed only of patients who underwent relatively minor thoracic surgery. In addition, our population was not biased by conduction blockade, such as spinal/epidural anaesthesia or analgesia, which is known to have a significant influence on the development of POUR [13–15]. Thus, in conjunction with the use of a strict protocol to detect and define POUR, the present study has the potential to contribute important information about the incidence and predictors of POUR in the field of thoracic surgery.
In our protocol shown in Fig. 1, POUR was defined as an inability to void or a post-void RU volume greater than 200 ml, when measured 4–6 h after preoperative voiding. The duration of POUR that can lead to persistent bladder dysfunction is not clear at this time. Some studies showed that, although normal bladder capacity ranges between 400 and 600 ml [11, 16], a transient filling volume between 500 and 1000 ml is not harmful if diagnosed and treated early within 1–2 h [16, 17]. On the other hand, an animal study showed that bladder overdistension for periods between 4 and 24 h reduced the bladder's ability to contract due to ischaemia, and can lead to persistent bladder dysfunction [6]. These results suggest that early catheterization decreases the incidence of long-term voiding dysfunction [6]. Based on the results of these studies, we adopted the aforementioned protocol in which the assessment of POUR is performed 4–6 h after preoperative voiding. Under this protocol, patients had enough time to void after surgery, because the mean operation time in our cohort was just about an hour (75 min in the POUR group and 70 min in the NO POUR group; Table 3). In terms of the clinically meaningful amount of POUR, many previous studies adopted the criteria of 500–600 ml of bladder volume or RU [2, 3, 7, 8, 11, 12]. However, in the present study, we focused on the voiding function, which is of greater clinical importance, than focusing on bladder volume alone. It is known that the patients with normal bladder function can empty their bladder even without a desire for micturition, if they are asked to. Thus, ‘post-void’ RU, the amount of RU in the bladder after a voluntary voiding, can be a useful screening test for evaluating bladder dysfunction. Although threshold values delineating what constitutes an abnormal post-void RU are poorly defined, most urologists agree that volumes of 50–100 ml in an adult, and 150 ml in old age, constitute the threshold for abnormal post-void RU volume [16]. In these respects, we adopted a post-void RU volume greater than 200 ml as the clinically meaningful amount of POUR.
In the present study, we found that the incidence of POUR tended to increase with increasing age. This finding is supported by another study where old age (>50 years) was determined to be an independent risk factor of POUR [12]. It has also been shown that older age tends to be associated with many types of bladder dysfunction such as decreased capacity, urinary flow rate and increased post-void RU volume. This can possibly be explained by age-related progressive degeneration of the nervous system [18]. It seems that such a predisposition in these patients may promote the development of POUR. Another consideration is the differences in the rates of POUR in male and female patients. In agreement with our findings, Tammela et al. [19] have reported a higher incidence of POUR in male patients compared with females. The differences in the anatomy of urinary outflow track between male and female may contribute to this finding. Furthermore, diagnosed or undiagnosed benign prostatic hypertrophy, which is gender-specific, could be a contributing cause of POUR in male patients [19]. In the present study, the risk of developing POUR was increased if the patients had coexisting DM. In a study covering several types of surgical procedures, Dreijer et al. [3] also found that diabetes was an independent risk factor for developing POUR. It is well known that diabetes predisposes patients to the development of urinary problems caused by diabetic neuropathy, which can affect all types of nerve fibres including those that innervate the bladder [20, 21].
It seems somewhat intuitive that old male patients having DM would have a higher likelihood of POUR. However, in the present study, we also found that lung resection increased the incidence of POUR. On reviewing the published data, there has been no study showing the causal relationship between lung resection and POUR. One of the most notable differences between lung resection and other thoracic procedures is the proportion of the patients to whom chest tubes were inserted at the completion of the procedure. For example, in the present study, while all patients undergoing lung resection had chest tubes, none were inserted into any patients undergoing sympathicotomy. It is natural that the patients to whom chest tubes were inserted feel more pain in the postoperative period than those without chest tubes. It is well known that postoperative pain activates the sympathetic pathways resulting in the relaxation of the detrusor and inhibiting relaxation of the internal urethral sphincter, and eventually leads to POUR [7, 8]. In agreement with this hypothesis, in our cohort, we found a positive correlation between chest tube insertion and the use of NSAIDs. The proportion of the patients who required NSAIDs was significantly higher in those with chest tubes than in those without (55.8 vs 22.0%, P < 0.001, data not shown). Considering this fact, it seems more reasonable to relate a high incidence of POUR in the lung resection group to increased pain, rather than to deduce that the procedure itself might have directly influenced the urinary system leading to POUR.
Although it did not reach statistical significance, POUR patients' mean hospital stay was 3 days longer than those who did not have POUR. Additionally, 14.7% (5/34) of all patients with POUR had to have indwelling urinary catheters placed. The average period of catheterization was 8 days. Most of these patients went on to specialized treatment by an urologist, and one of them developed a urinary tract infection. Thus, POUR may increase the morbidity rate, hospital stay and economic burden for the patients. To avoid adverse outcomes like these in clinical practice, a commonly accepted system that could standardize the selection of patients who need more careful observation for potential bladder dysfunction is required. Therefore, we developed an aggregate score to stratify the risk of POUR in thoracic surgery patients. From the results of this analysis, two risk classes were identified. It was readily apparent that patients in Class A had a lower risk of POUR, whereas patients in Class B had a higher risk of POUR (Table 4). Patients in Class B need more aggressive management to prevent bladder overdistension. In these high-risk patients, POUR-associated urinary complications may be prevented by aggressive strategies like the intraoperative placement of indwelling urinary catheter and maintenance for about 24 h postoperatively [22], or the prophylactic therapy with α-blocker in older men [23], or more frequent assessments of bladder volume by ultrasound at a short interval. This risk stratification system may therefore have a positive clinical and economic impact, reducing the incidence of postoperative urinary complications and hospital stay.
There are some potential limitations in this study. First, because we excluded patients who underwent intraoperative indwelling urinary catheterization, our cohort is mainly composed of patients who underwent relatively minor thoracic surgery, and is not representative of all thoracic surgery patients. Thus, the scoring system that we suggest cannot tell which of the patients who underwent intraoperative catheterization will be at higher risk of POUR. However, because intraoperative catheterization in itself may affect POUR by causing urethral trauma, prostatitis and patient discomfort [4, 5], and has the potential to bias the study population, it does not seem to be appropriate to analyse both groups (with or without intraoperative catheter) together. Thus, the risk analysis in the intraoperative-catheter group, which is beyond the scope of this study, should be performed separately. Despite this limitation, with the increase in the number of patients who undergo fast-track and minimally invasive thoracic surgical procedures, the data from our cohort can provide valuable information for perioperative management of these patients. Secondly, because our cohort consists of patients who were selected based on our strict preoperative protocol for POUR, our work has a limitation to be applied directly to other units which have no specific protocols like ours. However, the definition of POUR itself is inevitably arbitrary for now, and every single paper published about POUR has adopted different protocols. Although it is not certain whether our strict protocol affected the incidence of POUR or not, we think it is helpful for detecting POUR early, and eventually preventing the adverse outcomes from POUR such as delayed discharge, potential risk of systemic infection from urinary catheterization and possible long-term bladder dysfunction, etc. Thus, the result of this study, including our protocol, can be a good reference and a starting point for future studies. Third relates to how lung resection may influence POUR. As mentioned previously, POUR may result from increased pain from the chest tube, not because of the procedure itself. However, this interpretation cannot be confirmed because we did not assess the patients' postoperative pain directly, though we saw an increase in the use of NSAIDs in the patients with chest tubes. In addition, although chest tube insertion was a significant factor in univariable analysis (Table 2), the type of procedure was eventually the most powerful factor in multivariable analysis. Therefore, we cannot totally exclude the influence of other factors related directly to the lung resection procedure. With this in mind, future studies should be designed to take into account these factors; for example, by incorporating a pain scale or measuring the amount and/or duration of analgesics used by the patient. Finally, the scoring system in the present study was not validated in an independent patient sample. Although only reliable predictors, which were finally assessed by using a bootstrap resampling technique with 1000 samples, were used to contrast the final aggregate score, it should be emphasized that internal validity does not guarantee external validity. Future independent studies are needed to verify its applicability in other centres.
In our cohort, which mainly consists of minor thoracic surgery patients, 11.6% of patients suffered from POUR in their immediate postoperative period. The predictors of POUR were as follows: age over 40 years, male gender, history of DM and candidates for lung resection. The use of the developed scoring system may help in identifying those high-risk patients who need more aggressive management to prevent bladder overdistension and associated urinary complications.
Conflict of interest: none declared.





Comments
© The Author 2015. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved
Given the potential impact of postoperative urinary retention (POUR) on morbidity and duration of hospital stay [1-2], I read with great interest Kim et al. 's paper on the risk factors for urinary retention following minor thoracic surgery [3].
Of particular interest was the increased incidence of POUR in patients undergoing lung resection, a finding which has not been apparent in previous research [3]. Kim et al. attribute this to increased pain secondary to chest drain insertion, although they recognize that postoperative pain was not a specific outcome measure in their study [3]. We are informed that in their cohort, intravenous fluid was administered at a rate of 10 ml/h preoperatively. It seems relevant here to consider that increased perioperative fluid administration is a risk factor for lung injury following pulmonary resection [4]. In their randomized controlled trial on fluid management during video-assisted thoracoscopic surgery (VATS) for lung resection, Matot et al. conclude that intraoperative urinary output and postoperative renal function were not affected by fluid administration where Ringer's lactate was administered in the range of 2 to 8 ml/(kg · h) [5]. Particularly given that 76% of patients underwent VATS procedures in Kim et al. 's cohort [3], arguably it would be interesting to know the composition of fluid administered, the patient's weight, and whether any individuals experienced pulmonary complications.
As Kim et al. 's paper highlights, recognition of the risk factors for POUR is vital in order to identify at-risk groups preoperatively, with a view to reducing patient burden both in terms of a prolonged postoperative recovery and the potential for long-term implications on quality of life [3]. With increasing numbers of patients undergoing minimally invasive thoracic procedures, this paper provides a valuable contribution to the literature. Comparing these findings with patient outcomes following major and emergency thoracic surgery is recognized by the authors as an area for further research [3].
References
[1] Dreijer B, Moller MH, Bartholdy J. Post-operative urinary retention in a general surgical population. Eur J Anaesthesiol 2011;28:190-194.
[2] Brouwer TA, Rosier PF, Moons KG, Zuithoff NP, van Roon EN, Kalkman CJ. Postoperative bladder catheterization based on individual bladder capacity: a randomized trial. Anesthesiology 2014;doi: 10.1097/ALN.0000000000000507.
[3] Kim KW, Lee J-L, Kim JS, Lee Y-L, Cho W-J, Jung H et al. Risk factors for urinary retention following minor thoracic surgery. Interact CardioVasc Thorac Surg 6 January 2015;doi:10.1093/icvts/ivu445.
[4] Chau EH, Slinger P. Perioperative fluid management for pulmonary resection surgery and oesophagectomy. Semin Cardiothorac Vasc Anesth 2014;18:36-44.
[5] Matot I, Dery E, Bulgov Y, Cohen B, Paz J, Nesher N. Fluid management during video-assisted thoracoscopic surgery for lung resection: a randomized controlled trial of effects on urinary output and postoperative renal function. J Thorac Cardiovasc Surg 2013;146:461-466.
Conflict of interest: none declared