-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Röösli, Nino Künzli, Charlotte Braun-Fahrländer, Matthias Egger, Years of life lost attributable to air pollution in Switzerland: dynamic exposure–response model, International Journal of Epidemiology, Volume 34, Issue 5, October 2005, Pages 1029–1035, https://doi.org/10.1093/ije/dyi106
Close - Share Icon Share
Abstract
Background There is debate on how the effect of air pollution should be assessed. We propose an approach to estimate its impact on adult and infant mortality that integrates data from long-term epidemiological studies and studies of interventions to reduce pollution. We use the method to estimate the number of years of life lost (YLLs) attributable to air pollution during 1 year in Switzerland.
Methods A dynamic exposure–response model was implemented, which uses an exponential function (exp−kt) to model the change in mortality after cessation of air pollution. The model was populated with relative risk estimates and estimates of time constant k from the literature. Air pollution exposure in Switzerland was modelled using data from emission inventories. YLLs attributable to air pollution were calculated by taking the difference between observed survival probabilities in Switzerland in 2000 and modified survival probabilities, assuming no air pollution during the year 2000.
Results Meta-analyses of three studies of adult mortality and five studies of infant mortality gave relative risks of 1.059 (95% confidence interval (CI) 1.031–1.088) and 1.056 (95% CI 1.026–1.088) per 10 μg/m3 increase in PM10 concentration. Time constants k derived from two studies of the effects of the closing down of a steel mill in the Utah Valley and of the coal ban in Dublin were 0.88 and 0.11. Assuming a time constant k of 0.5 resulted in 42 400 (95% CI 22 600–63 600) YLLs, with 4.0% being ascribed to infant deaths. A total of 39% of the effect occurred in the same year and 80% within 5 years. The estimated number of YLLs was little affected by the choice of the time constant.
Conclusions In contrast to traditional steady-state models the dynamic model allows changes in mortality following short-term increases or decreases in air pollution levels to be quantified. This type of information is of obvious interest to policy makers.
There is increasing evidence from epidemiological studies that outdoor air pollution is a determinant of mortality at the population level, but there is debate on how exactly its impact should be assessed. Reliable estimation of the burden of air pollution on the health of the public is essential to inform environmental policy. Seminal early work focused on quantifying the acute, short-term effects of ambient air pollution.1 Today, health impact assessments are generally based on estimating morbidity and premature deaths2–5 as well as the number of years of life lost (YLLs),6–8 using concentration–response functions derived from cohort studies with long-term follow-up. These studies assume that levels of air pollution vary across different population groups but remain constant over time within groups. Estimates of the change in risk following an intervention that leads to sustained increases or decreases in air pollution levels are scarce, despite the fact that this type of information is of obvious interest to policy makers.
A Health Effects Institute working group recently concluded that observational studies of interventions aiming to reduce air pollution may be useful in the context of health impact assessments.9 This includes studies that examined the health effect of a sudden decrease or increase in exposure levels, for example, owing to a change in laws or regulations, or following the start or the end of operation of an air-polluting facility. The working group argued that data from such studies should inform future models of the health impact of air pollution.
We propose an approach to estimate the impact of air pollution on adult and infant mortality, which integrates data from long-term epidemiological studies and air pollution intervention studies. In this paper we describe the model, apply it to the estimation of the number of YLLs attributable to air pollution in Switzerland and compare results with those obtained with previously used methods.10,11
Methods
Based on the work by Leksell and Rabl,6 we developed a concentration–response model, which estimates the course of mortality after a sudden reduction of air pollution exposure. A reference scenario based on observed survival probabilities with the actual PM10 levels was compared with a hypothetical scenario where PM10 levels in Switzerland were reduced to 7.5 μg/m3 during 1 year (2000). Estimates of concentration–response associations between air pollution and mortality were obtained from the literature and used to modify the observed survival probabilities, taking into account that the effect of reduced levels during one year will wane with time. Life tables for the Swiss population were calculated using the modified and the observed survival probabilities. The difference between the two life tables is interpreted as the YLLs owing to the population's exposure to air pollution during the year 2000.
Identification and selection of relevant studies
We aimed to identify all population-based cohort studies of air pollution and adult mortality, which estimated the association between mortality and exposure to particulate matter (PM2.5, PM10, TSP, or black smoke). Cohort studies capture both short-term and long-term effects of exposure to air pollution. When several analyses from the same cohort were available, the most recent results were considered. For infant mortality, effects of long-term exposure are less relevant by definition, and effect estimates were derived from cohort studies as well as from case–control studies and time series analyses. Studies that reported the effect on mortality of a sudden sustained change in particulate matter (PM2.5, PM10, TSP, or black smoke) were considered eligible ‘intervention studies’. We searched Medline and Embase from inception to July 2003, the LUDOK specialist database (http://www.unibas.ch/ispmbs/LuG/welcome.html), and checked review articles and conference abstracts for eligible studies. We considered studies in any language.
Dynamic exposure–response model
We extracted estimates of the relative risk of death from all non-violent causes from studies in adults ≥30 years and studies of infant mortality. No studies were identified for the age group 1–30 years. For each study, risk ratios were standardized to a change of 10 μg/m3 in PM10 exposure. PM2.5 and black smoke concentration were converted into PM10 concentration using a conversion factor of 1.33.12,13 We used random-effects and fixed-effects meta-analysis to combine standardized risk ratios from different studies. We calculated the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance and performed standard tests of heterogeneity.14,15
Application to Switzerland
We used the model described above to estimate the number of YLLs attributable to air pollution in Switzerland in 2000. We estimated population exposure using a dispersion model that considers primary particulate matter, secondary particles formed in the atmosphere from precursor emissions, and transboundary large-scale PM10.16 Model inputs were emission inventories of PM10 as well as emission inventories or modelled exposure distributions of the precursor substances (NO2, SO2, VOC). We modelled population exposure distribution at a spatial resolution of 0.04 km2 (200 m × 200 m grid).17 The lowest exposure level was 7.5 μg/m3. The possible health impact of air pollution exposure below this concentration was not considered.
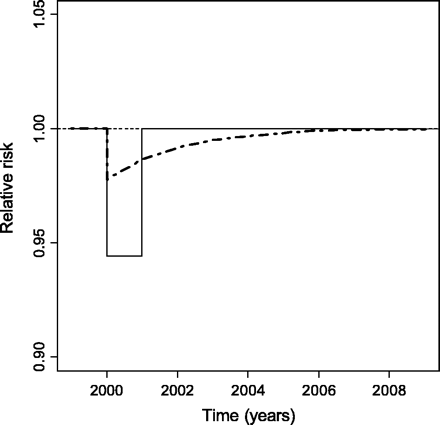
YLLs were calculated based on life tables, using the observed survival probabilities in Switzerland in the year 2000 as the reference scenario. Using modified survival functions the alternative scenario assumed that the population was not exposed to air pollution >7.5 μg/m3 during the year 2000; thereafter air pollution levels returned to previous values. According to the steady state model, reduction of air pollution will result in increased survival probabilities exclusively in the respective year. With the dynamic model survival probabilities will also be affected in subsequent years (Figure 1). The steady state model was used for infant mortality and the dynamic model for adult mortality. The difference between the life tables obtained from the reference and the alternative scenario is interpreted as the YLLs attributable to air pollution during the year 2000.
Time course of relative risk of death after a sudden decrease in air pollution exposure during the year 2000, assuming a steady state model (solid line) and a dynamic model (bold dashed line). The thin dashed line refers to the reference scenario
Results
Identification and selection of studies
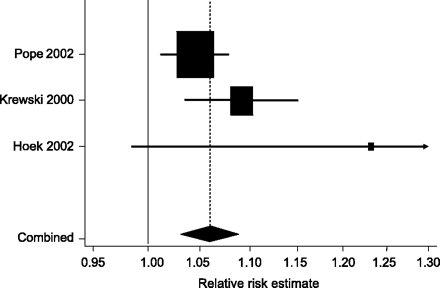
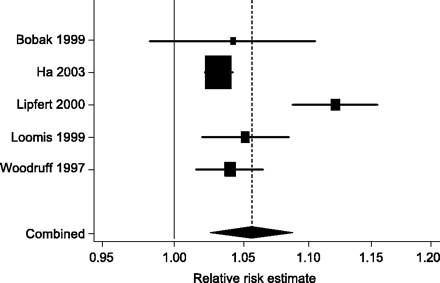
We identified four studies on the effect of air pollution on adult mortality: the Six-Cities study,18 the American Cancer Society study,19 the Netherlands Cohort study on Diet and Cancer,20 and the Adventists study.21 The last study was performed in non-smoking California Senventh-day Adventists who are not representative of the general population and was therefore excluded. Table 1 shows the characteristics of the three included cohort studies. Figure 2 presents the meta-analysis of estimates of the relative risk of death. There was moderate between study heterogeneity (I2 = 46%, P = 0.16 from test of heterogeneity). The combined relative risk from fixed-effect analysis was 1.059 (95% confidence interval (CI) 1.031–1.088) per 10 μg/m3 increase in average PM10 concentration. For infant mortality we identified two time series studies,22,23 two cohort studies,24,25 and one case–control study (Table 1).26 All these studies were included in the meta-analysis. Results were heterogeneous (I2 = 85%, P < 0.001), mainly owing to a study,24 which showed a greater relative risk than the other studies (Figure 3). The combined relative risk from random-effect meta-analysis was 1.056 (95% CI 1.026–1.088) per 10 μg/m3 increase in average PM10 exposure.
Fixed-effects meta-analysis of cohort studies of the effect of air pollution on mortality in adults. The combined relative risk is 1.059 (95% CI 1.031–1.088) per 10 μg/m3 increase in average PM10 concentration
Random-effects meta-analysis of cohort, case–control, and time-series studies of the effect of air pollution on infant mortality. The combined relative risk is 1.056 (95% CI 1.026–1.088) per 10 μg/m3 increase in average PM10 concentration
Characteristics of primary studies
| First author (year) . | Design . | Location . | Study period . | Number of deaths . | Age range . | Pollutant . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of adult mortality | ||||||||||||
| Krewski (2000) | Cohort | USA | 1974–1991 | 1430 | 25–74 years | PM10 | ||||||
| Pope (2002) | Cohort | USA | 1982–1998 | Not reported | >30 years | PM2.5 | ||||||
| Hoek (2002) | Cohort | The Netherlands | 1986–1994 | 489 | 55–69 years | Black smoke | ||||||
| Studies of infant mortality | ||||||||||||
| Woodruff (1997) | Cohort | USAa | 1989–1991 | 12 841 | 1–12 months | PM10 | ||||||
| Bobak (1999) | Case–control | Czech Republic | 1989–991 | 2006 | <1 year | TSP | ||||||
| Loomis (1999) | Time series | Mexico City | 1993–1995 | 2798 | <1 year | PM2.5 | ||||||
| Lipfert (2000) | Cohort | USA | 1990 | 13 041 | <1 year | PM10 | ||||||
| Ha (2003) | Time series | Seoul | 1995–1999 | 1045 | 1–12 months | PM10 | ||||||
| Intervention studies | ||||||||||||
| Pope (1992) | Time series | Utah Valley | 1985–1989 | 1736 | All ages | PM10 | ||||||
| Clancy (2002) | Time series | Dublin | 1984–1996 | 58 086 | All ages | Black smoke | ||||||
| First author (year) . | Design . | Location . | Study period . | Number of deaths . | Age range . | Pollutant . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of adult mortality | ||||||||||||
| Krewski (2000) | Cohort | USA | 1974–1991 | 1430 | 25–74 years | PM10 | ||||||
| Pope (2002) | Cohort | USA | 1982–1998 | Not reported | >30 years | PM2.5 | ||||||
| Hoek (2002) | Cohort | The Netherlands | 1986–1994 | 489 | 55–69 years | Black smoke | ||||||
| Studies of infant mortality | ||||||||||||
| Woodruff (1997) | Cohort | USAa | 1989–1991 | 12 841 | 1–12 months | PM10 | ||||||
| Bobak (1999) | Case–control | Czech Republic | 1989–991 | 2006 | <1 year | TSP | ||||||
| Loomis (1999) | Time series | Mexico City | 1993–1995 | 2798 | <1 year | PM2.5 | ||||||
| Lipfert (2000) | Cohort | USA | 1990 | 13 041 | <1 year | PM10 | ||||||
| Ha (2003) | Time series | Seoul | 1995–1999 | 1045 | 1–12 months | PM10 | ||||||
| Intervention studies | ||||||||||||
| Pope (1992) | Time series | Utah Valley | 1985–1989 | 1736 | All ages | PM10 | ||||||
| Clancy (2002) | Time series | Dublin | 1984–1996 | 58 086 | All ages | Black smoke | ||||||
TSP, total suspended particles; PM, particulate matter.
Excluding eight states (California, Indiana, Louisiana, Nebraska, New York, Oklahoma, South Dakota, and Washington).
Characteristics of primary studies
| First author (year) . | Design . | Location . | Study period . | Number of deaths . | Age range . | Pollutant . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of adult mortality | ||||||||||||
| Krewski (2000) | Cohort | USA | 1974–1991 | 1430 | 25–74 years | PM10 | ||||||
| Pope (2002) | Cohort | USA | 1982–1998 | Not reported | >30 years | PM2.5 | ||||||
| Hoek (2002) | Cohort | The Netherlands | 1986–1994 | 489 | 55–69 years | Black smoke | ||||||
| Studies of infant mortality | ||||||||||||
| Woodruff (1997) | Cohort | USAa | 1989–1991 | 12 841 | 1–12 months | PM10 | ||||||
| Bobak (1999) | Case–control | Czech Republic | 1989–991 | 2006 | <1 year | TSP | ||||||
| Loomis (1999) | Time series | Mexico City | 1993–1995 | 2798 | <1 year | PM2.5 | ||||||
| Lipfert (2000) | Cohort | USA | 1990 | 13 041 | <1 year | PM10 | ||||||
| Ha (2003) | Time series | Seoul | 1995–1999 | 1045 | 1–12 months | PM10 | ||||||
| Intervention studies | ||||||||||||
| Pope (1992) | Time series | Utah Valley | 1985–1989 | 1736 | All ages | PM10 | ||||||
| Clancy (2002) | Time series | Dublin | 1984–1996 | 58 086 | All ages | Black smoke | ||||||
| First author (year) . | Design . | Location . | Study period . | Number of deaths . | Age range . | Pollutant . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of adult mortality | ||||||||||||
| Krewski (2000) | Cohort | USA | 1974–1991 | 1430 | 25–74 years | PM10 | ||||||
| Pope (2002) | Cohort | USA | 1982–1998 | Not reported | >30 years | PM2.5 | ||||||
| Hoek (2002) | Cohort | The Netherlands | 1986–1994 | 489 | 55–69 years | Black smoke | ||||||
| Studies of infant mortality | ||||||||||||
| Woodruff (1997) | Cohort | USAa | 1989–1991 | 12 841 | 1–12 months | PM10 | ||||||
| Bobak (1999) | Case–control | Czech Republic | 1989–991 | 2006 | <1 year | TSP | ||||||
| Loomis (1999) | Time series | Mexico City | 1993–1995 | 2798 | <1 year | PM2.5 | ||||||
| Lipfert (2000) | Cohort | USA | 1990 | 13 041 | <1 year | PM10 | ||||||
| Ha (2003) | Time series | Seoul | 1995–1999 | 1045 | 1–12 months | PM10 | ||||||
| Intervention studies | ||||||||||||
| Pope (1992) | Time series | Utah Valley | 1985–1989 | 1736 | All ages | PM10 | ||||||
| Clancy (2002) | Time series | Dublin | 1984–1996 | 58 086 | All ages | Black smoke | ||||||
TSP, total suspended particles; PM, particulate matter.
Excluding eight states (California, Indiana, Louisiana, Nebraska, New York, Oklahoma, South Dakota, and Washington).
We identified three potentially eligible studies of interventions to reduce air pollution. One study27 was excluded because SO2 but not particulate matter was studied. The first of the included studies examined the effect of shutting down a steel mill in the Utah Valley on mortality during the following year.28 The average PM10 exposure level decreased by 15 μg/m3 and mortality by 3.2%. The second study investigated the impact of introducing the coal ban in Dublin.29 Following the new legislation the black smoke levels declined by 35.60 μg/m3 and mortality by 5.7%.
Modelling YLLs owing to air pollution in Switzerland
A linear approximation of the results from the steel mill study yielded a 2.1% decrease in mortality per 10 μg/m3 decrease in PM10 during 13 months. Using this figure and the combined relative risk of 1.059 from the meta-analysis of cohort studies, Equation (3) gave a time constant k of 0.88. The coal ban study showed a 1.6% decrease in mortality per 10 μg/m3 PM10 during 6 years. This corresponded to a time constant k of 0.11. Based on these results we determined YLLs for time constants k of 0.1, 0.2, 0.5, 1, 3 and infinity. Table 2 shows the effect on all-cause mortality in the period 2000–2009, assuming a k of 0.5. In this case 39% of the effect of air pollution during 2000 occurs in the same year and 63% within 2 years. The risk ratios for each year reflect the reduction in risk after reducing PM10 exposure by 10 μg/m3 during 2000, with exposure returning to the previous level in the following year. Multiplying the relative risks for each year yields the steady state relative risk of 0.944 (1/1.059) per 10 μg/m3 reduction in average PM10 exposure.
Distribution of the effect of a hypothetical reduction of 10 μg/m3 PM10 in 2000 on all-cause mortality 2000–2009 in Switzerland
| Year . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | 2006 . | 2007 . | 2008 . | 2009 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of total effect(%) | — | 39.3 | 23.9 | 14.5 | 8.8 | 5.3 | 3.2 | 2.0 | 1.2 | 0.7 | 0.4 |
| Relative risk (per 10 μg/m3 reduction in PM10) | 1.0 | 0.9775 | 0.9863 | 0.9917 | 0.9950 | 0.9969 | 0.9981 | 0.9989 | 0.9993 | 0.9996 | 0.9997 |
| Year . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | 2006 . | 2007 . | 2008 . | 2009 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of total effect(%) | — | 39.3 | 23.9 | 14.5 | 8.8 | 5.3 | 3.2 | 2.0 | 1.2 | 0.7 | 0.4 |
| Relative risk (per 10 μg/m3 reduction in PM10) | 1.0 | 0.9775 | 0.9863 | 0.9917 | 0.9950 | 0.9969 | 0.9981 | 0.9989 | 0.9993 | 0.9996 | 0.9997 |
Relative risk and proportion of total effect in each year are shown, assuming a time constant k of 0.5.
Distribution of the effect of a hypothetical reduction of 10 μg/m3 PM10 in 2000 on all-cause mortality 2000–2009 in Switzerland
| Year . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | 2006 . | 2007 . | 2008 . | 2009 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of total effect(%) | — | 39.3 | 23.9 | 14.5 | 8.8 | 5.3 | 3.2 | 2.0 | 1.2 | 0.7 | 0.4 |
| Relative risk (per 10 μg/m3 reduction in PM10) | 1.0 | 0.9775 | 0.9863 | 0.9917 | 0.9950 | 0.9969 | 0.9981 | 0.9989 | 0.9993 | 0.9996 | 0.9997 |
| Year . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | 2006 . | 2007 . | 2008 . | 2009 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of total effect(%) | — | 39.3 | 23.9 | 14.5 | 8.8 | 5.3 | 3.2 | 2.0 | 1.2 | 0.7 | 0.4 |
| Relative risk (per 10 μg/m3 reduction in PM10) | 1.0 | 0.9775 | 0.9863 | 0.9917 | 0.9950 | 0.9969 | 0.9981 | 0.9989 | 0.9993 | 0.9996 | 0.9997 |
Relative risk and proportion of total effect in each year are shown, assuming a time constant k of 0.5.
Modelling PM10 exposure in Switzerland yielded a population weighted average of 19.6 μg/m3 under the no-intervention scenario. Therefore, with our choice of 7.5 μg/m3 for the ‘no pollution’ alternative we quantify the impact of a contrast of 12.1 μg/m3. For time constant k of 0.5, life table calculations resulted in 42 400 (95% CI 22 600–63 600) YLLs owing to air pollution exposure in the year 2000, with 4.0% attributable to infant deaths (Table 3). This corresponds to 5882 (95% CI 3135–8822) YLLs per million of the Swiss population. A time constant of 0.1 (18% of excess deaths occur within 2 years) increased the number of YLLs to 46 200 (95% CI 24 500–68 000); a time constant of 3.0 (99.8% of deaths within 2 years) decreased YLLs to 40 700 (95% CI 21 900–59 300). Applying a steady state model (k = infinite) would result in 40 600 YLLs (95% CI 21 800–59 100).
YLLs attributable to air pollution in Switzerland during one year (2000), using different values of time constant k in a dynamic exposure–response model
| Time constant k . | 0.1 . | 0.2 . | 0.5 . | 3 . | ∞a . |
|---|---|---|---|---|---|
| Time period considered (years) | 30 | 20 | 10 | 10 | 1 |
| Proportion of effect within first year | 9.5% | 18.1% | 39.3% | 95.0% | 100.0% |
| Proportion effect within the first 2 years | 18.1% | 33.0% | 63.2% | 99.8% | 100.0% |
| Proportion effect within the first 5 years | 39.3% | 63.2% | 82% | 100.0% | 100.0% |
| Total number of years of life lost (YLLs) | 46 200 | 44 300 | 42 400 | 40 700 | 40 600 |
| 95% CI for YLLs | 24 500–68 000 | 23 500–65 100 | 22 600–63 600 | 21 900–59 300 | 21 800–59 100 |
| Proportion of YLLs attributable to infant deaths | 3.7% | 3.9% | 4.0% | 4.2% | 4.2% |
| Time constant k . | 0.1 . | 0.2 . | 0.5 . | 3 . | ∞a . |
|---|---|---|---|---|---|
| Time period considered (years) | 30 | 20 | 10 | 10 | 1 |
| Proportion of effect within first year | 9.5% | 18.1% | 39.3% | 95.0% | 100.0% |
| Proportion effect within the first 2 years | 18.1% | 33.0% | 63.2% | 99.8% | 100.0% |
| Proportion effect within the first 5 years | 39.3% | 63.2% | 82% | 100.0% | 100.0% |
| Total number of years of life lost (YLLs) | 46 200 | 44 300 | 42 400 | 40 700 | 40 600 |
| 95% CI for YLLs | 24 500–68 000 | 23 500–65 100 | 22 600–63 600 | 21 900–59 300 | 21 800–59 100 |
| Proportion of YLLs attributable to infant deaths | 3.7% | 3.9% | 4.0% | 4.2% | 4.2% |
Swiss population size: 7 209 000; infants (0–1 year): 77 800.
Corresponds to steady state model.
YLLs attributable to air pollution in Switzerland during one year (2000), using different values of time constant k in a dynamic exposure–response model
| Time constant k . | 0.1 . | 0.2 . | 0.5 . | 3 . | ∞a . |
|---|---|---|---|---|---|
| Time period considered (years) | 30 | 20 | 10 | 10 | 1 |
| Proportion of effect within first year | 9.5% | 18.1% | 39.3% | 95.0% | 100.0% |
| Proportion effect within the first 2 years | 18.1% | 33.0% | 63.2% | 99.8% | 100.0% |
| Proportion effect within the first 5 years | 39.3% | 63.2% | 82% | 100.0% | 100.0% |
| Total number of years of life lost (YLLs) | 46 200 | 44 300 | 42 400 | 40 700 | 40 600 |
| 95% CI for YLLs | 24 500–68 000 | 23 500–65 100 | 22 600–63 600 | 21 900–59 300 | 21 800–59 100 |
| Proportion of YLLs attributable to infant deaths | 3.7% | 3.9% | 4.0% | 4.2% | 4.2% |
| Time constant k . | 0.1 . | 0.2 . | 0.5 . | 3 . | ∞a . |
|---|---|---|---|---|---|
| Time period considered (years) | 30 | 20 | 10 | 10 | 1 |
| Proportion of effect within first year | 9.5% | 18.1% | 39.3% | 95.0% | 100.0% |
| Proportion effect within the first 2 years | 18.1% | 33.0% | 63.2% | 99.8% | 100.0% |
| Proportion effect within the first 5 years | 39.3% | 63.2% | 82% | 100.0% | 100.0% |
| Total number of years of life lost (YLLs) | 46 200 | 44 300 | 42 400 | 40 700 | 40 600 |
| 95% CI for YLLs | 24 500–68 000 | 23 500–65 100 | 22 600–63 600 | 21 900–59 300 | 21 800–59 100 |
| Proportion of YLLs attributable to infant deaths | 3.7% | 3.9% | 4.0% | 4.2% | 4.2% |
Swiss population size: 7 209 000; infants (0–1 year): 77 800.
Corresponds to steady state model.
Discussion
We used data from population-based cohort and case–control studies to obtain an average concentration–response function describing the steady state, and results from intervention studies to estimate the decrease in risk following termination of an exposure. These data were used to populate a dynamic model that allows estimation of the change in mortality following increases or decreases in air pollution levels. There is uncertainty regarding the choice of an appropriate time constant, but it seems likely that a substantial proportion of the benefit of reducing pollution levels manifests itself within a few years after the reduction has taken place. Of note, the estimation of the total number of YLLs was little affected by the choice of the time constant. Indeed, in this respect, the time constant was unimportant compared with the uncertainties associated with the relative risk estimates from epidemiological studies.
Strengths and weaknesses
The dynamic model builds on external evidence from interventions that reduce pollution. It allows more solid assessments of likely changes in mortality following defined reductions (or increases) in air pollution levels than the widely used models assuming steady-state conditions. It is this type of information that policy makers often need, for example, when deciding on whether an air polluting facility should be allowed to be built or closed down. In general, data from intervention-type studies complement the evidence from prospective studies on long-term effects and time-series studies on acute effects. Effect estimates from time series studies are an order of magnitude lower than those obtained from cohort studies, because by design they are concerned with effects following exposure within a few days.30 The two currently available intervention studies occupy the middle ground between cohort and time-series studies as they reported effects over 1 year28 and 6 years.29
Our case study for Switzerland illustrates that the approach can also be used to estimate the total impact of air pollution during a defined period of time, using a scenario and framework that may facilitate communication with policy makers and the public at large.
Our model and application also has a number of limitations. Most importantly, no empirical data exist that could ultimately confirm the accuracy of our model. For example, although an exponential decrease is often observed in biological systems, we cannot prove that assuming an exponential form of the curve is appropriate. Its shape may differ for different outcomes, for example, coronary heart disease and lung cancer.6 This could be examined empirically in very large intervention studies by estimating changes in risk at different time points and for different outcomes. Also, the approach we used to determine the time constant can only produce a solution if the intervention studies report smaller effect estimates than the cohort studies (see Equation 3), but that is expected if the cohort studies examine the long-term effects of cumulated exposure. The two intervention studies found a similar effect in the first year after exposure reduction, in the order of a 1–2% decrease in mortality per 10 μg/m3 reduction in PM10 concentration, which is compatible with the increase of mortality over several years of ∼6% per 10 μg/m3 increase in PM10 that was observed in the cohort studies.
In our case study for Switzerland, we assumed that levels of PM10 ≤7.5 μg/m3 are not harmful; however, for low and very low concentrations of PM10, the concentration–response function is uncertain, and it is unclear whether a no-effect threshold exists. Finally, we did not consider the impact of air pollution on mortality of age groups 1–30 years but it is clear that from ∼5 to 30 years the mortality is so low that the effect of pollution can be assumed to be negligible. Although response functions were very similar for adult and infant mortality, and YLLs are large for an infant death, infant mortality contributed only 4% of all YLLs. This is owing to the low infant mortality rates.
Comparison with other studies
Previous assessments of the impact of air pollution on health have generally been based on steady state models.2–5,10 The only exception, to our knowledge, is the study by Leksell and Rabl, who estimated the loss of life expectancy owing to air pollution for the European Union.6 Interestingly, the time constant was estimated from smoking cessation studies, rather than studies of air pollution, which may be considered problematic. Results were nevertheless comparable: Leksell and Rabl reported 0.22 days lost per 1 μg/m3 increase in PM2.5 per person and year of exposure. The corresponding figure for Switzerland, taking into account the size of the Swiss population and the different type of particulate matter (PM10), is 0.24 days. These similar figures underline that the choice of the time constant has little impact on the total number of YLLs estimated by the model. US Studies estimated about 22 000 smoking-attributable YLLs per million of the population,31,32 which is four times higher than our estimate for outdoor air pollution. In contrast to smoking the whole population is exposed to air pollution, and air pollution cessation is not a choice available to the individual.
Implications and future research
Our findings and those from the previous study6 demonstrate that steady-state and dynamic models will produce similar estimates of the total YLLs. Thus, if one is interested exclusively in the number of YLLs, the simpler steady-state approach will generally be appropriate. However, in many health impact assessment contexts the date of the prevented event is of interest. For example, the monetary value of a prevented death in the future will differ from the value of a death that is prevented in the present. Clearly, more data are needed from large epidemiological studies of mortality and air pollution, and particularly from studies of interventions that reduced or increased pollution levels. Future studies of interventions should ideally report results for the same indicators of total air pollution used in the studies that provide the concentration–response functions.
Data from long-term epidemiological studies and studies that examined sudden changes in exposure levels can be used to assess the health impact of ambient air pollution.
A dynamic exposure–response model based on an exponential function was developed to estimate YLLs attributable to ambient air pollution in Switzerland.
The model was insensitive to different assumptions regarding the course of mortality after cessation of air pollution.
In Switzerland an estimated 5882 YLLs per million of the population are attributable to air pollution each year.
We would like to thank Heini Sommer and Christoph Lieb for their contribution to this study, and Jürg Heldstab and Thomas Künzle who determined PM10 exposure levels in Switzerland. The study was funded by the Swiss Federal Department of Environment, Transport, Energy and Communications. N. K. is supported by the Southern California Environmental Health Sciences Center (NIEHS grant P30 ES07048).
References
Ostro B. Estimating the health effects of air pollutants. A method with an application to Jakarta. Policy Research Working Paper No. 1301. Washington DC: World Bank,
Coyle D, Stieb D, Burnett RT et al. Impact of particulate air pollution on quality-adjusted life expectancy in Canada.
Kan HD, Chen BH. Impact of long-term exposure to air particulate matter on life expectancy and survival rate of Shanghai residents.
Reshetin VP, Kazazyan VI. Public-health impact of outdoor air pollution in Russia.
Powe NA, Willis KG. Mortality and morbidity benefits of air pollution (SO2 and PM10) absorption attributable to woodland in Britain.
Leksell I, Rabl A. Air pollution and mortality: quantification and valuation of years of life lost.
Miller BG, Hurley JF. Life table methods for quantitative impact assessments in chronic mortality.
Rabl A. Interpretation of air pollution mortality: number of deaths or years of life lost?
HEI Accountability Working Group. Assessing Health Impact of Air Quality Regulations: Concepts and Methods for Accountability Research. Boston, MA: Health Effect Institute,
Künzli N, Kaiser R, Medina S et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment.
Sommer R, Neuenschwander R, Ackermann-Liebrich U et al. Monetarisierung der verkehrsbedingten externen Gesundheitskosten [Traffic related external health costs]. Bern: Dienst für Gesamtverkehrsfragen des Eidg. Verkehrs- und Energiewirtschaftsdepartementes,
Gehrig R, Buchmann B. Characterising seasonal variations and spatial distribution of ambient PM10 and PM2.5 concentrations based on long-term Swiss monitoring data.
Roemer WH, van Wijnen JH. Differences among black smoke, PM10 and PM1.0 levels at urban measurement sites.
Filliger P, Puybonnieux-Texier V, Schneider J. Health Costs Due to Road Traffic-Related Air Pollution—PM10 Population Exposure. Bern: Federal Department of Environment, Transport, Energy and Communications,
Sommer H, Lieb C, Heldstab J et al. Externe Gesundheitskosten durch verkehrsbedingte Luftverschmutzung [External costs due to traffic related air pollution]. Bern: Bundesamt für Raumentwicklung,
Krewski D, Burnett RT, Goldberg MS et al. Reanalysis of the Harvard Six Cities Study and American Cancer Society Study of Particulate Air Pollution and Mortality: Special Report. Cambridge, MA: Health Effect Institute,
Pope CAIII, Burnett RT, Thun MJ et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution.
Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study.
Abbey DE, Nishino N, McDonnell WF et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers.
Ha EH, Lee JT, Kim H et al. Infant susceptibility of mortality to air pollution in Seoul, South Korea.
Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City.
Lipfert FW, Zhang J, Wyzga RE. Infant mortality and air pollution: a comprehensive analysis of U.S. data for 1990.
Woodruff TJ, Grillo J, Schoendorf KC. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States.
Bobak M, Leon DA. The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period.
Hedley AJ, Wong CM, Thach TQ, Ma S, Lam TH, Anderson HR. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study.
Pope CAIII, Schwartz J, Ransom MR. Daily mortality and PM10 pollution in Utah Valley.
Clancy L, Goodman P, Sinclair H, Dockery DW. Effect of air-pollution control on death rates in Dublin, Ireland: an intervention study.
Künzli N, Medina S, Kaiser R, Quenel P, Horak F Jr, Studnicka M. Assessment of deaths attributable to air pollution: should we use risk estimates based on time series or on cohort studies?
Max W, Rice DP, Sung HY, Zhang X, Miller L. The economic burden of smoking in California.