-
PDF
- Split View
-
Views
-
Cite
Cite
Carol Brayne, Cherie McCracken, Fiona E Matthews, Cohort Profile: The Medical Research Council Cognitive Function and Ageing Study (CFAS), International Journal of Epidemiology, Volume 35, Issue 5, October 2006, Pages 1140–1145, https://doi.org/10.1093/ije/dyl199
Close - Share Icon Share
Origin of the study
Global ageing is a recent phenomenon. Its potential impact on social and economic aspects of more affluent countries highlighted ageing as a sufficiently important issue towards which to direct resources. Discussion between the Department of Health, Medical Research Council, and experts from the scientific and medical communities resulted in the decision that brain changes, most particularly cognitive decline, dementia, and their relation to disability were key topics requiring investigation at the population level. This prompted a decision to invest in research into this area and a working group was convened, which included those with epidemiological and biostatistical expertise relevant to such investigation. Out of this working group a successful bid for the study now known as the MRC Cognitive Function and Ageing Study emerged.
Study design
The study is a six-centre multidisciplinary multiphased longitudinal design (see map, Figure 1). There are five identical sites and one with a different sampling and interview structure. This centre (Liverpool) was already funded at the time of the discussions noted above and thus started earlier than the other five centres.1 The other five centres (Cambridgeshire, Gwynedd, Newcastle, Nottingham, and Oxford) were able to follow a standardized design and are referred to as the five identical sites. Their basic structure was a two-phase design with a screening interview followed by an assessment interview shortly afterwards, with a repeat at 2 years. The fieldwork began in 1991.2 There are many additional features, which are more fully described on the website (see below).
The aims of the study
The aims of the study have evolved over its existence and cover a wide range including descriptive epidemiology, neuropathology, policy, molecular epidemiology, and ethics.
The main descriptive epidemiological aims include (i) the estimation of the prevalence and incidence of cognitive decline and dementia, and geographical variation in those rates; (ii) the determination of the natural history of dementia, in particular the rate of progression of cognitive decline including the distribution of the interval between the identification of cognitive impairment and death, and (iii) the identification of factors associated with differing rates of cognitive decline and with the risk of dementia.
The principal neuropathological aim was to determine the contribution of different underlying pathologies to the rates of dementia and the geographic variation in these rates and to the burden of disability. Additional aims included to: (i) determine the prevalence and severity of pathological lesions in the brain of an unselected cohort of older people with and without cognitive impairment; (ii) determine the frequency of specific pathological diagnoses in people with cognitive impairment, and (iii) correlate severity of specific pathologies with patterns of cognition, function, and behaviour in life independently of clinical and pathological diagnostic categories.
The core aim related to policy was the evaluation of the degree of disability associated with cognitive decline and impairment, and the service needs this disability generates. These needs were to be compared with the needs generated by physical impairment. The study also sought to form the basis for longer-term studies of trends over time and by birth cohort of the prevalence and incidence of cognitive decline. In addition to these aims the breadth of the data collected has allowed the study to incorporate the investigation of expectation of life in various states of health, depression, and depressive symptomatology in the older population.
The DNA resource has been incorporated in a later phase of the study. The main molecular epidemiological aim has been to support genetics studies that have sought genes associated with all dementia, Alzheimer's disease, mixed and vascular dementia, cognitive impairment and decline.
A later aim of the study was to explore the ethical and legal aspects of brain donation within a population-based sample given changing perception surrounding organ donation.
The study also aimed to act as a core resource and provide a framework to support specific sub-studies in lone or joint centres. The Resource Implication Study3–9 utilized this framework to achieve the core policy aim (see above). Other sub-studies include the study of Healthy Ageing10–15 and the Network Study. This framework also extends to wider collaborations with CFAS centres contributing to European wide initiatives such as EURODEM16 and EURODEP.17
What does it cover?
Because the main study is focused on cognition and dementia it has collected the necessary dimensions of physical and mental health to arrive at a study diagnosis of dementia. It now has four major themes: (i) dementia (covering all aspects including cognition), (ii) depression, (iii) disability and healthy life expectancy, and (iv) health policy and health. In addition it has particular strengths in that it is one of the very few truly population based programmes with a donation programme—individuals in the study have indicated whether they wish to contribute to brain research through the donation of their brain after death (declaration of intention to donate).
Who is in the sample?
The first aim of the study was to estimate age-specific rates of prevalence of cognitive impairment and dementia among those aged 65 and over. The population is thus all those aged 65 years and over on the index date for centre (1990, 1991), living within a specified geographical location. Background information on the demographics of the populations sampled was collected from the Office of Population Census and Surveys (OPCS), 1990–91 census now Office of National Statistics (ONS), to relate to regional and national data.
Family Health Service Authority (FHSA) lists were used as the sampling frame. The frame would be incomplete if eligible members of the population were not registered with a GP. However individuals in long-stay hospitals remain registered with their GP 2 years after institutionalization so sampling from FHSA lists ensured their inclusion. Each centre looked into the practices of long-stay hospitals in their area to confirm this. The FHSA list of individuals was used for sampling on a geographical basis. Each centre defined this area, and the study population was drawn from all those who were resident within it. Problems of inaccuracy, patients who died or moved away but were still on the FHSA list, were resolved by asking GP surgeries to check the lists. On this basis, a sample of sufficient size to yield 2500 interviews of individuals aged 65 years and over, stratified by age (equal numbers aged 65–74 and 75 plus) was chosen from the FHSA lists for each selected area (in Liverpool this was 5000 interviews stratified by sex and 5 year age band). The population is flagged at ONS for mortality and the database is updated continuously.
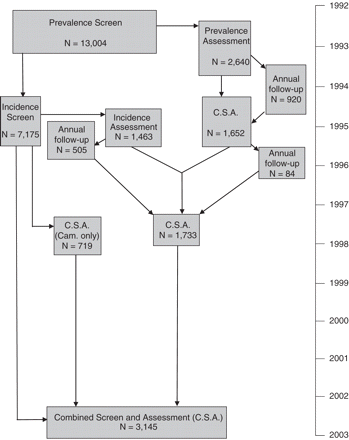
The follow-up has been determined by funding and the design of associated bolt-on studies. The main follow-up waves for the identical sites are captured in the audit trail shown in Figure 2, which shows the numbers for the main screen, assessment, 1 year follow-up and 2 year rescreen, new selection for assessment and further 1 year follow-up, 6 year follow-up of the assessed (with venepuncture), 8 year follow-up of those with intentions to donate, and 10 year follow-up of the total sample. In addition to this the main associated studies are the Resource Implication Study (4 centres—Cambridgeshire, Newcastle, Nottingham, Oxford), which followed those who provided care to the physically and cognitively frail at baseline, the ESRC funded Healthy Ageing Project, which interviewed in detail those who were not selected into the Resource Implication Study in Nottingham and Cambridgeshire, the Network Study conducted in Gwynedd and Liverpool to examine individuals' social networks, an embedded case–control study at 2 year incidence stage (Cambridgeshire), and the ongoing brain donation programme in all centres. This programme, in combination with the bloods taken at year 6 form the major components of the Biological Resource of the study.
Who is not in the sample?
Comprehensive analyses of those who were lost to follow-up have been conducted for all stages of the study. At baseline 19% of potential respondents refused, 6% had died, and 1% had moved out of the area. Similar percentages were found for all waves of the study. Individuals who had moved or refused had higher mortality than responders.18 CFAS has used this attrition for a detailed investigation of attrition effects in both short and longer time intervals.18,19
How, when, and which interviews were conducted?
All interviews were conducted in the respondent's place of residence, using portable computers with customized software. If the interviewer felt that the respondent was frail and tiring, or becoming agitated, the short ‘priority mode’ set of questions could be invoked manually. Screening interviews were undertaken by lay interviewers, recruited for the purpose and trained by both the local and national coordinator. Reliability checks were made by both the local and the national coordinator. Proxy screening interviews were conducted where an interview was not possible with the named participant, owing to, for example, extreme confusion or frailty. If after four attempts to contact, an interview was not arranged, the approach was abandoned. The screening of the entire sample took 2 years to complete.
The assessment (designed to be conducted 1 month after the screening interview) and annual (designed to be conducted 1 year after the screening interview) interviews were also undertaken using customized portable computers. These interviews were carried out by interviewers from professions allied to medicine, who had not undertaken the screening interview, also recruited and trained for the purpose. Interviewers did not know the outcome of the first interview. The interviews lasted from 45 to 90 min, again with a ‘priority mode’ route. The annual interview consisted of a combined screen and assessment, where information on changes since last interview was recorded. At the assessment and biannual follow-up interview permission was sought to approach and interview a relative or carer to ask for an objective account of the respondent's health and abilities.
Six years after the initial screening interview, all respondents in the assessed groups were interviewed using the combined screen and assessment interview and at the end of that interview signed permission was requested to take a sample of blood or saliva. Permission was also sought to access GP and hospital notes. At 8 years only those who had indicated an intention to donate brain tissue were re-interviewed with the combined screen and assessment interview.
At 10 years all survivors from the responding group of the complete study were recontacted for interview and if they agreed were interviewed using the combined screen and assessment interview.
What is collected at different interviews?
The screening interview contains questions on residence, marital status, education and occupation, living circumstances, contact with friends and family, health and social care contact, self-reported physical health, instrumental activities of daily living and activities of daily living, cognitive measures (Mini Mental State Examination with augmentation), and medication.
The assessment interview is mainly the Geriatric Mental State Examination (GMS) adapted for CFAS.20 This is a structured psychiatric interview, which collects sufficient information for algorithmic ‘diagnosis’ in the major psychiatric disorders of old age (dementia, depression, anxiety, and psychosis). This has been validated against clinical diagnosis and the instrument has been widely used in Europe and now forms part of the 10/66 international instrument. This interview has been augmented with questions from the CAMDEX (Cambridge Examination for Mental Disorders in the Elderly) including CAMCOG,21 the longer neuropsychological assessment. The relative or carer interview is mainly the History and Aetiological Schedule, the informant interview that accompanies the GMS.
The combined screen and assessment interview merges the two interviews but compresses some aspects of data collection. Complete versions of all the interviews including the interview questions and responses are available on the website.
The neuropathological assessment follows the standardized protocol of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD)22, with the exception that the neuropathologist is blind to the interview data. This covers in a semiquantitative form the main areas required for the assessment of neurodegenerative and cerebrovascular disorders. The forms are available on the website. The main genetic analyses have been on apoE and ACE.
Data collection in Liverpool was broadly similar to the other five sites except that the screening interview consisted of the GMS plus some of the screening interview questions listed above.
What has the study found?
The study has reported on prevalence and incidence of dementia, and lack of variation in these across the five identical sites.23–25 It has provided profiles of cognition for MMSE, extended MMSE, and CAMCOG, weighted back to the population.26,27 It has reported on risk for incident dementia including apoE and ACE.28–30 It has examined the relationship of cognition to mortality.18,31 It has reported on the mixed neuropathology found in the brains of the oldest old.32 In addition, the study has reported on a variety of impairments to healthy life in old age and their population burden.33–38 The Resource Implication Study has provided data for examination of carer burden and the costs of care for physically and cognitively frail.3–9,39 The data have been used for projection forward, for these vulnerable groups and also for the costs of long-term care. Liverpool has published on the prevalence of dementia, depression, and neurosis, together with incidence of dementia and schizophrenia.40–43
What are the main strengths?
The study is multisite and multidisciplinary. The population is truly representative with high response rates at each stage over diverse sites. Where there is no heterogeneity across sites the study is sufficiently large to provide indicative values for national estimates. The broad scope of measures has allowed the study to contribute to ageing research across a wide range of topics. There are repeat measures on cognition and function, which allows examination of trajectories. There are only two other population-based studies with brain donation in Europe.44,45 The study weighted the sample towards the over 75 age group at baseline, which has provided more robust data for the oldest old.
What are the main weaknesses?
It would be desirable to have higher response and lower drop out between waves, but analysis can adjust for loss between interviews. Blood taking and clinical assessment (including imaging) at baseline was not possible because of funding constraints, but venepuncture was included at year 6. The risk measures are self-report, using the available validated measures of the era.
Can I get hold of data?
The study actively encourages collaboration, and there are established mechanisms for approaching us via the themes mentioned above (see What does it cover?). Information is available on the website and also through contact with theme leads.
Where can I find out more?
The study website, www.cfas.ac.uk, configures information under themes, documentation, publications, and data. There is also a list of study contacts.
A full list of the study investigators and contributors is available on the website
We are grateful to our respondents, their families, and their primary care teams. The study has only been possible because of the dedication of a large number of individuals over the years who are listed on the website. The MRC CFA Study has been supported by major awards from the Medical Research Council and the Department of Health. Thanks are also due to the Biological Resource Advisory Group for overseeing this aspect of study and to Lu Gao for preparation of the manuscript.
References
Saunders PA, Copeland JR, Dewey ME, Larkin BA, Scott A. Alpha: the Liverpool MRC Study of the incidence of dementia and cognitive decline.
Chadwick C. The MRC Multicentre Study of Cognitive Function and Ageing: a EURODEM incidence study in progress.
Buck D, Gregson BA, Bamford CH et al. Psychological distress among informal supporters of frail older people at home and in institutions. The Resource Implications Study Group of the MRC Cognitive Function and Ageing Study.
McNamee P, Gregson BA, Wright K, Buck D, Bamford CH, Bond J. Estimation of a multiproduct cost function for physically frail older people.
McNamee P, Gregson BA, Buck D, Bamford CH, Bond J, Wright K. Costs of formal care for frail older people in England: the resource implications study of the MRC cognitive function and ageing study (RIS MRC CFAS).
Bond J, Farrow G, Gregson BA et al. Informal caregiving for frail older people at home and in long-term care institutions: who are the key supporters?
Melzer D, McWilliams B, Brayne C, Johnson T, Bond J. Profile of disability in elderly people: estimates from a longitudinal population study.
Psychological morbidity among informal caregivers of older people: a 2-year follow-up study. The Resource Implications Study Group of the MRC study of cognitive function and ageing (RIS MRC CFAS).
McNamee P, Bond J, Buck D. Costs of dementia in England and Wales in the 21st century.
Solomou W, Richards MPM, Huppert FA, Brayne C, Morgan K. Divorce, current marital status and well-being in an elderly population.
Huppert FA, Solomou W, O'Connor S, Morgan K, Sussams P, Brayne C. Aging and lymphocyte subpopulations: whole-blood analysis of immune markers in a large population sample of healthy elderly individuals.
Morgan K, Armstrong GK, Huppert FA, Brayne C, Solomou W. Healthy ageing in urban and rural Britain: a comparison of exercise and diet.
Huppert FA, Pinto EM, Morgan K, Brayne C. Survival in a population sample is predicted by proportions of lymphocyte subsets.
Huppert FA, Pinto E, Morgan K, MRC CFAS, Brayne C. Immune measures which predict 9-year survival in an elderly population sample. Basic Biology and Clinical Impact of Immunosenescence, Advances in Cell Aging and Gerontology
Pinto EM, Huppert FA, Morgan K, Mrc C, Brayne C. Neutrophil counts, monocyte counts and cardiovascular disease in the elderly.
Rocca WA, Hofman A, Brayne C et al. The prevalence of vascular dementia in Europe: facts and fragments from 1980–1990 studies. EURODEM-Prevalence Research Group.
Copeland JR, Beekman AT, Braam AW et al. Depression among older people in Europe: the EURODEP studies.
Matthews FE, Chatfield M, Freeman C, McCracken C, Brayne C. Attrition and bias in the MRC cognitive function and ageing study: an epidemiological investigation.
Matthews FE, Chatfield M, Brayne C. An investigation of whether factors associated with short-term attrition change or persist over ten years: data from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS).
Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT.
Huppert FA, Brayne C, Gill C, Paykel ES, Beardsall L. CAMCOG–a concise neuropsychological test to assist dementia diagnosis: socio-demographic determinants in an elderly population sample.
Mirra SS, Heyman A, McKeel D et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease.
Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS).
Matthews F, Brayne C. The incidence of dementia in England and Wales: findings from the five identical sites of the MRC CFA Study.
Williams JG, Huppert FA, Matthews FE, Nickson J. Performance and normative values of a concise neuropsychological test (CAMCOG) in an elderly population sample.
Huppert FA, Cabelli ST, Matthews FE. Brief cognitive assessment in a UK population sample—distributional properties and the relationship between the MMSE and an extended mental state examination.
Yip AG, Brayne C, Matthews FE. Risk factors for incident dementia in England and Wales: The Medical Research Council Cognitive Function and Ageing Study. A population-based nested case-control study.
Yip AG, Brayne C, Easton D, Rubinsztein DC. An investigation of ACE as a risk factor for dementia and cognitive decline in the general population.
Yip AG, Brayne C, Easton D, Rubinsztein DC. Apolipoprotein E4 is only a weak predictor of dementia and cognitive decline in the general population.
Neale R, Brayne C, Johnson AL. Cognition and survival: an exploration in a large multicentre study of the population aged 65 years and over.
Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS).
Brayne C, Gao L, Matthews F. Challenges in the epidemiological investigation of the relationships between physical activity, obesity, diabetes, dementia and depression.
Jagger C, Matthews F. Gender differences in life expectancy free of impairment at older ages.
McGee MA, Johnson AL, Kay DW. The description of activities of daily living in five centres in England and Wales. Medical Research Council Cognitive Function and Ageing Study.
Melzer D, McWilliams B, Brayne C, Johnson T, Bond J. Socioeconomic status and the expectation of disability in old age: estimates for England.
Parker CJ, Morgan K, Dewey ME. Physical illness and disability among elderly people in England and Wales: the Medical Research Council Cognitive Function and Ageing Study. The Analysis Group.
Spiers NA, Matthews RJ, Jagger C et al. Diseases and impairments as risk factors for onset of disability in the older population in England and Wales: findings from the Medical Research Council Cognitive Function and Ageing Study.
Comas-Herrera A, Wittenberg R, Pickard L, Knapp M, MRC-CFAS. Cognitive impairment in older people: its implications for future demand for services and costs. Mental Health Research Review
Copeland JR, Chen R, Dewey ME et al. Community-based case-control study of depression in older people. Cases and sub-cases from the MRC-ALPHA Study.
Copeland JR, McCracken CF, Dewey ME et al. Undifferentiated dementia, Alzheimer's disease and vascular dementia: age- and gender-related incidence in Liverpool. The MRC-ALPHA Study.
Copeland JR, Dewey ME, Scott A et al. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity, and outcome.
Saunders PA, Copeland JR, Dewey ME et al. The prevalence of dementia, depression and neurosis in later life: the Liverpool MRC-ALPHA Study.
Polvikoski T, Sulkava R, Myllykangas L et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study.