-
PDF
- Split View
-
Views
-
Cite
Cite
Carolin Feterowski, Alexander Novotny, Simone Kaiser-Moore, Peter F. Mühlradt, Tanja Roßmann-Bloeck, Martina Rump, Bernhard Holzmann, Heike Weighardt, Attenuated pathogenesis of polymicrobial peritonitis in mice after TLR2 agonist pre-treatment involves ST2 up-regulation, International Immunology, Volume 17, Issue 8, August 2005, Pages 1035–1046, https://doi.org/10.1093/intimm/dxh282
Close - Share Icon Share
Abstract
The innate immune system uses Toll-like receptors (TLRs) to activate and instruct immune responses against microbial pathogens. Administration of TLR agonists to mice induces a state of hyporesponsiveness, or tolerance, characterized by reduced cytokine production upon subsequent second challenge. The present study examined the effects of pre-treatment of mice with TLR2-dependent stimuli on the host defense against acute polymicrobial infection. Immune priming of mice with macrophage-activating lipopeptide-2 (MALP-2) 4 days prior to infection greatly improves survival and bacterial clearance in a model of polymicrobial septic peritonitis which is associated with enhanced accumulation of effector neutrophils in the peritoneal cavity. Further, the systemic and local production of both myeloid differentiation factor 88 (MyD88)-dependently and MyD88-independently produced cytokines was substantially diminished, but not completely absent, in TLR2-treated mice. While pre-treatment with MALP-2 does not involve differential expression of TLR and IL-1R-associated kinase proteins, ST2, a negative regulator of TLR signaling, was up-regulated after treatment of mice with either MALP-2 or N-α-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-L-cysteine. Therefore, ST2 may be a mechanism, among others, to attenuate the sepsis-induced cytokine burst. Thus, these results suggest that immune protection in mice after pre-treatment with TLR2-dependent stimuli involves the induction of enhanced pathogen defense by neutrophils. In addition, up-regulation of ST2 could contribute to the diminished cytokine production.
Introduction
Conserved molecular patterns of microbial pathogens are recognized by the innate immune systems via germ line-encoded pattern-recognition receptors. Recent work has shown that Toll-like receptors (TLRs) function as mammalian pattern-recognition receptors signaling the presence of microbial components to innate immune cells (1). At least 10 members of the mammalian TLR family have been reported, with each TLR showing a distinct specificity for molecular patterns of microbes (2). TLR2 mediates cellular responses to a large number of microbial products including peptidoglycan, bacterial lipopeptides, lipoteichoic acid, mycobacterial lipoarabinomannan and yeast cell wall components (2). The ligand specificity and signal transduction capacity of TLR2 are determined by heterodimerization with other TLRs, such as TLR1 and TLR6, thereby providing a possible explanation for the diverse specificity of TLR2 (3–7). Whereas heterodimerization of TLR2 and TLR6 is required for macrophage-activating lipopeptide-2 (MALP-2) responsiveness (8), stimulation with N-α-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-L-cysteine (Pam3Cys) involves heterodimerization of TLR1 and TLR2 (9).
Signaling of TLRs proceeds via myeloid differentiation factor 88 (MyD88), IL-1R-associated kinase (IRAK) proteins and TNF receptor-associated factor-6 resulting in activation of mitogen-activated kinase (MAP) kinases and NF-κB (2). These pathways are evolutionary conserved and lead to cellular responses such as cytokine production or dendritic cell maturation. Due to the usage of common signaling pathways, activation of innate immune cells via different TLRs may cause synergistic effects (10). It is conceivable that during polymicrobial infections, synergistic TLR activities may decrease the activation threshold of the innate immune system. However, in the presence of large numbers of pathogens, as observed during polymicrobial sepsis, synergistic actions of TLRs could contribute to the development of inflammatory organ damage. In addition to the common MyD88-dependent pathway, TLR4 and TLR3 activate an MyD88-independent pathway involving the adaptor protein TIR domain-containing adaptor inducing IFN-β (TRIF), leading to the production of IFN-β and several IFN-β-dependent genes including IP-10 (11, 12).
Pre-exposure to LPS reduces the sensitivity to a second challenge with LPS resulting in a diminished production of numerous cytokines both in rodents and humans, a phenomenon called endotoxin tolerance (13, 14). Studies revealed that different phases of endotoxin tolerance exist (15). Whereas the so-called non-specific phase shows up hours after endotoxin administration until several days, the late phase is characterized by the production of anti-LPS antibodies indicating that different mechanisms are responsible for tolerance effects during the time course of tolerance induction.
Induction of endotoxin tolerance is dependent on the presence of functional LPS-sensitive macrophages (16, 17). Treatment of murine macrophages with TLR2-dependent agonists such as lipoteichoic acid or MALP-2 also induces tolerance against a subsequent challenge with the same stimulus (10, 18). Stimulation of cells through TLR2 was also shown to attenuate the subsequent response to LPS, suggesting that signaling pathways common to multiple TLRs are affected in tolerant cells in vitro (10, 18) as well as in vivo (19).
The molecular mechanisms of TLR-induced tolerance may involve the down-regulation of the TLR4–MD2 complex (20) which may lead to diminished TLR signaling. Furthermore, expression of IRAK-M and IRAK-1 as well as supressor of cytokine signaling (SOCS-1) was shown to be related to endotoxin tolerance. IRAK-M induction was observed after LPS stimulation and IRAK-M-deficient macrophages show reduced endotoxin tolerance while the expression of TLR-induced cytokines was enhanced (21). IRAK-1 was shown to be down-regulated in endotoxin-tolerant THP-1 macrophages (22). Expression of SOCS-1 could be induced by LPS and SOCS-1-deficient macrophages did not develop endotoxin tolerance (23, 24). Recent data show an important role for the toll/IL-1 receptor (TIR) family member ST2 in the induction of endotoxin tolerance (25). ST2 is expressed by Th2 cells, mast cells and macrophages (26). It was shown to be up-regulated after LPS stimulation and regulates cytokine expression in a model of acute lung injury (27). ST2 negatively regulates TLR4 and IL-1R signaling via sequestration of MyD88. ST2-deficient macrophages could not be tolerized to LPS in vitro and ST2-deficient mice did not develop endotoxin tolerance in response to LPS in vivo (25, 27).
In the present study, we investigated the effects of pre-treatment of mice by the TLR2 agonists MALP-2 and Pam3Cys on the immune defense against polymicrobial septic peritonitis in mice. Injection of TLR2 ligands caused, in contrast to injection with the TLR4 ligand LPS, no elevation of systemic tumor necrosis factor (TNF) and IL-10. Pre-treatment of mice with the TLR2 ligands MALP-2 and Pam3Cys prior to sepsis induction results in improved survival and increased bacterial clearance. Furthermore, attenuated production of sepsis-induced cytokines and chemokines could be observed systemically and locally. Priming with MALP-2 does not alter the expression of TLR2 and 4 as well as the expression of members of the IRAK family. In contrast, ST2, a negative regulator of TLR-induced signaling, was shown to be up-regulated in spleens after MALP-2 pre-treatment. These results suggest that attenuation of systemic and local cytokine production involving ST2, as well as enhanced accumulation of inflammatory neutrophils in the peritoneal cavity, may contribute to the protective effects during polymicrobial peritonitis after TLR2-pre-treatment.
Methods
Treatment of mice
Mice were injected intra-peritoneally (i.p.) with 2 and 8 μg MALP-2, 50 and 200 μg Pam3Cys (EMC Microcollections, Tübingen, Germany) or 80 and 320 μg Escherichia coli LPS, serotype 0127:B8 (Sigma Chemical Co., St Louis, MO, USA), in PBS. Mice were sacrificed either 2 or 6 h after injection. For in vivo immune priming, mice were injected with the same amounts of MALP-2 and Pam3Cys 4 days before colon ascendens stent peritonitis (CASP) surgery. The control group was injected i.p. with the same volume of PBS. MALP-2 was synthesized and purified as described previously (28). The regimen for in vivo treatment with MALP-2 was chosen because it was shown that i.p. injection of 2 μg MALP-2 into mice is sufficient to cause transient local and systemic inflammatory reactions (19). The concentration of Pam3Cys was chosen equally to those of the manufacturer's recommendations for the induction of the adjuvant effect of Pam3Cys. The dosage for LPS was used as described previously (29).
Analysis of cytokine production in serum and peripheral organs
Peripheral blood, peritoneal lavage fluid, spleen, liver and lung were collected either prior to CASP at the indicated time points or at 3, 6 and 12 h after CASP after extensive perfusion of mice with PBS. Serum and peritoneal lavage fluid were frozen at −80°C. Peripheral organs were snap frozen in liquid nitrogen. Organs were homogenized after thawing in 1 ml PBS containing complete protease inhibitors (Roche Diagnostics, Mannheim, Germany). Organ extracts were centrifuged (6000 × g for 20 min at 4°C) and supernatants were collected. Cytokine concentrations were measured by ELISA specific for TNF, IL-10, IL-12p40 and IL-18 (all from R&D Systems, Minneapolis, MN, USA). Cytokine levels in peripheral organs were normalized against the protein concentration in each organ extract. Protein concentrations were determined by the bicinchoninic acid (BCA) method according to the manufacturer's instructions (Pierce, Rockford, IL, USA).
CASP model of polymicrobial septic peritonitis
Female C57BL/6 mice were maintained under specific pathogen-free conditions and used at 8–12 weeks of age. The technique used for induction of CASP was performed as described previously (29, 30). Briefly, the colon ascendens was exteriorized and a 7/0 ethilon thread (Ethicon, Nordersted, Germany) was stitched through the anti-mesenteric portion of the colon ascendens ∼10 mm distal of the ileocecal valve. A 16-gauge venous catheter was punctured anti-mesenterically through the colonic wall into the intestinal lumen, directly proximal of the pre-tied knot and fixed. To ensure proper intra-luminal position of the stent, stool was milked from the cecum into the colon ascendens until a small drop appeared. Fluid resuscitation of the animals was performed by flushing 0.5 ml sterile saline into the peritoneal cavity prior to closure of the abdominal wall.
Determination of bacterial numbers
Mice were sacrificed 12 h after CASP surgery and peritoneal lavage fluid was collected. Serial dilutions of lavage fluids were plated on McConkey and colistin naladixic acid agar or blood agar plates (Becton Dickinson, Heidelberg, Germany) to determine the colony-forming units (CFUs) of gram-negative and gram-positive bacteria, respectively. CFUs were counted after incubation at 37°C for 24 h and calculated as CFUs per whole peritoneal cavity.
Flow cytometry
For analysis of leukocyte recruitment to the peritoneal cavity after CASP, peritoneal lavage cells were collected at various time points after CASP and erythrocytes were lysed for 5 min in lysis buffer (155 mM ammonium chloride, 15 mM sodium bicarbonate, 1 mM EDTA). Peritoneal leukocytes were incubated with fluorochrome-labeled antibodies against Mac-1 (M1/70), Ly-6G/Gr-1 (RB6-8C5) or appropriate isotype-matched controls (all from BD PharMingen, San Diego, CA, USA) for 30 min at 4°C in PBS containing 1% BSA. After washing the cells with PBS, fluorescence was analyzed on an Epics XL Cytometer (Coulter Immunotech, Hialeah, FL, USA).
Oxidative burst
Production of reactive oxygen metabolites was accessed by a flow cytometry method as described (31). Briefly, peritoneal exudate cells were collected 6 h after CASP surgery from MALP-2- and PBS-injected mice. Peripheral blood cells from untreated mice served as control. Erythrocytes were lysed as described above (‘flow cytometry’). Leukocytes were washed in HBSS and loaded in the dark for 15 min at 37°C with 60 μM dihydrorhodamine 123. After adding 2.5 μM sodium azide and 0.05 mM cytochalasin B, cells were incubated for 20 min at 37°C in the dark. Flow cytometry analysis was performed immediately thereafter using an Epics XL cytometer.
Western blot analysis
For western blotting, spleen extracts as described above were used. Lysates were cleared by centrifugation at 10 000 × g for 10 min, boiled in SDS sample buffer, resolved on a 10% SDS gel and blotted onto nitrocellulose membranes (Immobilon P; Schleicher and Schuell GmbH, Dassel, Germany). Membranes were probed with antibodies against IRAK-1 and IRAK-M (Abcam, Cambridge, UK) and p38 MAP-kinase (New England Biolabs, Frankfurt, Germany) as loading control, following incubation with secondary HRP-conjugated antibodies (New England Biolabs) and and visualized using enhanced chemiluminescence (Amersham, Upsala, Sweden) for detection.
Quantitation of mRNA levels by reverse transcription–PCR
Spleens of mice pre-treated for 4 days with either MALP-2 or PBS as control were removed and single-cell suspensions were prepared. RNA extractions were carried out with the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. First-strand cDNA was synthesized from 20 μg of total RNA using a mixture of oligo(dT)12–18 and random hexamer primers and Superscript reverse transcriptase (Invitrogen, Karlsruhe, Germany). The reaction was incubated for 60 min at 42°C and terminated by heating to 95°C for 5 min. Serial 1: 2 dilutions of at least two independent cDNA preparations for each cellular fraction were used as template in reverse transcription (RT)–PCR reactions. The final cDNA dilution yielding detectable amplification products was scored for each sample. To normalize mRNA levels the cDNA titers for TLR genes were divided by the β-actin titers obtained from the same cDNA template.
Primers for amplification of specific cDNA fragments are listed as follows: β-actin sense (5′ATGGATGACGATATCGCT 3′) and β-actin antisense (5′ATGAGGGTAGTCTGTCAGGT 3′), Tlr2 sense (5′AGAGAAAGTACTTACTGCATTCTCTCTGAT 3′) and Tlr2 antisense (5′TTGAGCCAGAATCATTTGAGATCAATGCAA 3′), Tlr4 sense (5′GACACCCTCCATAGACTT 3′) and Tlr4 antisense (5′GGTATTCATCTCTACAGG 3′), Tlr1 sense (5′CCTTCCGAGCCCTTCGCTTTG 3′) and Tlr1 antisense (5′AGCTCACTGAACGATTTCCAGTGG 3′), Tlr6 sense (5′GCTGAGAGCATATTGGT-GTTG3′) and Tlr6 antisense (5′TGCAGTTCCCTTAGAGCTTTC3′).
Real-time PCR analysis
For real-time quantitative RT–PCR, 1 μg of total RNA of spleens of mice pre-treated with MALP-2, Pam3Cys or PBS for 4 days was reverse transcribed as described above. Primers were designed using PrimerExpress software (Applied Biosystems, Foster City, CA, USA). SYBR-green master mix was used to detect accumulation of PCR product during cycling on the SDS7700 (Applied Biosystems). Expression of ST2 of MALP-2-treated animals was normalized to β-actin and displayed as fold-change relative to samples of PBS-treated mice used as the calibrator (set to 1). Primers for amplification of specific cDNA fragments are listed as follows: β-actin sense (5′ACCCACACTGTGCCCATCTAC 3′), β-actin antisense (5′AGCCAAGTCCAGACGCAGG 3′); ST2 sense: (5′GCTTGTCCAGGCAGAGCT 3′), ST2 antisense (5′GAGCCGTCCTACTGTCCA 3′).
Statistical analysis
Statistical analysis of the data was performed using the Mann–Whitney U-test or the Student's t-test where appropriate. Survival data were analyzed using the log-rank test. All data are presented as mean ± SEM. The level of significance was P < 0.05.
Results
Acute immune reaction after injection of TLR2 and TLR4 ligands
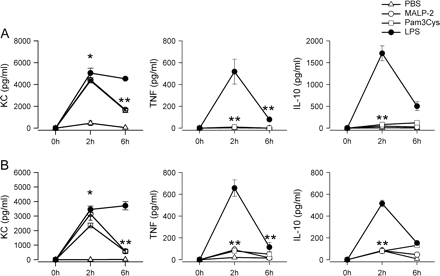
It has been shown previously that the host defense against bacterial infections is greatly improved by pre-treatment of mice with LPS or immunostimulatory CpG-oligodeoxynucleotide (ODN), which signal through TLR4 and TLR9, respectively (29, 31). Injection of LPS and CpG leads to the induction of proinflammatory cytokines early after injection. Therefore, the induction of an acute immune reaction after injection of LPS was compared with that of TLR2 agonists MALP-2 and Pam3Cys. MALP-2 is known to stimulate immune cells through a pathway dependent on TLR2 and TLR6 (8, 9), whereas Pam3Cys induces signal transduction through TLR2 and TLR1 (8). Systemic levels of KC, TNF and IL-10 were determined 2 and 6 h after i.p. injection of 2 and 8 μg MALP-2, 50 and 200 μg Pam3Cys or 80 and 320 μg LPS, referred to as low or high mediator concentration. To rule out dose-dependent effects, mice were injected with two doses of agonists. Figure 1 shows that injection of MALP-2, Pam3Cys and LPS leads to the induction of the chemokine KC after 2 h for both agonist concentrations used, with the different agonists showing comparable effects. Marked production of the cytokines TNF and IL-10 were only detectable in LPS-treated mice, not after injection of MALP-2 and Pam3Cys. Six hours after injection, the systemic KC levels in MALP-2- and Pam3Cys-treated mice were significantly attenuated compared with the early time point (2.7-fold reduction for both agonists in the low concentration, 4.4-fold for MALP-2 and 2-fold at the high concentration used), whereas the systemic KC levels of LPS-treated mice stayed markedly elevated at this time point. The systemic levels of TNF and IL-10, however, decreased markedly in LPS-treated mice.
Induction of acute systemic immune response in mice treated with MALP-2, Pam3Cys and LPS. Mice were injected i.p. with 2 μg MALP-2 (circles), 50 μg Pam3Cys (squares) or 80 μg LPS (filled circles) or PBS (triangles). Serum samples were obtained 2 and 6 h after injection and were analyzed for production of KC, TNF and IL-10 by ELISA. Results are derived from six mice per group and time point. (B) Mice were injected i.p. with 8 μg MALP-2 (circles), 200 μg Pam3Cys (squares) or 320 μg LPS (filled circles) or PBS (triangles). Serum samples were obtained 2 and 6 h after injection and were analyzed for production of KC, TNF and IL-10 by ELISA. Results are derived from six mice per group and time point. *P < 0.05 (MALP-2- or Pam3Cys-treated mice versus controls), **P < 0.05 (LPS-treated mice versus MALP-2- or Pam3Cys-treated mice).
Thus, pre-treatment of mice with LPS leads to a sustained induction of cyto- and chemokine responses, whereas the acute immune reaction induced by MALP-2 and Pam3Cys is only marginal.
Priming of mice with MALP-2 and Pam3Cys improves survival in polymicrobial septic peritonitis
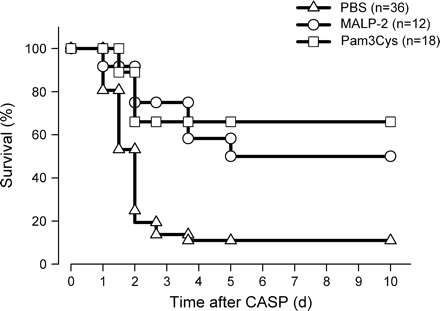
To further analyze the effects of immune priming by TLR2 ligands in vivo, mice were treated i.p. with 2 μg MALP-2 or 50 μg Pam3Cys and septic peritonitis was induced by the CASP procedure 4 days later. The results depicted in Fig. 2 demonstrate that pre-treatment of mice with both TLR2 ligands MALP-2 and Pam3Cys significantly improved survival of septic peritonitis compared with control mice (P < 0.001 for both treatment groups).
Improved survival of acute polymicrobial peritonitis in mice treated with MALP-2 or Pam3Cys. Mice were injected i.p. with PBS (triangles, n = 36) or with 2 μg MALP-2 (open circles, n = 12) or 50 μg Pam3Cys (open squares, n = 18) for 4 days before CASP. Survival was monitored over 10 days. The data were pooled from four independent experiments each demonstrating an improved survival of mice primed with TLR2 ligands compared with controls. Statistical significance was determined by log-rank test. P < 0.005 Malp-2 and PAM3Cys treated mice versus controls.
Analysis of host defense mechanisms in MALP-2-treated mice
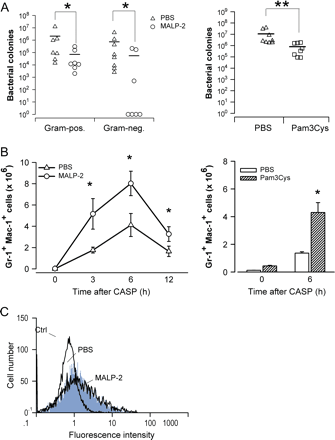
To analyze the mechanisms of TLR2 agonist-induced protection against polymicrobial peritonitis, antibacterial effector mechanisms which influence the host defense were analyzed (32, 33). Peritoneal lavage fluid was harvested 12 h after CASP surgery and bacterial counts were determined. As depicted in Fig. 3(A), mice pre-treated with MALP-2 or Pam3Cys showed significantly lower numbers of viable bacteria in the peritoneal cavity when compared with control mice. Thus, pre-treatment of mice with the TLR2 agonists MALP-2 or Pam3Cys enhances bacterial clearance.
Enhanced antibacterial defense in MALP-2-treated mice. (A) For determination of bacterial load at the site of infection, peritoneal lavage fluid was obtained 20 h after CASP surgery from mice pre-treated with MALP-2 (circles) or PBS (triangles) and Pam3Cys (squares) and PBS (triangles). Serial dilutions of peritoneal lavage fluid were plated on McConkey and CNA agar to determine colony numbers of gram-negative and gram-positive bacteria, respectively. For determination of bacterial load of Pam3Cys-treated mice, peritoneal lavage fluid was plated on blood agar plates. Horizontal bars represent mean bacterial numbers in each group. Results are derived from six–eight independent mice per group. *P < 0.05 (MALP-2-treated mice versus controls), **P < 0.05 (Pam3Cys-treated mice versus controls). (B) Mice were injected i.p. with 2 μg MALP-2 (circles), Pam3Cys or PBS (triangles) and 4 days later peritoneal cells were harvested either immediately before CASP (0 h) or at various time points thereafter. Neutrophils were identified by expression of Gr-1 and Mac-1 as described (35). Absolute neutrophil numbers per peritoneal cavity were determined from six–eight independent mice per group and time point. *P < 0.05 (MALP-2-treated or Pam3Cys-treated mice versus controls). (C) Peritoneal lavage was performed 6 h after CASP in mice pre-treated with MALP-2 or PBS. The production of reactive oxygen intermediates was determined directly ex vivo without additional in vitro stimulation of the cells. Unstimulated peripheral blood neutrophils from untreated mice were used for control (ctrl). Neutrophils were identified by their forward and side scatter profile as described (31). The histograms shown are representative of four mice per group.
Neutrophils represent pivotal effector cells in septic peritonitis which efficiently phagocytose bacteria and produce antimicrobial agents such as reactive oxygen and nitrogen intermediates (32–34). We therefore investigated the accumulation of neutrophils in the peritoneal cavities of MALP-2-treated and control mice at various time points after CASP surgery. Neutrophils were identified by high expression of both Gr-1 and Mac-1 (35). A marked increase of peritoneal neutrophil numbers could be observed as early as 3 h after CASP in both PBS- and MALP-2-treated mice (Fig. 3B). However, during the entire observation period, absolute neutrophil numbers were significantly greater in MALP-2-primed mice than in PBS-injected controls (Fig. 3B). The enhanced peritoneal neutrophil accumulation in MALP-2-pre-treated mice was most pronounced early after CASP (2.9-, 1.9- and 2.0-fold at 3, 6 and 12 h, respectively). Since cell recruitment after sepsis induction was most apparent 6 h after sepsis induction, infiltration of effector granulocytes was analyzed in Pam3Cys-treated mice 6 h after sepsis induction. Pre-treatment of mice with Pam3Cys, as the pre-treatment with MALP-2, leads to enhanced accumulation of Gr-1/Mac-1-positive effector granulocytes.
To investigate the antimicrobial activity of peritoneal neutrophils, cells were isolated from MALP-2-primed mice or from PBS controls 6 h after CASP and the production of reactive oxygen intermediates was measured. Experiments were conducted without additional in vitro stimulation of neutrophils to directly examine the activation by in vivo exposure to intestinal bacteria. The results in Fig. 3(C) clearly demonstrate that the generation of reactive oxygen metabolites by peritoneal neutrophils from septic MALP-2-treated mice and from septic PBS controls was substantially elevated when compared with neutrophils from non-septic mice. Importantly, neutrophil production of reactive oxygen intermediates was comparable in MALP-2-primed and control mice subjected to CASP (Fig. 3C). These results suggest that neutrophils accumulating in the peritoneal cavity of MALP-2-pre-treated mice are activated and exhibit normal antimicrobial activity.
Influence of pre-treamtment of mice with MALP-2 or Pam3Cys on the systemic cytokine response during septic peritonitis
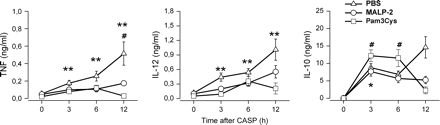
To further elucidate potential effects of the immune priming induced by TLR2 ligation during septic peritonitis, the systemic cytokine response was examined. Mice were pre-treated 4 days prior to sepsis induction via CASP surgery either with MALP-2 or Pam3Cys. The results depicted in Fig. 4 show that serum concentrations of TNF, IL-12 and IL-10 were strongly increased during the course of septic peritonitis in control mice with the highest levels being observed at 12 h after CASP. In contrast, systemic levels of TNF, IL-12 and IL-10 were reduced in mice pre-treated with either MALP-2 or Pam3Cys as compared with controls during the entire observation period. The marked increase of serum TNF and IL-12, which was observed in control mice between 6 and 12 h after CASP, was greatly attenuated or even absent in MALP-2- and Pam3Cys-treated mice. Serum levels of IL-10 showed a marked increase during the first 3 h after peritonitis induction, which was present in all treatment groups and was most pronounced for Pam3Cys-treated mice. Whereas the systemic IL-10 production in control mice further increases, the IL-10 production in MALP-2- and Pam3Cys-treated mice does not. It should be noted, however, that despite this significant reduction of systemic cytokines following priming with MALP-2 or Pam3Cys, serum levels of TNF, IL-12 and IL-10 were elevated in septic as compared with non-septic mice at different time points after CASP (Fig. 4). Thus, pre-treatment with TLR2 agonists leads to attenuated systemic cytokine response after sepsis induction.
Reduced serum cytokines levels during septic peritonitis after treatment of mice with MALP-2 or Pam3Cys. Mice were pre-treated with MALP-2 (circles), Pam3Cys (squares) or PBS (triangles) 4 days before CASP surgery. Serum samples were obtained either from mice prior to CASP (0 h) or from mice 3, 6 and 12 h after CASP. The content of TNF, IL-10 and IL-12p40 was determined by ELISA. Results are derived from 9–18 mice per group and time point. *P < 0.05 and **P < 0.01 (MALP-2-treated or Pam3Cys-treated mice versus controls), #P < 0.05 (MALP-2-treated mice at 0 h versus later time points).
Because treatment of mice with either MALP-2 or Pam3Cys seems to involve similar mechanisms the further analysis of the sepsis pathology of mice was carried out after MALP-2 pre-treatment.
Analysis of local cytokine and chemokine production in MALP-2-treated mice
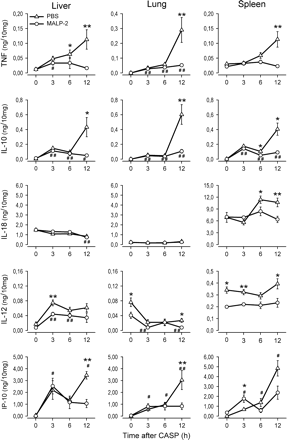
To examine the effects of MALP-2 priming on the cytokine response during septic peritonitis in more detail, the concentrations of TNF, IL-10, IL-12, IL-18 and IP-10 were measured in protein extracts of various peripheral organs (Fig. 5). In septic control mice, both TNF and IL-10 levels were up-regulated in liver, lung and spleen. Pre-treatment of mice with MALP-2, however, resulted in a consistent reduction of TNF and IL-10 protein levels in all organs examined. The sepsis-induced elevation of IL-18 observed in the spleen of control mice was also markedly reduced in MALP-2-primed mice. In contrast, IL-18 levels in liver and lung were not affected by septic peritonitis, irrespective of whether mice were pre-treated with MALP-2 or not. In control mice, septic peritonitis induced an increase of IL-12 protein in the liver, whereas IL-12 was markedly decreased in the lung and remained at high constitutive levels in the spleen. These observations suggest the existence of pre-formed IL-12 in lung and spleen, which may be released in response to the infectious challenge. Nonetheless, MALP-2-pre-treated mice exhibited significantly lower IL-12 protein concentrations than control mice in all organs tested.
Diminished cytokine levels in peripheral organs of MALP-2-treated mice during septic peritonitis. Mice were pre-treated with MALP-2 (circles) or PBS (triangles) 4 days before CASP. Lung, liver and spleen were removed either from mice prior to CASP (0 h) or from mice 3, 6 and 12 h after CASP after extensive perfusion with PBS. Organs were homogenized and protein extracts were prepared. The content of TNF, IL-10, IL-12p40, IL-18 and IP-10 was measured by ELISA. In each sample, cytokine levels were normalized against the total protein concentration. Results are derived from 8–12 mice per group and time point. *P < 0.05 and **P < 0.001 (MALP-2-treated mice versus controls), #P < 0.05 and ##P < 0.001 (MALP-2-treated mice at 0 h versus later time points).
Signaling induced by TLR agonists involves MyD88-dependent and -independent pathways. Whereas TLR2 signaling involves only MyD88, TLR4 induces both MyD88-dependent and MyD88-independent pathways (11, 12). To assess the influence of priming with the TLR2 agonist MALP-2 on MyD88-independently regulated cytokines, the production of IP-10 was analyzed. IP-10 was shown to be induced during septic peritonitis in both MALP-2-treated and control mice, but the expression level was significantly reduced during the observation period in liver, lung and spleen of MALP-2-treated mice compared with control mice. IP-10 showed an early increase in both experimental groups, which was more pronounced in the spleens of MALP-2-treated animals but in contrast to control mice there was no further increase at later time points.
These results therefore demonstrate that MALP-2 priming strongly attenuates the local cytokine production during septic peritonitis. This attenuation involves both MyD88-dependent and -independent signaling pathways and was most pronounced for the late time points. However, pre-treatment of mice with MALP-2 did not completely abolish cytokine production as significantly elevated concentrations of TNF, IL-10, IP-10 and IL-12, but not IL-18, were detected in various organs of septic as compared with non-septic MALP-2-primed mice (Fig. 5).
Expression of TLR2, TLR1, TLR6 and TLR4 by MALP-2 treatment
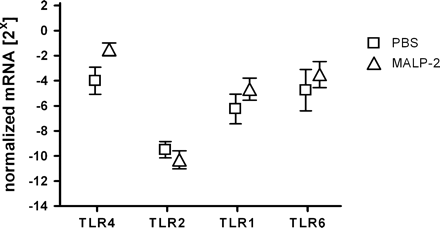
To elucidate mechanisms that contribute to the diminished cytokine production in TLR2-treated mice, expression of TLR4, TLR2, TLR1 and TLR6 mRNA was analyzed. Down-regulation of TLR4 was shown to be involved in endotoxin tolerance in vivo and in vitro (20) and therefore could correlate with diminished production of cytokines in endotoxin tolerance. To assess TLR expression after MALP-2 treatment, spleens were isolated 4 days after MALP-2 priming. Expression of TLR was determined by semi-quantitative RT–PCR. As depicted in Fig. 6, expression levels of the investigated TLRs were not significantly altered in the spleen after MALP-2 treatment. TLR4 levels, however, were shown to be slightly enhanced compared with controls (P = 0.801). These data indicate that MALP-2 treatment for 4 days does not lead to down-regulation of TLR4, TLR2, TLR1 and TLR6 and can therefore not explain the MALP-2-induced reduction of cytokine responses.
Modulation of mRNA levels of TLRs in MALP-2-treated mice. Mice were pre-treated with MALP-2 or PBS for 4 days. RNA was prepared from spleen cell suspensions. Semi-quantitative PCR analysis was performed for tlr2 and tlr4 using expression of glycerinaldehyde 3-phosphate dehydrogenase as control. Results are derived from 8–12 mice per group.
Expression of IRAK-1 and IRAK-M in mice pre-treated with TLR2 agonists
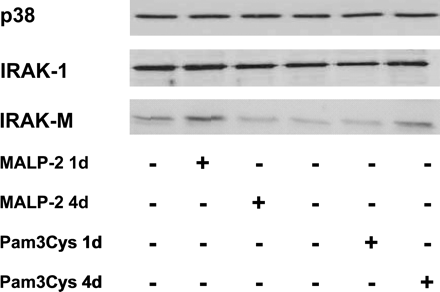
It was shown that changes in the expression levels of IRAK proteins are involved in LPS-induced immune responses (21, 22). IRAK-1, which is involved in TLR signaling, is greatly diminished in endotoxin-tolerant THP-1 cells (22). IRAK-M negatively regulates LPS-induced cytokine expression and is related to the development of endotoxin tolerance in vitro (21). Therefore, we examined the expression levels of IRAK proteins after MALP-2 priming. Spleen homogenates were analyzed 4 days after MALP-2 injection without peritonitis induction. Expression levels of p38 MAP-kinase total protein served as a control. As depicted in Fig. 7, the expression levels of IRAK-1 were comparable in MALP-2-treated and control mice. Expression levels of IRAK-M seem to be enhanced after 1 day of priming and return to control levels after 4 days, whereas expression levels of IRAK-M after Pam3Cys priming were up-regulated after 4 days compared with the early time point. Since Pam3Cys and MALP-2 both induce protection during septic peritonits and show reduced cytokine levels 4 days after priming, these results may not be in line with these findings. Thus, differences in the expression levels of IRAK-1 and IRAK-M do not seem to be involved in the altered cytokine response 4 days after MALP-2 treatment.
Expression of IRAK-1, IRAK-2 and IRAK-M in mice treated with MALP-2 or Pam3Cys. Mice were pre-treated with MALP-2, Pam3Cys or PBS 4 days before CASP surgery. Spleens were removed from mice prior to CASP (0 h) after extensive perfusion with PBS. Organs were homogenized and protein extracts were prepared. Expression of IRAK-1 and IRAK-M were analyzed by western blot. Expression of p38 MAP-kinase was used as control. The histogram shown is a representative result of four independent experiments.
Up-regulation of ST2 after priming with MALP-2
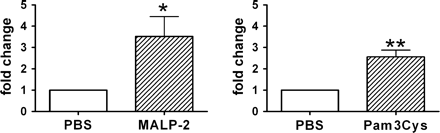
Recent data show that the TIR family member ST2 plays an important role in the induction of endotoxin tolerance (25). ST2-deficient macrophages show enhanced cytokine expression after TLR-induced stimulation (25). Expression of ST2 mRNA was analyzed using real-time PCR in spleens of 4-day MALP-2-primed and Pam3Cys-primed mice compared with control mice. This analysis revealed a 3.8-fold up-regulation of ST2 mRNA in MALP-2-primed and a 2.5-fold up-regulation in Pam3Cys-primed mice relatively compared with control mice (Fig. 8). These data demonstrate that priming with the TLR2 agonists MALP-2 and Pam3Cys for 4 days induces up-regulation of ST2 mRNA in vivo, indicating an involvement of ST2 in cytokine regulation after MALP-2 pre-treatment.
Up-regulation of ST2 in MALP-2-primed and Pam3Cys-treated mice. Mice were pre-treated with MALP-2, Pam3Cys or PBS for 4 days. Real-time PCR using SYBR-green dye inclusion was used to amplifiy ST2 cDNA in splenic samples. Relative quantification of ST2 expression in MALP-2-primed or Pam3Cys-primed mice compared with PBS control mice was calculated using β-actin as control. Data were given as fold induction of ST2 mRNA compared with PBS-treated mice which were used as calibrator and was set to 1. Results are derived from six–nine mice per group. *P < 0.05 (MALP-2-treated mice versus controls), **P < 0.05 (Pam3Cys-treated mice versus controls).
Discussion
The present report demonstrates that pre-treatment of mice with the TLR2-dependent agonists MALP-2 and Pam3Cys significantly improves survival and bacterial clearance in polymicrobial septic peritonitis. The protective effects of priming with TLR2 agonists were associated with an increased effector neutrophil response and a mitigated production of both pro- and anti-inflammatory cytokines during septic peritonitis. Both effects seem to contribute to the enhanced survival of TLR2-treated mice. Recently, Wang et al. (36) showed that tolerance induced by the TLR2 agonist Pam3Cys protects against polymicrobial peritonitis induced by cecal ligation and puncture. In contrast to our model, these data describe short-term tolerance induced by the injection of Pam3Cys 24 h before challenge using 4-fold higher doses of Pam3Cys. In both models, enhanced recruitment of inflammatory neutrophils into the site of inflammation and reduced systemic cytokine levels could be demonstrated. Wang et al. (36) demonstrate over-expression of CR3 and FcγIII receptors which may contribute to the enhanced microbicidal acitivites in short-term tolerance. In our model, however, up-regulation of these receptors could not be demonstrated (data not shown). In addition, we could show that after 4 days of pre-treatment with MALP-2, cytokine levels in peripheral organs were greatly diminished. While regulation of TLRs as well as the expression of IRAK proteins do not contribute to the diminished systemic and peripheral cytokine burst after sepsis induction, up-regulation of ST2 may contribute to the attenuated cytokine production after 4 days of priming with TLR2 agonists.
Our previous studies showed that pre-treatment with LPS and CpG for 4 and 6 days, respectively, also improves survival of septic peritonitis (29, 31). When comparing these studies, however, both common and unique effects of these TLR agonists in modulating immune responses become apparent. Similar to MALP-2 priming, administration of LPS and CpG-ODN also increased accumulation of effector neutrophils at the site of infection. These findings are consistent with the more general view that priming through either TLR2, TLR4 or TLR9 may improve host defense against pathogens that are efficiently cleared by neutrophils. In contrast to the effects on neutrophil responses, pre-treatment of mice with different TLR agonists differentially affects cytokine response during septic peritonitis. Whereas production of all pro- and anti-inflammatory cytokines examined was greatly reduced in MALP-2-primed and Pam3Cys-primed mice, pre-treatment with CpG-ODN did not alter systemic cytokine levels during septic peritonitis (31). Moreover, induction of LPS pre-treatment attenuated production of TNF and IL-12, but not of IL-10 and IL-18, in septic mice (29). When comparing systemic cytokine production of Pam3Cys-primed mice to MALP-2-primed mice, however, slight differences became apparent. Cytokine levels in Pam3Cys-treated mice fell 12 h after sepsis induction compared with the earlier time points, whereas cytokines in MALP-2-treated mice did not change over the observation period. Also systemic levels of IL-10 were higher in Pam3Cys-treated mice compared with MALP-2 treatment. These differences in the kinetics of cytokine induction could indicate that the differential receptor usage of MALP-2 (TLR1/TLR6) and Pam3Cys (TLR1/TLR2) (8, 9) results in slightly diverse signal induction events, but treatment with both TLR2 agonists are able to reduce the sepsis-induced cytokine burst. Thus, pre-treatment with TLR2 agonists largely attenuates the sepsis-induced hyperinflammatory reaction.
To compare acute effects caused by the administration of TLR2 and TLR4 agonists, the induction of inflammatory cytokines was examined. To rule out dose-dependent effects, injection of agonists was done with the dose which was used for in vivo priming, and also a 4-fold higher dose was applied. Injection of MALP-2 and Pam3Cys agonists does not induce an acute cytokine burst in contrast to LPS at both concentrations used. Since both the TLR4 agonist LPS and the TLR2 agonists MALP-2 and Pam3Cys induce protection against polymicrobial peritonitis, but only LPS causes a significant acute cytokine response after injection, these immediate effects seem not to be important for the protective effects.
Recruitment of neutrophils to the local site of infection is an essential mechanism to eliminate certain classes of pathogens. Independent reports have shown that depletion of neutrophils severely impairs resistance against intra-abdominal bacterial infection both in humans and experimental animals (32, 33, 37). The present report shows that pre-treatment of mice with either MALP-2 or Pam3Cys substantially augments and accelerates peritoneal neutrophil accumulation during septic peritonitis. Moreover, neutrophils isolated from the infected peritoneal cavities of MALP-2-primed mice exhibited a normal production of reactive oxygen metabolites indicating that the total antimicrobial activity at the local site of infection was increased. This also was reflected by the significantly reduced bacterial load in the peritoneal cavity in both MALP-2- and Pam3Cys-treated mice. Considered together with previous work (29), our results therefore suggest that the improved resistance against septic peritonitis after MALP-2 priming is related to an augmented effector neutrophil response.
In MALP-2-treated septic mice, diminished production of cytokines could be observed. In particular, high cytokine levels observed at 12 h after induction of septic peritonitis were strongly reduced or absent in MALP-2-treated mice. This attenuated cytokine response was observed for systemic levels as well as for the cytokine content of individual organs. Priming of mice with MALP-2 may therefore prevent excessive production of inflammatory cytokines, which is considered to play an important role in the development of organ injury and septic shock (38–40). Besides the MyD88-dependenly regulated cytokines TNF, IL-10 and IL-12, the TRIF-dependent cytokine IP-10 was also down-regulated indicating that both the MyD88-dependent and -independent TLR signaling pathways are mitigated after MALP-2 treatment followed by challenge of mice with an acute polymicrobial infection. Thus, MALP-2-induced signaling before polymicrobial peritonitis influences several TLR-induced pathways.
It is important to note, however, that cytokine production was only reduced, but not completely abolished, by priming of mice with MALP-2. These results are therefore consistent with the view that, although significantly attenuating the cytokine response, MALP-2 priming of mice preserves the production of these mediators at levels sufficient for protective pathogen defense. Thus, immune modulation by TLR2 agonists may represent an important process involved in attenuating the cytokine response to infection.
The molecular mechanisms of endotoxin tolerance operate both at the receptor level and at the signaling cascades. Down-regulation of the TLR4–MD2 complex in endotoxin tolerance (20) as well as TLR2 in lipoprotein-induced tolerance (41) was described in vitro. Here we could not observe any down-regulation of TLR2, TLR1, TLR6 and TLR4 mRNA after 4 days of MALP-2 treatment. Indeed, Nomura et al. showed re-expression of TLR4 after 24 h of endotoxin tolerance, suggesting that down-regulation of TLR may be a short-time effect after administration of the tolerogenic TLR agonist. In our experiments, however, the second stimulus was set after 4 days, so this mechanism may not contribute to tolerance at later time points. Since in cell lines over-expressing TLR2 and TLR4 endotoxin tolerance can be induced, also mechanisms other than the regulation of TLR expresssion must be involved (42). In addition, members of the IRAK family as IRAK-1 and IRAK-M, as well as SOCS-1, have been identified to be involved in the regulation of TLR-induced signaling during endotoxin tolerance (21, 22, 24). In our experiments differences in the expession levels of either IRAK-1 and IRAK-M after 4 days of priming with MALP-2 or Pam3Cys could not explain the attenuated cytokine response. IRAK-M may mediate short-time endotoxin tolerance effects since IRAK-M- deficient macrophages retain a substantial capacity to develop endotoxin tolerance after priming for 24 h (21). IRAK-1 is rapidly degraded after LPS stimulation (43). In vitro, IRAK-1 degradation within hours contributes to endotoxin tolerance (22, 44). Therefore, in vitro short-term tolerance and in vivo priming for a longer time might involve different mechanisms. SOCS-1 also seems to be rapidly induced after LPS stimulation and seems to involve rapid signaling mechanisms like degradation of IκB kinases (24) and therefore may also be involved in short-time endotoxin tolerance mechanisms. Since MALP-2 treatment induces cytokine suppression after 4 days, such short-time effects involving IRAK proteins or SOCS-1 may not be operative.
Recently, ST2, an orphan member of the TIR family of receptors, has been described as crucial mediator of endotoxin tolerance. In contrast to several other molecules involved in TLR signaling it is induced late upon LPS challenge (25) and its expression could be shown for up to 48 h (27). ST2 was shown to negatively regulate TLR signaling since ST2-deficent macrophages show enhanced cytokine response after TLR-induced stimulation (25). Here, we show that, in contrast to the expression of TLR and IRAK proteins, ST2 mRNA was up-regulated in spleens of MALP-2-treated and Pam3Cys-treated mice after 4 days of treatment. However, up-regulation in MALP-2-treated animals was more pronounced compared with Pam3Cys treatment. This again could indicate that triggering of TLR2/TLR6 by MALP-2 could be more profound or could induce differentially induced pathways compared with the activation of TLR2/TLR1 by Pam3Cys. It is therefore conceivable that up-regulation of ST2 contributes to the suppression of cytokine induction in septic MALP-2-pre-treated mice.
In summary, the present report demonstrates that treatment of mice with the TLR2-dependent agonist MALP-2 several days before pathogen exposure markedly increases the resistance against polymicrobial septic peritonitis. Immune protection in these mice was associated with an enhanced effector neutrophil response and attenuated cytokine production. Up-regulation of the negative regulatory receptor ST2 4 days after injection of MALP-2 may contribute to the diminished cytokine production and thereby to immune protection in MALP-2-pre-treated mice.
Transmitting editor: E. Vivier
This work was supported by by the Deutsche Forschungsgemeinschaft through SFB 576 (project A7).
References
Kopp, E. B. and Medzhitov, R.
Akira, S., Takeda, K. and Kaisho, T.
Bulut, Y., Faure, E., Thomas, L., Equils, O. and Arditi, M.
Hajjar, A. M., O'Mahony, D. S., Ozinsky, A. et al.
Ozinsky, A., Underhill, D. M., Fontenot, J. D. et al.
Takeuchi, O., Takeda, K., Hoshino, K., Adachi, O., Ogawa, T. and Akira, S.
Wyllie, D. H., Kiss-Toth, E., Visintin, A. et al.
Takeuchi, O., Kawai, T., Mühlradt, P. F. et al.
Takeuchi, O., Kaufmann, A., Grote, K. et al.
Sato, S., Nomura, F., Kawai, T. et al.
Hoebe, K., Du, X., Goergel, P. et al.
Yamamoto, M., Sato, S., Mori, K. et al.
Christman, J. W., Lancaster, L. H. and Blackwell, T. S.
Granowitz, E. V., Porat, R., Mier, J. W. et al.
Greisman, S. E., Edward, M. D., Young, J. and Woodward, W. E.
Freudenberg, M. A., Keppler, D. and Galanos, C.
Freudenberg, M. A. and Galanos, C.
Lehner, M. D., Morath, S., Michelsen, K. S., Schumann, R. R. and Hartung, T.
Deiters, U., Gumenscheimer, M., Galanos, C. and Mühlradt, P. F.
Nomura, F., Akashi, S., Sakao, Y. et al.
Kobayashi, K., Hernandez, L. D., Galán, J. E., Janeway, C. A., Medzhitov, R. and Flavell, R. A.
Li, L., Cousart, S., Hu, J. and McCall, C. E.
Kinjyo, I., Hanada, T., Inagaki-Ohara, K. et al.
Nakagawa, R., Naka, T., Tsutsui, H. et al.
Brint, E. K., Xu, D., Liu, H. et al.
Trajkovic, V., Sweet, M. J. and Xu, D.
Oshikawa, K., Yanagisawa, K., Tominaga, S. and Sugiyama, Y.
Mühlradt, P. F., Kieß, M., Meyer, H., Süßmuth, R. and Jung, G.
Feterowski, C., Weighardt, H., Emmanuilidis, K., Hartung, T. and Holzmann, B.
Zantl, N., Uebe, A., Neumann, B. et al.
Weighardt, H., Feterowski, C., Veit, M., Rump, M., Wagner, H. and Holzmann, B.
Dalrymple, S. A., Slattery, R., Aud, D. M., Krishna, M., Lucian, L. A. and Murray, R.
Haziot, A., Hijiya, N., Gangloff, S. C., Silver, J. and Goyert, S. M.
Matsumoto, T., Tateda, K., Miyazaki, S. et al.
Lagasse, E. and Weissman, I. L.
Wang, J.-H., Doyle, M., Manning, B. J. et al.
Hawkins, H. K., Heffelfinger, S. C. and Anderson, D. C.
Beutler, B. and Poltorak, A.
Bone, R. C., Grodzin, C. J. and Balk, R. A.
Dinarello, C. A.
Wang, J.-H., Doyle, M., Manning, B. J., Di Wu, Q., Blankson, S. and Redmond, H. P.
Medvedev, A. E., Henneke, P., Schromm, A. et al.
Noubir, S., Hmama, Z. and Reiner, N. E.