-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew F. Shorr, Mark J. Kunkel, Marin Kollef, Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies, Journal of Antimicrobial Chemotherapy, Volume 56, Issue 5, November 2005, Pages 923–929, https://doi.org/10.1093/jac/dki355
Close - Share Icon Share
Abstract
Objectives: To compare outcomes in patients with Staphylococcusaureus bacteraemia treated with linezolid with those of vancomycin-treated patients.
Methods: We pooled and analysed five randomized studies comparing linezolid with vancomycin, focusing on the 144 adults with S. aureus bacteraemia, which was secondary in >70% of patients. Efficacy variables were clinical cure of primary infection, microbiological success (eradication of S. aureus from blood or presumed eradication based on clinical cure of primary infection), survival, and outcome predictors identified by multivariate logistic regression.
Results: Of 99 clinically evaluable patients, primary infection was cured in 28 (55%) of 51 linezolid recipients and 25 (52%) of 48 vancomycin recipients [odds ratio (OR) for cure with linezolid versus vancomycin, 1.12; 95% confidence interval (CI), 0.51–2.47]. There were no between-group differences in the meta-analysis (OR, 1.16; 95% CI, 0.5–2.65). Of 53 evaluable patients with methicillin-resistant S. aureus (MRSA) bacteraemia, clinical cure occurred in 14 (56%) of 25 linezolid recipients and 13 (46%) of 28 vancomycin recipients (OR, 1.47; 95% CI, 0.50–4.34). Microbiological success occurred in 41 (69%) of 59 linezolid recipients and 41 (73%) of 56 vancomycin recipients (OR, 0.83; 95% CI, 0.37–1.87). Fifty-five (74%) of 74 linezolid recipients survived versus 52 (74%) of 70 vancomycin recipients (OR, 1.00; 95% CI, 0.47–2.12). In the multivariate analysis, treatment group was not a significant predictor of clinical cure or survival.
Conclusions: Linezolid was associated with outcomes that were not inferior to those of vancomycin in patients with secondary S. aureus bacteraemia.
Introduction
Bacteraemia due to Staphylococcus aureus is a growing problem. S. aureus bacteraemia occurs frequently in both the hospital and community, where it causes substantial morbidity, increases the cost of medical care, and contributes to mortality. Increasing antibiotic resistance complicates the approach to S. aureus bacteraemia; however, bacteraemia caused by methicillin-susceptible S. aureus (MSSA) remains problematic as well. In intensive care units (ICUs) of hospitals participating in the National Nosocomial Infections Surveillance (NNIS), 57% of S. aureus isolates from any site were resistant to methicillin (MRSA), representing a 13% increase in 2002 compared with the previous 5 years.1 Methicillin resistance was also common in other areas of participating NNIS hospitals, accounting for 42% of S. aureus isolates from non-ICU inpatient areas and for 26% of those from outpatient areas.1S. aureus bacteraemia, whether due to MSSA or MRSA, increased the duration and cost of hospitalization at Duke University Medical Center in the United States.2 Specifically, MSSA bacteraemia increased the median attributable total cost of hospitalization by ∼$10 000 compared with matched controls without bacteraemia, and MRSA bacteraemia increased the cost by ∼$27 000.2 The mean mortality rates were 23.4% for MSSA bacteraemia and 36.4% for MRSA bacteraemia [odds ratio (OR), 1.93; P < 0.001] in a meta-analysis of 31 cohort studies.3
Therapeutic options for patients with S. aureus bacteraemia are limited. Vancomycin is an initial option when MRSA infection is suspected; however, findings from retrospective4,5 and prospective studies6–8 suggest that vancomycin may provide suboptimal results, especially compared with β-lactam antibiotics in patients with MSSA infections.6,8 The availability of linezolid offers an alternative to vancomycin for treatment of S. aureus infection. Linezolid has in vitro activity against both MSSA and MRSA. Clinical activity has been confirmed in many types of infections including nosocomial pneumonia,9,10 ventilator-associated pneumonia,11 complicated skin and soft tissue infections,12 and MRSA infections.13,14 Linezolid has been suggested as an alternative to vancomycin in patients with S. aureus bacteraemia,6 but few data are available describing the efficacy of linezolid in this setting. Consequently, some clinicians may have concerns about the efficacy of linezolid when the blood culture is positive for S. aureus. We hypothesized that linezolid remains effective when used as treatment of secondary S. aureus bacteraemia as measured by traditional safety end points as well as by infection outcome. To test this hypothesis, we conducted a pooled analysis of prospective, randomized, controlled studies comparing linezolid with vancomycin and focused on the subset with S. aureus bacteraemia. Our study objectives were to compare clinical outcomes in the linezolid group with those in the vancomycin group, and to evaluate the safety of linezolid in patients with secondary S. aureus bacteraemia.
Methods
Study design
To determine outcomes in adult patients with S. aureus bacteraemia treated with linezolid and to compare them with those in patients treated with vancomycin, we pooled and retrospectively analysed data from all randomized studies comparing linezolid with vancomycin in the sponsor's database. We confirmed that there were no additional studies by searching PubMed, using the publication type limit ‘randomized controlled trial’ and using the keywords linezolid, vancomycin, and either bacteremia or bacteraemia. We also hand-searched the last 5 years' abstracts from the Infectious Diseases Society of America (IDSA), Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), and European Congress of Clinical Microbiology and Infectious Diseases (ECMID). These studies have been described previously9,10,12–14 and were generally similar except as described herein. All five studies were prospective, randomized, and multicentre; two were double blind.9,10 Two authors (A.F.S. and M.H.K.) examined each study and graded its quality using the system developed by Jadad et al.15
Adults were eligible for enrolment in the initial prospective studies if they had signs and symptoms of pneumonia acquired 48 h after hospital admission,9,10 complicated skin and soft tissue infections,12 or MRSA infections.13,14 Most infections, including the MRSA infections, were pneumonia or skin and soft tissue infections. Isolated pathogens were submitted to a central laboratory for identification to ensure consistency of results. Patients were excluded if they had infections caused by pathogens resistant to study drugs. To be included in the pooled analysis, patients had to have blood cultures positive for S. aureus and receive at least one dose of study drug. Secondary bacteraemia was defined as a bloodstream infection in a patient with a documented primary infection elsewhere. Each initial prospective study was approved by each investigator's Institutional Review Board. Patients were enrolled from July 1998 to March 2003 with appropriate informed consent.
Interventions
Treatment protocols were similar among the initial prospective studies. Patients were randomly assigned to receive linezolid 600 mg or vancomycin 1 g, each every 12 h. Linezolid was administered intravenously for at least 7 days and could then be changed to the bioequivalent oral formulation. Vancomycin dosage adjustments were required for patients with renal impairment and were permitted for other patients according to the local standard of care. In the blinded studies, a research pharmacist or equivalent non-study personnel monitored vancomycin dosages. In the nosocomial pneumonia studies,9,10 patients received concurrent aztreonam 1 to 2 g every 8 h, which could be discontinued if no Gram-negative pathogens were identified. In the other studies, aztreonam or gentamicin was allowed. In the study of skin and soft tissue infections,12 vancomycin could be switched to a penicillinase-resistant penicillin if S. aureus was determined to be susceptible to methicillin. The duration of linezolid or vancomycin treatment was predefined depending on the type of infection and ranged from a minimum of 7 days for urinary tract infection to a maximum of 28 days for bacteraemia of unknown origin.
Efficacy variables
We examined the following efficacy variables: cure of primary infection, microbiological eradication of S. aureus bacteraemia, and overall survival. The primary efficacy variable was clinical outcome of the primary infection after the end of therapy [i.e. test of cure (TOC)], which ranged from a minimum of 7 days after the end of therapy for urinary tract infection to a maximum of 35 days for bacteraemia of unknown origin. In the initial prospective studies, clinical cure was defined as resolution of baseline signs and symptoms of primary infection, with improvement or lack of progression of radiographic, laboratory, and other objective findings; failure was defined as persistence or progression of signs and symptoms. Patients had to have received at least 5 days and 10 doses of study drug to be assessed as having clinical cure, and at least 2 days and four doses to be assessed as having clinical failure; otherwise, they were assessed as having indeterminate clinical outcome. In the pooled analysis, microbiological outcome of bacteraemia was determined in all patients who had repeat blood cultures, or who were assessed as having clinical cure or failure of primary infection. Microbiological success was defined as documented eradication of S. aureus from blood or, if blood culture was not repeated, presumed eradication based on clinical cure of primary infection. Microbiological failure was defined as documented persistence of S. aureus in blood; reinfection; or, if blood culture was not repeated, presumed persistence based on clinical failure of primary infection. Overall survival was determined at final follow-up evaluation, which ranged from 15 days after end of treatment of MRSA infections in one study14 to 98 days for bacteraemia of unknown origin.
Adverse event analysis
All adverse events were recorded regardless of relationship to study drug. Investigators assessed each event as serious or not serious. All patients who had received at least one dose of study drug were included in the safety analysis, which consisted of standard reporting of adverse clinical events throughout the study and changes in platelet counts. To be included in the platelet analysis, patients had to have received at least 1 day of study drug and have a baseline and at least one follow-up platelet count. Platelet counts were determined by a laboratory that was central for each study. Platelet counts were measured at baseline and at end of therapy in all studies and at other predetermined intervals in four of five studies (usually on days 3, 9, and 15 and at ∼2 weeks after end of therapy).
Statistics
We performed the following separate analyses: (i) extraction of patient-level data from each study followed by pooling of the data; (ii) assessment of outcomes across all studies by meta-analysis; and (iii) identification of significant predictors of clinical cure and survival by multivariate analysis. For the patient-level analysis, continuous variables were compared between treatment groups with Student's t-test, and categorical variables were compared with the χ2 test. For the meta-analysis conducted to assess the effect of drug treatment on clinical cure of primary infection, we relied on the risk differences based on odds ratios from each of the randomized studies and their respective 95% confidence intervals (CIs). Heterogeneity was assessed visually with Galbraith plots as well as with Q statistics (χ2) using the methods of Mantel and Haenszel. We employed a random-effects model and calculated a summary odds ratio to describe the relationship between treatment group and outcome.
For the multivariate logistic regression conducted to identify predictors of clinical cure and survival in patients with bacteraemia, we used a random-effects model and placed the following baseline variables in the model and adjusted for co-linearity: treatment (linezolid or vancomycin); pathogen (MRSA or MSSA); sex; race (white or other); age (< or ≥65 years); presence or absence of nosocomial pneumonia, skin and soft tissue infection, or any of the following comorbidities: diabetes, congestive heart failure, renal insufficiency (creatinine ≤ or >1.5 mg/dL), malignancy, or any comorbidity. Missing data were left as missing instead of being imputed. Because of the number of subjects with missing values, the Acute Physiology and Chronic Health Evaluation (APACHE II) score (as a continuous variable) was included as a separate result through simple logistic regression. We also placed ‘individual study’ in the initial model, but this variable was not significant and therefore was not retained. Stepwise analyses used significance levels of 0.30 for entry into the model and 0.30 for staying in the model; statistical significance was assessed by the likelihood ratio test. Odds ratios, 95% CIs, and P values for baseline variables associated with clinical cure and survival were calculated for the most parsimonious logistic regression model. In addition, treatment effect alone was assessed using a simple logistic regression. Statistics were calculated using Statistical Analysis System (SAS) Version 6.12 (SAS Institute Inc, Cary, NC, USA). A P value of ≤0.05 was considered statistically significant.
Results
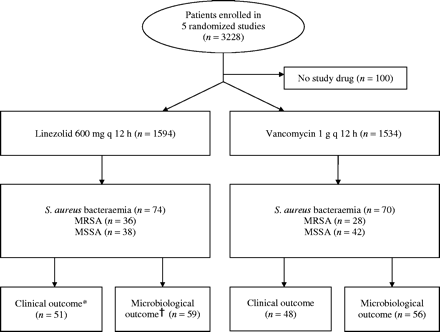
Of 3228 patients enrolled in five studies, 144 (4.5%) had S. aureus bacteraemia and received treatment with linezolid (n = 74) or vancomycin (n = 70) (Figure 1). The median Jadad score for all five studies was 7 of 8 possible points (range, 6–8). Seventy-three (50.7%) of 144 patients had MRSA infections (Table 1). More patients were less than 65 years of age in the vancomycin group [n = 39 (55.7%)] than in the linezolid group [n = 29 (39.2%); P = 0.05], but there were no differences in mean ages between treatment groups (mean age, 59.3 versus 63.5 years; P = 0.16). Otherwise, patients were evenly distributed between treatment groups on the basis of demographic and disease characteristics at baseline and overall duration of treatment. The only other difference was the shorter duration of intravenous treatment with linezolid than with vancomycin (mean duration, 8.6 versus 11.7 days; P = 0.004).
Flow diagram for patients with bacteraemia enrolled in randomized studies comparing linezolid and vancomycin. MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; q, every. *Determination of clinical outcome of primary infection. †Determination of microbiological outcome of bacteraemia.
Characteristics of patients with S. aureus bacteraemia in randomized studies comparing linezolid and vancomycin
| . | Number of patients (%), unless otherwise stated . | . | . | |||
|---|---|---|---|---|---|---|
| Characteristics . | linezolid (n = 74) . | vancomycin (n = 70) . | P valuea . | |||
| Age | ||||||
| mean ± SD, years | 63.5 ± 17.1 | 59.3 ± 18.9 | 0.163 | |||
| <65 years | 29 (39.2) | 39 (55.7) | 0.047 | |||
| Sex, men | 51 (68.9) | 41 (58.6) | 0.196 | |||
| APACHE II score available | 41 (55.4) | 41 (58.6) | 0.915 | |||
| APACHE II score (mean ± SD) | 13.9 ± 6.8 | 14.1 ± 7.5 | 0.915 | |||
| Mechanical ventilation | 25 (33.8) | 26 (37.1) | 0.660 | |||
| Comorbidities | ||||||
| congestive heart failure | 12 (16.2) | 13 (18.6) | 0.709 | |||
| diabetes | 26 (35.1) | 19 (27.1) | 0.301 | |||
| malignancy, active | 13 (17.6) | 7 (10.0) | 0.189 | |||
| renal insufficiency (creatinine > 1.5 mg/dL) | 32 (43.2) | 22 (31.4) | 0.155 | |||
| Primary infection | 0.924 | |||||
| pneumonia | 33 (44.6) | 27 (38.6) | ||||
| skin and soft tissue infection | 22 (29.7) | 21 (30.0) | ||||
| bacteraemia | 8 (10.8) | 8 (11.4) | ||||
| urinary tract infection | 1 (1.4) | 1 (1.4) | ||||
| other, not specified | 10 (13.5) | 13 (18.6) | ||||
| MRSA infection | 36 (48.6) | 37 (52.9) | 0.614 | |||
| Duration of therapy (days) (mean ± SD) | ||||||
| intravenous therapy | 8.6 ± 5.5 | 11.7 ± 6.8 | 0.004 | |||
| intravenous and oral therapy | 12.1 ± 6.5 | 11.7 ± 6.8 | 0.666 | |||
| . | Number of patients (%), unless otherwise stated . | . | . | |||
|---|---|---|---|---|---|---|
| Characteristics . | linezolid (n = 74) . | vancomycin (n = 70) . | P valuea . | |||
| Age | ||||||
| mean ± SD, years | 63.5 ± 17.1 | 59.3 ± 18.9 | 0.163 | |||
| <65 years | 29 (39.2) | 39 (55.7) | 0.047 | |||
| Sex, men | 51 (68.9) | 41 (58.6) | 0.196 | |||
| APACHE II score available | 41 (55.4) | 41 (58.6) | 0.915 | |||
| APACHE II score (mean ± SD) | 13.9 ± 6.8 | 14.1 ± 7.5 | 0.915 | |||
| Mechanical ventilation | 25 (33.8) | 26 (37.1) | 0.660 | |||
| Comorbidities | ||||||
| congestive heart failure | 12 (16.2) | 13 (18.6) | 0.709 | |||
| diabetes | 26 (35.1) | 19 (27.1) | 0.301 | |||
| malignancy, active | 13 (17.6) | 7 (10.0) | 0.189 | |||
| renal insufficiency (creatinine > 1.5 mg/dL) | 32 (43.2) | 22 (31.4) | 0.155 | |||
| Primary infection | 0.924 | |||||
| pneumonia | 33 (44.6) | 27 (38.6) | ||||
| skin and soft tissue infection | 22 (29.7) | 21 (30.0) | ||||
| bacteraemia | 8 (10.8) | 8 (11.4) | ||||
| urinary tract infection | 1 (1.4) | 1 (1.4) | ||||
| other, not specified | 10 (13.5) | 13 (18.6) | ||||
| MRSA infection | 36 (48.6) | 37 (52.9) | 0.614 | |||
| Duration of therapy (days) (mean ± SD) | ||||||
| intravenous therapy | 8.6 ± 5.5 | 11.7 ± 6.8 | 0.004 | |||
| intravenous and oral therapy | 12.1 ± 6.5 | 11.7 ± 6.8 | 0.666 | |||
APACHE, Acute Physiology and Chronic Health Evaluation; MRSA, methicillin-resistant S. aureus; SD, standard deviation.
P value determined by χ2 test.
Characteristics of patients with S. aureus bacteraemia in randomized studies comparing linezolid and vancomycin
| . | Number of patients (%), unless otherwise stated . | . | . | |||
|---|---|---|---|---|---|---|
| Characteristics . | linezolid (n = 74) . | vancomycin (n = 70) . | P valuea . | |||
| Age | ||||||
| mean ± SD, years | 63.5 ± 17.1 | 59.3 ± 18.9 | 0.163 | |||
| <65 years | 29 (39.2) | 39 (55.7) | 0.047 | |||
| Sex, men | 51 (68.9) | 41 (58.6) | 0.196 | |||
| APACHE II score available | 41 (55.4) | 41 (58.6) | 0.915 | |||
| APACHE II score (mean ± SD) | 13.9 ± 6.8 | 14.1 ± 7.5 | 0.915 | |||
| Mechanical ventilation | 25 (33.8) | 26 (37.1) | 0.660 | |||
| Comorbidities | ||||||
| congestive heart failure | 12 (16.2) | 13 (18.6) | 0.709 | |||
| diabetes | 26 (35.1) | 19 (27.1) | 0.301 | |||
| malignancy, active | 13 (17.6) | 7 (10.0) | 0.189 | |||
| renal insufficiency (creatinine > 1.5 mg/dL) | 32 (43.2) | 22 (31.4) | 0.155 | |||
| Primary infection | 0.924 | |||||
| pneumonia | 33 (44.6) | 27 (38.6) | ||||
| skin and soft tissue infection | 22 (29.7) | 21 (30.0) | ||||
| bacteraemia | 8 (10.8) | 8 (11.4) | ||||
| urinary tract infection | 1 (1.4) | 1 (1.4) | ||||
| other, not specified | 10 (13.5) | 13 (18.6) | ||||
| MRSA infection | 36 (48.6) | 37 (52.9) | 0.614 | |||
| Duration of therapy (days) (mean ± SD) | ||||||
| intravenous therapy | 8.6 ± 5.5 | 11.7 ± 6.8 | 0.004 | |||
| intravenous and oral therapy | 12.1 ± 6.5 | 11.7 ± 6.8 | 0.666 | |||
| . | Number of patients (%), unless otherwise stated . | . | . | |||
|---|---|---|---|---|---|---|
| Characteristics . | linezolid (n = 74) . | vancomycin (n = 70) . | P valuea . | |||
| Age | ||||||
| mean ± SD, years | 63.5 ± 17.1 | 59.3 ± 18.9 | 0.163 | |||
| <65 years | 29 (39.2) | 39 (55.7) | 0.047 | |||
| Sex, men | 51 (68.9) | 41 (58.6) | 0.196 | |||
| APACHE II score available | 41 (55.4) | 41 (58.6) | 0.915 | |||
| APACHE II score (mean ± SD) | 13.9 ± 6.8 | 14.1 ± 7.5 | 0.915 | |||
| Mechanical ventilation | 25 (33.8) | 26 (37.1) | 0.660 | |||
| Comorbidities | ||||||
| congestive heart failure | 12 (16.2) | 13 (18.6) | 0.709 | |||
| diabetes | 26 (35.1) | 19 (27.1) | 0.301 | |||
| malignancy, active | 13 (17.6) | 7 (10.0) | 0.189 | |||
| renal insufficiency (creatinine > 1.5 mg/dL) | 32 (43.2) | 22 (31.4) | 0.155 | |||
| Primary infection | 0.924 | |||||
| pneumonia | 33 (44.6) | 27 (38.6) | ||||
| skin and soft tissue infection | 22 (29.7) | 21 (30.0) | ||||
| bacteraemia | 8 (10.8) | 8 (11.4) | ||||
| urinary tract infection | 1 (1.4) | 1 (1.4) | ||||
| other, not specified | 10 (13.5) | 13 (18.6) | ||||
| MRSA infection | 36 (48.6) | 37 (52.9) | 0.614 | |||
| Duration of therapy (days) (mean ± SD) | ||||||
| intravenous therapy | 8.6 ± 5.5 | 11.7 ± 6.8 | 0.004 | |||
| intravenous and oral therapy | 12.1 ± 6.5 | 11.7 ± 6.8 | 0.666 | |||
APACHE, Acute Physiology and Chronic Health Evaluation; MRSA, methicillin-resistant S. aureus; SD, standard deviation.
P value determined by χ2 test.
Clinical cure
Clinical outcome of the primary infection was determined in 99 patients. Clinical cure occurred in 28 (55%) of 51 linezolid-treated patients and in 25 (52%) of 48 vancomycin-treated patients (OR for cure with linezolid compared with vancomycin, 1.12; 95% CI, 0.51–2.47). In the intent-to-treat analysis of all patients, including those with missing or indeterminate outcomes (coded as failures), clinical cure occurred in 28 (38%) of 74 linezolid-treated patients and in 25 (36%) of 70 vancomycin-treated patients. In those with MRSA bacteraemia and determination of clinical outcome, clinical cure occurred in 14 (56%) of 25 linezolid-treated patients and in 13 (46%) of 28 vancomycin-treated patients (OR for cure with linezolid compared with vancomycin, 1.47; 95% CI, 0.50–4.34).
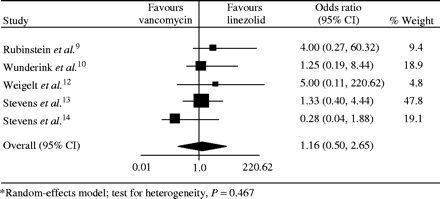
There were no differences between treatment groups in the individual studies or in the meta-analysis (OR for cure with linezolid compared with vancomycin, 1.16; 95% CI, 0.50–2.65) (Figure 2).
Meta-analysis* of clinical cure in randomized studies comparing linezolid and vancomycin.
All 144 patients were included in the multivariate analysis of clinical cure; patients with missing data were coded as failures. The only variables that were significant predictors of clinical cure were absence of congestive heart failure (OR, 0.23; 95% CI, 0.06–0.84) and absence of renal insufficiency (OR, 0.32; 95% CI, 0.11–0.95). Treatment group (OR for cure with linezolid compared with vancomycin, 1.05; 95% CI, 0.42–2.66), baseline demographic characteristics, MRSA infection, and other comorbidities were not significant predictors of clinical cure in the full model and were therefore excluded from the stepwise analysis. The trend suggesting that APACHE II score (as a continuous variable) was a predictor of clinical cure was not significant in a simple logistic regression analysis (OR, 0.94; 95% CI, 0.875–1.007).
Microbiological success
Microbiological outcome of bacteraemia was determined in 115 patients. In the linezolid group, S. aureus was eradicated from the blood in 26 patients and presumed to be eradicated because of clinical cure of primary infection in 15, resulting in microbiological success in 41 (69%) of 59 patients. Of the patients with documented eradication of S. aureus, three had superinfections. One patient had clinical failure of underlying nosocomial pneumonia and Enterococcus faecalis in blood. The other two patients had clinical cure of primary infection and either Acinetobacter baumannii or E. faecalis combined with Klebsiella pneumoniae in blood. Of the 18 patients with microbiological failure, 10 had documented persistence of S. aureus during treatment, except for two cases that occurred within 2 weeks after the end of treatment. The primary infections in the patients with documented persistence were bacteraemia (n = 3); other, not specified (n = 3); pneumonia (n = 2); and urinary tract infection (n = 1). The remaining eight had presumed persistence based on clinical failure of pneumonia (n = 3); skin and soft tissue infection (n = 2); other, not specified (n = 2); and bacteraemia (n = 1).
In the vancomycin group, S. aureus was eradicated from the blood in 25 patients and presumed to be eradicated in 16, resulting in microbiological success in 41 (73%) of 56 patients (OR for microbiological success with linezolid, 0.83; 95% CI, 0.37–1.87). Of the patients with documented eradication of S. aureus, three had superinfections. One had indeterminate clinical outcome of primary infection and Pseudomonas aeruginosa in blood. The other two had clinical failure of primary infection and either P. aeruginosa or A. baumannii in blood. Of the 15 patients with microbiological failure, eight had documented persistence of S. aureus during treatment, except for one case that occurred 3 weeks after the end of treatment. The primary infections in the patients with documented persistence were pneumonia (n = 5); bacteraemia (n = 1); other, not specified (n = 1); and skin and soft tissue infection (n = 1). The remaining seven had presumed persistence based on clinical failure of other, not specified (n = 4); pneumonia (n = 1); bacteraemia (n = 1); and skin and soft tissue infection (n = 1).
In both groups, the primary infections in patients with microbiological failures were distributed across all diagnoses present at baseline. In addition, the distribution of primary infections in patients with microbiological failure was similar between treatment groups. To the best of our knowledge, none of these patients had endocarditis or other sequelae.
Survival
Survival status at end of study was determined in all patients with S. aureus bacteraemia and in the subset with MRSA bacteraemia. Fifty-five (74%) of 74 linezolid-treated patients survived compared with 52 (74%) of 70 vancomycin-treated patients (OR for survival with linezolid compared with vancomycin, 1.00; 95% CI, 0.47–2.12). In those with MRSA bacteraemia, 24 (67%) of 36 linezolid-treated patients survived compared with 24 (65%) of 37 vancomycin-treated patients (OR for survival with linezolid compared with vancomycin, 1.08; 95% CI, 0.41–2.85).
All 144 patients were included in the multivariate analysis of survival. The only variables that were significant predictors of survival were any comorbidity (OR, 5.82; 95% CI, 1.24–27.44), absence of malignancy (OR, 0.15; 95% CI, 0.03–0.70), and absence of congestive heart failure (OR, 0.10; 95% CI, 0.02–0.43). The trend suggesting MRSA infection was a predictor of survival was not significant (OR, 0.44; 95% CI, 0.18–1.07). Treatment group (OR for cure with linezolid compared with vancomycin, 0.91; 95% CI, 0.39–2.14), baseline demographic characteristics, and other comorbidities were not significant predictors of survival in the full model and were therefore excluded from the stepwise analysis. APACHE II score was a predictor of survival in a simple logistic regression analysis (OR, 0.91; 95% CI, 0.85–0.99).
Safety
All treated patients were included in the safety analysis (Table 2). The incidence of adverse events was similar between treatment groups, including any adverse event (79.7% for linezolid versus 69.6% for vancomycin), serious adverse events (47.3% versus 37.1%), and discontinuation from treatment (36.5% versus 38.6%) (all P values >0.1). In the linezolid group, the mean platelet count was 243 × 109 platelets/L at baseline (n = 56) and 277 × 109 platelets/L at end of treatment (n = 44). In the vancomycin group, the mean platelet count was 255 × 109 platelets/L at baseline (n = 55) and 293 × 109 platelets/L at end of treatment (n = 51). After adjusting for baseline values, there were no between-group differences in mean changes from baseline among patients whose platelet counts were determined at both baseline and end of treatment (mean change, 20 versus 27 × 109 platelets/L, P = 0.78). The percentage of patients who experienced new-onset thrombocytopenia (decrease from baseline of ≥150 × 109 platelets/L to <150 × 109 platelets/L) was higher in the linezolid group than in the vancomycin group (13.9% versus 0%, P = 0.02).
Safety in randomized studies comparing linezolid and vancomycin
| . | Number of patients (%) . | . | . | |
|---|---|---|---|---|
| Type of adverse event . | linezolid (n = 74) . | vancomycin (n = 70) . | P value . | |
| Any adverse event, including those not related to study drug | 59/74 (79.7) | 48/69 (69.6) | 0.16 | |
| Serious adverse event, as assessed by investigators | 35/74 (47.3) | 26/70 (37.1) | 0.22 | |
| Discontinuation from treatment | 27/74 (36.5) | 27/70 (38.6) | 0.80 | |
| New-onset thrombocytopeniaa | 5/36 (13.9) | 0 (0) | 0.02 | |
| . | Number of patients (%) . | . | . | |
|---|---|---|---|---|
| Type of adverse event . | linezolid (n = 74) . | vancomycin (n = 70) . | P value . | |
| Any adverse event, including those not related to study drug | 59/74 (79.7) | 48/69 (69.6) | 0.16 | |
| Serious adverse event, as assessed by investigators | 35/74 (47.3) | 26/70 (37.1) | 0.22 | |
| Discontinuation from treatment | 27/74 (36.5) | 27/70 (38.6) | 0.80 | |
| New-onset thrombocytopeniaa | 5/36 (13.9) | 0 (0) | 0.02 | |
New-onset thrombocytopenia = decrease from baseline of ≥150 × 109 platelets/L to <150 × 109 platelets/L.
Safety in randomized studies comparing linezolid and vancomycin
| . | Number of patients (%) . | . | . | |
|---|---|---|---|---|
| Type of adverse event . | linezolid (n = 74) . | vancomycin (n = 70) . | P value . | |
| Any adverse event, including those not related to study drug | 59/74 (79.7) | 48/69 (69.6) | 0.16 | |
| Serious adverse event, as assessed by investigators | 35/74 (47.3) | 26/70 (37.1) | 0.22 | |
| Discontinuation from treatment | 27/74 (36.5) | 27/70 (38.6) | 0.80 | |
| New-onset thrombocytopeniaa | 5/36 (13.9) | 0 (0) | 0.02 | |
| . | Number of patients (%) . | . | . | |
|---|---|---|---|---|
| Type of adverse event . | linezolid (n = 74) . | vancomycin (n = 70) . | P value . | |
| Any adverse event, including those not related to study drug | 59/74 (79.7) | 48/69 (69.6) | 0.16 | |
| Serious adverse event, as assessed by investigators | 35/74 (47.3) | 26/70 (37.1) | 0.22 | |
| Discontinuation from treatment | 27/74 (36.5) | 27/70 (38.6) | 0.80 | |
| New-onset thrombocytopeniaa | 5/36 (13.9) | 0 (0) | 0.02 | |
New-onset thrombocytopenia = decrease from baseline of ≥150 × 109 platelets/L to <150 × 109 platelets/L.
Discussion
This retrospective pooled analysis of patient-level data and simultaneous meta-analysis of five prospective, randomized, controlled studies demonstrates that linezolid is associated with outcomes that are not inferior to those of vancomycin in 144 patients with S. aureus bacteraemia. Most of our patients had secondary bacteraemia associated with serious primary infections, usually nosocomial pneumonia or complicated skin and soft tissue infection; however, 11% had primary bacteraemia. An additional 16%, all with MRSA infections,13,14 could have had primary bacteraemia because their diagnoses were ‘other, not specified.’ In the pooled analysis, we did not find any differences between treatment groups as measured by clinical outcome of primary infection in patients with S. aureus infection and in the subset with MRSA infection, by microbiological outcome of secondary bacteraemia, and by survival. Treatment group (i.e. linezolid or vancomycin) was not a significant predictor of clinical cure of primary infection or survival in the meta-analysis or multivariate analysis.
Others have reported that vancomycin provided suboptimal results in patients with S. aureus bacteraemia.4–8 In a prospective, observational analysis of primary or secondary MSSA bacteraemia, Chang and colleagues6 observed that initial treatment with vancomycin as opposed to nafcillin was a significant predictor of microbiological failure (OR, 6.5; 95% CI, 1.0–52.8; P < 0.05). In a similar study, Fowler and colleagues7 estimated that use of vancomycin for primary S. aureus bacteraemia was associated with a greater chance of relapse (OR, 4.1; 95% CI, 1.5–11.6; P = 0.008). Finally, González and colleagues8 prospectively followed 86 patients with S. aureus pneumonia complicated by bacteraemia; use of vancomycin as opposed to cloxacillin for MSSA bacteraemia strongly correlated with death (OR, 14.5; 95% CI, 1.4–145.6).8 We, though, did not find a relationship between vancomycin use and treatment failure. However, given our limited sample size (71 subjects with MSSA bacteraemia), our statistical power to examine this question was severely constrained. Another factor that may have affected our ability to detect a difference between treatment groups was the high survival rate (74%), presumably due to the presence of skin and soft tissue infections in nearly a third of our patients and absence of reported episodes of endocarditis.
Independent groups recently identified reduced susceptibility to vancomycin as a factor contributing to clinical treatment failure in patients with MRSA bacteraemia. Charles and colleagues16 reported that failure was more likely to occur if bacteraemia was due to heterogeneous vancomycin-intermediate S. aureus (VISA; MIC, 8–16 mg/L), defined as one organism of VISA per 105 to 106 organisms of MRSA (P < 0.001), than due to vancomycin-susceptible MRSA. Sakoulas and colleagues17 reported that failure was more likely to occur if vancomycin MIC values were 1–2 mg/L than if they were ≤0.5 mg/L (P = 0.01). Vancomycin serum concentrations were adequate in the second study,17 but inadequate concentrations might have been a factor in the first study16 because failure was associated with troughs of <10 mg/L during the first week of treatment. However, adjusting the dose to ensure appropriate troughs did not improve the outcome, and prompt response occurred in most patients after initiating linezolid. No isolates of VISA were reported in our study.
Pharmacokinetic data were not collected in the prospective studies comparing linezolid with vancomycin.9,10,12–14 Studies indicating the potential for pharmacokinetic monitoring to improve clinical outcome, primarily when combined with pharmacodynamic modelling,18,19 were not available when our prospective studies were designed. At that time, the main reason for pharmacokinetic monitoring was to prevent toxicity, especially in patients with rapidly changing renal function or other risk factors.20 Consequently, our study protocols permitted dosage adjustments and pharmacokinetic monitoring according to the local standard of care. Furthermore, the initial vancomycin dosage, 1 g every 12 h, is the approved dosage, the dosage recommended in a standard text,21 and identical with the maximum allowed in a recent randomized study of neutropenic patients with persistent fever and cancer.22
We did not detect any safety differences between linezolid and vancomycin as measured by the incidences of any adverse event, serious adverse events, and treatment discontinuations. We did not perform a thorough safety analysis or evaluate specific adverse events, except for thrombocytopenia, because they were reported in individual studies9,10,12–14 and in a pooled analysis of comparator-controlled studies.23 In our pooled analysis, linezolid was associated with more episodes of new-onset thrombocytopenia; however, the criterion, decrease from baseline of ≥150 × 109 platelets/L to <150 × 109 platelets/L, is not clinically relevant in patients with baseline counts of 150 × 109 platelets/L. In contrast with our findings in 144 patients, Nasraway and colleagues24 did not detect any differences between linezolid and vancomycin in a retrospective analysis of 686 patients with nosocomial pneumonia9,10 including 38 of those in our analysis. In addition to the variables in our study, they evaluated substantially low counts (i.e. <75% of the lower limit of normal), percentage change in platelet counts, and shifts in platelet count category from baseline in all patients and in the subset at high risk because of low baseline values. There is some evidence that the risk of thrombocytopenia may be related to duration of linezolid therapy and is more likely to occur after 2 or 3 weeks.25,26 The mean duration of linezolid therapy in our study was only 12.1 ± 6.5 days and therefore may have been too short to affect the probability of thrombocytopenia. Rao and colleagues,27 moreover, recently reported a paucity of haematological effects associated with linezolid use. Their analysis was prospective and focused on patients given linezolid or vancomycin as long-term therapy for orthopaedic infections.
Our study has several important limitations in addition to the previously mentioned debate over the ideal vancomycin dosage. First, our study was retrospective; however, unlike traditional retrospective approaches, we used prospectively collected data from five high-quality randomized studies.9,10,12–14 Furthermore, our end points were not prone to bias in interpretation because they were prospectively defined (e.g. clinical cure) or highly objective (e.g. survival). Although clinical cure is not the usual end point for bacteraemia, the primary infection is unlikely to be cured if bacteraemia persists. Additionally, to be conservative and to address methodological limitations, we extracted patient-level data and evaluated them by both accepted meta-analytic techniques and regression analysis (to control for potential confounders and interactions). These analyses mutually reinforced each other because they reached similar conclusions. Second, the studies were heterogeneous with respect to requirements for follow-up and surveillance blood cultures. Consequently, repeat blood cultures were not obtained in 40% of patients, and microbiological outcome was based on microbiological outcome of the primary infection site. Again, use of a random-effects model for meta-analysis helps to address this concern. Third, the initial prospective studies were not designed for the treatment of patients with bacteraemia, except in the MRSA infection studies that allowed enrolment of patients with bacteraemia. Otherwise, the duration of treatment was based on the primary infection, not bacteraemia. Patients were not routinely monitored for late relapse, except in the MRSA infection studies. Endocarditis was defined by positive surgical specimen or by persistently positive blood cultures plus either vascular phenomena or predisposing heart disease; echocardiography and other tests were not required. Using these criteria, investigators did not report any episodes of endocarditis at enrolment or during the predefined observation and follow-up periods. The duration of observation depended on the type of infection and, for clinical outcome, ranged from 7 days after the end of treatment of urinary tract infection to 35 days for bacteraemia of unknown origin. Fourth, concomitant gentamicin therapy could have been a confounding factor because of its anti-staphylococcal activity. To rule out this possibility, we investigated the largest of the three studies in which it was allowed and confirmed that its use was rare, was comparable between groups, and did not affect clinical or microbiological outcomes.13 Fifth, our sample size was limited, as noted above, and we did not prospectively estimate the sample size needed to support our hypothesis, which restricts the strength of our conclusions. With a larger sample size, we might have been able to detect clinically significant differences; however, no trends were evident favouring vancomycin. Furthermore, to date, ours represents one of the largest comparative studies of antibiotic options for S. aureus bacteraemia and the only one of linezolid for secondary bacteraemia.
In conclusion, the choice of antibiotic therapy for patients with secondary S. aureus bacteraemia requires a risk–benefit analysis. The results of our pooled analysis of five prospective, randomized, controlled studies support our hypothesis that linezolid is associated with clinical, microbiological, and survival outcomes that are not inferior to those of vancomycin in patients with secondary S. aureus bacteraemia. Furthermore, linezolid appears to be well tolerated. Although we did not find conclusive evidence of an increased risk of thrombocytopenia in patients with S. aureus bacteraemia, it is prudent to monitor complete blood cell counts during linezolid therapy. If other risk factors for myelosuppression are present or prolonged therapy is required, vigilance for adverse events should be increased. More studies are needed to confirm our findings. Until such studies become available, we do not believe that linezolid causes any harm in patients with secondary S. aureus bacteraemia.
Transparency declarations
Conflict of interest (authors). A. F. S. is a member of the speakers' bureau for Pfizer Inc. M. J. K. is employed by Pfizer Inc. M. H. K. has received honoraria from Pfizer Inc. for lecturing at national conferences. Conflict of interest (acknowledged contributors). A. R., H. B. and D. R. are employed by Pfizer Inc. C. W. H. is a consultant for Pfizer Inc.
We thank Arlene Reisman, MPH, Helen Bhattacharyya, PhD, and Diane Ruzzi, BS, for performing statistical analyses; and Cindy W. Hamilton, PharmD, for assisting with manuscript preparation. The randomized, prospective studies were supported by grants from Pfizer Inc., Peapack, NJ. The pooled analysis of those studies was initiated by the investigators. The authors had complete access to all data, and had final control over the content, review, and submission of the manuscript.
References
Solomon S, Horan T, Andrus M et al. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003.
Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs?
Cosgrove SE, Sakoulas G, Perencevich EN et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis.
Hartstein AI, Mulligan ME, Morthland VH et al. Recurrent Staphylococcus aureus bacteremia.
Johnson LB, Almoujahed MO, Ilg K et al. Staphylococcus aureus bacteremia: compliance with standard treatment, long-term outcome and predictors of relapse.
Chang FY, Peacock JE Jr, Musher DM et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study.
Fowler VG Jr, Kong LK, Corey GR et al. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients.
González C, Rubio M, Romero-Vivas J et al. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms.
Rubinstein E, Cammarata S, Oliphant T et al. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study.
Wunderink RG, Cammarata SK, Oliphant TH et al. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia.
Kollef MH, Rello J, Cammarata SK et al. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin.
Weigelt JA, Itani KM, Lau WK et al. Linezolid vs vancomycin in the treatment of complicated skin and soft tissue infections (CSSTI): clinically significant outcome differences. In: Programs and Abstracts of the 40th Annual Meeting of the Infectious Disease Society of America, Chicago, IL,
Stevens DL, Herr D, Lampiris H et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections.
Stevens DL, Harrison M, Graham DR et al. Linezolid (LZD) IV/PO vs. Vancomycin (Vanco) IV for the treatment of suspected or proven methicillin-resistant Staphylococcus spp. (MRSS) infections: a second randomized, open-label, clinical trial. In: Programs and Abstracts of the 40th Annual Meeting of the Infectious Disease Society of America, Chicago, IL,
Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?
Charles PG, Ward PB, Johnson PD et al. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus.
Sakoulas G, Moise-Broder PA, Schentag J et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia.
Moise PA, Forrest A, Bhavnani SM et al. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus.
Schentag JJ. Antimicrobial action and pharmacokinetics/pharmacodynamics: the use of AUIC to improve efficacy and avoid resistance.
Moellering RC Jr. Monitoring serum vancomycin levels: climbing the mountain because it is there?
Gilbert DN, Moellering RC Jr, Sande MA. The Sanford Guide to Antimicrobial Therapy, 31st edn. Hyde Park, VT: Antimicrobial Therapy, Inc,
Cometta A, Kern WV, De Bock R et al. Vancomycin versus placebo for treating persistent fever in patients with neutropenic cancer receiving piperacillin–tazobactam monotherapy.
Rubinstein E, Isturiz R, Standiford HC et al. Worldwide assessment of linezolid's clinical safety and tolerability: comparator-controlled phase III studies.
Nasraway SA, Shorr AF, Kuter DJ et al. Linezolid does not increase the risk of thrombocytopenia in patients with nosocomial pneumonia: comparative analysis of linezolid and vancomycin use.
Kuter DJ, Tillotson GS. Hematologic effects of antimicrobials: focus on the oxazolidinone linezolid.
Birmingham MC, Rayner CR, Meagher AK et al. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program.
Author notes
1Pulmonary, Critical Care & Respiratory Services, Washington Hospital Center, 110 Irving Street NW, Room 2A38-D, Washington, DC 20010, USA; 2Pfizer Inc., 235 E. 42nd Street, New York, NY 10017, USA; 3Washington University School of Medicine, 660 South Euclid Avenue, St Louis, MO 63110, USA