-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel A. Shelburne, Martin Montes, Richard J. Hamill, Immune reconstitution inflammatory syndrome: more answers, more questions, Journal of Antimicrobial Chemotherapy, Volume 57, Issue 2, February 2006, Pages 167–170, https://doi.org/10.1093/jac/dki444
Close - Share Icon Share
Abstract
The institution of highly active antiretroviral therapy (HAART) in HIV-infected patients restores protective immune responses against a wide variety of pathogens and dramatically decreases mortality. In a subset of patients receiving HAART, immune reconstitution is associated with a pathological inflammatory response leading to substantial short-term morbidity and even mortality. The past several years have seen marked advances in our clinical understanding of the immune reconstitution inflammatory syndrome (IRIS), but many questions remain. This article summarizes recent data on clinical risk factors for the development of IRIS. A consistent finding from multiple groups is that IRIS develops in a substantial percentage of HIV-infected patients who have an underlying opportunistic infection and receive HAART. As the use of HAART stands to markedly increase over the next several years, optimal care of patients receiving HAART will need to incorporate monitoring for and treating complications of IRIS.
Introduction
The development of highly active antiretroviral therapy (HAART) has markedly improved the outlook for patients infected with HIV.1 While the receipt of HAART engenders protective immune responses against a wide variety of pathogens, for some patients a profound, pathological inflammatory reaction ensues targeted at either subclinical or previously recognized microbes.2–4 The inflammatory response can result in a spectrum of presentations ranging from clinical worsening of a treated opportunistic infection (OI), atypical appearance of an unrecognized OI to even autoimmune disorders such as Graves' disease.5–7 A multitude of names have been applied to these situations including immune restoration disease and immune reconstitution inflammatory syndrome (IRIS).2–5
Most of the original descriptions of IRIS consisted of case reports or case series of small numbers of patients.8–10 More recently, numerous groups have published cohort studies regarding incidence, risk factors and timing of onset of IRIS among patients receiving HAART.11–20 These investigations have provided the required information for clinicians treating HIV-infected patients, but much uncertainty remains. Moreover, the dearth of pathological data means that our understanding of the mechanisms of IRIS remains at a rudimentary level.
Historical view of IRIS in HIV- and non-HIV-infected patients
While recognition of IRIS did not become widespread until after the introduction of HAART in the mid-1990s, prior to this point there was ample appreciation that improvement in immune function could result in pathological inflammation. The so-called paradoxical responses were well described among non-HIV-infected patients treated for Mycobacterium tuberculosis (MTB) infection.21 Clinical worsening in these patients following initiation of anti-MTB therapy had been attributed to a reversal of the immunosuppression that MTB infection induces and was associated with conversion of MTB skin tests from negative to positive.21 Inflammatory reactions during treatment are also routine in patients infected with Mycobacterium leprae.22 Finally, recovery of immune cells following bone marrow transplantation or chemotherapy has been clearly associated with clinical deterioration for some patients.23
That such inflammatory responses might occur among HIV patients was first recognized when zidovudine monotherapy resulted in atypical, localized presentations of Mycobacterium avium intracellulare (MAI) infection.24 The introduction of HAART was quickly followed by numerous reports of patients in whom recovery of immune responses led to clinical worsening.8,10,25 Initially, the induction of immunity engendered by HAART was most clearly demonstrated in the case of hepatitis B virus infection where serial antibody measurements could be correlated with the clinical course.10 The measurement of pathogen-specific responses using delayed hypersensitivity testing or more advanced T-cell techniques demonstrated that IRIS often occurred in the setting of a measurable increase in immune response to an underlying OI.20,26
Movement to define IRIS
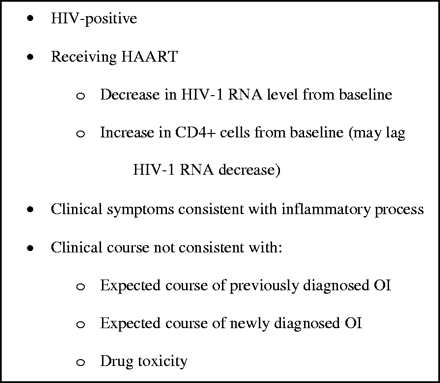
A major obstacle to quality research on IRIS has been the difficulty in establishing a definition. The problems in developing a clinically useful definition included the wide variety of underlying OIs associated with IRIS, the need to incorporate both unmasking of clinically silent infections and worsening of previously diagnosed OIs, and the difficulty in establishing that a new microbial process or drug toxicity was not the cause of a particular patient's clinical presentation. Despite the complexities, several definitions of IRIS have been utilized (Figure 1), each incorporating the general concept that cases of IRIS need to have an inflammatory component occurring in the setting of immune reconstitution that cannot be explained by drug toxicity or a new OI.4,5,16,17 As noted below, falling HIV-1 RNA levels rather than rising CD4+ cell counts may be a more sensitive finding during IRIS, and therefore has assumed a more prominent role in some definitions.4,13
Proposed criteria for the diagnosis of IRIS.5 IRIS, immune reconstitution inflammatory syndrome; HAART, highly active antiretroviral therapy; OI, opportunistic infection.
Systematic investigation of IRIS
As a workable definition of IRIS has evolved, studies have moved from case reports and case series to more systematic investigations that have shed insight on incidence, risk factors and clinical presentations.12–20 All of the studies have come from single centres, and the majority have chosen to focus on those patients assumed to be at the highest risk for developing IRIS, i.e. patients who initiate HAART following the diagnosis of a specific OI. Therefore, the relative percentages of OIs that underlie IRIS in all patients initiating HAART are not clear. In the three studies that have examined a cohort of all patients receiving HAART, the majority of IRIS cases have been associated with underlying viral infections (cytomegalovirus, herpes simplex virus, varicella-zoster virus, and hepatitis C virus) and mycobacteria (either MAI or M. tuberculosis).12,14,18 Given that the prevalence of specific AIDS-related OIs is quite distinct based on locale, it is likely that similar geographical variance will be observed in IRIS cases.
When systematic investigations of IRIS are examined, the percentage of patients developing IRIS and the timing of IRIS onset following HAART initiation have been surprisingly similar. For patients with an underlying OI, between 15 and 45% of patients developed IRIS with the majority of cases presenting within 60 days of initiating antiretroviral therapy.13,15,16,19,20 When all patients receiving HAART were analysed, between 15 and 25% of patients developed IRIS, with most cases again occurring in the first 3 months of therapy.12,14,18 The reproducibility of the results generated by the various investigations is demonstrated by the remarkably similar finding that in two different studies the incidence of developing IRIS in patients co-infected with MTB was 15.1 and 15.2 per 100-patient-years of HAART.13,15
While the timing of symptoms and percentages of patients has been quite similar across multiple studies, establishing reproducible risk factors at baseline for the development of IRIS has been more elusive. In two studies where all patients initiating HAART were examined, having a lower CD4+ T-lymphocyte count prior to starting HAART was associated with subsequent development of IRIS.12,14 Such an association makes sense in that lower CD4+ counts increase the likelihood of having dissemination of a microbe with which a restored immune system can react following initiation of antiretroviral therapy. Perhaps not surprisingly, when patients who already had a documented OI were studied, baseline CD4+ counts were not predictive of developing IRIS, probably due to the low starting values for all members of the cohort.13,15–17,19 The predictive values of baseline HIV-1 RNA levels have also been examined with one study finding an association and another finding a trend (P = 0.07) towards higher HIV-1 RNA levels in patients who developed IRIS after initiating HAART.13,16 Finally, multiple studies have looked at whether the occurrence of IRIS is related to the duration between initiating treatment for an underlying OI and starting HAART. While four studies have found that initiating HAART in closer proximity to starting therapy for an OI does increase the risk of developing IRIS, other investigations have not found such a relationship.13,15–17,19,20
In addition to baseline characteristics, investigators have also focused on the relationship between response to therapy and the development of IRIS. Given that the vast majority of adherent patients respond to HAART in terms of rising CD4+ cell counts and falling HIV-1 RNA levels, trying to discern differences in response to therapy among patients who do or do not develop IRIS depends heavily on sample size. For example, in one study where a total of 47 patients were examined, the rise in CD4+ count from baseline was 99 cells/mm3 among patients who developed IRIS and 35 cells/mm3 for those who did not, but the difference was not statistically significant (P = 0.07).16 In contrast, when 180 patients were studied a rise in CD4+ cells after 90 days of therapy was statistically associated with the development of IRIS.13 Interestingly, in this cohort the change in CD4+ cells over the first 3 months of HAART was not associated with IRIS suggesting that longer time periods of observation may be needed to detect immunological changes associated with IRIS. Importantly, a rapid fall in the HIV-1 RNA level during the first 3 months of therapy was highly associated with the development of IRIS. This finding suggests that clinicians suspicious of IRIS shortly after the initiation of HAART should be more attentive to the change in HIV-1 RNA levels rather than CD4+ counts when using response to therapy as a criterion for diagnosing IRIS.
Taken together, the data generated by many independent investigators is beginning to clarify our clinical picture of IRIS. Patients at highest risk for developing IRIS after starting HAART are those with low baseline CD4+ counts or with an underlying OI, two scenarios that are directly related. Most cases of IRIS can be expected to occur within the first few months of initiating therapy. A brisk immune recovery, in terms of rising CD4+ counts, seems to be associated with IRIS development, although the CD4+ rise may not occur until well after a dramatic fall in HIV-1 RNA levels. Importantly, we still lack a definitive answer on whether initiating antiretroviral therapy in close proximity to starting treatment for an OI increases the risk of subsequent development of IRIS. Also, all IRIS treatments have been anecdotal in nature, which precludes any recommendations about therapy. Finally, the aforementioned studies suffer from their retrospective nature. Ongoing prospective studies may be able to clarify some of the outstanding issues.
Pathological basis of IRIS
To understand the immunopathogenesis of IRIS it will be crucial to elucidate the intrinsic dynamics of immune cell recovery after initiation of HAART. The numerical increase in circulatory CD4+ T-cells has been well described, but much remains to be learned about the characteristics, specificity and function of these cells. Initial descriptions showed that activated memory cells (CD4+CD45RO+) account for the early incremental phase of CD4+ cell recovery following effective HAART.27 Recovery of lost responses to specific antigens also occurs during this early phase, probably because of cellular redistribution rather than a de novo specific CD4+ cell proliferation since naive activated CD4+ T cells (CD4+CD45RA+CD62L+) do not recover until after several months of therapy. The nature and strength of this antigen-specific immune response may be responsible for at least the early-onset cases of IRIS.28
Whether IRIS is the result of a response to a high antigen burden, an excessive response by the recovering immune system, exacerbated production of pro-inflammatory cytokines or a lack of immune regulation due to inability to produce regulatory cytokines remains to be determined.7 Disease susceptibility genes for specific subsets of IRIS have been identified including TNFA-308*2 with mycobacterial disease and HLA-B44 with herpesvirus-related IRIS.29,30 In addition, a CD4+ T-cell subset known as T-regulatory cells (CD4+CD25+FoxP3+) have an intrinsic ability to down-regulate immune responses and may play a role in the pathogenesis of IRIS.31 These T-regulatory cells are susceptible to HIV infection32 and their numbers are also decreased in AIDS.33 Studies on T-regulatory cells in tuberculosis-related IRIS are in progress.
Conclusions
Clinicians treating patients with AIDS need to be aware that HAART-engendered immune recovery may result in pathological inflammation in a subset of patients. Vigilance needs to be especially high during the first several months of therapy when the incidence of IRIS peaks, but cases continue to occur even after 1 or 2 years of therapy. Despite making large strides in the past several years on many aspects of IRIS, important questions remain. Further systematic investigations, especially in the areas of pathogenesis and treatment, are required to optimally care for IRIS patients.
Transparency declarations
None to declare.
Support for this work was provided by a National Institutes of Health Mentored Clinical Investigator Award (K12 RR17665) to S. A. S and in part by the Department of Veterans Affairs.
References
Mocroft A, Ledergerber B, Katlama C et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study.
DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy.
Hirsch HH, Kaufmann G, Sendi P et al. Immune reconstitution in HIV-infected patients.
French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy.
Shelburne SA, III, Hamill RJ, Rodriguez-Barradas MC et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy.
Chen F, Day SL, Metcalfe RA et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease.
Price P, Mathiot N, Krueger R et al. Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy.
Race EM, Adelson-Mitty J, Kriegel GR et al. Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease.
Blanche P, Gombert B, Ginsburg C et al. HIV combination therapy: immune restitution causing cryptococcal lymphadenitis dramatically improved by anti-inflammatory therapy.
Carr A, Cooper DA. Restoration of immunity to chronic hepatitis B infection in HIV-infected patient on protease inhibitor.
Shelburne SA, III, Darcourt J, White AC, Jr. et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy.
Jevtovic DJ, Salemovic D, Ranin J et al. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy.
Shelburne SA, Visnegarwala F, Darcourt J et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy.
French MA, Lenzo N, John M et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy.
Kumarasamy N, Chaguturu S, Mayer KH et al. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India.
Breton G, Duval X, Estellat C et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy.
Lortholary O, Fontanet A, Memain N et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France.
de Boer MG, Kroon FP, Kauffmann RH et al. Immune restoration disease in HIV-infected individuals receiving highly active antiretroviral therapy: clinical and immunological characteristics.
Navas E, Martin-Davila P, Moreno L et al. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy.
Narita M, Ashkin D, Hollender ES et al. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS.
Cheng VC, Ho PL, Lee RA et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients.
Manandhar R, Shrestha N, Butlin CR et al. High levels of inflammatory cytokines are associated with poor clinical response to steroid treatment and recurrent episodes of type 1 reactions in leprosy.
Cheng VC, Yuen KY, Chan WM et al. Immunorestitution disease involving the innate and adaptive response.
French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients.
Woods ML, II, MacGinley R, Eisen DP et al. HIV combination therapy: partial immune restitution unmasking latent cryptococcal infection.
French MA. Antiretroviral therapy. Immune restoration disease in HIV-infected patients on HAART.
Autran B, Carcelain G, Li TS et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease.
Stone SF, Price P, Brochier J et al. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy.
Price P, Keane NM, Stone SF et al. MHC haplotypes affect the expression of opportunistic infections in HIV patients.
Price P, Morahan G, Huang D et al. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases.
Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3.
Oswald-Richter K, Grill SM, Shariat N et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells.
Author notes
1Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, USA; 2Department of Medicine, Section of Infectious Diseases (111G), Michael E. DeBakey Veterans Affairs Medical Center, 2002 Holcombe Boulevard, Houston, TX 77030, USA