-
PDF
- Split View
-
Views
-
Cite
Cite
S. Pournaras, A. Markogiannakis, A. Ikonomidis, L. Kondyli, K. Bethimouti, A. N. Maniatis, N. J. Legakis, A. Tsakris, Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit, Journal of Antimicrobial Chemotherapy, Volume 57, Issue 3, March 2006, Pages 557–561, https://doi.org/10.1093/jac/dkl004
Close - Share Icon Share
Abstract
Objectives: To investigate the resistance mechanisms and the genetic relationship of imipenem-resistant Acinetobacter baumannii isolates recovered in the intensive care unit (ICU) of a tertiary care hospital.
Methods: Imipenem-resistant A. baumannii clinical and environmental isolates were collected in the ICU of the Red Cross General Hospital, Athens, Greece between March and October 2002. The isolates were tested by Etest MBL, PCR, RT–PCR and sequencing for carbapenemase-encoding genes, PFGE and synergy experiments using meropenem and the efflux pump inhibitor carbonyl cyanide chlorophenylhydrazone.
Results: During the study period, 15 clinical and two environmental imipenem-resistant (MIC 8 to >128 mg/L) A. baumannii isolates were recovered. PFGE showed six different clones that included both clinical and environmental isolates. All 17 isolates were negative by Etest MBL and PCR for genes blaIMP, blaVIM, blaSPM, blaOXA-23-like and blaOXA-24-like. Genes blaOXA-51-like and blaOXA-58-like were amplified from 15 and 14 isolates, respectively. Sequencing of blaOXA-51-like amplicons identified blaOXA-66 (nine cases) and blaOXA-69 (six cases), whereas blaOXA-58-like sequences were classical blaOXA-58. Reverse transcriptase-PCR showed that blaOXA-51-like genes were expressed in 12 and blaOXA-58 in 10 isolates; in these isolates, inhibition of OXA enzymes by 200 mM of NaCl reduced carbapenem MICs by up to 4-fold. Overexpression of proton-gradient dependent efflux pumps did not contribute to carbapenem resistance in any isolate. Similarly, although AmpC expression was demonstrated in eight isolates, inhibition of AmpC with cloxacillin did not reduce the MICs of carbapenems significantly.
Conclusions: These findings indicate wide dissemination of OXA-58 carbapenemase, which contributes, at least partially, to the imipenem resistance of unrelated A. baumannii isolates in our ICU.
Introduction
Carbapenems have a potent activity against acinetobacters and are often used as a last resort for the treatment of infections due to multiresistant Acinetobacter baumannii isolates. However, acinetobacters may develop resistance to carbapenems through various combined mechanisms, including AmpC stable derepression, decreased permeability, altered penicillin-binding proteins (PBPs) and, rarely, efflux pump overexpression.1,2 Moreover, carbapenem resistance among acinetobacters has been sporadically attributed to the production of carbapenemases such as class B IMP-type and VIM-type metallo-β-lactamases and class D OXA-type carbapenemases.3 Recently, two novel class D oxacillinases with carbapenemase properties, designated OXA-51 and OXA-58, have been described among A. baumannii isolates4,5; at least six variants of blaOXA-51 have been detected in acinetobacters from various regions worldwide.6
In our region imipenem-non-susceptible A. baumannii are being isolated with increasing frequency from clinical sources.7 Preliminary susceptibility data in our hospital indicated that several infections among patients hospitalized in our intensive care unit (ICU) were caused by imipenem-resistant A. baumannii that exhibited cross-resistance to almost all alternative antimicrobials. The similar antimicrobial susceptibility patterns of these isolates prompted an investigation of their genetic relationship and carbapenem resistance mechanisms.
Materials and methods
Bacterial isolates
The study included all imipenem-resistant (MICs of imipenem ≥ 8 mg/L) A. baumannii non-repetitive isolates recovered consecutively from clinical infections of separate patients in the general ICU of the Red Cross General Hospital, Athens, Greece, between March and October 2002. We also included imipenem-resistant environmental A. baumannii isolates that were recovered during the study period by swabbing the environment and equipment adjacent to the patients as well as by culturing samples from the hands of hospital care workers (HCWs). The isolates were provisionally identified to the genus level by the Vitek 2 automated system (bioMérieux, Marcy l′Étoile, France) and the API 20NE system (bioMérieux), and the identification of A. baumannii was performed by a simplified identification scheme.
Susceptibility testing
Imipenem and meropenem MICs were determined using an agar dilution method, whereas susceptibility testing against other antimicrobials (amikacin, ciprofloxacin, cefepime, gentamicin, kanamycin, ofloxacin, piperacillin and piperacillin/tazobactam) was performed by disc diffusion. The isolates were also tested by Etest MBL (AB Biodisk, Solna, Sweden) for possible metallo-β-lactamase production. Pseudomonas aeruginosa ATCC 27853 was used as control in susceptibility testing and a VIM-type carbapenemase-producing P. aeruginosa strain8 was used as a control for Etest MBL.
Pulsed-field gel electrophoresis
PFGE of ApaI-digested genomic DNA of A. baumannii isolates was performed with a CHEF-DRIII system (Bio-Rad, Hemel Hempstead, UK).
PCR amplification
PCR testing of the isolates for carbapenemase-encoding genes (blaIMP, blaVIM, blaSPM, blaOXA-23-like, blaOXA-24-like, blaOXA-58-like) was done using consensus primers that were specific for each enzyme group.5,7–10 The isolates were also screened for the blaOXA-51-like genes using primers that amplify a 353 bp internal fragment (kindly provided by Dr S. Brown, University of Edinburgh, UK), as well as partially degenerate primers (sense: 5′-TGAACATTAAAICACTCTT-3′, antisense: 5′-CTATAAAATACCTAATTGTT-3′) that were designed to amplify an 825 bp product in all blaOXA-51-like alleles. Cycling conditions were: an initial denaturation step at 94°C for 5 min, amplification steps of 94°C for 1 min, 54°C for 1 min, 72°C for 45 s for a total of 35 cycles and a final extension at 72°C for 7 min.
RT–PCR and inhibition experiments
RT–PCR was performed for blaOXA-51-like and blaOXA-58-like genes as described previously.11 RT–PCR was also performed for ampC genes12 to investigate the contribution of AmpC expression to imipenem resistance. To evaluate the expression of bla genes, the mRNA of the single copy housekeeping gene, recA, was amplified using primers that amplify a 425 bp product. The relative contribution of OXA-58 enzyme to carbapenem resistance was checked by estimating carbapenem MICs with and without 200 mM of NaCl.5 Contribution of AmpC β-lactamase was tested by determining carbapenem MICs in agar with and without 200 mg/L of cloxacillin.
Plasmid analysis
Plasmid analysis was performed using an alkaline lysis method. Plasmid and residual chromosomal DNA bands were extracted separately from 0.8% agarose gels using a QIAquick Gel Extraction kit (Qiagen, Hilden, Germany). The respective DNA extracts were subjected to PCR for genes blaOXA-51-like and blaOXA-58-like to check the location of each gene. Plasmid curing experiments used ethidium bromide at the maximum concentration that allowed the growth of isolates (400 mg/L).
Synergy experiments for testing overexpression of efflux pumps
Synergy experiments were performed using imipenem and the efflux pump inhibitor carbonyl cyanide chlorophenylhydrazone (CCCP).11 Susceptibility testing of imipenem was performed by disc diffusion and agar dilution using Mueller–Hinton agar with and without 12.5 μM CCCP.
Results
During the study period 15 non-repetitive imipenem-resistant A. baumannii isolates were recovered from clinical infections of separate patients; eight were from cases of pneumonia, five from bacteraemia and two from urinary tract infections. In addition, two imipenem-resistant A. baumannii isolates were recovered from 62 environmental samples; one was recovered from the surface of a patient's bed rails and a second one was recovered from the hands of an HCW. The MICs of imipenem and meropenem for the 17 isolates are shown in Table 1. The isolates were multidrug-resistant exhibiting resistance to all other β-lactams (ampicillin/sulbactam, aztreonam, ceftazidime, cefepime, piperacillin and piperacillin/tazobactam), aminoglycosides (amikacin, gentamicin, netilmicin and tobramycin) and fluoroquinolones (ciprofloxacin and ofloxacin); they were susceptible only to colistin.
PFGE of the clinical and environmental imipenem-resistant isolates showed six distinct genotypes, with two each containing two subtypes (Table 1). The two environmental isolates belonged to the same genotype as two clinical isolates. The infection control team of the hospital implemented restriction of carbapenem usage and strict antiseptic techniques, which included the rigorous use of alcohol-chlorhexidine solutions before and between patient and equipment contact and before leaving the unit.
The Etest MBL was negative in all 17 imipenem-resistant isolates. The isolates were also negative for blaIMP, blaVIM, blaSPM, blaOXA-23-like and blaOXA-24-like genes. The blaOXA-51-like and blaOXA-58-like genes were amplified in 15 and 14 isolates, respectively. Sequencing of blaOXA-51-like amplicons identified blaOXA-66 (nine cases) and blaOXA-69 (six cases), while all blaOXA-58-like alleles encoded classical OXA-58 enzyme.
Plasmids were analysed in six isolates (one each of PFGE types I, IIa, IIb, IIIa, IIIb and VI) that harboured both blaOXA-51-like and blaOXA-58 genes; three isolates carried single plasmids of ∼24–70 MDa; one isolate had two plasmids of ∼45 and 3 MDa; two isolates had no detectable plasmids. The large plasmids and the chromosomal band of each isolate were extracted from the gel and used as templates in PCR for blaOXA-51-like and blaOXA-58. Results suggested that plasmids carried blaOXA-58 in isolates of type I, IIIa, IIIb and VI, and blaOXA-51-like in type I and IIIb. Consistent with this, blaOXA-51-like was cured from the isolates of type I and IIIb, and blaOXA-58 from types I, IIIa, IIIb and VI, indicating that in types I and IIIb the same plasmid might harbour both genes.
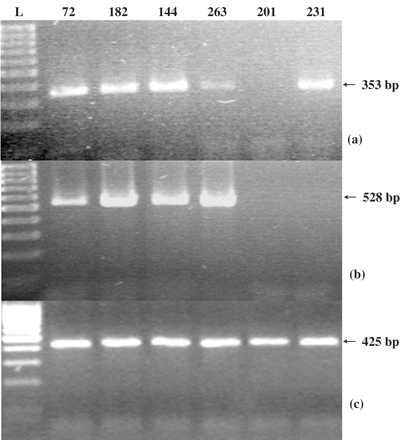
RT-PCR showed that blaOXA-51-like genes were expressed in 12 isolates, and blaOXA-58 in 10 isolates (Figure 1); in these isolates, inhibition of oxacillinases with 200 mM of NaCl reduced carbapenem MICs by up to 4-fold (Table 1). Interestingly, three isolates that expressed neither blaOXA-51-like nor blaOXA-58 showed no reduction in carbapenem MICs in the presence of 200 mM of NaCl (Table 1). AmpC expression was demonstrated by RT–PCR in eight isolates, but inhibition of AmpC with cloxacillin did not reduce carbapenem MICs significantly (Table 1). None of the isolates exhibited significant synergy between imipenem and CCCP, indicating that overexpression of proton-gradient dependent efflux pumps did not contribute to the imipenem resistance. Presence or absence of OXA-58 did not affect patient mortality (data not shown).
RT–PCR products showing expression of the blaOXA-51 (a), blaOXA-58 (b) and recA (c) genes of A. baumannii isolates representative of each PFGE type that carry both blaOXA-51 and blaOXA-58. Lane L, 100 bp DNA ladder. Sizes of the amplified fragments are indicated by arrows. The index numbers of the isolates are those listed in Table 1.
PFGE types, MICs (mg/L), synergy test results and PCR results for the 17 carbapenem-resistant A. baumannii isolates
| . | . | . | . | . | Cloxacillin (200 mg/L) . | NaCl (200 mM) . | . | PCR results . | . | . | RT–PCR results . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | Site of isolation . | PFGE type . | MIC of IPM . | MIC of MEM . | MIC of IPM . | MIC of IPM . | MIC of MEM . | blaampC . | blaoxa-51 . | blaOXA-58 . | blaampC . | blaOXA-51 . | blaOXA-58 . | |||||
| 72 | Blood | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 111 | Hands of an HCW | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 155 | Patient's bed | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 189 | Bronchial | I | 8 | 2 | 8 | 4 | 1 | + | − | + | + | − | + | |||||
| 132 | Bronchial | IIa | 16 | 2 | 16 | 8 | 1 | + | + | − | + | + | − | |||||
| 182 | Blood | IIa | 16 | 2 | 16 | 8 | 1 | + | + | + | − | + | + | |||||
| 144 | Blood | IIb | 64 | 16 | 64 | 64 | 4 | + | + | + | + | + | + | |||||
| 157 | Bronchial | IIb | 64 | 16 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 176 | Bronchial | IIIa | 32 | 4 | 16 | 8 | 1 | + | + | + | + | + | + | |||||
| 223 | Bronchial | IIIa | 8 | 2 | 8 | 4 | 1 | − | − | + | − | − | + | |||||
| 231 | Blood | &#IIIa | >128 | >128 | >128 | 128 | 128 | + | + | + | + | + | − | |||||
| 201 | Urine | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | + | − | − | |||||
| 229 | Blood | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | − | − | − | |||||
| 242 | Bronchial | IIIb | 32 | 8 | 16 | 32 | 8 | + | + | + | + | − | − | |||||
| 208 | Bronchial | IV | 16 | 4 | 16 | 16 | 4 | − | + | − | − | + | − | |||||
| 255 | Urine | V | >128 | >128 | >128 | 128 | 128 | + | + | − | + | + | − | |||||
| 263 | Bronchial | VI | 8 | 2 | 8 | 8 | 1 | + | + | + | − | + | + | |||||
| . | . | . | . | . | Cloxacillin (200 mg/L) . | NaCl (200 mM) . | . | PCR results . | . | . | RT–PCR results . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | Site of isolation . | PFGE type . | MIC of IPM . | MIC of MEM . | MIC of IPM . | MIC of IPM . | MIC of MEM . | blaampC . | blaoxa-51 . | blaOXA-58 . | blaampC . | blaOXA-51 . | blaOXA-58 . | |||||
| 72 | Blood | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 111 | Hands of an HCW | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 155 | Patient's bed | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 189 | Bronchial | I | 8 | 2 | 8 | 4 | 1 | + | − | + | + | − | + | |||||
| 132 | Bronchial | IIa | 16 | 2 | 16 | 8 | 1 | + | + | − | + | + | − | |||||
| 182 | Blood | IIa | 16 | 2 | 16 | 8 | 1 | + | + | + | − | + | + | |||||
| 144 | Blood | IIb | 64 | 16 | 64 | 64 | 4 | + | + | + | + | + | + | |||||
| 157 | Bronchial | IIb | 64 | 16 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 176 | Bronchial | IIIa | 32 | 4 | 16 | 8 | 1 | + | + | + | + | + | + | |||||
| 223 | Bronchial | IIIa | 8 | 2 | 8 | 4 | 1 | − | − | + | − | − | + | |||||
| 231 | Blood | &#IIIa | >128 | >128 | >128 | 128 | 128 | + | + | + | + | + | − | |||||
| 201 | Urine | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | + | − | − | |||||
| 229 | Blood | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | − | − | − | |||||
| 242 | Bronchial | IIIb | 32 | 8 | 16 | 32 | 8 | + | + | + | + | − | − | |||||
| 208 | Bronchial | IV | 16 | 4 | 16 | 16 | 4 | − | + | − | − | + | − | |||||
| 255 | Urine | V | >128 | >128 | >128 | 128 | 128 | + | + | − | + | + | − | |||||
| 263 | Bronchial | VI | 8 | 2 | 8 | 8 | 1 | + | + | + | − | + | + | |||||
HCW, hospital care worker; IPM, imipenem; MEM, meropenem.
PFGE types, MICs (mg/L), synergy test results and PCR results for the 17 carbapenem-resistant A. baumannii isolates
| . | . | . | . | . | Cloxacillin (200 mg/L) . | NaCl (200 mM) . | . | PCR results . | . | . | RT–PCR results . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | Site of isolation . | PFGE type . | MIC of IPM . | MIC of MEM . | MIC of IPM . | MIC of IPM . | MIC of MEM . | blaampC . | blaoxa-51 . | blaOXA-58 . | blaampC . | blaOXA-51 . | blaOXA-58 . | |||||
| 72 | Blood | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 111 | Hands of an HCW | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 155 | Patient's bed | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 189 | Bronchial | I | 8 | 2 | 8 | 4 | 1 | + | − | + | + | − | + | |||||
| 132 | Bronchial | IIa | 16 | 2 | 16 | 8 | 1 | + | + | − | + | + | − | |||||
| 182 | Blood | IIa | 16 | 2 | 16 | 8 | 1 | + | + | + | − | + | + | |||||
| 144 | Blood | IIb | 64 | 16 | 64 | 64 | 4 | + | + | + | + | + | + | |||||
| 157 | Bronchial | IIb | 64 | 16 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 176 | Bronchial | IIIa | 32 | 4 | 16 | 8 | 1 | + | + | + | + | + | + | |||||
| 223 | Bronchial | IIIa | 8 | 2 | 8 | 4 | 1 | − | − | + | − | − | + | |||||
| 231 | Blood | &#IIIa | >128 | >128 | >128 | 128 | 128 | + | + | + | + | + | − | |||||
| 201 | Urine | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | + | − | − | |||||
| 229 | Blood | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | − | − | − | |||||
| 242 | Bronchial | IIIb | 32 | 8 | 16 | 32 | 8 | + | + | + | + | − | − | |||||
| 208 | Bronchial | IV | 16 | 4 | 16 | 16 | 4 | − | + | − | − | + | − | |||||
| 255 | Urine | V | >128 | >128 | >128 | 128 | 128 | + | + | − | + | + | − | |||||
| 263 | Bronchial | VI | 8 | 2 | 8 | 8 | 1 | + | + | + | − | + | + | |||||
| . | . | . | . | . | Cloxacillin (200 mg/L) . | NaCl (200 mM) . | . | PCR results . | . | . | RT–PCR results . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | Site of isolation . | PFGE type . | MIC of IPM . | MIC of MEM . | MIC of IPM . | MIC of IPM . | MIC of MEM . | blaampC . | blaoxa-51 . | blaOXA-58 . | blaampC . | blaOXA-51 . | blaOXA-58 . | |||||
| 72 | Blood | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 111 | Hands of an HCW | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 155 | Patient's bed | I | 64 | 8 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 189 | Bronchial | I | 8 | 2 | 8 | 4 | 1 | + | − | + | + | − | + | |||||
| 132 | Bronchial | IIa | 16 | 2 | 16 | 8 | 1 | + | + | − | + | + | − | |||||
| 182 | Blood | IIa | 16 | 2 | 16 | 8 | 1 | + | + | + | − | + | + | |||||
| 144 | Blood | IIb | 64 | 16 | 64 | 64 | 4 | + | + | + | + | + | + | |||||
| 157 | Bronchial | IIb | 64 | 16 | 64 | 32 | 4 | + | + | + | − | + | + | |||||
| 176 | Bronchial | IIIa | 32 | 4 | 16 | 8 | 1 | + | + | + | + | + | + | |||||
| 223 | Bronchial | IIIa | 8 | 2 | 8 | 4 | 1 | − | − | + | − | − | + | |||||
| 231 | Blood | &#IIIa | >128 | >128 | >128 | 128 | 128 | + | + | + | + | + | − | |||||
| 201 | Urine | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | + | − | − | |||||
| 229 | Blood | IIIb | 16 | 16 | 16 | 16 | 16 | + | + | + | − | − | − | |||||
| 242 | Bronchial | IIIb | 32 | 8 | 16 | 32 | 8 | + | + | + | + | − | − | |||||
| 208 | Bronchial | IV | 16 | 4 | 16 | 16 | 4 | − | + | − | − | + | − | |||||
| 255 | Urine | V | >128 | >128 | >128 | 128 | 128 | + | + | − | + | + | − | |||||
| 263 | Bronchial | VI | 8 | 2 | 8 | 8 | 1 | + | + | + | − | + | + | |||||
HCW, hospital care worker; IPM, imipenem; MEM, meropenem.
Discussion
Several clones of imipenem-resistant A. baumannii producing OXA-58-carbapenem-hydrolysing oxacillinase and naturally occurring OXA-51-type oxacillinases have emerged in a general ICU of a Greek tertiary hospital. This spread could have been facilitated by the extensive use of carbapenems for treating the multidrug-resistant Gram-negative bacteria that are commonly isolated in our unit. In addition, the detection of a common clone among environmental and clinical imipenem-resistant A. baumannii indicates that environmental contamination contributes to the difficulty in restricting spread of this organism in our hospital. Surveillance cultures and strict antiseptic techniques possibly reduced the further spread of these bacteria.
Our blaOXA-58-bearing acinetobacters had alleles identical to that described in Toulouse5 and other southern European regions,13 showing wide geographical spread of this gene. Also, our blaOXA-51 alleles resembled those described previously among A. baumannii from different regions (Spain, Hong Kong, Singapore and Turkey).6 It has been recently shown that blaOXA-51-like alleles are very poor carbapenemases and naturally present in almost all A. baumannii isolates, regardless of their susceptibility or resistance to carbapenems.14 The negative amplification of this gene in two isolates of the present study might be due to point mutations, even though our partially degenerate primers amplify all known blaOXA-51-like alleles.
In accordance with previous findings,13blaOXA-58 was plasmid-mediated in many of our isolates. Héritier et al.1 demonstrated that OXA-58 exhibits weak carbapenemase activity and plays a role in carbapenem resistance in A. baumannii, particularly when blaOXA-58 is highly expressed. These authors also suggested that overexpression of efflux pumps might contribute to higher levels of carbapenem resistance, but proton-gradient dependent efflux pumps appeared not to contribute to the carbapenem resistance of our isolates. Although blaOXA-58 was present and expressed by most of the isolates, NaCl reduced carbapenem MICs no more than 4-fold, with imipenem MICs remaining in the resistant category. Similarly, inhibition of AmpC by cloxacillin did not affect MICs of carbapenems significantly. This suggests that much of the resistance in our isolates may depend on impermeability or other combined mechanisms that additionally affected resistance to other antibiotics such as expanded-spectrum cephalosporinases.2 This is also supported by the finding that two isolates with the highest carbapenem MICs (>128 mg/L) did not express blaOXA-58, but only blaOXA-51-like gene. Further studies are needed to determine whether imipenem-resistant A. baumannii expressing OXA-58 carbapenemase exist in other Greek hospitals and to evaluate the dissemination of these and other class D carbapenemases among acinetobacters with variable carbapenem MICs.
Transparency declarations
We have no conflicts to declare.
References
Héritier C, Poirel L, Lambert T et al. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii.
Quale J, Bratu S, Landman D et al. Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City.
Livermore DM. The impact of carbapenemases on antimicrobial development and therapy.
Brown S, Young HK, Amyes SGB. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina.
Poirel L, Marqué S, Héritier C et al. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii.
Brown S, Amyes SGB. The sequences of seven class D β-lactamases isolated from carbapenem-resistant Acinetobacter baumannii from four continents.
Tsakris A, Tsioni C, Pournaras S et al. Spread of low-level carbapenem-resistant Acinetobacter baumannii in a tertiary care Greek hospital.
Tsakris A, Pournaras S, Woodford N et al. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece.
Senda K, Arakawa Y, Ichiyama S et al. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams.
Toleman MA, Simm AM, Murphy TA et al. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme.
Pournaras S, Maniati M, Spanakis N et al. Spread of efflux pump-overexpressing, non-metallo-β-lactamase-producing, meropenem-resistant but ceftazidime-susceptible Pseudomonas aeruginosa in a region with blaVIM endemicity.
Corvec S, Caroff N, Espaze E et al. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains.
Marqué S, Poirel L, Héritier C et al. Regional occurrence of plasmid-mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter spp. in Europe.
Author notes
1Department of Microbiology, Medical School, University of Thessaly, Mezourlo, Larissa, Greece; 2Department of Microbiology, Medical School, University of Athens, 11527 Athens, Greece; 3Department of Clinical Microbiology, Red Cross Hospital, Athens, Greece