-
PDF
- Split View
-
Views
-
Cite
Cite
Stephanie K. Henderson-Begg, David M. Livermore, Lucinda M. C. Hall, Effect of subinhibitory concentrations of antibiotics on mutation frequency in Streptococcus pneumoniae, Journal of Antimicrobial Chemotherapy, Volume 57, Issue 5, May 2006, Pages 849–854, https://doi.org/10.1093/jac/dkl064
Close - Share Icon Share
Abstract
Objectives: To investigate the effect of subinhibitory concentrations of ciprofloxacin, streptomycin, trimethoprim, ampicillin and erythromycin on mutation frequency in Streptococcus pneumoniae.
Methods: Frequency of mutation to rifampicin resistance was determined in three clinical isolates grown with or without antibiotic treatment. dinB was analysed using PCR and DNA sequence determination.
Results: Subinhibitory levels of ciprofloxacin and streptomycin increased the frequency of mutation to rifampicin resistance between 2- and 5-fold for all three isolates, which is comparable to the increase seen in mismatch repair mutants of this species. These increases appeared not to be dependent on the function of the error-prone DNA polymerase encoded by dinB, since one of the isolates was a naturally occurring deletion mutant for this gene. Trimethoprim increased the mutation frequency for two isolates, but not the dinB mutant; ampicillin and erythromycin had no significant effect on mutation frequencies for any isolate.
Conclusions: Exposure to quinolones and aminoglycosides at subinhibitory concentrations may result in increased mutability in pneumococci, as well as selecting for resistance per se.
Introduction
The ability of bacteria to generate mutations with increased frequency under conditions of environmental stress may confer a selective advantage. A number of stress response mechanisms that increase mutation frequency have been characterized, mostly for Escherichia coli. The presence of antibiotics, even at low concentrations, may constitute an environmental stress, and several studies have described the induction of a hypermutator phenotype in E. coli by sublethal concentrations of antibiotics. For example, low concentrations of quinolone induce the SOS response, resulting in increased mutation largely through the induction of error-prone DNA polymerases.1,2 In addition, SOS-independent induction of error-prone DNA polymerase IV, encoded by dinB, was recently reported to result in an increased mutation rate in the presence of β-lactam antibiotics.3 A further mechanism, dependent on recABC, has been described to explain an increased mutation frequency in the presence of streptomycin.4,5 The potential for antibiotics to promote increased rates of mutation has clear implications for the evolution of antibiotic resistance among bacterial pathogens, but it is currently unclear whether these data for E. coli can be extrapolated to bacteria from other taxa.
Streptococcus pneumoniae lacks the classical SOS response as defined by phenotype, in that UV light does not result in increased mutation.6 Furthermore, no homologue of the LexA repressor, which is central to induction of the SOS response in E. coli,7 nor of DinR, the LexA analogue in Bacillus subtilis,8 can be identified in the genome sequence.9,10 The S. pneumoniae genome does include a gene identified as dinB, predicted to encode DNA polymerase IV, but not umuDC, which would encode the error-prone DNA polymerase V considered to be mainly responsible for the increased mutation seen in the E. coli SOS response.11,12
In the present study we investigated the effect of subinhibitory concentrations of five antibiotics on mutation frequency and transformation frequency in three clinical isolates of S. pneumoniae, including a natural deletion mutant of dinB.
Materials and methods
Bacterial isolates and culture conditions
Isolates of S. pneumoniae PN94/661 (serotype 9V), PN94/361 (6B) and PN93/1719 (14) were selected from a collection described previously; PN94/661 and PN93/1719 represent clones that are commonly isolated in the UK.13,14 R6Rif was selected as a rifampicin-resistant mutant of the laboratory strain R615 by spreading ∼108 cfu on to plates containing 2 mg/L rifampicin. Liquid cultures were grown in brain heart infusion (BHI) broth (Oxoid, Basingstoke, UK) with addition of 2% horse serum (Oxoid), unless otherwise specified. Plate cultures were grown on Iso-Sensitest agar (Oxoid) containing 5% defibrinated horse blood (Oxoid). All cultures were incubated at 37°C in 5% atmospheric CO2; when colonies were to be counted, plates were incubated for 48 h.
Determination of mutation frequency
Overnight growth from a blood agar plate was suspended in BHI broth and diluted to produce 10 mL cultures with an OD620 of 0.1. After incubation for 6–8 h, until OD620 exceeded 0.4, 1 mL aliquots were mixed with 0.3 mL amounts of sterile glycerol and frozen at −70°C to provide a stock for mutation frequency experiments. The viable count in the stock and the number of pre-existing rifampicin-resistant mutants were determined prior to freezing. To determine frequency of mutation to rifampicin resistance, 10 mL BHI broths were inoculated with 0.1 mL of a 10−2 dilution of the stock starter culture. Cultures were incubated at 37°C until no further increase in OD620 was detected (∼7–8 h); then the viable count and number of rifampicin-resistant mutants were determined by spreading triplicate 0.2 mL samples on to plates containing 2 mg/L rifampicin, and appropriately diluted samples were plated on to non-selective plates. For determination of mutation frequency with subinhibitory concentrations of antibiotic, ampicillin, ciprofloxacin, erythromycin, streptomycin or trimethoprim was added to the BHI broths prior to incubation at the maximum concentration found to allow normal growth for each isolate, as monitored by optical density (see Table 1).
Concentrations of antibiotics used to determine effects on mutation frequency
| Isolate . | Antibiotic . | MIC (mg/L) . | Concentration (mg/L) . |
|---|---|---|---|
| PN94/661 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 8 | 2 | |
| PN94/361 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 32 | 8 | |
| PN93/1719 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.5 | 0.25 | |
| erythromycin | 2 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 4 | 2 |
| Isolate . | Antibiotic . | MIC (mg/L) . | Concentration (mg/L) . |
|---|---|---|---|
| PN94/661 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 8 | 2 | |
| PN94/361 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 32 | 8 | |
| PN93/1719 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.5 | 0.25 | |
| erythromycin | 2 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 4 | 2 |
Concentrations of antibiotics used to determine effects on mutation frequency
| Isolate . | Antibiotic . | MIC (mg/L) . | Concentration (mg/L) . |
|---|---|---|---|
| PN94/661 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 8 | 2 | |
| PN94/361 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 32 | 8 | |
| PN93/1719 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.5 | 0.25 | |
| erythromycin | 2 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 4 | 2 |
| Isolate . | Antibiotic . | MIC (mg/L) . | Concentration (mg/L) . |
|---|---|---|---|
| PN94/661 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 8 | 2 | |
| PN94/361 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.25 | 0.1875 | |
| erythromycin | 0.03 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 32 | 8 | |
| PN93/1719 | ampicillin | 0.007 | 0.00525 |
| ciprofloxacin | 0.5 | 0.25 | |
| erythromycin | 2 | 0.0075 | |
| streptomycin | 128 | 32 | |
| trimethoprim | 4 | 2 |
Determination of transformation frequency
DNA for transformation was extracted as described previously16 from R6Rif. Stock starter cultures (as described above) were inoculated into 3 mL of CAT medium at a 10−2 dilution. Where appropriate this medium was supplemented with subinhibitory concentrations of ampicillin, ciprofloxacin, erythromycin, streptomycin or trimethoprim (Table 1). After 3 h of incubation at 37°C, each culture was split into three 1 mL aliquots and (i) treated with 1 μg of colony stimulating peptide (CSP;17 kindly provided by D. Morrison, University of Illinois at Chicago) and 10 μL of 1 M NaOH, (ii) treated with 10 μL of 1 M NaOH or (iii) left untreated. All cultures were incubated for a further 20 min at 37°C, after which donor DNA (10 μL) was added to aliquots (i) and (ii). All three cultures were then incubated at 30°C for 30 min and then at 37°C for a further 2 h. The frequency of rifampicin-resistant transformants (or mutants) was determined as above by plating appropriate dilutions on to selective and non-selective agar.
Characterization of dinB
Fragments incorporating dinB were amplified using PCR from S. pneumoniae isolates by standard methods. Crude DNA extracts were prepared by heating cell suspensions to 99°C for 10 min and sedimenting cell debris. Amplification was performed using ReddyMix 1.1 (Abgene, Epsom, UK), with 30 cycles of 1 min of denaturation at 95°C, 1 min of annealing at 55°C and 1 min of polymerase extension at 72°C. Primers for amplification of the complete gene were: dinBLF1, CAGCCATACGGATACCACC, −750 to −730; and dinBRR1, GTGAAAGCCGGAGAACTCTT, +1417 to +1397 (numbered relative to the start codon). Additional primers used for sequencing were: dinBRF1, ATCGAGATCTTCGGTATCAAGTCAGCGGTC, +388 to +408; dinBLR1, CCTTGGGCCCGCTCGAATCTGGAGTCCCAC, +311 to +291; dinBmidIIF1, ACCTGTAGCTATGAGGCTCGA, +174 to +195; dinBmidIIR1, GATTGAGAGCGACTTTTTCTG, +868 to +847; dinBmidF1, CCAGGCCGTCTTTATCTCAG, +248 to +268; and dinBmidR1, AAAACGCCCATTTGATGAAG, +641 to +621. DNA sequences were determined at the Genome Centre of Barts and the London School of Medicine and Dentistry on a Prism 3700 sequencing apparatus (Applied Biosystems, Warrington, UK), using the end terminator chemistry kit BigDye v2.0 (Applied Biosystems).
Statistical analysis
Mutation and transformation frequency data, which are non-parametric, were analysed using the Mann–Whitney U-test, performed with the StatsDirect 2.3 software package.
Nucleotide sequence accession number
The nucleotide sequence data reported in this study have been assigned GenBank accession number DQ263607.
Results
Effect of subinhibitory concentrations of antibiotics on mutation frequency
Mutation to rifampicin resistance represents a convenient assay for spontaneous point mutation that has been widely applied to both Gram-negative and Gram-positive bacteria, including S. pneumoniae.18,19 Resistance in pneumococci can be conferred by a number of missense mutations within rpoB.20,21 Five antibiotics were examined as possible modulators of mutation frequency, selected to represent different mechanisms of action: ampicillin, ciprofloxacin, erythromycin, streptomycin and trimethoprim. Isolates were initially inoculated into broths with a range of antibiotic concentrations below MIC, and growth was monitored by optical density. The highest concentration giving a growth rate and maximum OD close to normal was chosen for testing the impact on mutation frequency, except that for PN93/1719, which was macrolide resistant, erythromycin was used at the same concentration as in the other two isolates (Table 1). Microscopic examination of the cultures grown in the presence of antibiotics did not reveal any morphological abnormalities, such as extended chain length, that might lead to anomalous results. In each experimental run for determination of mutation frequency, a single aliquot of the starter culture was used to inoculate six broths to which ampicillin, ciprofloxacin, erythromycin, streptomycin, trimethoprim or no antibiotic was added. For each isolate 14 or 15 runs were performed. The probability of pre-existing mutants being present in the inoculum was below 0.08 in all cases, as determined from their frequency in the starter culture.
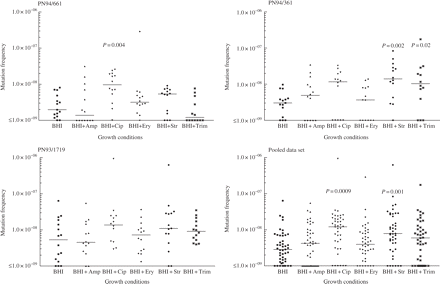
Mutation frequencies observed are plotted in Figure 1, and median mutation frequencies are given in Table 2 (the use of medians rather than means prevents skewing by ‘jackpot’ cultures). When the data for all three isolates were pooled, ciprofloxacin and streptomycin were found to increase the median frequency of rifampicin-resistant mutants 3.4- and 2.7-fold, respectively. Statistical analysis demonstrated that these differences in distribution were highly significant (P < 0.01). A similar pattern was observed when each isolate was examined individually: median mutation frequencies were elevated at least 2-fold in the presence of either ciprofloxacin or streptomycin, although statistical significance was not always achieved. The effect of trimethoprim appeared to differ between isolates: median mutation frequency to rifampicin resistance was slightly decreased in PN94/661, but increased in PN94/361 and PN93/1719. The increase was significant for isolate PN94/361 (P = 0.02), and for the pool of PN94/361 and PN93/1719 (P = 0.0035). Median mutation frequencies were little changed in the presence of ampicillin or erythromycin.
Frequency of mutation to rifampicin resistance observed after growth in BHI alone or with addition of antibiotics at subinhibitory concentrations (Table 1). Horizontal bars indicate median values. P values are shown for distributions that were significantly different from the distribution with BHI alone.
Median mutation frequencies observed after growth in BHI alone or with the addition of antibiotics at subinhibitory concentrations (Table 1)
| . | Median mutation frequencya . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Condition of culture growth . | PN94/661 . | PN94/361 . | PN93/1719 . | pooled data . | |||
| BHI | 1.96 × 10−9 | 3.01 × 10−9 | 3.75 × 10−9 | 2.85 × 10−9 | |||
| BHI + ampicillin | 1.38 × 10−9 | 4.91 × 10−9 | 4.54 × 10−9 | 4.21 × 10−9 | |||
| BHI + ciprofloxacin | 9.65 × 10−9 | 1.16 × 10−8 | 7.62 × 10−9 | 9.81 × 10−9 | |||
| BHI + erythromycin | 3.18 × 10−9 | 3.65 × 10−9 | 7.26 × 10−9 | 3.95 × 10−9 | |||
| BHI + streptomycin | 5.35 × 10−9 | 1.40 × 10−8 | 1.10 × 10−8 | 7.81 × 10−9 | |||
| BHI + trimethoprim | 1.21 × 10−9 | 1.03 × 10−8 | 9.14 × 10−9 | 5.97 × 10−9 | |||
| . | Median mutation frequencya . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Condition of culture growth . | PN94/661 . | PN94/361 . | PN93/1719 . | pooled data . | |||
| BHI | 1.96 × 10−9 | 3.01 × 10−9 | 3.75 × 10−9 | 2.85 × 10−9 | |||
| BHI + ampicillin | 1.38 × 10−9 | 4.91 × 10−9 | 4.54 × 10−9 | 4.21 × 10−9 | |||
| BHI + ciprofloxacin | 9.65 × 10−9 | 1.16 × 10−8 | 7.62 × 10−9 | 9.81 × 10−9 | |||
| BHI + erythromycin | 3.18 × 10−9 | 3.65 × 10−9 | 7.26 × 10−9 | 3.95 × 10−9 | |||
| BHI + streptomycin | 5.35 × 10−9 | 1.40 × 10−8 | 1.10 × 10−8 | 7.81 × 10−9 | |||
| BHI + trimethoprim | 1.21 × 10−9 | 1.03 × 10−8 | 9.14 × 10−9 | 5.97 × 10−9 | |||
Median of at least 14 replicas for each isolate and condition.
Median mutation frequencies observed after growth in BHI alone or with the addition of antibiotics at subinhibitory concentrations (Table 1)
| . | Median mutation frequencya . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Condition of culture growth . | PN94/661 . | PN94/361 . | PN93/1719 . | pooled data . | |||
| BHI | 1.96 × 10−9 | 3.01 × 10−9 | 3.75 × 10−9 | 2.85 × 10−9 | |||
| BHI + ampicillin | 1.38 × 10−9 | 4.91 × 10−9 | 4.54 × 10−9 | 4.21 × 10−9 | |||
| BHI + ciprofloxacin | 9.65 × 10−9 | 1.16 × 10−8 | 7.62 × 10−9 | 9.81 × 10−9 | |||
| BHI + erythromycin | 3.18 × 10−9 | 3.65 × 10−9 | 7.26 × 10−9 | 3.95 × 10−9 | |||
| BHI + streptomycin | 5.35 × 10−9 | 1.40 × 10−8 | 1.10 × 10−8 | 7.81 × 10−9 | |||
| BHI + trimethoprim | 1.21 × 10−9 | 1.03 × 10−8 | 9.14 × 10−9 | 5.97 × 10−9 | |||
| . | Median mutation frequencya . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Condition of culture growth . | PN94/661 . | PN94/361 . | PN93/1719 . | pooled data . | |||
| BHI | 1.96 × 10−9 | 3.01 × 10−9 | 3.75 × 10−9 | 2.85 × 10−9 | |||
| BHI + ampicillin | 1.38 × 10−9 | 4.91 × 10−9 | 4.54 × 10−9 | 4.21 × 10−9 | |||
| BHI + ciprofloxacin | 9.65 × 10−9 | 1.16 × 10−8 | 7.62 × 10−9 | 9.81 × 10−9 | |||
| BHI + erythromycin | 3.18 × 10−9 | 3.65 × 10−9 | 7.26 × 10−9 | 3.95 × 10−9 | |||
| BHI + streptomycin | 5.35 × 10−9 | 1.40 × 10−8 | 1.10 × 10−8 | 7.81 × 10−9 | |||
| BHI + trimethoprim | 1.21 × 10−9 | 1.03 × 10−8 | 9.14 × 10−9 | 5.97 × 10−9 | |||
Median of at least 14 replicas for each isolate and condition.
In addressing factors that could distort the interpretation of the results, we considered the impact of ‘zero’ results, where cultures produced no resistant colonies. The viable counts of cultures grown in the presence of antibiotics were consistently lower than those of cultures with no antibiotic (mean viable counts for the three isolates combined were 5.4 × 108 with ciprofloxacin, 6.1 × 108 with streptomycin and 9.1 × 108 with no antibiotic). Consequently, the threshold mutation frequency for detection was higher with antibiotics than without, meaning that antibiotic treatment could produce a zero result even with a real mutation frequency that exceeded the measured frequencies in the absence of antibiotic. Any distortion of the data from this factor would increase the value of P in comparisons where antibiotics increased mutation frequency, underrating rather than exaggerating the statistical significance.
Effect of subinhibitory concentrations of antibiotic on transformation frequency
Isolates PN94/361 and PN94/661, but not PN93/1719, were found to be induced to competence by the addition of CSP,17 so the impact of antibiotics on transformation frequency was investigated for the first two isolates only (none of the three could be transformed without CSP). R6Rif was used as the source of donor DNA. Since growth phase affects the induction of competence, growth of the isolates was monitored by OD620 in CAT medium supplemented with antibiotics at the concentrations used to determine mutation frequency (Table 1); it was confirmed that these conditions did not alter the growth curves of the isolates. Nevertheless, when transformation was attempted, cultures grown with ampicillin (with and without addition of CSP, NaOH or DNA) consistently failed to reach normal cell densities, so transformation frequencies could not be determined with this compound.
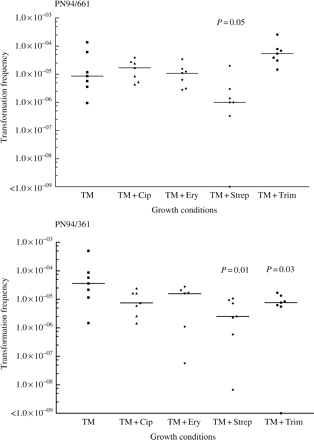
Figure 2 illustrates the transformation frequencies to rifampicin resistance in the presence of subinhibitory concentrations of antibiotic as determined in seven experimental runs for each isolate. These results suggest a difference between the two isolates in the response to ciprofloxacin, erythromycin and trimethoprim: median transformation frequency was decreased for PN94/361, whereas there was little change for PN94/661 in the presence of ciprofloxacin and erythromycin, and there was a 6-fold increase for trimethoprim (P = 0.07). For both isolates transformation frequency appeared considerably reduced in the presence of streptomycin, with a >6-fold reduction in the median values (P ≤ 0.05). Control cultures without DNA, as well as previously determined mutation frequencies, confirmed that point mutation did not contribute significantly to the number of rifampicin-resistant colonies obtained. None of the antibiotics used induced competence, since transformation was not detected without the addition of CSP.
Graphs of frequency of transformation to rifampicin resistance observed after growth in CAT alone or with addition of antibiotics at subinhibitory concentrations (Table 1). Horizontal bars indicate median values. P values are shown for distributions that were significantly different from the distribution with CAT alone.
Characterization of dinB in clinical isolates
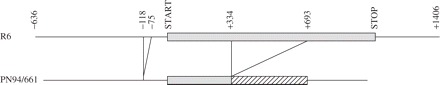
The amplification product of dinB from PN94/661 was ∼400 bp shorter than expected, while PN94/361 and PN93/1719 gave products of the size predicted from the whole genome sequences.9,10 Sequence determination of the dinB locus from PN94/661 revealed a deletion of 359 bp from nucleotides 334–692 of the 1071 bp R6 (wild-type) coding region, and a deletion of 45 bp upstream of the start codon (Figure 3). Any protein produced from the deleted dinB would share only the N-terminal 111 amino acids with the 357-amino-acid wild-type DinB, precluding any functional polymerase activity.
Deletions observed in dinB of PN94/661 relative to wild-type dinB from the sequenced genome of S. pneumoniae R6.
Discussion
The combined results for three clinical isolates demonstrated that subinhibitory concentrations of ciprofloxacin and streptomycin caused an increase in the mutation frequency of S. pneumoniae. While the increase was relatively modest in scale (3.4-fold for ciprofloxacin and 2.7-fold for streptomycin), it was statistically significant and was comparable to the 4-fold increase in frequency of mutation to rifampicin resistance reported for isogenic hex (mismatch repair) mutants of S. pneumoniae.22,23
Pitfalls in equating increased mutant numbers to an increased mutation frequency were considered. Any inhibition in cell division or separation, for example through d-Ala-d-Ala transpeptidase inhibition by β-lactam antibiotics, could result in colony forming units representing a larger number of genomes, which would in turn tend to overrepresent the proportion of resistant mutants. Microscopic examination of cultures grown in the presence of antibiotics demonstrated no evidence of morphological abnormalities or increases in the numbers of conjoined cells. Our experimental protocol did not rule out the possibility that growth in the presence of subinhibitory concentrations of ciprofloxacin or streptomycin might allow the organisms to grow and divide after plating on to rifampicin for a longer time than untreated cells, giving more opportunity for mutation to arise. However, while it has been reported that rifampicin may diminish the rate of killing of pneumococci by fluoroquinolones,24,25 the inverse does not appear to be true in that fluoroquinolones do not reduce the killing activity of rifampicin alone.24
hex mutants of S. pneumoniae demonstrate elevated transformation frequency as well as increased mutation frequency, since correction of mismatched recombination intermediates is impaired.26,27 In this study the presence of ciprofloxacin at levels that increased mutation frequency had no concomitant effect on transformation frequency. Streptomycin decreased the frequency of transformation for both isolates tested; it is plausible that even low concentrations of streptomycin may hinder the synthesis of new proteins required for the development of competence.
By analogy to mechanisms responsible for increased mutation frequency in response to antibiotics in E. coli,2,3 we proposed to test the hypothesis that the predicted error-prone DNA polymerase of pneumococci, DinB, would be required to evoke an increase in mutation frequency. Fortuitously, it transpired that one of the isolates under study, PN94/661, was a natural deletion mutant of dinB. This organism nevertheless demonstrated very similar responses to ciprofloxacin and streptomycin to the two dinB+ isolates, implying that induction of DinB is unlikely to explain the increased mutation frequencies observed with these agents.
In contrast, the effect of trimethoprim did differ among the isolates tested, with an elevated mutation frequency for the two dinB+ clinical isolates and a reduced mutation frequency for PN94/661. Transformation results also suggested a different response to trimethoprim among the isolates: transformation frequency was decreased in PN94/361 but increased in the dinB mutant PN94/661. Trimethoprim has not previously been implicated in promoting mutation, to our knowledge. The significance of dinB deletion to these results requires further investigation.
This work adds to a growing body of evidence that several antibiotics, and ciprofloxacin in particular, can promote an increase in mutation frequency at subinhibitory concentrations in diverse bacteria, now including S. pneumoniae as well as E. coli, Pseudomonas aeruginosa, Staphylococcus aureus and Mycobacterium fortuitum.1,28,29 By analogy to E. coli, it is often assumed that these responses are effected through induction of error-prone DNA polymerases in an SOS-type response. However, this cannot be the case in pneumococci since key elements of the SOS response are absent and, as we have shown here, dinB is not required for the responses to ciprofloxacin or streptomycin, although it is implicated in the response to trimethoprim. Our results emphasize that E. coli is not a universal paradigm for understanding factors that regulate mutation frequency in other bacteria.
Antimicrobial therapy aims to target an infecting organism with lethal or inhibitory concentrations of antibiotic. However the antibiotic concentration in vivo often falls below the inhibitory level for some proportion of the dosage interval, and may fail to reach an inhibitory level in some body compartments. The increase in mutation frequency observed in the presence of subinhibitory concentrations of antibiotic implies that not only will antibiotic use select for resistant mutants, but it may also increase the likelihood that resistant mutants will arise. Currently, the emergence among S. pneumoniae of resistance to fluoroquinolones themselves, mediated by mutations (as well as lateral gene transfer) in chromosomal topoisomerase genes gyrA and parC, is of particular concern.30,31 Furthermore, since pneumococci are frequently carried asymptomatically in the nasopharynx of young children, they may be exposed to multiple rounds of antibiotics directed at the treatment of other infections. An effect of trimethoprim may be particularly significant in this context, since it is heavily used and has inherently marginal activity against pneumococci.
Transparency declarations
None to declare.
We thank Bryn Bridges for helpful discussions in the course of this work. We are grateful to the St Bartholomew's and the Royal London Charitable Foundation for funding.
References
Phillips I, Culebras E, Moreno F et al. Induction of the SOS response by new 4-quinolones.
Ysern P, Clerch B, Castano M et al. Induction of SOS genes in Escherichia coli and mutagenesis in Salmonella typhimurium by fluoroquinolones.
Perez-Capilla T, Baquero MR, Gomez-Gomez JM et al. SOS-independent induction of dinB transcription by β-lactam-mediated inhibition of cell wall synthesis in Escherichia coli.
Balashov S, Humayun MZ. Mistranslation induced by streptomycin provokes a RecABC/RuvABC-dependent mutator phenotype in Escherichia coli cells.
Ren L, Rahman MS, Humayun MZ. Escherichia coli cells exposed to streptomycin display a mutator phenotype.
Gasc AM, Sicard N, Claverys JP et al. Lack of SOS repair in Streptococcus pneumoniae.
Little JW, Mount DW, Yanisch-Perron CR. Purified lexA protein is a repressor of the recA and lexA genes.
Miller MC, Resnick JB, Smith BT et al. The Bacillus subtilis dinR gene codes for the analogue of Escherichia coli LexA. Purification and characterization of the DinR protein.
Hoskins J, Alborn WE Jr, Arnold J et al. Genome of the bacterium Streptococcus pneumoniae strain R6.
Tettelin H, Nelson KE, Paulsen IT et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae.
Tang M, Shen X, Frank EG et al. UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V.
Bagg A, Kenyon CJ, Walker GC. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli.
Hall LM, Whiley RA, Duke B et al. Genetic relatedness within and between serotypes of Streptococcus pneumoniae from the United Kingdom: analysis of multilocus enzyme electrophoresis, pulsed-field gel electrophoresis, and antimicrobial resistance patterns.
Lawrence ER, Arias CA, Duke B et al. Evaluation of serotype prediction by cpsA-cpsB gene polymorphism in Streptococcus pneumoniae.
Avery OT, MacLeod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types.
Maskell JP, Sefton AM, Hall, LM. Mechanism of sulfonamide resistance in clinical isolates of Streptococcus pneumoniae.
Havarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae.
Gustafsson I, Sjolund M, Torell E et al. Bacteria with increased mutation frequency and antibiotic resistance are enriched in the commensal flora of patients with high antibiotic usage.
Morosini MI, Baquero MR, Sanchez-Romero JM et al. Frequency of mutation to rifampin resistance in Streptococcus pneumoniae clinical strains: hexA and hexB polymorphisms do not account for hypermutation.
Enright M, Zawadski P, Pickerill P et al. Molecular evolution of rifampicin resistance in Streptococcus pneumoniae.
Padayachee T, Klugman KP. Molecular basis of rifampin resistance in Streptococcus pneumoniae.
Negri MC, Morosini MI, Baquero MR et al. Very low cefotaxime concentrations select for hypermutable Streptococcus pneumoniae populations.
Tiraby JG, Fox MS. Marker discrimination in transformation and mutation of pneumococcus.
Fitoussi F, Doit C, Geslin P et al. Killing activities of trovafloxacin alone and in combination with β-lactam agents, rifampin, or vancomycin against Streptococcus pneumoniae isolates with various susceptibilities to extended-spectrum cephalosporins at concentrations clinically achievable in cerebrospinal fluid.
Giron KP, Gross ME, Musher DM et al. In vitro antimicrobial effect against Streptococcus pneumoniae of adding rifampin to penicillin, ceftriaxone, or 1-ofloxacin.
Claverys JP, Mejean V, Gasc AM et al. Mismatch repair in Streptococcus pneumoniae: relationship between base mismatches and transformation efficiencies.
Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation.
Fung-Tomc J, Kolek B, Bonner DP. Ciprofloxacin-induced, low-level resistance to structurally unrelated antibiotics in Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus.
Gillespie SH, Basu S, Dickens AL et al. Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates.
Doern GV, Richter SS, Miller A et al. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes?
Author notes
1Centre for Infectious Disease, Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary, University of London, 4 Newark Street, London E1 2AT, UK; 2Antibiotic Resistance Monitoring and Reference Laboratory, Health Protection Agency Centre for Infections, 61 Colindale Avenue, London NW9 5HT, UK