-
PDF
- Split View
-
Views
-
Cite
Cite
Vanya A. Gant, Michael W. D. Wren, Michael S. M. Rollins, Annette Jeanes, Stephen S. Hickok, Tony J. Hall, Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms, Journal of Antimicrobial Chemotherapy, Volume 60, Issue 2, August 2007, Pages 294–299, https://doi.org/10.1093/jac/dkm201
Close - Share Icon Share
Abstract
We investigated three novel highly charged copper-based inorganic biocidal formulations for their activity against organisms highly relevant to healthcare-associated infection.
The three copper-based formulations were tested: (i) against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA), Legionella pneumophila, Acinetobacter calcoaceticus/baumannii (ACCB), glycopeptide-resistant Enterococcus and spores of Clostridium difficile in time–kill assays; (ii) for their ability to decontaminate ultramicrofibre (UMF) cloths; and (iii) for their cytotoxicity to human skin and intestinal epithelial cells.
All three copper-based formulations were potently biocidal down to concentrations of 1 ppm for both stationary- and log-phase organisms, and they were all active against C. difficile spores. At 150 ppm, they achieved a complete (>6 log10) kill of MRSA and ACCB mostly within 1 h. This biocidal activity was not achieved by copper sulphate or the inorganic binders used in the formulations. All three copper-based formulations completely decontaminated UMF cloths containing MRSA, ACCB or C. difficile spores, suggesting that any of these copper-based formulations would be highly beneficial in the healthcare environment. All three copper-based formulations and copper sulphate were not cytotoxic to human epithelial cells up to concentrations of 100–200 ppm.
All three of the novel copper-based biocidal formulations, but not their components (copper sulphate and inorganic binders), have potent activity against organisms highly relevant to healthcare-associated infections.
Introduction
Healthcare-associated infection (HAI) remains a major challenge. Healthcare institutions have responded by setting out minimal standards for the quality of both infection control and the decontamination of the healthcare environment itself. In the UK, individual institutions have in addition been directed by several Government documents setting out a framework for implementation of defined minimum standards.1,2
The healthcare environment becomes rapidly contaminated by pathogenic organisms which can survive for weeks and months on inanimate surfaces. While the overall degree to which such environmental sources of infection contribute to HAIs continues to be debated, many infections can be directly related to environmental sources.3 Consequently, considerable effort has recently been invested in hospital cleaning and decontamination, at least partly fuelled by public pressure.
Decontamination agents fall into three broad groups, namely oxygen-rich agents such as hydrogen peroxide, halide-containing agents, and quaternary ammonium compounds. These compounds degrade the environment, are hazardous to health, and are relatively weak biocides, respectively.4,5 Furthermore, recent evidence suggests that quaternary ammonium compounds and triclosan may drive antibiotic resistance.6,7
Copper is an element which is essential to human health as it contributes to the function of numerous essential metabolic processes.8,9 It also demonstrates considerable biocidal toxicity for microorganisms at concentrations well below those toxic for human tissues and cells.10 The biocidal action of copper is thought to relate to damage to plasma membranes, site-specific Fenton reaction-mediated damage to nucleic acids, and disruption of essential sulphydryl group-containing proteins.11 More recent work also demonstrates the biocidal activity of solid copper surfaces for both HAI organisms and mycobacteria.12–14
A potent liquid formulation containing copper might therefore be of potential use in the control of pathogens found in the healthcare environment. Accordingly, we have investigated the biocidal activity of three novel highly charged inorganic copper-based formulations—CuAL42, CuPC33 and CuWB50—which are composed of copper sulphate and one of three inorganic binder components (AL42, PC33, WB50) for their ability to kill microorganisms responsible for HAIs, and their potential toxicity for two human cell lines. Finally, we examined the potential use of these compounds in disinfecting contaminated ultramicrofibre (UMF) cleaning cloths.
Materials and methods
Materials
The three copper-based formulations—CuPC33, CuAL42 and CuWB50—were provided by Remedy Research Ltd. These formulations are composed of copper sulphate and two inorganic compounds that form the metallo-ion binders. The binders comprise an ammonium salt and an inorganic acid—ammonium phosphate and phosphoric acid in PC33, ammonium sulphate and sulphuric acid in AL42, and ammonium chloride and hydrochloric acid in WB50. These compounds are available for research purposes upon request to Remedy Research Ltd. The concentration of elemental copper in each stock solution was 30.43 g/L. A control solution of copper sulphate made to the same concentration in distilled water and the three binders (PC33, AL42 and WB50) alone were also provided.
Blood agar, nutrient broth and BYCE medium were purchased from Oxoid Ltd (UK). All cytotoxicity assay reagents and tissue culture media were purchased from Sigma-Aldrich.
Microbiological studies
We used methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter calcoaceticus/baumannii (ACCB), glycopeptide-resistant Enterococcus (GRE), and Legionella pneumophila—that were isolated from inpatient cases at UCLH. These strains are available upon request to Dr Gant. Clostridium difficile ribotype 001 was obtained from Dr J. Brazier, Anaerobe Reference Unit, University Hospital of Wales, Cardiff, UK.
MRSA, ACCB and GRE were grown in pure culture on blood agar and a single colony transferred to nutrient broth and incubated with shaking for 6 h at 37°C. The 6 h broth cultures (logarithmic phase cells) were then centrifuged to deposit the cells, the broth discarded and the bacterial cells washed and centrifuged three times using phosphate buffered saline (PBS) at pH 7.2. The final suspension was made in PBS and the viable cell count adjusted to the required concentration for the experiments (1.5 × 108 cfu/mL for MRSA and ACCB and 1 × 107 cfu/mL for GRE). These log phase cells were then exposed to the copper formulations under test at a final concentration of 1.0 ppm. Samples from these exposed cells were taken at 15, 30, 60 and 120 min and the viable count determined by the Miles and Misra technique.15 A control culture of PBS samples at 15 and 120 min was performed to ensure viability and stability of the inocula.
The above experiments were repeated using stationary phase MRSA, ACCB and GRE by taking cells from 24 h agar plate cultures and suspending them directly into PBS at 1.5 × 108 cells/mL after an initial PBS wash, without a 6 h broth culture step. The results shown in Tables 1 and 2 are representative of three similar experiments.
Effect of three copper formulations (1 ppm) on the ability of bacteria in logarithmic phase growth, and C. difficile spores, to form colonies
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1.5 × 108) | 4 × 107 | 2 × 107 | 5 × 106 | 4 × 105 |
| ACCB (1.5 × 108) | 3 × 107 | 4 × 106 | 5 × 105 | 5 × 105 |
| GRE (1 × 107) | 1 × 105 | 1.5 × 104 | 2.5 × 103 | 1 × 103 |
| L. pneumophila (5 × 106) | 1 × 105 | 7.5 × 104 | 7.5 × 104 | 4 × 104 |
| C. difficile spores (5 × 105) | 2.5 × 104 | 2.5 × 104 | 1.5 × 104 | 1.6 × 103 |
| CuPC33 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 2.5 × 107 | 9 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 2.5 × 107 | 2 × 107 | 1 × 106 | 5 × 105 |
| GRE (1 × 107) | 1 × 106 | 1 × 105 | 1 × 105 | 2 × 104 |
| L. pneumophila (5 × 106) | 3 × 104 | 3 × 104 | 1 × 104 | 8 × 103 |
| C. difficile spores (5 × 105) | 2 × 104 | 1.5 × 103 | 1 × 103 | 1 × 103 |
| CuWB50 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 5 × 106 | 5 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 5 × 107 | 1 × 107 | 5 × 105 | 9 × 104 |
| GRE (1 × 107) | 2.5 × 106 | 3.5 × 106 | 7.5 × 105 | 7 × 104 |
| L. pneumophila (5 × 106) | 3 × 105 | 2 × 105 | 5 × 104 | 2.5 × 104 |
| C. difficile spores (5 × 105) | 6.5 × 103 | 1 × 103 | 3.5 × 102 | 3.5 × 102 |
| Control (PBS) | ||||
| MRSA (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| ACCB (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| GRE (1 × 107) | 1 × 107 | — | — | 1 × 107 |
| L. pneumophila (5 × 106) | 5 × 106 | — | — | 5 × 106 |
| C. difficile spores (5 × 105) | 5 × 105 | — | — | 5 × 105 |
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1.5 × 108) | 4 × 107 | 2 × 107 | 5 × 106 | 4 × 105 |
| ACCB (1.5 × 108) | 3 × 107 | 4 × 106 | 5 × 105 | 5 × 105 |
| GRE (1 × 107) | 1 × 105 | 1.5 × 104 | 2.5 × 103 | 1 × 103 |
| L. pneumophila (5 × 106) | 1 × 105 | 7.5 × 104 | 7.5 × 104 | 4 × 104 |
| C. difficile spores (5 × 105) | 2.5 × 104 | 2.5 × 104 | 1.5 × 104 | 1.6 × 103 |
| CuPC33 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 2.5 × 107 | 9 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 2.5 × 107 | 2 × 107 | 1 × 106 | 5 × 105 |
| GRE (1 × 107) | 1 × 106 | 1 × 105 | 1 × 105 | 2 × 104 |
| L. pneumophila (5 × 106) | 3 × 104 | 3 × 104 | 1 × 104 | 8 × 103 |
| C. difficile spores (5 × 105) | 2 × 104 | 1.5 × 103 | 1 × 103 | 1 × 103 |
| CuWB50 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 5 × 106 | 5 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 5 × 107 | 1 × 107 | 5 × 105 | 9 × 104 |
| GRE (1 × 107) | 2.5 × 106 | 3.5 × 106 | 7.5 × 105 | 7 × 104 |
| L. pneumophila (5 × 106) | 3 × 105 | 2 × 105 | 5 × 104 | 2.5 × 104 |
| C. difficile spores (5 × 105) | 6.5 × 103 | 1 × 103 | 3.5 × 102 | 3.5 × 102 |
| Control (PBS) | ||||
| MRSA (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| ACCB (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| GRE (1 × 107) | 1 × 107 | — | — | 1 × 107 |
| L. pneumophila (5 × 106) | 5 × 106 | — | — | 5 × 106 |
| C. difficile spores (5 × 105) | 5 × 105 | — | — | 5 × 105 |
Effect of three copper formulations (1 ppm) on the ability of bacteria in logarithmic phase growth, and C. difficile spores, to form colonies
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1.5 × 108) | 4 × 107 | 2 × 107 | 5 × 106 | 4 × 105 |
| ACCB (1.5 × 108) | 3 × 107 | 4 × 106 | 5 × 105 | 5 × 105 |
| GRE (1 × 107) | 1 × 105 | 1.5 × 104 | 2.5 × 103 | 1 × 103 |
| L. pneumophila (5 × 106) | 1 × 105 | 7.5 × 104 | 7.5 × 104 | 4 × 104 |
| C. difficile spores (5 × 105) | 2.5 × 104 | 2.5 × 104 | 1.5 × 104 | 1.6 × 103 |
| CuPC33 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 2.5 × 107 | 9 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 2.5 × 107 | 2 × 107 | 1 × 106 | 5 × 105 |
| GRE (1 × 107) | 1 × 106 | 1 × 105 | 1 × 105 | 2 × 104 |
| L. pneumophila (5 × 106) | 3 × 104 | 3 × 104 | 1 × 104 | 8 × 103 |
| C. difficile spores (5 × 105) | 2 × 104 | 1.5 × 103 | 1 × 103 | 1 × 103 |
| CuWB50 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 5 × 106 | 5 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 5 × 107 | 1 × 107 | 5 × 105 | 9 × 104 |
| GRE (1 × 107) | 2.5 × 106 | 3.5 × 106 | 7.5 × 105 | 7 × 104 |
| L. pneumophila (5 × 106) | 3 × 105 | 2 × 105 | 5 × 104 | 2.5 × 104 |
| C. difficile spores (5 × 105) | 6.5 × 103 | 1 × 103 | 3.5 × 102 | 3.5 × 102 |
| Control (PBS) | ||||
| MRSA (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| ACCB (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| GRE (1 × 107) | 1 × 107 | — | — | 1 × 107 |
| L. pneumophila (5 × 106) | 5 × 106 | — | — | 5 × 106 |
| C. difficile spores (5 × 105) | 5 × 105 | — | — | 5 × 105 |
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1.5 × 108) | 4 × 107 | 2 × 107 | 5 × 106 | 4 × 105 |
| ACCB (1.5 × 108) | 3 × 107 | 4 × 106 | 5 × 105 | 5 × 105 |
| GRE (1 × 107) | 1 × 105 | 1.5 × 104 | 2.5 × 103 | 1 × 103 |
| L. pneumophila (5 × 106) | 1 × 105 | 7.5 × 104 | 7.5 × 104 | 4 × 104 |
| C. difficile spores (5 × 105) | 2.5 × 104 | 2.5 × 104 | 1.5 × 104 | 1.6 × 103 |
| CuPC33 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 2.5 × 107 | 9 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 2.5 × 107 | 2 × 107 | 1 × 106 | 5 × 105 |
| GRE (1 × 107) | 1 × 106 | 1 × 105 | 1 × 105 | 2 × 104 |
| L. pneumophila (5 × 106) | 3 × 104 | 3 × 104 | 1 × 104 | 8 × 103 |
| C. difficile spores (5 × 105) | 2 × 104 | 1.5 × 103 | 1 × 103 | 1 × 103 |
| CuWB50 | ||||
| MRSA (1.5 × 108) | 4.5 × 107 | 5 × 106 | 5 × 106 | 3 × 105 |
| ACCB (1.5 × 108) | 5 × 107 | 1 × 107 | 5 × 105 | 9 × 104 |
| GRE (1 × 107) | 2.5 × 106 | 3.5 × 106 | 7.5 × 105 | 7 × 104 |
| L. pneumophila (5 × 106) | 3 × 105 | 2 × 105 | 5 × 104 | 2.5 × 104 |
| C. difficile spores (5 × 105) | 6.5 × 103 | 1 × 103 | 3.5 × 102 | 3.5 × 102 |
| Control (PBS) | ||||
| MRSA (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| ACCB (1.5 × 108) | 1.5 × 108 | — | — | 1.5 × 108 |
| GRE (1 × 107) | 1 × 107 | — | — | 1 × 107 |
| L. pneumophila (5 × 106) | 5 × 106 | — | — | 5 × 106 |
| C. difficile spores (5 × 105) | 5 × 105 | — | — | 5 × 105 |
Effect of three copper formulations at 1 ppm on the ability of bacteria in stationary phase growth, and C. difficile spores, to form colonies
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1 × 108) | 6 × 107 | 2 × 107 | 4 × 106 | 3 × 105 |
| ACCB (1 × 108) | 5 × 107 | 8 × 106 | 7 × 105 | 5 × 105 |
| GRE (1 × 107) | 5 × 106 | 3 × 105 | 1 × 105 | 7 × 104 |
| CuWB50 | ||||
| MRSA (1 × 108) | 5 × 107 | 2 × 107 | 5 × 106 | 2 × 105 |
| ACCB (1 × 108) | 4 × 107 | 1 × 107 | 8 × 105 | 1 × 105 |
| GRE (1 × 107) | 2 × 106 | 1 × 106 | 7 × 105 | 9 × 104 |
| CuPC33 | ||||
| MRSA (1 × 108) | 4 × 107 | 3 × 107 | 1 × 107 | 8 × 105 |
| ACCB (1 × 108) | 3 × 107 | 2 × 107 | 6 × 106 | 8 × 105 |
| GRE (1 × 107) | 6 × 106 | 5 × 105 | 3 × 105 | 8 × 104 |
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1 × 108) | 6 × 107 | 2 × 107 | 4 × 106 | 3 × 105 |
| ACCB (1 × 108) | 5 × 107 | 8 × 106 | 7 × 105 | 5 × 105 |
| GRE (1 × 107) | 5 × 106 | 3 × 105 | 1 × 105 | 7 × 104 |
| CuWB50 | ||||
| MRSA (1 × 108) | 5 × 107 | 2 × 107 | 5 × 106 | 2 × 105 |
| ACCB (1 × 108) | 4 × 107 | 1 × 107 | 8 × 105 | 1 × 105 |
| GRE (1 × 107) | 2 × 106 | 1 × 106 | 7 × 105 | 9 × 104 |
| CuPC33 | ||||
| MRSA (1 × 108) | 4 × 107 | 3 × 107 | 1 × 107 | 8 × 105 |
| ACCB (1 × 108) | 3 × 107 | 2 × 107 | 6 × 106 | 8 × 105 |
| GRE (1 × 107) | 6 × 106 | 5 × 105 | 3 × 105 | 8 × 104 |
Effect of three copper formulations at 1 ppm on the ability of bacteria in stationary phase growth, and C. difficile spores, to form colonies
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1 × 108) | 6 × 107 | 2 × 107 | 4 × 106 | 3 × 105 |
| ACCB (1 × 108) | 5 × 107 | 8 × 106 | 7 × 105 | 5 × 105 |
| GRE (1 × 107) | 5 × 106 | 3 × 105 | 1 × 105 | 7 × 104 |
| CuWB50 | ||||
| MRSA (1 × 108) | 5 × 107 | 2 × 107 | 5 × 106 | 2 × 105 |
| ACCB (1 × 108) | 4 × 107 | 1 × 107 | 8 × 105 | 1 × 105 |
| GRE (1 × 107) | 2 × 106 | 1 × 106 | 7 × 105 | 9 × 104 |
| CuPC33 | ||||
| MRSA (1 × 108) | 4 × 107 | 3 × 107 | 1 × 107 | 8 × 105 |
| ACCB (1 × 108) | 3 × 107 | 2 × 107 | 6 × 106 | 8 × 105 |
| GRE (1 × 107) | 6 × 106 | 5 × 105 | 3 × 105 | 8 × 104 |
| . | cfu . | |||
|---|---|---|---|---|
| Organism (inoculum) . | 15 min . | 30 min . | 60 min . | 120 min . |
| CuAL42 | ||||
| MRSA (1 × 108) | 6 × 107 | 2 × 107 | 4 × 106 | 3 × 105 |
| ACCB (1 × 108) | 5 × 107 | 8 × 106 | 7 × 105 | 5 × 105 |
| GRE (1 × 107) | 5 × 106 | 3 × 105 | 1 × 105 | 7 × 104 |
| CuWB50 | ||||
| MRSA (1 × 108) | 5 × 107 | 2 × 107 | 5 × 106 | 2 × 105 |
| ACCB (1 × 108) | 4 × 107 | 1 × 107 | 8 × 105 | 1 × 105 |
| GRE (1 × 107) | 2 × 106 | 1 × 106 | 7 × 105 | 9 × 104 |
| CuPC33 | ||||
| MRSA (1 × 108) | 4 × 107 | 3 × 107 | 1 × 107 | 8 × 105 |
| ACCB (1 × 108) | 3 × 107 | 2 × 107 | 6 × 106 | 8 × 105 |
| GRE (1 × 107) | 6 × 106 | 5 × 105 | 3 × 105 | 8 × 104 |
Suspensions of L. pneumophila were made from 5 day cultures on BCYE medium in PBS. After 6 h of growth in broth to obtain log phase cells as described above, the cells suspension was adjusted to 5 × 106 cells/mL in PBS.
Spore suspensions of C. difficile were made by suspending a 5 day culture of the organism on blood agar (incubated anaerobically) in 50:50 alcohol/saline. A Miles and Misra count was then performed on this suspension to determine the final concentration of viable spores and the concentration was finally adjusted to 5 × 105 spores/mL for experiments.
For the 16 h time–kill curves with the three copper-based formulations (copper sulphate with three different binders), copper sulphate and the inorganic binders alone, logarithmic-phase MRSA and ACCB were prepared as described above. In order to maintain log phase growth over a 16 h period, the time–kill experiments were carried out in RPMI 1640 culture medium using 150 ppm of the copper-based formulations, and equivalent concentrations of copper sulphate and the inorganic binders.
In all experiments, 1 mL samples taken at various times were mixed with 1 mL of Ringer's solution and plated on appropriate agar plates and incubated for 24 h at 37°C, when cfu were counted.
Decontamination of laminate surfaces with UMF cloths
Laminated surfaces in common use in hospitals were inoculated with 100 µL of sterile PBS containing 2 × 106 cfu of MRSA or ACCB or 3 × 105 spores/mL of C. difficile, spread with a sterile flat spreader over a 100 cm2 area, and allowed to dry. The area was then sampled with contact plates to ensure the viability of the inoculum. The area was then cleaned with UMF cloths moistened to the recommended limit with sterile water (control) or with a 150 ppm solution of the copper formulations diluted in water. The area was then contact plated again to assess the removal of the inoculum by the UMF cloths. The UMF cloths were then bagged in mini-grip bags and left at room temperature for 16 h to simulate travel to the hospital laundry or static storage on the ward. After 16 h, the UMF cloths were placed into 100 mL of PBS and agitated in a Stomacher (Seward Ltd, UK) for 3 min at 250 rpm.
Viable counts were performed on the eluent. In addition, 10 mL samples of eluent were centrifuged at 2000 g for 10 min and the deposit cultured onto blood agar and incubated for 24 h at 37°C when cfu were counted. Background environmental bacterial counts on the boards were assessed by contact plating unused parts of the boards. The results shown in Table 3 are representative of three similar experiments.
Cleaning of contaminated surfaces using UMF cloths wetted with copper-based formulations or water (control UMF)
| UMF with Cu formulation (150 ppm) . | Inoculum per 100 cm2 . | cfu on contaminated surfaces detected with contact plates . | cfu from UMF cloths after 16 h . | Board control cfu . | |
|---|---|---|---|---|---|
| Pre-clean . | Post-clean . | ||||
| CuAL42 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuPC33 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuWB50 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| Control UMF | |||||
| MRSA | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 3 × 105 | 0 |
| UMF with Cu formulation (150 ppm) . | Inoculum per 100 cm2 . | cfu on contaminated surfaces detected with contact plates . | cfu from UMF cloths after 16 h . | Board control cfu . | |
|---|---|---|---|---|---|
| Pre-clean . | Post-clean . | ||||
| CuAL42 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuPC33 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuWB50 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| Control UMF | |||||
| MRSA | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 3 × 105 | 0 |
Cleaning of contaminated surfaces using UMF cloths wetted with copper-based formulations or water (control UMF)
| UMF with Cu formulation (150 ppm) . | Inoculum per 100 cm2 . | cfu on contaminated surfaces detected with contact plates . | cfu from UMF cloths after 16 h . | Board control cfu . | |
|---|---|---|---|---|---|
| Pre-clean . | Post-clean . | ||||
| CuAL42 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuPC33 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuWB50 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| Control UMF | |||||
| MRSA | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 3 × 105 | 0 |
| UMF with Cu formulation (150 ppm) . | Inoculum per 100 cm2 . | cfu on contaminated surfaces detected with contact plates . | cfu from UMF cloths after 16 h . | Board control cfu . | |
|---|---|---|---|---|---|
| Pre-clean . | Post-clean . | ||||
| CuAL42 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuPC33 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| CuWB50 | |||||
| MRSA | 2 × 106 | >500 | 0 | 0 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 0 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 0 | 0 |
| Control UMF | |||||
| MRSA | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| ACCB | 2 × 106 | >500 | 0 | 2 × 106 | 0 |
| C. difficile spores | 3 × 105 | >500 | 0 | 3 × 105 | 0 |
Cytotoxicity assay
Two human cell lines (both obtained from the ATCC) were used for these studies; HT-29 is an intestinal epithelial cell line and A431 is a squamous epithelial skin cell line. HT-29 was cultured in McCoy's modified medium supplemented with 10% fetal calf serum (FCS), 2 g/L sodium bicarbonate and 2 mM l-glutamine. A431 was cultured in RPMI 1640 medium supplemented with 10% FCS, 2 g/L sodium bicarbonate and 2 mM l-glutamine. The cells were cultured in a humidified incubator at 37°C with a 5% CO2 in air atmosphere. For cytotoxicity experiments, the cells were plated into the wells of flat-bottom 96-well plates at 2 × 104 cells per well in the appropriate medium and allowed to settle overnight. Samples of the copper-based formulations or copper sulphate at various concentrations in the appropriate complete media were then added and the cells cultured for a further 24 h. After examination by light microscopy, the cells were then fixed and stained to quantitatively determine cytotoxicity as described later.
The sulforhodamine B (SRB) cytotoxicity assay has been described in detail previously.16 Briefly, the cells were washed twice with RPMI-1640 medium and then fixed with 10% trichloroacetic acid for 1 h at 4°C. After washing twice with tap water, the cells were stained with SRB (0.4% w/v SRB in 1% acetic acid) for 30 min at room temperature. After washing twice with tap water, the remaining stain was dissolved in 10 mM Tris base and the absorbance of the wells was measured on a Dynatech Multiplate ELISA reader at 540 nm. Percent cell survival was calculated by dividing the test A540 by control A540 and multiplying by 100.
Results
All three copper-based formulations (CuAL42, CuPC33 and CuWB50) reduced bacterial numbers in a dose-dependent fashion (Table 1). At a concentration of 1 ppm, all three copper-based formulations achieved an ∼2 to 3 log10 reduction of cfu of MRSA, ACCB and GRE. CuAL42 and CuWB50 achieved a 2 log10 reduction and CuPC33 a 3 log10 reduction in cfu of L. pneumophila. CuAL42 and CuPC33 achieved a 2 log10 reduction in cfu of C. difficile spores, while CuWB50 achieved a 3 log10 reduction in cfu of C. difficile spores. Control bacterial cultures with PBS showed no reduction in cfu formation over the 2 h incubation period.
As shown in Table 2, the inhibitory effect of the three copper-based formulations on cfu of stationary phase MRSA, ACCB and GRE was similar to the results obtained with bacteria in logarithmic-phase growth (Table 1).
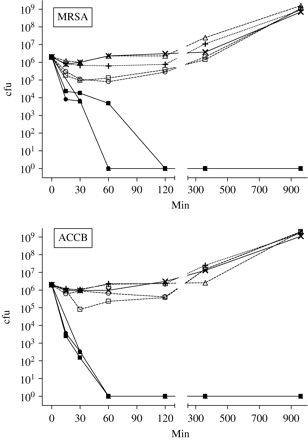
Time–kill curves were performed on MRSA and ACCB in order to compare the relative effects on bacterial numbers of the three copper-based formulations and their components—copper sulphate and the three different inorganic binders: AL42, PC33 and WB50. For these experiments, we used a final concentration of 150 ppm of the three copper-based formulations and copper sulphate, and the equivalent concentrations of the inorganic binders. Figure 1 shows that exposure to all three copper-based compounds completely inhibited cfu formation by ACCB and MRSA (>6 log10 kill) in 60 min, except for CuPC33 which required 2 h to completely inhibit MRSA cfu formation. In contrast, copper sulphate and the three inorganic binders alone had little or no effect on cfu formation by ACCB and MRSA, and by 6–16 h bacterial cfu formation in the presence of these compounds was very similar to that in control cultures.
The effect of three copper formulations (150 ppm) and their components (copper sulphate and inorganic binders) on the ability of bacteria in logarithmic phase growth to form colonies. Crosses (X) with solid line, control; filled triangles, CuAL42; filled square, CuPC33; filled circles, CuWB50; plus symbols (+), copper sulphate; open triangles, AL42 binder; open squares PC33 binder; open circles, WB50 binder. The results shown are the means of triplicate observations and are representative of three similar experiments. Error bars representing standard deviations (generally < 10%) are not shown for reasons of clarity.
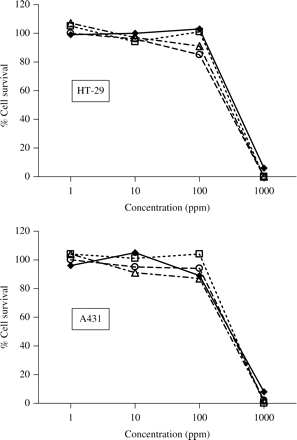
We assessed the cytotoxic effects of the three copper-based formulations and copper sulphate using two human cell lines. Figure 2 shows that the three copper-based formulations and copper sulphate were not significantly cytotoxic to HT-29 or A431 cells at concentrations from 1 to 100 ppm. At 1000 ppm, however, all three copper-based formulations and copper sulphate were significantly cytotoxic (90–100%) to both cell lines, and under the light microscope the cells appeared rounded and granular, suggesting an apoptotic effect. The three inorganic binders were also tested on A431 cells and showed no cytotoxicity at concentrations up to and including 1000 ppm (data not shown).
Cytotoxicity of three copper formulations and copper sulphate to HT-29 and A431 cells. Open squares, CuAL42; open triangles, CuPC33; open circles, CuWB50; filled diamonds and solid lines, copper sulphate. The results shown are the means of triplicate observations from duplicate experiments. Error bars representing standard deviations (generally < 10%) are not shown for reasons of clarity.
In order to investigate potential applications of the copper-based formulations, we elected to measure their ability to decontaminate UMF cloths which had been used to remove MRSA, ACCB or C. difficile spores from boards with laminate surfaces. As shown in Table 3, the UMF cloths completely removed the bacteria and spores from the boards whether they were wetted with water (control) or copper-based formulations (150 ppm). Eluent samples from the control UMF cloths contained as many bacterial and spore cfu as were initially used to contaminate the boards, showing that the organisms survived the overnight storage. In contrast, no cfu of MRSA, ACCB or C. difficile were recovered from any of the UMF cloths treated with the copper-based formulations. No background contamination of the boards was detected by contact plating (Table 3).
Discussion
The results presented here show that all three copper-based formulations demonstrate considerable bactericidal activity at a concentration of 1 ppm. Interestingly, the copper–based formulations were equally active against growing bacteria and bacteria in stationary phase (Tables 1 and 2), suggesting a mechanism of action which does not rely on bacterial metabolic processes involved in cell cycling but rather a cytotoxic effect on the bacterial cells.
In time–kill studies with ACCB and MRSA, we observed that all three copper-based formulations (150 ppm) killed both strains within 1–2 h, while equivalent concentrations of the components of these products, copper sulphate and the three inorganic binders, had little effect on bacterial growth and were equivalent to control cultures at 6–16 h (Figure 1). At a concentration of 20 ppm, the three copper-based compounds also completely inhibited colony formation of ACCB and MRSA, but this required 16 h of exposure (data not shown).
The mechanism by which the inorganic binders so effectively enhance copper's biocidal activity is currently under investigation. Preliminary experiments using scanning electron microscopy indicate that exposure of ACCB to CuAL42 (150 ppm) for 20 min causes extensive membrane ‘blebbing’.
The results shown in Figure 2 demonstrate equivalent toxicity of copper for two different human cell lines, whether it is present as the sulphate salt or formulated with the inorganic binders. These results with these human cells are in distinct contrast to the bacterial time–kill results, as discussed earlier. We conclude that the three copper-based formulations used in this study possess selective toxicity to bacteria as opposed to human cells. This is in addition to the recognized ability of eukaryotic cells to withstand copper toxicity through well characterized, often highly complex mechanisms of intracellular protein-dependent coordination, chelation and transport, to well evolved extracellular binding pathways in multicellular higher organisms.9 Notwithstanding this, enhancement of biocidal activity allows for bacterial killing at much lower concentrations of copper.
It is well established that UMF cloths are very effective for cleaning and for removing microorganisms from surfaces.17,18 In a healthcare environment, however, this can lead to an accumulation of heavily contaminated cloths. Since we found that the copper-based formulations were very effective biocides against a variety of bacteria, we decided to investigate their potential use in the decontamination of UMF cloths.
Table 3 shows that UMF cloths wetted with water or any one of the copper-based formulations (150 ppm) are very effective at removing MRSA, ACCB and C. difficile spores from laminate surfaces. Storage of the cloths for 16 h at room temperature did not affect survival of the bacteria or spores, since all of the bacteria and spores used to contaminate the board surfaces were recovered as cfu from the UMF cloths wetted with water. In contrast, the UMF cloths wetted with any of the copper-based formulations contained no viable MRSA, ACCB or C. difficile spores since no cfu developed from the eluents of these cloths. These results suggest that pre-treatment of UMF cloths with any of these copper-based formulations would reduce the risk of inadvertent healthcare-related contamination.
Our results demonstrate that all three copper-based formulations are potently biocidal for several organisms highly relevant to HAI. The biocidal activity of copper has been appreciated for several thousands of years and it continues to be used in Bordeaux mixture for the protection of late season harvest grapes from fungi, such as Coniella petrakii and Guignardia bidwellii.19 These biocidal properties have prompted further work to adapt copper for use in healthcare environments. Thus, Keevil et al.12–14 have reported on the biocidal properties of solid copper surfaces for M tuberculosis, MRSA and Escherichia coli, and a novel technology for weaving copper into textiles now also exists.20
Several mechanisms and target sites of action have been proposed for this very broad spectrum of biocidal activity. Firstly, copper is a highly redox-active metallo-ion that promotes membrane lipid peroxidation which in turn damages bacterial plasma membrane integrity.21 Secondly, copper binds and disorders helical DNA by engaging this structure at two different binding sites, explaining its activity against a wide range of viruses.22 Thirdly, copper facilitates free radical-mediated degradation of proteins, which includes on the one hand oxidation of specific amino acid residues, and on the other hand degradation of sulphydryl moieties.23 It has been reported, however, that copper does not cause significant oxidative damage to E. coli DNA.24 We are currently investigating the relative contribution of each of these mechanisms to our results. In this respect, we have found using flow cytometry that CuAL42 causes a rapid collapse of ACCB membrane potential.
Copper added to animal feed can promote resistance.25 Accordingly, we are undertaking experiments examining the potential of these copper formulations to induce resistance.
Regardless of these currently incompletely understood biocidal mechanisms, we believe that these three copper-based formulations may have several important applications in mitigating against HAIs. This would seem all the more relevant in view of their demonstrated activity against C. difficile spores, and the high probability that they have virucidal activity.22
Acknowledgements
Remedy Research Ltd contracted and funded this research on its proprietary and patented copper-based formulations.
Transparency declarations
S. S. H. and T. J. H. are employees of Remedy Research Ltd. S. S. H. owns shares in Remedy Research Ltd.