-
PDF
- Split View
-
Views
-
Cite
Cite
Mercedes Gonzalez Moreno, Andrej Trampuz, Mariagrazia Di Luca, Synergistic antibiotic activity against planktonic and biofilm-embedded Streptococcus agalactiae, Streptococcus pyogenes and Streptococcus oralis, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 11, November 2017, Pages 3085–3092, https://doi.org/10.1093/jac/dkx265
Close - Share Icon Share
Abstract
To determine the antimicrobial activity against streptococcal biofilm in species mostly isolated from implant-associated infections and examine the effect of enzyme treatment of biofilm on the antimicrobial activity of different antibiotics.
The activities of fosfomycin, rifampicin, benzylpenicillin, daptomycin, gentamicin, levofloxacin, proteinase K and their combinations on planktonic and/or biofilm-embedded standard laboratory strains of Streptococcus agalactiae, Streptococcus pyogenes and Streptococcus oralis were investigated in vitro by standard methods and isothermal microcalorimetry.
MIC values obtained for the tested antimicrobials against planktonic bacteria ranged from 0.016 to 128 mg/L for the three species tested. Higher antibiotic concentrations were usually required to reduce biofilm in comparison with planktonic bacteria, with the exception of gentamicin, for which similar concentrations (4–16 mg/L) exerted an effect on both planktonic and biofilm cells. A synergistic effect against the streptococcal biofilm of the three species was observed when gentamicin was combined with benzylpenicillin or with rifampicin. Moreover, antibiotic concentrations comparable to the MIC observed against planktonic cells induced a strong reduction of viable bacteria in proteinase K pre-treated biofilm.
This study shows that the combination of gentamicin with either benzylpenicillin or rifampicin exerts a synergistic effect against biofilms produced by the tested streptococci strains in vitro. Our results also suggest that coupling a dispersal agent with conventional antibiotics may facilitate their access to the bacteria within the biofilm. In vivo and clinical studies are needed in order to confirm whether such a strategy may be effective in the treatment of implant-associated infections caused by streptococci.
Introduction
Implant-associated infections remain a major complication in modern surgical procedures.1 Infections involving medical implants present unique challenges because bacteria adherent to a surface can form a complex structure, known as the matrix, which protects the organisms from the host immune system and from antibiotic activity.1 Current therapeutic approaches, such as radical surgical debridement and prolonged antibiotic therapy, often result in high morbidity for the patients and in significant increase in healthcare costs.1
Streptococci account for ∼10% of prosthetic joint infections (PJIs),2 and the frequency is expected to rise further due to the general increase in streptococcal infections in adults.3,4 Streptococci are most frequently isolated in haematogenous PJI and originate from the oral cavity after dental manipulation or remote infections of the respiratory, abdominal or urogenital system.5 There is no universal agreement on the best choice of antibiotic therapy against streptococcal biofilms. In addition, the reported treatment outcomes of streptococcal PJIs are often contradictory.6,7 In general, intravenous benzylpenicillin or ceftriaxone is recommended for 2–4 weeks, followed by oral amoxicillin. Some authors recommend addition of rifampicin to amoxicillin for the treatment of rifampicin-susceptible streptococcal PJI.8 Antibiotic combinations were tested against Streptococcus spp. isolated from blood or throat swabs, including rifampicin.9,10 However, it remains unclear whether the addition of amoxicillin to rifampicin is superior for eradication of streptococcal biofilms compared with amoxicillin alone.
Here, we have investigated the in vitro activity of fosfomycin, rifampicin, benzylpenicillin, daptomycin, gentamicin, levofloxacin and their combinations against planktonic and biofilm S.agalactiae, S.pyogenes and S.oralis. Additionally, we evaluated whether the enzymatic treatment of S. agalactiae, S. pyogenes and S. oralis biofilms with proteinase K could affect the susceptibility of the bacteria to different antibiotics.
Materials and methods
Bacterial strains
S. agalactiae (ATCC 13813), S. oralis (ATCC 35037) and S. pyogenes (ATCC 19615) strains were used for this study. Bacteria were stored using a cryovial bead preservation system (Microbank; Pro-Lab Diagnostics, Canada) at −80°C. A standard 5 × 105 cfu/mL final inoculum was used for all the antimicrobial tests.
Etest
Etest (bioMerieux SA, France) was performed in Mueller–Hinton agar (MHA) with 5% sheep blood (Becton, Dickinson and Company, Germany) as suggested by the Etest Application Guide. After incubation at 37°C with 5% CO2 for 24 h, the MIC was determined as the concentration at which the inhibition ellipse intersected the scale of the strip.
Antimicrobial assay by microcalorimetry and sonication/colony counting
The measurements were performed in an isothermal 48-channel batch calorimeter (thermal activity monitor, model 3102 TAM III; TA Instruments, USA), as described in previous studies.11,12 Briefly, for the evaluation of the antimicrobial activity versus planktonic bacteria, 5 × 105 cfu/mL cells were inoculated in microcalorimetry ampoules containing CAMHB (Becton, Dickinson and Company, USA) supplemented with 2.5% lysed horse blood (LHB) (TCS Bioscience, UK), in the presence of 2-fold serial dilutions of antibiotics. The heat production was recorded during 24 h and the results were plotted as heat flow (μW) versus time. For anti-biofilm tests, 1.0 McFarland bacterial suspension was diluted 1:10 in CAMHB/2.5% LHB and incubated in the presence of porous sintered glass beads (ROBU®, Germany) at 37°C for 24 h. The ratio between beads and diluted bacterial suspension was 1 bead:1 mL. Then, beads were washed three times with sterile 0.9% saline and exposed to 2-fold serial dilutions of antibiotics in 1 mL of CAMHB/2.5% LHB. A growth control, in which the biofilm was incubated in the absence of treatment, was also included. After a 24 h incubation at 37°C, beads were rinsed three times with sterile 0.9% saline and placed in microcalorimetry ampoules containing 3 mL of CAMHB/2.5% LHB, in order to monitor the heat produced by the biofilm cells still viable following the antibiotic treatment. Sterile beads were also included as a negative control. The heat production was measured at 37°C for 48 h. For both the planktonic and biofilm assays, the growth medium for testing daptomycin and fosfomycin was supplemented with calcium chloride (40 mg/L) and glucose 6-phosphate (25 mg/L), respectively. All experiments were performed in triplicate.
The minimum heat inhibitory concentration (MHIC) for planktonic bacteria was defined as the lowest antimicrobial concentration inhibiting growth-related heat production after 24 h, as previously reported.13 The minimum biofilm bactericidal concentration (MBBC) measured by calorimetry was defined as the lowest antimicrobial concentration leading to lack of heat production related to the absence of bacterial re-growth after 48 h of incubation in the microcalorimeter. The evaluation of the synergistic effect of antibiotic combinations against planktonic and biofilm streptococci was performed by microcalorimetry, as described above. The minimum biofilm eradicating concentration (MBEC) of antibiotic combinations, defined as the lowest concentration of antibiotic required to eradicate the biofilm (0 cfu/bead on plate counts), was evaluated by cfu counting of the sonicated beads, as previously described.14 The synergistic activity was evaluated by calculation of the fractional inhibitory concentration index (FICI). The two antimicrobial agents in combination exert synergistic, indifferent or antagonistic effect if FICI is ≤0.5, >0.5 to ≤4, or >4, respectively.15 FICI for all the combinations was determined as [(minimum concentration of drug A in combination) / (minimum concentration of drug A alone)] + [(minimum concentration of drug B in combination) / (minimum concentration of drug B alone)]. The obtained MHICs, MBBC and MBEC values were used to calculate the FICI of antibiotic combinations related to: (i) inhibition of planktonic bacteria growth; (ii) bactericidal activity versus streptococcal sessile cells; and (iii) eradicating activity against streptococcal biofilms, respectively.
Evaluation of antimicrobial activity against biofilms pre-treated with proteinase K
Biofilms aged 24 h, which were grown on the beads as previously described, were incubated in 500 μL of CAMHB/2.5% LHB containing proteinase K (New England Biolabs, USA) at final concentrations of 25, 50 and 100 mg/L at 37°C, according to the protocol described by Doern et al.16 Following 1 h of incubation, 500 μL of CAMHB/2.5% LHB containing increasing concentrations of antibiotics was added, and the samples were incubated at 37°C for a further 24 h. The beads were then washed three times with sterile 0.9% saline, placed in ampoules containing 3 mL of CAMHB/2.5% LHB and analysed by isothermal microcalorimetry for 48 h.
Results
Antimicrobial susceptibility testing
Table 1 summarizes the susceptibility of S. agalactiae, S. pyogenes and S. oralis to different antibiotics by Etest and microcalorimetry (Figures S1–S3 available as Supplementary data at JAC Online).
Antimicrobial susceptibility of Streptococcus spp. by Etest and microcalorimetry in planktonic and biofilm forms
| . | MIC (mg/L) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | S. agalactiae (ATCC 13813) . | S. pyogenes (ATCC 19615) . | S. oralis (ATCC 35037) . | ||||||
| . | . | MHIC/MBBC . | . | MHIC/MBBC . | . | MHIC/MBBC . | |||
| Antimicrobial . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . |
| Fosfomycin | 64 | 64 | >1024 | 64 | 128 | >1024 | 96 | 128 | >1024 |
| Rifampicin | 0.064 | 0.128 | 1024 | 0.023 | 0.064 | 256 | 0.125 | 0.128 | 512 |
| Benzylpenicillin | 0.047 | 0.064 | 64 | 0.016 | 0.016 | 32 | 0.064 | 0.064 | 64 |
| Daptomycin | 0.25 | 0.5 | 64 | 0.23 | 0.125 | 16 | 0.75 | 2 | 1024 |
| Gentamicin | 3 | 4 | 8 | 1 | 4 | 4 | 4 | 8 | 16 |
| Levofloxacin | 0.75 | 1 | 1024 | 0.38 | 0.5 | 1024 | 1.5 | 2 | 1024 |
| . | MIC (mg/L) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | S. agalactiae (ATCC 13813) . | S. pyogenes (ATCC 19615) . | S. oralis (ATCC 35037) . | ||||||
| . | . | MHIC/MBBC . | . | MHIC/MBBC . | . | MHIC/MBBC . | |||
| Antimicrobial . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . |
| Fosfomycin | 64 | 64 | >1024 | 64 | 128 | >1024 | 96 | 128 | >1024 |
| Rifampicin | 0.064 | 0.128 | 1024 | 0.023 | 0.064 | 256 | 0.125 | 0.128 | 512 |
| Benzylpenicillin | 0.047 | 0.064 | 64 | 0.016 | 0.016 | 32 | 0.064 | 0.064 | 64 |
| Daptomycin | 0.25 | 0.5 | 64 | 0.23 | 0.125 | 16 | 0.75 | 2 | 1024 |
| Gentamicin | 3 | 4 | 8 | 1 | 4 | 4 | 4 | 8 | 16 |
| Levofloxacin | 0.75 | 1 | 1024 | 0.38 | 0.5 | 1024 | 1.5 | 2 | 1024 |
Antimicrobial susceptibility of Streptococcus spp. by Etest and microcalorimetry in planktonic and biofilm forms
| . | MIC (mg/L) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | S. agalactiae (ATCC 13813) . | S. pyogenes (ATCC 19615) . | S. oralis (ATCC 35037) . | ||||||
| . | . | MHIC/MBBC . | . | MHIC/MBBC . | . | MHIC/MBBC . | |||
| Antimicrobial . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . |
| Fosfomycin | 64 | 64 | >1024 | 64 | 128 | >1024 | 96 | 128 | >1024 |
| Rifampicin | 0.064 | 0.128 | 1024 | 0.023 | 0.064 | 256 | 0.125 | 0.128 | 512 |
| Benzylpenicillin | 0.047 | 0.064 | 64 | 0.016 | 0.016 | 32 | 0.064 | 0.064 | 64 |
| Daptomycin | 0.25 | 0.5 | 64 | 0.23 | 0.125 | 16 | 0.75 | 2 | 1024 |
| Gentamicin | 3 | 4 | 8 | 1 | 4 | 4 | 4 | 8 | 16 |
| Levofloxacin | 0.75 | 1 | 1024 | 0.38 | 0.5 | 1024 | 1.5 | 2 | 1024 |
| . | MIC (mg/L) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | S. agalactiae (ATCC 13813) . | S. pyogenes (ATCC 19615) . | S. oralis (ATCC 35037) . | ||||||
| . | . | MHIC/MBBC . | . | MHIC/MBBC . | . | MHIC/MBBC . | |||
| Antimicrobial . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . | Etest . | planktonic . | biofilm . |
| Fosfomycin | 64 | 64 | >1024 | 64 | 128 | >1024 | 96 | 128 | >1024 |
| Rifampicin | 0.064 | 0.128 | 1024 | 0.023 | 0.064 | 256 | 0.125 | 0.128 | 512 |
| Benzylpenicillin | 0.047 | 0.064 | 64 | 0.016 | 0.016 | 32 | 0.064 | 0.064 | 64 |
| Daptomycin | 0.25 | 0.5 | 64 | 0.23 | 0.125 | 16 | 0.75 | 2 | 1024 |
| Gentamicin | 3 | 4 | 8 | 1 | 4 | 4 | 4 | 8 | 16 |
| Levofloxacin | 0.75 | 1 | 1024 | 0.38 | 0.5 | 1024 | 1.5 | 2 | 1024 |
The Etest analysis showed that S. agalactiae, S. pyogenes and S. oralis strains were susceptible to benzylpenicillin, according to the EUCAST breakpoint recommendations. In addition, S. agalactiae and S. pyogenes were also susceptible to levofloxacin, daptomycin and rifampicin.17 In all these cases, the MHICs obtained by calorimetry for planktonic bacteria were consistent with Etest MIC values, with the only exception of rifampicin. Indeed, although S. agalactiae was susceptible to rifampicin based on the Etest method, the MHIC value was one dilution higher than the MIC, and the strain was intermediate according to microcalorimetry analysis.
The analysis of the susceptibility of S. oralis to rifampicin, daptomycin and levofloxacin was less immediate due to the lack of indication on such breakpoint values for viridans group streptococci by EUCAST recommendations. The daptomycin breakpoint for S. oralis was considered to be 1 mg/L based on the work of Streit et al.18 As a result, this strain was found susceptible to daptomycin according to the Etest method, but resistant based on calorimetric analysis for planktonic bacteria. Based on the literature,19 we considered an MIC <2 mg/L as reference for the susceptibility of S. oralis to rifampicin; in this case both the Etest and the calorimetric analysis identified this strain as susceptible. S. oralis was considered susceptible to levofloxacin when the MIC and MHIC values were found to be ≤2 mg/L, following the CLSI recommendations for viridans group streptococci.20 For the evaluation of the susceptibility of Streptococcus spp. to fosfomycin we used the breakpoints for Enterococcus spp. proposed by the CLSI, a practice that was also followed by authors of similar studies.21,22 Both the Etest and the calorimetric approach identified S. agalactiae and S. oralis as susceptible and intermediate to fosfomycin, respectively. However, the two techniques provided a different outcome in the case of S. pyogenes, which was found to be susceptible or intermediate when the Etest and microcalorimetry were used, respectively. Susceptibility testing on these strains is not usually recommended for gentamicin, although it can be used to screen for high-level aminoglycoside resistance (MIC >128 mg/L) in viridans group streptococci. In our study, S. oralis exhibited a low level of intrinsic gentamicin resistance.
Activity of antibiotics against S. agalactiae, S. pyogenes and S. oralis biofilms
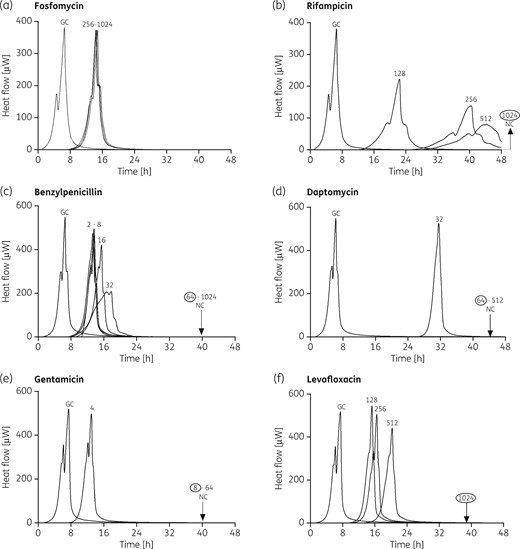
The antimicrobial activity of different antibiotics was evaluated by microcalorimetry measuring: (i) the complete inhibition of heat production, correlating with a strong reduction of viable bacterial cells or with the complete eradication of biofilm; and (ii) the delay/decrease in the heat flow peak compared with the growth control. The three strains tested were susceptible only to higher concentrations of the tested antibiotics (mostly ranging from 64 to 1024 mg/L) when grown as biofilms (Table 1 and Figure 1 and Figures S4 and S5) if compared with the MHIC value obtained for planktonic bacteria (Table 1 and Figures S1–S3). Fosfomycin, which was tested up to 1024 mg/L, showed no inhibition of heat flow production (Table 1, Figure 1a and Figures S4a and S5a), indicating that complete eradication could not be achieved, despite the presence of high concentrations of antibiotic. However, 256 mg/L fosfomycin tested against S. agalactiae and S. oralis biofilms (Figure 1a and Figure S5a) caused a delay of ∼8 h in the production of heat, compared with untreated controls, suggesting that the treatment could result in a reduction in the number of viable sessile cells, although not in complete biofilm eradication. In the case of S. pyogenes, a similar concentration of fosfomycin was able to reduce the heat produced by the cells by 50% (Figure S4a). Moreover, biofilm cells of Streptococcus group A/B and viridans streptococci showed a different response to treatment with daptomycin, as already observed in the case of planktonic cells. Indeed, daptomycin showed lower activity against S. oralis biofilm (MBBC = 1024 mg/L) compared with S. agalactiae (MBBC = 64 mg/L) and S. pyogenes (MBBC = 16 mg/L) (Figures S5d, 1d and S4d, respectively). The three streptococcal strains shared comparable behaviour following treatment of their biofilms with rifampicin (Figures 1b, S4b and S5b), benzylpenicillin (Figures 1c, S4c and S5c), gentamicin (Figures 1e, S4e and S5e) and levofloxacin (Figures 1f, S4f and S5f).
Microcalorimetry analysis of S. agalactiae (ATCC 13813) biofilm treated with different antibiotic concentrations. Each curve shows the heat produced by viable bacteria present in the biofilm after 24 h of antibiotic treatment or no treatment. Numbers represent concentrations (in mg/L) of fosfomycin (a), rifampicin (b), benzylpenicillin (c), daptomycin (d), gentamicin (e) and levofloxacin (f). Circled values represent the MBBC, defined as the lowest antimicrobial concentration leading to absence of bacterial regrowth after 48 h. GC, growth control; NC, negative control.
A concentration-dependent activity was observed in the case of rifampicin and benzylpenicillin, in which the delay in heat production at increasing concentrations was linked to a decrease in the heat flow peak. Notably, gentamicin was the most active antibiotic against the biofilm produced by the three streptococcal species. In this case, the values of MBBC ranged from 4 to 16 mg/L (Figures 1e, S4e and S5e and Table 1), and they were similar to those obtained for planktonic bacteria (Figures S1e, S2e and S3e and Table 1).
Antibiotic activity after pre-treatment with proteinase K against S. agalactiae, S. pyogenes and S. oralis biofilms
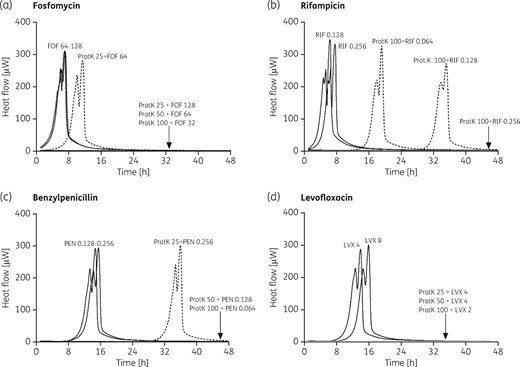
The presence of the exopolymeric matrix has long been held to have a role in limiting the penetration of antimicrobials to cells deeply embedded within biofilms. Thus, disruption of such an essential component could revert the physical tolerance of the bacteria in the biofilm. To test this hypothesis, we treated S. oralis, S. agalactiae and S. pyogenes biofilms for 1 h with 25, 50 and 100 mg/L proteinase K, and then we evaluated the susceptibility of sessile cells to different antibiotics. As shown in Figure 2, following incubation with proteinase K, fosfomycin, rifampicin, benzylpenicillin and levofloxacin all caused a complete suppression of heat production at concentrations lower than those required to exert the same effect in the absence of enzymatic treatment (32, 0.256, 0.064 and 2 mg/L, respectively). Antibiotics at these concentrations showed little or no effect against biofilms not treated with proteinase K. In addition, total inhibition of heat production was also observed when biofilm was incubated with 128 mg/L fosfomycin and 4 mg/L levofloxacin, following a pre-treatment with lower concentration of proteinase K (25 mg/L). Similar results were obtained when the effect of the treatment with proteinase K was evaluated on S. agalactiae and S. pyogenes biofilms (Figure S6). Control experiments performed by incubating S. oralis biofilms with 100 mg/L proteinase K confirmed that the enzymatic treatment does not affect bacterial viability (Figure S7).
Evaluation of antimicrobial activity of fosfomycin (a), rifampicin (b), benzylpenicillin (c) and levofloxacin (d) on enzymatically treated S. oralis biofilms by microcalorimetry. Numbers represent concentrations (in mg/L). ProtK, proteinase K; FOF, fosfomycin; RIF, rifampicin; PEN, benzylpenicillin; LVX, levofloxacin. Bacterial growth-related heat was suppressed by sub-inhibitory concentrations of the antibiotic when combined with biofilms treated with proteinase K at different concentrations (25 mg/L, 50 mg/L and 100 mg/L).
Synergistic effect of different combinations of antibiotics against S. agalactiae, S. pyogenes and S. oralis by microcalorimetry and/or colony counting
Different combinations of antibiotics with benzylpenicillin and rifampicin were tested against S. oralis biofilm by isothermal microcalorimetry. The FICI values, calculated taking into account the MBBC of the antibiotics (FICIMBBC), are showed in Table 2.
MBBC and FICIMBBC of antibiotic combinations against S. oralis biofilm evaluated by microcalorimetry
| Antibiotic combination . | MBBC (mg/L) . | FICIMBBC (interpretation) . |
|---|---|---|
| Benzylpenicillin + fosfomycin | >32+1024 | >1 (NI) |
| Benzylpenicillin + rifampicin | 32+64 | 0.625 (NI) |
| Benzylpenicillin + daptomycin | 4+512 | 0.563 (NI) |
| Benzylpenicillin + gentamicin | 4+1 | 0.125 (S) |
| Benzylpenicillin + levofloxacin | >32+512 | >1 (NI) |
| Rifampicin + fosfomycin | >526+1024 | >1 (NI) |
| Rifampicin + daptomycin | 256+512 | 1 (NI) |
| Rifampicin + gentamicin | 16+2 | 0.156 (S) |
| Rifampicin + levofloxacin | 256+256 | 0.75 (NI) |
| Antibiotic combination . | MBBC (mg/L) . | FICIMBBC (interpretation) . |
|---|---|---|
| Benzylpenicillin + fosfomycin | >32+1024 | >1 (NI) |
| Benzylpenicillin + rifampicin | 32+64 | 0.625 (NI) |
| Benzylpenicillin + daptomycin | 4+512 | 0.563 (NI) |
| Benzylpenicillin + gentamicin | 4+1 | 0.125 (S) |
| Benzylpenicillin + levofloxacin | >32+512 | >1 (NI) |
| Rifampicin + fosfomycin | >526+1024 | >1 (NI) |
| Rifampicin + daptomycin | 256+512 | 1 (NI) |
| Rifampicin + gentamicin | 16+2 | 0.156 (S) |
| Rifampicin + levofloxacin | 256+256 | 0.75 (NI) |
NI, no interaction; S, synergism.
MBBC and FICIMBBC of antibiotic combinations against S. oralis biofilm evaluated by microcalorimetry
| Antibiotic combination . | MBBC (mg/L) . | FICIMBBC (interpretation) . |
|---|---|---|
| Benzylpenicillin + fosfomycin | >32+1024 | >1 (NI) |
| Benzylpenicillin + rifampicin | 32+64 | 0.625 (NI) |
| Benzylpenicillin + daptomycin | 4+512 | 0.563 (NI) |
| Benzylpenicillin + gentamicin | 4+1 | 0.125 (S) |
| Benzylpenicillin + levofloxacin | >32+512 | >1 (NI) |
| Rifampicin + fosfomycin | >526+1024 | >1 (NI) |
| Rifampicin + daptomycin | 256+512 | 1 (NI) |
| Rifampicin + gentamicin | 16+2 | 0.156 (S) |
| Rifampicin + levofloxacin | 256+256 | 0.75 (NI) |
| Antibiotic combination . | MBBC (mg/L) . | FICIMBBC (interpretation) . |
|---|---|---|
| Benzylpenicillin + fosfomycin | >32+1024 | >1 (NI) |
| Benzylpenicillin + rifampicin | 32+64 | 0.625 (NI) |
| Benzylpenicillin + daptomycin | 4+512 | 0.563 (NI) |
| Benzylpenicillin + gentamicin | 4+1 | 0.125 (S) |
| Benzylpenicillin + levofloxacin | >32+512 | >1 (NI) |
| Rifampicin + fosfomycin | >526+1024 | >1 (NI) |
| Rifampicin + daptomycin | 256+512 | 1 (NI) |
| Rifampicin + gentamicin | 16+2 | 0.156 (S) |
| Rifampicin + levofloxacin | 256+256 | 0.75 (NI) |
NI, no interaction; S, synergism.
Gentamicin in combination with either benzylpenicillin or rifampicin showed a synergistic effect against S. oralis biofilm (Figures S8b and S9b), whereas the same combinations did not show any interaction against planktonic bacteria (FICI of 0.625 and 0.531 respectively; Figures S8a and S9a). In contrast, benzylpenicillin/rifampicin and rifampicin/fosfomycin combinations were synergistic against planktonic S. oralis (Figures S10a and S11a) but not against biofilm (Figures S10b and S11b). All the other combinations tested on biofilms showed no interaction (Table 2). In order to evaluate whether the combinations gentamicin/benzylpenicillin and gentamicin/rifampicin could effect complete eradication of the biofilm on the beads, we tested different concentrations of these antibiotics against S. agalactiae, S. pyogenes and S. oralis. The sonication fluids obtained from the beads were plated and the cfu counted. The eradicating concentration and the FICI for biofilm eradication (FICIMBEC) are reported in Table 3. Both gentamicin/benzylpenicillin and gentamicin/rifampicin combinations showed a synergistic effect (with FICI ≤0.258) against the three tested species.
MBEC and FICIMBEC of antibiotic combinations against Streptococcus spp. biofilms evaluated by sonication and plating
| Antimicrobial . | S. agalactiae . | S. pyogenes . | S. oralis . | |||
|---|---|---|---|---|---|---|
| (ATCC 13813) . | (ATCC 19615) . | (ATCC 35037) . | ||||
| MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | |
| Rifampicin | 2048 | 512 | 2048 | |||
| Benzylpenicillin | 2048 | 512 | >2048 | |||
| Gentamicin | 8 | 4 | 32 | |||
| Rifampicin + gentamicin | 8+1 | 0.129 (S) | ≤4+1 | ≤0.258 (S) | 16+2 | 0.070 (S) |
| Benzylpenicillin + gentamicin | 4+0.5 | 0.064 (S) | ≤2+0.25 | ≤0.066 (S) | ≤4+0.5 | <0.018 (S) |
| Antimicrobial . | S. agalactiae . | S. pyogenes . | S. oralis . | |||
|---|---|---|---|---|---|---|
| (ATCC 13813) . | (ATCC 19615) . | (ATCC 35037) . | ||||
| MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | |
| Rifampicin | 2048 | 512 | 2048 | |||
| Benzylpenicillin | 2048 | 512 | >2048 | |||
| Gentamicin | 8 | 4 | 32 | |||
| Rifampicin + gentamicin | 8+1 | 0.129 (S) | ≤4+1 | ≤0.258 (S) | 16+2 | 0.070 (S) |
| Benzylpenicillin + gentamicin | 4+0.5 | 0.064 (S) | ≤2+0.25 | ≤0.066 (S) | ≤4+0.5 | <0.018 (S) |
S, synergism.
MBEC and FICIMBEC of antibiotic combinations against Streptococcus spp. biofilms evaluated by sonication and plating
| Antimicrobial . | S. agalactiae . | S. pyogenes . | S. oralis . | |||
|---|---|---|---|---|---|---|
| (ATCC 13813) . | (ATCC 19615) . | (ATCC 35037) . | ||||
| MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | |
| Rifampicin | 2048 | 512 | 2048 | |||
| Benzylpenicillin | 2048 | 512 | >2048 | |||
| Gentamicin | 8 | 4 | 32 | |||
| Rifampicin + gentamicin | 8+1 | 0.129 (S) | ≤4+1 | ≤0.258 (S) | 16+2 | 0.070 (S) |
| Benzylpenicillin + gentamicin | 4+0.5 | 0.064 (S) | ≤2+0.25 | ≤0.066 (S) | ≤4+0.5 | <0.018 (S) |
| Antimicrobial . | S. agalactiae . | S. pyogenes . | S. oralis . | |||
|---|---|---|---|---|---|---|
| (ATCC 13813) . | (ATCC 19615) . | (ATCC 35037) . | ||||
| MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | MBEC (mg/L) . | FICIMBEC . | |
| Rifampicin | 2048 | 512 | 2048 | |||
| Benzylpenicillin | 2048 | 512 | >2048 | |||
| Gentamicin | 8 | 4 | 32 | |||
| Rifampicin + gentamicin | 8+1 | 0.129 (S) | ≤4+1 | ≤0.258 (S) | 16+2 | 0.070 (S) |
| Benzylpenicillin + gentamicin | 4+0.5 | 0.064 (S) | ≤2+0.25 | ≤0.066 (S) | ≤4+0.5 | <0.018 (S) |
S, synergism.
Discussion
The management of streptococcal implant-associated infections is challenging due to the lack of evidence for an effective therapy against biofilms. Few studies have investigated the antimicrobial activity in vitro and in vivo on streptococcal PJIs.23 Therefore, we compared the in vitro activities of different classes of antibiotics, alone and in combinations, against planktonic and biofilm laboratory standard strains of S. agalactiae, S. pyogenes and S. oralis, as representative of three different streptococcal groups causing PJIs.7
Microcalorimetry was used for the analysis of the antibiotic activity. This highly sensitive method was previously widely used for the evaluation of antimicrobial susceptibility both in planktonic and biofilm form.12,13 Similar values of MIC were usually obtained by microcalorimetry when compared with Etest. Nonetheless, a 0.5-fold difference in the MHIC of rifampicin against S. agalactiae tested by microcalorimetry, as compared with the Etest MIC, relocated the susceptibility of this strain to an intermediate level. This may be due to the fact that in this case the MIC corresponded exactly to the breakpoint value, so that a difference of one dilution between MIC and MHIC observed with the two techniques was enough to result in a different definition of the planktonic S. agalactiae phenotype. Consistent results were obtained also when the synergism activity of antibiotic combinations was tested by calorimetry compared with colony counting. Therefore, microcalorimetry represents a reliable method that can be applied to assess the effect of antimicrobials, or their combinations, on the viability of a microorganism. This technique proves even more helpful for conducting tests on biofilms, due to the possibility of analysing sessile bacterial growth on different biomaterials.
As previously reported in the literature for many microorganisms,12 we observed that, for all tested antibiotics, considerably higher concentrations (≥125-fold higher) were necessary to eradicate streptococcal biofilms in comparison with the killing of planktonic bacteria; however, these concentrations cannot be reached in clinical practice. Among all the antibiotics tested against streptococcal sessile cells, only gentamicin was able to completely eradicate the biofilm at low MBEC (4–16 mg/L).
The combination of antibiotics with dispersal agents appears to be a promising alternative strategy to treat biofilm-forming infections. Indeed, an increased antibiotic susceptibility has been observed with most dispersal agents, including many industrially produced enzymes, such as dispersin B, proteinase K and DNaseI.24–26 In our study we also observed an increased susceptibility of enzymatically treated biofilm to the subsequent action of four different antibiotics. Indeed, a 1 h treatment of a 24 h-old streptococcal biofilm with proteinase K led to decreases in the MBBCs of fosfomycin, rifampicin, benzylpenicillin and levofloxacin to values that were comparable to the MHICs obtained for planktonic bacteria. Although this preliminary finding confirms the hypothesis that bacteria detached from the matrix re-enter in a planktonic state and regain normal antibiotic susceptibility,25 the actual suitability of the use of proteinase K as an adjuvant in the treatment of biofilm-related infections remains to be evaluated. Indeed, adverse effects on host extracellular matrix proteins, or even the risk of septic shock, cannot be excluded in an in vivo physiological environment. Nonetheless, our results could be seen as a proof of concept encouraging the use of this experimental setting to further expand studies to different potential dispersal agents, in order to find a combination treatment that could be effective and safe at the same time.
The main issue with the currently used antibiotic combinations is that they lack antibiofilm activity against streptococcal biofilms. Benzylpenicillin is normally the antibiotic of choice for the treatment of streptococcal implant-associated infections.2 Although the use of rifampicin-based combinations was described in a few case reports related to PJIs caused by streptococci,27,28 in the absence of clinical evidence the effect of the use of this antibiotic in combination remains unclear. Thus, in our study we tested combinations of benzylpenicillin with rifampicin and each of these antibiotics with fosfomycin, daptomycin, gentamicin or levofloxacin at sub-inhibitory concentrations to evaluate synergistic effects against planktonic and biofilm S. oralis. When rifampicin was used in combination with benzylpenicillin, a synergistic effect was observed on planktonic S. oralis, but not on biofilm bacteria. This result is consistent with the retrospective study of Akgun et al.,29 in which no differences were found in the treatment outcome of biofilm-related PJIs caused by streptococci when the patients’ treatment regimen either included or did not include rifampicin.
On the other hand, when gentamicin was combined with benzylpenicillin or rifampicin, an improved antimicrobial activity was observed against biofilm S. oralis, but not against planktonic bacteria. This result reinforces the view that findings obtained on planktonic cells cannot be translated to biofilms. In a recent study, Allison et al.30 found that in the presence of specific metabolites (such as glucose) gentamicin rapidly killed Escherichiacoli and Staphylococcusaureus persister cells. Persister cells are a subpopulation within a biofilm that is phenotypically tolerant to antibiotics, thus remains viable over the course of drug exposure and repopulates the biofilm when the levels of antibiotic drop. It could be speculated that, while rifampicin or benzylpenicillin kills the majority of the cells in streptococcal biofilm with the exception of persister cells, gentamicin could selectively target them, resulting in complete biofilm eradication.
In addition, our data indicate that the combination of gentamicin/benzylpenicillin is active not only against S. oralis, but also against S. pyogenes and S. agalactiae. Based on clinical evidence, the intravenous administration of benzylpenicillin in combination with gentamicin for 2–4 weeks, followed by amoxicillin, was already suggested by Zimmerli et al.31 for the treatment of PJI due to S. agalactiae. Moreover, a recent study evaluated the in vitro activity of the penicillin/gentamicin combination against biofilm of group B streptococci. The results showed that after a 12 h treatment of biofilms produced by clinical isolates with 3 mg/L penicillin combined with 4 mg/L gentamicin the number of cfu in biofilm was reduced by >3 log units compared with the untreated control in one of the four tested strains.23 These data are in agreement with the results that we obtained for S. agalactiae.
Despite both in vivo32 and in vitro33 studies showing a synergistic effect when combining rifampicin and gentamicin against planktonic staphylococci, the activity of such an antibiotic combination had not been investigated in either planktonic or biofilm streptococci until now.
To the best of our knowledge, we demonstrated for the first time that the addition of gentamicin to rifampicin has a synergistic effect in eradicating the biofilm of the three tested streptococcal species.
The exact mechanism underlying the synergism of gentamicin with benzylpenicillin or rifampicin against streptococci remains to be elucidated, whereas for enterococci it has been proposed that combinations of either benzylpenicillin or ampicillin with gentamicin or streptomycin facilitate the intracellular uptake of the aminoglycoside, which causes the subsequent bactericidal effect against the enterococci.34 In contrast, in viridans group streptococci, benzylpenicillin had no effect on streptomycin uptake and a minimal effect on bactericidal rate when compared with either drug alone.35
In conclusion, this study suggests that the gentamicin/benzylpenicillin or gentamicin/rifampicin combinations display a synergistic effect against biofilms of streptococcal species in vitro. Moreover, the degradation of the protein component of the streptococcal biofilm matrix improves the antibiotic activity against sessile bacteria. Further in vivo and clinical studies are required to determine the potential treatment regimen based on benzylpenicillin/gentamicin or rifampicin/gentamicin combination for patients with implant-associated infections caused by streptococci.
Acknowledgements
We would like to thank Prof Arianna Tavanti, Dr Lisa Lombardi and Mr Matteo Ugolini for helpful discussion and Dr Colin Gerard Egan for revising the manuscript for English language.
This study was presented in part at the 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, 2016 (Poster P1705).
Funding
This work was supported by PRO-IMPLANT Foundation (www.pro-implant-foundation.org) in Berlin. InfectoPharm supported this study by an educational grant.
Transparency declarations
None to declare.
Supplementary data
Figures S1 to S11 appear as Supplementary data at JAC Online.