-
PDF
- Split View
-
Views
-
Cite
Cite
Fessessework Guale, Shahriar Shahreza, Jeffrey P. Walterscheid, Hsin-Hung Chen, Crystal Arndt, Anna T. Kelly, Ashraf Mozayani, Validation of LC–TOF-MS Screening for Drugs, Metabolites, and Collateral Compounds in Forensic Toxicology Specimens, Journal of Analytical Toxicology, Volume 37, Issue 1, January/February 2013, Pages 17–24, https://doi.org/10.1093/jat/bks084
Close - Share Icon Share
Abstract
Liquid chromatography time-of-flight mass spectrometry (LC–TOF-MS) analysis provides an expansive technique for identifying many known and unknown analytes. This study developed a screening method that utilizes automated solid-phase extraction to purify a wide array of analytes involving stimulants, benzodiazepines, opiates, muscle relaxants, hypnotics, antihistamines, antidepressants and newer synthetic “Spice/K2” cannabinoids and cathinone “bath salt” designer drugs. The extract was applied to LC–TOF-MS analysis, implementing a 13 min chromatography gradient with mobile phases of ammonium formate and methanol using positive mode electrospray. Several common drugs and metabolites can share the same mass and chemical formula among unrelated compounds, but they are structurally different. In this method, the LC–TOF-MS was able to resolve many isobaric compounds by accurate mass correlation within 15 ppm mass units and a narrow retention time interval of less than 10 s of separation. Drug recovery yields varied among spiked compounds, but resulted in overall robust area counts to deliver an average match score of 86 when compared to the retention time and mass of authentic standards. In summary, this method represents a rapid, enhanced screen for blood and urine specimens in postmortem, driving under the influence, and drug facilitated sexual assault forensic toxicology casework.
Introduction
The path of forensic toxicology analyses is dictated by findings from initial screens and investigative reports. These initial screens are often based on immunological assays or gas chromatography–mass spectrometry (GC–MS) library matches (1). Although these methods can provide a comprehensive view of the analytes involved in impairment or death, there are inherent shortcomings when new drugs become available (2). New antibodies must be developed, and there can be a false negative if the drug is susceptible to thermal decomposition during GC–MS acquisitions. Liquid chromatography–tandem mass spectrometry (LC–MS-MS) is a sensitive and specific instrument that can be programmed to detect hundreds to thousands of common drugs and poisons (3–5). However, liquid chromatography time-of-flight mass spectrometry (LC–TOF-MS) analysis provides an expansive technique for determining many known and unknown analyte possibilities (6, 7).
Toxicological screens are routinely performed by using immunoassays or GC–MS library matching. The immunoassay is a quick and affordable technique for identifying classes of drugs, but it cannot easily be adapted to new analytes without a great deal of biotechnology support. GC–MS has proven to be an excellent platform for the sensitive and selective detection of a wide range of drugs and poisons. However, there are known shortcomings in dealing with metabolites (8), thermolabile compounds or artifacts (9–11) and co-eluting chromatographic peaks (12).
Although it has been shown that LC–MS-MS technology overcomes many of these deficiencies and allows for extremely sensitive and selective detection of analytes, one must instruct the instrument to acquire data on specific ion transitions at particular collision energies. The instrument will seek what it is meant to scan and dismiss ions that fall outside its parameters (13, 14). Therefore, LC–MS-MS is not considered the best choice as a primary screening tool if one must initially anticipate every possible analyte.
The commercialization of LC–TOF-MS permits the use of gentle electrospray ionization while providing accurate mass and retention time data to identify analytes. A wide array of drugs, metabolites, adulterants and other exotic compounds can be monitored by this technique (15–19). This type of analysis invokes the powerful potential for forensic toxicology investigations by providing scientific evidence of the use of a drug, its origins, manner of absorption and relative amounts. Although solid-phase extraction (SPE) and LC–TOF-MS procedures have already been described in previous publications (20, 21), this work details a validated integrated method for the extraction and analysis of a wide array of forensic toxicology relevant substances involving stimulants, benzodiazepines, opiates, muscle relaxants, hypnotics, antihistamines, antidepressants and newer synthetic “Spice/K2” cannabinoids and cathinone “bath salt” designer drugs.
Methods
Reagents
Reference standards for drug compounds were obtained from Cerilliant Corporation (Round Rock, TX), Cayman Chemical (Ann Arbor, MI) or Sigma-Aldrich (St. Louis, MO). The internal standard SKF-525A/Proadifien, salts, solvents and other liquids such as ammonium formate, sodium phosphate, hexane, isopropanol, methylene chloride, ammonium hydroxide and acetic acid were purchased from Sigma-Aldrich. GC/GC–MS grade methanol was obtained from Honeywell Burdick and Jackson (Morristown, NJ). Test tubes were obtained from Fisher Scientific (Pittsburgh, PA) and Strata-X Drug B SPE columns were provided by Phenomenex (Torrance, CA).
Sample preparation
Controls were prepared for extraction by spiking drugs at final levels of 20 ng/mL for common analytes such as cocaine, amphetamines, benzodiazepines, opiates, bath salts and Spice compounds, 50 ng/mL for common antihistamines and antidepressants and 200 ng/mL of SKF-525A internal standard into 1 mL blood or urine contained in 13 × 100 mm screw top test tubes. The mixture was diluted with 4 mL of deionized water, capped, vortexed and then centrifuged for 10 min at 3,500 rpm. The supernatant was transferred to fresh 12 × 75 mm test tubes, and an additional 2 mL of 100 mM sodium phosphate buffer, pH 6.0, was mixed into each tube.
Automated SPE
A 10-module Caliper RapidTrace extraction instrument was used to automate solid-phase purification of the analytes from the biological specimen. The modules were loaded with Strata-X-drug B extraction columns and programmed to run a stepwise protocol for conditioning, loading, washing and eluting the extract. In the first step, the pipettor cannula was purged with 5 mL of methanol at a rate of 42 mL/min, followed by a rinse with 5 mL of deionized water, also at 42 mL/min. The column was conditioned with 3 mL of methanol at 5 mL/min, followed by the application of 3 mL deionized water at 5 mL/min and a final rinse using 1 mL of 100 mM sodium phosphate, pH 6.0, to prepare the column for sample loading.
Controls and case samples were applied to extraction columns (SPE cartridges) by dispensing two rounds of 3.5 mL sample volume at 2 mL/min. The cannula was first rinsed by purging with 5 mL of deionized water at 42 mL/min, and then used to rinse the column with 3 mL of methanol at 5 mL/min, followed by a final rinse using 1 mL of 100 mM acetic acid at 5 mL/min. The column was further dried of aqueous residue by applying a stream of compressed nitrogen gas for 2 min.
The acidic and basic extracts were collected in two steps using mixed organic solvents. The first elution was accomplished by delivering 3 mL of a hexane–ethyl acetate (1:1) mixture at 2 mL/min. After a 3 mL methanol rinse at 5 mL/min, the column was dried with compressed nitrogen gas for 2 min. The second fraction was obtained by delivering 3 mL of a methylene chloride–isopropanol–ammonium hydroxide (78:20:2) mixture at 2 mL/min. The two organic solvent extracts were combined and evaporated to dryness using compressed nitrogen gas. The residue was reconstituted in 200 µL of 10% methanol–water and transferred to autosampler vials for injection.
LC–TOF-MS
The liquid chromatograph was powered by an Agilent 1290 Infinity system consisting of binary pumps, degasser, column heater and autosampler. Based on manufacturer recommendations, the pumps were programmed to deliver an increasing gradient of methanol against an aqueous mobile phase of 5 mM ammonium formate over the following time course: 5% methanol from 0 to 0.5 min, increasing to 30% methanol at 1.5 min, 60% methanol at 4.5 min, to 95% methanol at 6.5 min, and a reset to 5% methanol as acquisition stopped at 10 min with a 3 min post-run. Separations were performed using an Agilent Eclipse Plus C18 1.8 µm, 3.0 × 100 mm column. The autosampler injected 4 µL of sample per run, with automated needle washes in between. The column flow rate was kept at 0.6 mL/min with the heater at a constant 50°C.
The mass analyzer was an Agilent 6230 TOF-MS operated in positive ion scan mode with mass scanning from 100 to 1000 m/z. The ion source was upgraded from the original Agilent Jet Stream (AJS) source to the dual-sprayer version for improved reference mass delivery. The instrument acquired data using the following parameters: drying gas temperature, 350°C; drying gas flow, 8.0 L/min; nebulizer, 35 psi; sheath gas temperature, 400°C; sheath gas flow, 11 L/min; VCap. 3.500 V; nozzle, 0 V; fragmentor, 125 V; skimmer, 65 V; and octopole RF peak, 750. A constant flow of Agilent TOF reference solution through the reference nebulizer allowed the system to continuously correct for any mass drift by using the reference mass ions purine at 121.05087 and HP-921 at 922.00979 m/z.
Data Analysis
Target compounds were detected and reported from accurate-mass scan data using Agilent MassHunter Qualitative and Personal Compound Database and Library (PCDL) software. The PCDL database contains target compound names, assigned compound classes, molecular formula, retention time and additional information including CAS number, structure and other internet-searchable resources such as ChemSpider. For the purposes of this study, the retention time data were obtained and entered into the database by injecting solutions of pure reference drug standards at a cutoff concentration level for each drug.
The Qualitative Analysis software identifies target compounds using a “Find by Formula” algorithm that can run automatically at the completion of each acquisition. First, it generates a narrow-mass extracted ion chromatogram (EIC) for one or more expected ions (typically only M+H under these LC conditions) in a time window around the expected retention time for the target compound. Next, it integrates the EIC and retrieves an averaged spectrum across the peak if the area exceeds the minimum area threshold. It interrogates the spectrum and compares to theoretical values for the target formula, mass error for the main adduct, mass errors for the isotopic peak spacing and relative abundances of the isotopic peaks.
Based on manufacturer recommendations, compounds with retention time differences less than or equal to 0.15 min from the reference standard retention time and mass errors within 15 ppm are reported. By summarizing the accumulated data, it calculates a score out of a possible 100% match. To consider a compound as identified, the target compound should score greater than 50 out of 100. These criteria are actually quite broad, because compounds recovered from specimens typically displayed retention time differences of 0.05 min, mass errors less than 5 ppm and scores exceeding 80 out of 100.
Results
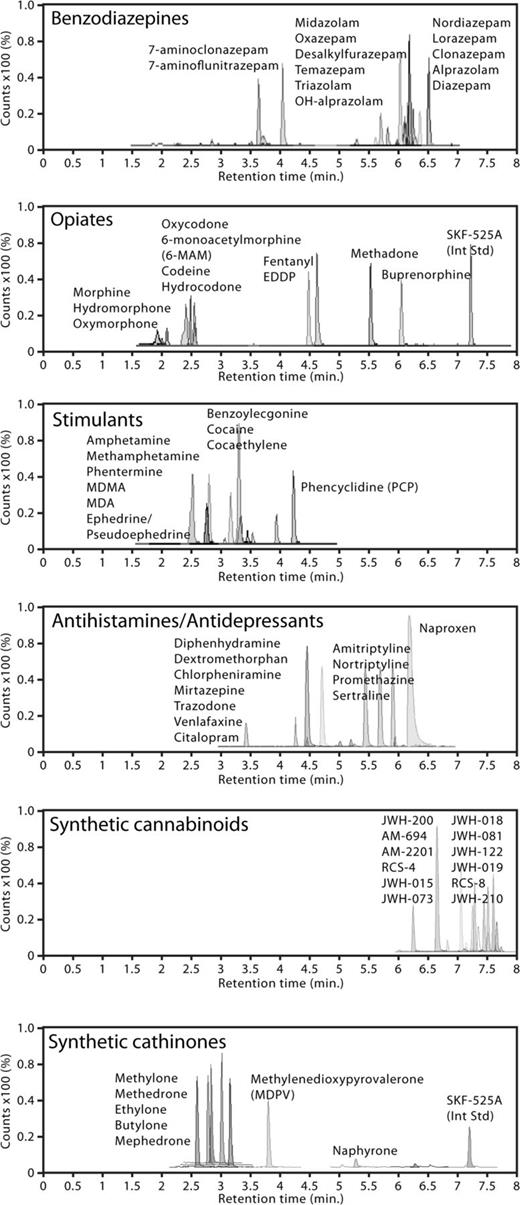
A series of tests was performed to model the types of drugs and efficient extractions needed to evaluate forensic toxicology casework. Using sheep blood spiked with various drugs, several controls were developed and subjected to SPE, and then analyzed by LC–TOF-MS. Sheep blood is used for controls in the laboratory due to the variability of human blood, its health risks and the possible presence of undeclared drugs. A representative set of chromatograms displaying typical drug class separations from spiked blood extractions is shown in Figure 1.
Representative base peak corrected, ion-extracted chromatograms of drug standards separated within classes such as benzodiazepines, opiates, stimulants, antidepressants, synthetic cannabinoids and cathinones.
After performing several experimental runs of compounds and reconciling with recommendations from the manufacturer, the following criteria were determined to consider a compound as identified: retention time difference ±0.15 min, mass difference for the main adduct ±15 ppm and target compound score greater than 50 out of 100. These criteria are actually quite broad, because compounds typically displayed retention times within ≤0.05 min, mass errors well within ±10 ppm and average scores of 86/100. By applying these criteria, false positive identification of drugs with similar molecular mass and formulas of the target compounds was eliminated. The results of the drug compounds extracted and analyzed are shown in Tables IA–C.
Drug Compounds Identified by Retention Time, Accurate Mass, Score, and Potential Isobaric/Isomeric Interfering Compounds in a Representative Run
| Name . | Formula . | RT (min) . | Mass . | Mass (Tgt) . | Difference (ppm) . | Score . | Area . | Isobaric/isomer . |
|---|---|---|---|---|---|---|---|---|
| A) Stimulants, Benzodiazepines, Opiates and Muscle Relaxants | ||||||||
| Benzodiazepines | ||||||||
| 7-Aminoclonazepam | C15H12ClN3O | 3.63 | 285.06742 | 285.06689 | 1.86 | 99 | 3634238 | |
| 7-Aminoflunitrazepam | C16H14FN3O | 4.03 | 283.11256 | 283.11209 | 1.67 | 95 | 4776594 | |
| α-Hydroxyalprazolam | C17H13ClN4O | 5.79 | 324.07880 | 324.07779 | 3.11 | 97 | 976185 | |
| Alprazolam | C17H13ClN4 | 6.00 | 308.08351 | 308.08287 | 2.07 | 96 | 4959375 | |
| Chlordiazepoxide | C16H14ClN3O | 6.22 | 299.08356 | 299.08254 | 3.42 | 53 | 2049696 | |
| Clonazepam | C15H10ClN3O3 | 5.58 | 315.04196 | 315.04107 | 2.82 | 68 | 465799 | |
| Desalkylflurazepam | C15H10ClFN2O | 6.07 | 288.04695 | 288.04657 | 1.32 | 99 | 1519946 | |
| Diazepam | C16H13ClN2O | 6.47 | 284.07215 | 284.07164 | 1.79 | 98 | 4644744 | |
| Flunitrazepam | C16H12FN3O3 | 5.67 | 313.08731 | 313.08627 | 3.32 | 86 | 1829333 | |
| Lorazepam | C15H10Cl2N2O2 | 5.94 | 320.01432 | 320.01193 | 7.46 | 61 | 320460 | |
| Midazolam | C18H13ClFN3 | 6.16 | 325.07850 | 325.07820 | 0.90 | 97 | 6045516 | |
| Nordiazepam | C15H11ClN2O | 6.33 | 270.05713 | 270.05599 | 4.20 | 93 | 1756261 | |
| Oxazepam | C15H11ClN2O2 | 5.96 | 286.05055 | 286.05091 | –1.24 | 75 | 298293 | |
| Temazepam | C16H13ClN2O2 | 6.12 | 300.06778 | 300.06656 | 4.08 | 92 | 2226199 | |
| Triazolam | C17H12Cl2N4 | 5.95 | 342.04184 | 342.04390 | –6.03 | 90 | 5155100 | |
| Opiates/opioids | ||||||||
| 6-Acetylmorphine | C19H21NO4 | 2.53 | 327.14784 | 327.14706 | 2.39 | 90 | 1594081 | Naloxone |
| Buprenorphine | C29H41NO4 | 6.10 | 467.30399 | 467.30356 | 0.93 | 99 | 3938671 | |
| Morphine | C17H19NO3 | 1.87 | 285.13741 | 285.13649 | 3.20 | 77 | 1706304 | Hydromorphone |
| Hydromorphone | C17H19NO3 | 2.05 | 285.13751 | 285.13649 | 3.58 | 75 | 928698 | Morphine |
| Oxycodone | C18H21NO4 | 2.45 | 315.14930 | 315.14706 | 7.12 | 70 | 2070280 | |
| Oxymorphone | C17H19NO4 | 1.95 | 301.13224 | 301.13141 | 2.76 | 63 | 478331 | |
| Codeine | C18H21NO3 | 2.37 | 299.15311 | 299.15214 | 3.22 | 88 | 3426320 | Hydrocodone |
| Hydrocodone | C18H21NO3 | 2.51 | 299.15266 | 299.15214 | 1.71 | 91 | 2574367 | Codeine |
| Methadone | C21H27NO | 5.57 | 309.21014 | 309.20926 | 2.82 | 97 | 5113103 | |
| 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) | C20H23N | 4.64 | 277.18157 | 277.18305 | –5.35 | 89 | 6308463 | Amitriptyline |
| Fentanyl | C22H28N2O | 4.50 | 336.21862 | 336.22016 | –4.60 | 83 | 4629074 | |
| Tramadol | C16H25NO2 | 3.48 | 263.18810 | 263.18853 | 1.63 | 85 | 23521235 | O-desmethylvenlafaxine |
| Muscle relaxants | ||||||||
| Carisoprodol | C12H24N2O4 | 5.90 | 260.17231 | 260.17361 | –5.00 | 86 | 3693290 | |
| Meprobamate | C9H18N2O4 | 4.43 | 218.12684 | 218.12666 | 0.84 | 83 | 1348511 | |
| Cyclobenzaprine | C20H21N | 5.49 | 275.16828 | 275.16740 | 3.18 | 88 | 25722239 | |
| B) OTC Medications, Antidepressants, Antiepileptics and Hypnotics | ||||||||
| Stimulants | ||||||||
| Amphetamine | C9H13N | 2.76 | 135.10439 | 135.10480 | –3.06 | 98 | 2944608 | |
| Methamphetamine | C10H15N | 2.79 | 149.11999 | 149.12045 | –3.10 | 91 | 4458551 | Phentermine |
| Phentermine | C10H15N | 3.17 | 149.12006 | 149.12045 | –2.63 | 96 | 3246484 | Methamphetamine |
| MDMA | C11H15NO2 | 2.84 | 193.11048 | 193.11028 | 1.04 | 89 | 4508459 | Methedrone, 5-Methyl MDA |
| Methylenedioxy-amphetamine (MDA) | C10H13NO2 | 2.76 | 179.09564 | 179.09463 | 5.63 | 85 | 2926006 | |
| Ephedrine/pseudoephedrine | C10H15NO | 2.51 | 165.11625 | 165.11536 | 5.39 | 85 | 5913477 | p-Methoxyamphetamine |
| Cocaethylene | C18H23NO4 | 3.96 | 317.16290 | 317.16271 | 0.61 | 96 | 1661569 | |
| Cocaine | C17H21NO4 | 3.45 | 303.14774 | 303.14706 | 2.26 | 87 | 725916 | Scopalamine |
| Benzoylecgonine | C16H19NO4 | 3.31 | 289.13166 | 289.13141 | 0.88 | 98 | 8511535 | |
| Lidocaine | C14H22N2O | 3.23 | 234.17065 | 234.17321 | –10.93 | 50 | 95330 | |
| Phencyclidine (PCP) | C17H25N | 4.25 | 243.19764 | 243.19870 | –4.38 | 90 | 4676077 | |
| Methylphenidate | C14H19NO2 | 3.54 | 233.14192 | 233.14158 | 1.44 | 91 | 583504 | |
| Ritalinic acid | C13H17NO2 | 3.34 | 219.12702 | 219.12593 | 4.96 | 85 | 2444074 | |
| OTC/antihistamines | ||||||||
| Acetaminophen | C8H9NO2 | 2.32 | 151.06386 | 151.06333 | 3.51 | 80 | 1938626 | |
| Salicylic acid | C7H6O3 | 2.88 | 138.03159 | 138.03169 | –0.74 | 100 | 164003 | |
| Ibuprofen | C13H18O2 | 6.94 | 206.12960 | 206.13068 | –5.15 | 92 | 7325473 | |
| Naproxen | C14H14O3 | 6.22 | 230.09420 | 230.09429 | –0.62 | 99 | 1202612 | |
| Diphenhydramine | C17H21NO | 4.75 | 255.16152 | 255.16231 | –3.11 | 91 | 24757875 | Atomoxetine |
| Chlorpheniramine | C16H19ClN2 | 4.49 | 274.12361 | 274.12368 | –0.24 | 95 | 29133002 | |
| Promethazine | C17H20N2S | 5.24 | 284.13637 | 284.13472 | 5.81 | 85 | 1773708 | Promazine |
| Dextromethorphan | C18H25NO | 4.70 | 271.19384 | 271.19361 | –0.85 | 89 | 24551563 | |
| Antidepressants | ||||||||
| Mirtazapine | C17H19N3 | 3.87 | 265.16070 | 265.15790 | –10.56 | 84 | 8601329 | |
| Trazodone | C19H22ClN5O | 4.58 | 371.15230 | 371.15129 | –2.72 | 72 | 9515116 | |
| Sertraline | C17H17Cl2N | 5.96 | 305.07354 | 305.07381 | –0.88 | 95 | 19711190 | |
| Venlafaxine | C17H27NO2 | 4.43 | 277.20370 | 277.20418 | 1.73 | 87 | 25447867 | |
| Citalopram | C20H21FN2O | 4.62 | 324.16210 | 324.16379 | 5.21 | 90 | 24889357 | |
| Amitriptyline | C20H23N | 5.67 | 277.18318 | 277.18305 | –0.47 | 79 | 21923939 | EDDP |
| Nortriptyline | C19H21N | 5.74 | 263.16861 | 263.16740 | 4.61 | 91 | 23096205 | |
| Antiepileptics | ||||||||
| Phenytoin | C15H12N2O2 | 5.24 | 252.08940 | 252.08988 | –1.91 | 96 | 12299940 | Oxcarbazepine |
| Topiramate | C12H21NO8S | 4.59 | 339.09840 | 339.09879 | –1.10 | 87 | 34664699 | |
| Hypnotics | ||||||||
| Doxylamine | C17H22N2O | 3.78 | 270.17340 | 270.17321 | –0.70 | 95 | 22908918 | 5-MeO-DALT |
| Zaleplon | C17H15N5O | 5.06 | 305.12896 | 305.12766 | 4.25 | 81 | 1163895 | |
| Zolpidem | C19H21N3O | 4.30 | 307.16669 | 307.16846 | –5.78 | 66 | 4611269 | |
| Zopiclone | C17H17ClN6O3 | 3.46 | 388.10590 | 388.10507 | 2.16 | 92 | 5444754 | |
| C) Synthetic Cannabinoid Spice Compounds and Synthetic Cathinone Bath Salts | ||||||||
| Synthetic cannabinoids | ||||||||
| RCS-4 | C21H23NO2 | 7.29 | 321.17395 | 321.17288 | 3.34 | 83 | 4670598 | |
| JWH-073 | C23H21NO | 7.33 | 327.16322 | 327.16231 | 2.76 | 92 | 6744872 | JWH-015 |
| JWH-019 | C25H25NO | 7.64 | 355.19398 | 355.19361 | 1.03 | 90 | 8203283 | JWH-007, JWH-122 |
| JWH-122 | C25H25NO | 7.64 | 355.19399 | 355.19361 | 1.04 | 98 | 8180911 | JWH-007, JWH-019 |
| AM-2201 | C24H22FNO | 7.10 | 359.16917 | 359.16854 | 1.75 | 97 | 8283323 | |
| JWH-210 | C26H27NO | 7.77 | 369.20959 | 369.20926 | 0.87 | 86 | 467841 | JWH-020 |
| JWH-081 | C25H25NO2 | 7.55 | 371.18892 | 371.18853 | 1.05 | 99 | 6697934 | JWH-018-6-MeO |
| RCS-8 | C25H29NO2 | 7.69 | 375.22051 | 375.21983 | 1.82 | 99 | 3012613 | |
| JWH-200 | C25H24N2O2 | 6.69 | 384.18418 | 384.18378 | 1.04 | 93 | 16634958 | |
| JWH-251 | C22H25NO | 7.43 | 319.19418 | 319.19361 | 1.78 | 75 | 7669899 | |
| JWH-015 | C23H21NO | 7.23 | 327.16331 | 327.16231 | 3.05 | 94 | 10038079 | JWH-073 |
| JWH-250 | C22H25NO2 | 7.33 | 335.18924 | 335.18853 | 2.13 | 88 | 8064283 | JWH-201, JWH-302 |
| JWH-203 | C21H22ClNO | 7.42 | 339.13989 | 339.13899 | 2.64 | 86 | 220661 | |
| JWH-022 | C24H21NO | 7.35 | 339.16313 | 339.16231 | 2.40 | 78 | 563953 | |
| JWH-018 | C24H23NO | 7.46 | 341.18006 | 341.17796 | 6.13 | 64 | 122556 | JWH-016 |
| JWH-016 | C24H23NO | 7.42 | 341.17868 | 341.17796 | 2.09 | 83 | 727863 | JWH-018 |
| JWH-007 | C25H25NO | 7.56 | 355.19409 | 355.19361 | 1.35 | 97 | 2276986 | JWH-019, JWH-122 |
| JWH-018-6-MeO | C25H25NO2 | 7.47 | 371.18949 | 371.18853 | 2.58 | 84 | 179250 | JWH-081 |
| Pravadoline WIN-48098 | C23H26N2O3 | 6.58 | 378.19627 | 378.19434 | 5.10 | 43 | 137908 | |
| JWH-098 | C26H27NO2 | 7.61 | 385.20562 | 385.20418 | 3.75 | 61 | 89646 | |
| AM-1241 | C22H22IN3O3 | 5.45 | 503.07024 | 503.07058 | –0.68 | 83 | 863232 | |
| AM-694 | C20H19FINO | 6.87 | 435.04974 | 435.04954 | 0.46 | 99 | 1099238 | |
| Synthetic cathinones | ||||||||
| Mephedrone | C11H15NO | 3.15 | 177.11515 | 177.11536 | –1.22 | 63 | 116712 | Phenmetrazine, AMMI |
| Methedrone | C11H15NO2 | 2.84 | 193.11062 | 193.11028 | 1.76 | 54 | 296298 | MDMA, 5-methyl-MDA |
| 4'-Methyl-α-pyrrolidino-propiophenone (MPPP) | C14H19NO | 3.42 | 217.14606 | 217.14666 | –2.80 | 93 | 352269 | |
| Butylone | C12H15NO3 | 3.01 | 221.10554 | 221.10519 | 1.57 | 79 | 433487 | Ethylone, metaxalone |
| Ethylone | C12H15NO3 | 2.78 | 221.10605 | 221.10519 | 3.89 | 74 | 268832 | Butylone, metaxalone |
| Methylenedioxy-pyrovalerone (MDPV) | C16H21NO3 | 3.80 | 275.15267 | 275.15214 | 1.92 | 91 | 564883 | |
| Methylone | C11H13NO3 | 2.61 | 207.09019 | 207.08954 | –3.12 | 94 | 1233166 | |
| Pentedrone | C12H17NO | 3.57 | 191.13125 | 191.13101 | –1.27 | 72 | 9596955 | Phendimetrazine, 4-MEC, DMMC |
| αpyrrolidino-propiophenone (α-PPP) | C13H17NO | 2.78 | 203.13121 | 203.13101 | –0.97 | 94 | 7569091 | |
| Naphyrone | C19H23NO | 5.28 | 281.17842 | 281.17796 | 1.63 | 91 | 1244472 | |
| Name . | Formula . | RT (min) . | Mass . | Mass (Tgt) . | Difference (ppm) . | Score . | Area . | Isobaric/isomer . |
|---|---|---|---|---|---|---|---|---|
| A) Stimulants, Benzodiazepines, Opiates and Muscle Relaxants | ||||||||
| Benzodiazepines | ||||||||
| 7-Aminoclonazepam | C15H12ClN3O | 3.63 | 285.06742 | 285.06689 | 1.86 | 99 | 3634238 | |
| 7-Aminoflunitrazepam | C16H14FN3O | 4.03 | 283.11256 | 283.11209 | 1.67 | 95 | 4776594 | |
| α-Hydroxyalprazolam | C17H13ClN4O | 5.79 | 324.07880 | 324.07779 | 3.11 | 97 | 976185 | |
| Alprazolam | C17H13ClN4 | 6.00 | 308.08351 | 308.08287 | 2.07 | 96 | 4959375 | |
| Chlordiazepoxide | C16H14ClN3O | 6.22 | 299.08356 | 299.08254 | 3.42 | 53 | 2049696 | |
| Clonazepam | C15H10ClN3O3 | 5.58 | 315.04196 | 315.04107 | 2.82 | 68 | 465799 | |
| Desalkylflurazepam | C15H10ClFN2O | 6.07 | 288.04695 | 288.04657 | 1.32 | 99 | 1519946 | |
| Diazepam | C16H13ClN2O | 6.47 | 284.07215 | 284.07164 | 1.79 | 98 | 4644744 | |
| Flunitrazepam | C16H12FN3O3 | 5.67 | 313.08731 | 313.08627 | 3.32 | 86 | 1829333 | |
| Lorazepam | C15H10Cl2N2O2 | 5.94 | 320.01432 | 320.01193 | 7.46 | 61 | 320460 | |
| Midazolam | C18H13ClFN3 | 6.16 | 325.07850 | 325.07820 | 0.90 | 97 | 6045516 | |
| Nordiazepam | C15H11ClN2O | 6.33 | 270.05713 | 270.05599 | 4.20 | 93 | 1756261 | |
| Oxazepam | C15H11ClN2O2 | 5.96 | 286.05055 | 286.05091 | –1.24 | 75 | 298293 | |
| Temazepam | C16H13ClN2O2 | 6.12 | 300.06778 | 300.06656 | 4.08 | 92 | 2226199 | |
| Triazolam | C17H12Cl2N4 | 5.95 | 342.04184 | 342.04390 | –6.03 | 90 | 5155100 | |
| Opiates/opioids | ||||||||
| 6-Acetylmorphine | C19H21NO4 | 2.53 | 327.14784 | 327.14706 | 2.39 | 90 | 1594081 | Naloxone |
| Buprenorphine | C29H41NO4 | 6.10 | 467.30399 | 467.30356 | 0.93 | 99 | 3938671 | |
| Morphine | C17H19NO3 | 1.87 | 285.13741 | 285.13649 | 3.20 | 77 | 1706304 | Hydromorphone |
| Hydromorphone | C17H19NO3 | 2.05 | 285.13751 | 285.13649 | 3.58 | 75 | 928698 | Morphine |
| Oxycodone | C18H21NO4 | 2.45 | 315.14930 | 315.14706 | 7.12 | 70 | 2070280 | |
| Oxymorphone | C17H19NO4 | 1.95 | 301.13224 | 301.13141 | 2.76 | 63 | 478331 | |
| Codeine | C18H21NO3 | 2.37 | 299.15311 | 299.15214 | 3.22 | 88 | 3426320 | Hydrocodone |
| Hydrocodone | C18H21NO3 | 2.51 | 299.15266 | 299.15214 | 1.71 | 91 | 2574367 | Codeine |
| Methadone | C21H27NO | 5.57 | 309.21014 | 309.20926 | 2.82 | 97 | 5113103 | |
| 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) | C20H23N | 4.64 | 277.18157 | 277.18305 | –5.35 | 89 | 6308463 | Amitriptyline |
| Fentanyl | C22H28N2O | 4.50 | 336.21862 | 336.22016 | –4.60 | 83 | 4629074 | |
| Tramadol | C16H25NO2 | 3.48 | 263.18810 | 263.18853 | 1.63 | 85 | 23521235 | O-desmethylvenlafaxine |
| Muscle relaxants | ||||||||
| Carisoprodol | C12H24N2O4 | 5.90 | 260.17231 | 260.17361 | –5.00 | 86 | 3693290 | |
| Meprobamate | C9H18N2O4 | 4.43 | 218.12684 | 218.12666 | 0.84 | 83 | 1348511 | |
| Cyclobenzaprine | C20H21N | 5.49 | 275.16828 | 275.16740 | 3.18 | 88 | 25722239 | |
| B) OTC Medications, Antidepressants, Antiepileptics and Hypnotics | ||||||||
| Stimulants | ||||||||
| Amphetamine | C9H13N | 2.76 | 135.10439 | 135.10480 | –3.06 | 98 | 2944608 | |
| Methamphetamine | C10H15N | 2.79 | 149.11999 | 149.12045 | –3.10 | 91 | 4458551 | Phentermine |
| Phentermine | C10H15N | 3.17 | 149.12006 | 149.12045 | –2.63 | 96 | 3246484 | Methamphetamine |
| MDMA | C11H15NO2 | 2.84 | 193.11048 | 193.11028 | 1.04 | 89 | 4508459 | Methedrone, 5-Methyl MDA |
| Methylenedioxy-amphetamine (MDA) | C10H13NO2 | 2.76 | 179.09564 | 179.09463 | 5.63 | 85 | 2926006 | |
| Ephedrine/pseudoephedrine | C10H15NO | 2.51 | 165.11625 | 165.11536 | 5.39 | 85 | 5913477 | p-Methoxyamphetamine |
| Cocaethylene | C18H23NO4 | 3.96 | 317.16290 | 317.16271 | 0.61 | 96 | 1661569 | |
| Cocaine | C17H21NO4 | 3.45 | 303.14774 | 303.14706 | 2.26 | 87 | 725916 | Scopalamine |
| Benzoylecgonine | C16H19NO4 | 3.31 | 289.13166 | 289.13141 | 0.88 | 98 | 8511535 | |
| Lidocaine | C14H22N2O | 3.23 | 234.17065 | 234.17321 | –10.93 | 50 | 95330 | |
| Phencyclidine (PCP) | C17H25N | 4.25 | 243.19764 | 243.19870 | –4.38 | 90 | 4676077 | |
| Methylphenidate | C14H19NO2 | 3.54 | 233.14192 | 233.14158 | 1.44 | 91 | 583504 | |
| Ritalinic acid | C13H17NO2 | 3.34 | 219.12702 | 219.12593 | 4.96 | 85 | 2444074 | |
| OTC/antihistamines | ||||||||
| Acetaminophen | C8H9NO2 | 2.32 | 151.06386 | 151.06333 | 3.51 | 80 | 1938626 | |
| Salicylic acid | C7H6O3 | 2.88 | 138.03159 | 138.03169 | –0.74 | 100 | 164003 | |
| Ibuprofen | C13H18O2 | 6.94 | 206.12960 | 206.13068 | –5.15 | 92 | 7325473 | |
| Naproxen | C14H14O3 | 6.22 | 230.09420 | 230.09429 | –0.62 | 99 | 1202612 | |
| Diphenhydramine | C17H21NO | 4.75 | 255.16152 | 255.16231 | –3.11 | 91 | 24757875 | Atomoxetine |
| Chlorpheniramine | C16H19ClN2 | 4.49 | 274.12361 | 274.12368 | –0.24 | 95 | 29133002 | |
| Promethazine | C17H20N2S | 5.24 | 284.13637 | 284.13472 | 5.81 | 85 | 1773708 | Promazine |
| Dextromethorphan | C18H25NO | 4.70 | 271.19384 | 271.19361 | –0.85 | 89 | 24551563 | |
| Antidepressants | ||||||||
| Mirtazapine | C17H19N3 | 3.87 | 265.16070 | 265.15790 | –10.56 | 84 | 8601329 | |
| Trazodone | C19H22ClN5O | 4.58 | 371.15230 | 371.15129 | –2.72 | 72 | 9515116 | |
| Sertraline | C17H17Cl2N | 5.96 | 305.07354 | 305.07381 | –0.88 | 95 | 19711190 | |
| Venlafaxine | C17H27NO2 | 4.43 | 277.20370 | 277.20418 | 1.73 | 87 | 25447867 | |
| Citalopram | C20H21FN2O | 4.62 | 324.16210 | 324.16379 | 5.21 | 90 | 24889357 | |
| Amitriptyline | C20H23N | 5.67 | 277.18318 | 277.18305 | –0.47 | 79 | 21923939 | EDDP |
| Nortriptyline | C19H21N | 5.74 | 263.16861 | 263.16740 | 4.61 | 91 | 23096205 | |
| Antiepileptics | ||||||||
| Phenytoin | C15H12N2O2 | 5.24 | 252.08940 | 252.08988 | –1.91 | 96 | 12299940 | Oxcarbazepine |
| Topiramate | C12H21NO8S | 4.59 | 339.09840 | 339.09879 | –1.10 | 87 | 34664699 | |
| Hypnotics | ||||||||
| Doxylamine | C17H22N2O | 3.78 | 270.17340 | 270.17321 | –0.70 | 95 | 22908918 | 5-MeO-DALT |
| Zaleplon | C17H15N5O | 5.06 | 305.12896 | 305.12766 | 4.25 | 81 | 1163895 | |
| Zolpidem | C19H21N3O | 4.30 | 307.16669 | 307.16846 | –5.78 | 66 | 4611269 | |
| Zopiclone | C17H17ClN6O3 | 3.46 | 388.10590 | 388.10507 | 2.16 | 92 | 5444754 | |
| C) Synthetic Cannabinoid Spice Compounds and Synthetic Cathinone Bath Salts | ||||||||
| Synthetic cannabinoids | ||||||||
| RCS-4 | C21H23NO2 | 7.29 | 321.17395 | 321.17288 | 3.34 | 83 | 4670598 | |
| JWH-073 | C23H21NO | 7.33 | 327.16322 | 327.16231 | 2.76 | 92 | 6744872 | JWH-015 |
| JWH-019 | C25H25NO | 7.64 | 355.19398 | 355.19361 | 1.03 | 90 | 8203283 | JWH-007, JWH-122 |
| JWH-122 | C25H25NO | 7.64 | 355.19399 | 355.19361 | 1.04 | 98 | 8180911 | JWH-007, JWH-019 |
| AM-2201 | C24H22FNO | 7.10 | 359.16917 | 359.16854 | 1.75 | 97 | 8283323 | |
| JWH-210 | C26H27NO | 7.77 | 369.20959 | 369.20926 | 0.87 | 86 | 467841 | JWH-020 |
| JWH-081 | C25H25NO2 | 7.55 | 371.18892 | 371.18853 | 1.05 | 99 | 6697934 | JWH-018-6-MeO |
| RCS-8 | C25H29NO2 | 7.69 | 375.22051 | 375.21983 | 1.82 | 99 | 3012613 | |
| JWH-200 | C25H24N2O2 | 6.69 | 384.18418 | 384.18378 | 1.04 | 93 | 16634958 | |
| JWH-251 | C22H25NO | 7.43 | 319.19418 | 319.19361 | 1.78 | 75 | 7669899 | |
| JWH-015 | C23H21NO | 7.23 | 327.16331 | 327.16231 | 3.05 | 94 | 10038079 | JWH-073 |
| JWH-250 | C22H25NO2 | 7.33 | 335.18924 | 335.18853 | 2.13 | 88 | 8064283 | JWH-201, JWH-302 |
| JWH-203 | C21H22ClNO | 7.42 | 339.13989 | 339.13899 | 2.64 | 86 | 220661 | |
| JWH-022 | C24H21NO | 7.35 | 339.16313 | 339.16231 | 2.40 | 78 | 563953 | |
| JWH-018 | C24H23NO | 7.46 | 341.18006 | 341.17796 | 6.13 | 64 | 122556 | JWH-016 |
| JWH-016 | C24H23NO | 7.42 | 341.17868 | 341.17796 | 2.09 | 83 | 727863 | JWH-018 |
| JWH-007 | C25H25NO | 7.56 | 355.19409 | 355.19361 | 1.35 | 97 | 2276986 | JWH-019, JWH-122 |
| JWH-018-6-MeO | C25H25NO2 | 7.47 | 371.18949 | 371.18853 | 2.58 | 84 | 179250 | JWH-081 |
| Pravadoline WIN-48098 | C23H26N2O3 | 6.58 | 378.19627 | 378.19434 | 5.10 | 43 | 137908 | |
| JWH-098 | C26H27NO2 | 7.61 | 385.20562 | 385.20418 | 3.75 | 61 | 89646 | |
| AM-1241 | C22H22IN3O3 | 5.45 | 503.07024 | 503.07058 | –0.68 | 83 | 863232 | |
| AM-694 | C20H19FINO | 6.87 | 435.04974 | 435.04954 | 0.46 | 99 | 1099238 | |
| Synthetic cathinones | ||||||||
| Mephedrone | C11H15NO | 3.15 | 177.11515 | 177.11536 | –1.22 | 63 | 116712 | Phenmetrazine, AMMI |
| Methedrone | C11H15NO2 | 2.84 | 193.11062 | 193.11028 | 1.76 | 54 | 296298 | MDMA, 5-methyl-MDA |
| 4'-Methyl-α-pyrrolidino-propiophenone (MPPP) | C14H19NO | 3.42 | 217.14606 | 217.14666 | –2.80 | 93 | 352269 | |
| Butylone | C12H15NO3 | 3.01 | 221.10554 | 221.10519 | 1.57 | 79 | 433487 | Ethylone, metaxalone |
| Ethylone | C12H15NO3 | 2.78 | 221.10605 | 221.10519 | 3.89 | 74 | 268832 | Butylone, metaxalone |
| Methylenedioxy-pyrovalerone (MDPV) | C16H21NO3 | 3.80 | 275.15267 | 275.15214 | 1.92 | 91 | 564883 | |
| Methylone | C11H13NO3 | 2.61 | 207.09019 | 207.08954 | –3.12 | 94 | 1233166 | |
| Pentedrone | C12H17NO | 3.57 | 191.13125 | 191.13101 | –1.27 | 72 | 9596955 | Phendimetrazine, 4-MEC, DMMC |
| αpyrrolidino-propiophenone (α-PPP) | C13H17NO | 2.78 | 203.13121 | 203.13101 | –0.97 | 94 | 7569091 | |
| Naphyrone | C19H23NO | 5.28 | 281.17842 | 281.17796 | 1.63 | 91 | 1244472 | |
Drug Compounds Identified by Retention Time, Accurate Mass, Score, and Potential Isobaric/Isomeric Interfering Compounds in a Representative Run
| Name . | Formula . | RT (min) . | Mass . | Mass (Tgt) . | Difference (ppm) . | Score . | Area . | Isobaric/isomer . |
|---|---|---|---|---|---|---|---|---|
| A) Stimulants, Benzodiazepines, Opiates and Muscle Relaxants | ||||||||
| Benzodiazepines | ||||||||
| 7-Aminoclonazepam | C15H12ClN3O | 3.63 | 285.06742 | 285.06689 | 1.86 | 99 | 3634238 | |
| 7-Aminoflunitrazepam | C16H14FN3O | 4.03 | 283.11256 | 283.11209 | 1.67 | 95 | 4776594 | |
| α-Hydroxyalprazolam | C17H13ClN4O | 5.79 | 324.07880 | 324.07779 | 3.11 | 97 | 976185 | |
| Alprazolam | C17H13ClN4 | 6.00 | 308.08351 | 308.08287 | 2.07 | 96 | 4959375 | |
| Chlordiazepoxide | C16H14ClN3O | 6.22 | 299.08356 | 299.08254 | 3.42 | 53 | 2049696 | |
| Clonazepam | C15H10ClN3O3 | 5.58 | 315.04196 | 315.04107 | 2.82 | 68 | 465799 | |
| Desalkylflurazepam | C15H10ClFN2O | 6.07 | 288.04695 | 288.04657 | 1.32 | 99 | 1519946 | |
| Diazepam | C16H13ClN2O | 6.47 | 284.07215 | 284.07164 | 1.79 | 98 | 4644744 | |
| Flunitrazepam | C16H12FN3O3 | 5.67 | 313.08731 | 313.08627 | 3.32 | 86 | 1829333 | |
| Lorazepam | C15H10Cl2N2O2 | 5.94 | 320.01432 | 320.01193 | 7.46 | 61 | 320460 | |
| Midazolam | C18H13ClFN3 | 6.16 | 325.07850 | 325.07820 | 0.90 | 97 | 6045516 | |
| Nordiazepam | C15H11ClN2O | 6.33 | 270.05713 | 270.05599 | 4.20 | 93 | 1756261 | |
| Oxazepam | C15H11ClN2O2 | 5.96 | 286.05055 | 286.05091 | –1.24 | 75 | 298293 | |
| Temazepam | C16H13ClN2O2 | 6.12 | 300.06778 | 300.06656 | 4.08 | 92 | 2226199 | |
| Triazolam | C17H12Cl2N4 | 5.95 | 342.04184 | 342.04390 | –6.03 | 90 | 5155100 | |
| Opiates/opioids | ||||||||
| 6-Acetylmorphine | C19H21NO4 | 2.53 | 327.14784 | 327.14706 | 2.39 | 90 | 1594081 | Naloxone |
| Buprenorphine | C29H41NO4 | 6.10 | 467.30399 | 467.30356 | 0.93 | 99 | 3938671 | |
| Morphine | C17H19NO3 | 1.87 | 285.13741 | 285.13649 | 3.20 | 77 | 1706304 | Hydromorphone |
| Hydromorphone | C17H19NO3 | 2.05 | 285.13751 | 285.13649 | 3.58 | 75 | 928698 | Morphine |
| Oxycodone | C18H21NO4 | 2.45 | 315.14930 | 315.14706 | 7.12 | 70 | 2070280 | |
| Oxymorphone | C17H19NO4 | 1.95 | 301.13224 | 301.13141 | 2.76 | 63 | 478331 | |
| Codeine | C18H21NO3 | 2.37 | 299.15311 | 299.15214 | 3.22 | 88 | 3426320 | Hydrocodone |
| Hydrocodone | C18H21NO3 | 2.51 | 299.15266 | 299.15214 | 1.71 | 91 | 2574367 | Codeine |
| Methadone | C21H27NO | 5.57 | 309.21014 | 309.20926 | 2.82 | 97 | 5113103 | |
| 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) | C20H23N | 4.64 | 277.18157 | 277.18305 | –5.35 | 89 | 6308463 | Amitriptyline |
| Fentanyl | C22H28N2O | 4.50 | 336.21862 | 336.22016 | –4.60 | 83 | 4629074 | |
| Tramadol | C16H25NO2 | 3.48 | 263.18810 | 263.18853 | 1.63 | 85 | 23521235 | O-desmethylvenlafaxine |
| Muscle relaxants | ||||||||
| Carisoprodol | C12H24N2O4 | 5.90 | 260.17231 | 260.17361 | –5.00 | 86 | 3693290 | |
| Meprobamate | C9H18N2O4 | 4.43 | 218.12684 | 218.12666 | 0.84 | 83 | 1348511 | |
| Cyclobenzaprine | C20H21N | 5.49 | 275.16828 | 275.16740 | 3.18 | 88 | 25722239 | |
| B) OTC Medications, Antidepressants, Antiepileptics and Hypnotics | ||||||||
| Stimulants | ||||||||
| Amphetamine | C9H13N | 2.76 | 135.10439 | 135.10480 | –3.06 | 98 | 2944608 | |
| Methamphetamine | C10H15N | 2.79 | 149.11999 | 149.12045 | –3.10 | 91 | 4458551 | Phentermine |
| Phentermine | C10H15N | 3.17 | 149.12006 | 149.12045 | –2.63 | 96 | 3246484 | Methamphetamine |
| MDMA | C11H15NO2 | 2.84 | 193.11048 | 193.11028 | 1.04 | 89 | 4508459 | Methedrone, 5-Methyl MDA |
| Methylenedioxy-amphetamine (MDA) | C10H13NO2 | 2.76 | 179.09564 | 179.09463 | 5.63 | 85 | 2926006 | |
| Ephedrine/pseudoephedrine | C10H15NO | 2.51 | 165.11625 | 165.11536 | 5.39 | 85 | 5913477 | p-Methoxyamphetamine |
| Cocaethylene | C18H23NO4 | 3.96 | 317.16290 | 317.16271 | 0.61 | 96 | 1661569 | |
| Cocaine | C17H21NO4 | 3.45 | 303.14774 | 303.14706 | 2.26 | 87 | 725916 | Scopalamine |
| Benzoylecgonine | C16H19NO4 | 3.31 | 289.13166 | 289.13141 | 0.88 | 98 | 8511535 | |
| Lidocaine | C14H22N2O | 3.23 | 234.17065 | 234.17321 | –10.93 | 50 | 95330 | |
| Phencyclidine (PCP) | C17H25N | 4.25 | 243.19764 | 243.19870 | –4.38 | 90 | 4676077 | |
| Methylphenidate | C14H19NO2 | 3.54 | 233.14192 | 233.14158 | 1.44 | 91 | 583504 | |
| Ritalinic acid | C13H17NO2 | 3.34 | 219.12702 | 219.12593 | 4.96 | 85 | 2444074 | |
| OTC/antihistamines | ||||||||
| Acetaminophen | C8H9NO2 | 2.32 | 151.06386 | 151.06333 | 3.51 | 80 | 1938626 | |
| Salicylic acid | C7H6O3 | 2.88 | 138.03159 | 138.03169 | –0.74 | 100 | 164003 | |
| Ibuprofen | C13H18O2 | 6.94 | 206.12960 | 206.13068 | –5.15 | 92 | 7325473 | |
| Naproxen | C14H14O3 | 6.22 | 230.09420 | 230.09429 | –0.62 | 99 | 1202612 | |
| Diphenhydramine | C17H21NO | 4.75 | 255.16152 | 255.16231 | –3.11 | 91 | 24757875 | Atomoxetine |
| Chlorpheniramine | C16H19ClN2 | 4.49 | 274.12361 | 274.12368 | –0.24 | 95 | 29133002 | |
| Promethazine | C17H20N2S | 5.24 | 284.13637 | 284.13472 | 5.81 | 85 | 1773708 | Promazine |
| Dextromethorphan | C18H25NO | 4.70 | 271.19384 | 271.19361 | –0.85 | 89 | 24551563 | |
| Antidepressants | ||||||||
| Mirtazapine | C17H19N3 | 3.87 | 265.16070 | 265.15790 | –10.56 | 84 | 8601329 | |
| Trazodone | C19H22ClN5O | 4.58 | 371.15230 | 371.15129 | –2.72 | 72 | 9515116 | |
| Sertraline | C17H17Cl2N | 5.96 | 305.07354 | 305.07381 | –0.88 | 95 | 19711190 | |
| Venlafaxine | C17H27NO2 | 4.43 | 277.20370 | 277.20418 | 1.73 | 87 | 25447867 | |
| Citalopram | C20H21FN2O | 4.62 | 324.16210 | 324.16379 | 5.21 | 90 | 24889357 | |
| Amitriptyline | C20H23N | 5.67 | 277.18318 | 277.18305 | –0.47 | 79 | 21923939 | EDDP |
| Nortriptyline | C19H21N | 5.74 | 263.16861 | 263.16740 | 4.61 | 91 | 23096205 | |
| Antiepileptics | ||||||||
| Phenytoin | C15H12N2O2 | 5.24 | 252.08940 | 252.08988 | –1.91 | 96 | 12299940 | Oxcarbazepine |
| Topiramate | C12H21NO8S | 4.59 | 339.09840 | 339.09879 | –1.10 | 87 | 34664699 | |
| Hypnotics | ||||||||
| Doxylamine | C17H22N2O | 3.78 | 270.17340 | 270.17321 | –0.70 | 95 | 22908918 | 5-MeO-DALT |
| Zaleplon | C17H15N5O | 5.06 | 305.12896 | 305.12766 | 4.25 | 81 | 1163895 | |
| Zolpidem | C19H21N3O | 4.30 | 307.16669 | 307.16846 | –5.78 | 66 | 4611269 | |
| Zopiclone | C17H17ClN6O3 | 3.46 | 388.10590 | 388.10507 | 2.16 | 92 | 5444754 | |
| C) Synthetic Cannabinoid Spice Compounds and Synthetic Cathinone Bath Salts | ||||||||
| Synthetic cannabinoids | ||||||||
| RCS-4 | C21H23NO2 | 7.29 | 321.17395 | 321.17288 | 3.34 | 83 | 4670598 | |
| JWH-073 | C23H21NO | 7.33 | 327.16322 | 327.16231 | 2.76 | 92 | 6744872 | JWH-015 |
| JWH-019 | C25H25NO | 7.64 | 355.19398 | 355.19361 | 1.03 | 90 | 8203283 | JWH-007, JWH-122 |
| JWH-122 | C25H25NO | 7.64 | 355.19399 | 355.19361 | 1.04 | 98 | 8180911 | JWH-007, JWH-019 |
| AM-2201 | C24H22FNO | 7.10 | 359.16917 | 359.16854 | 1.75 | 97 | 8283323 | |
| JWH-210 | C26H27NO | 7.77 | 369.20959 | 369.20926 | 0.87 | 86 | 467841 | JWH-020 |
| JWH-081 | C25H25NO2 | 7.55 | 371.18892 | 371.18853 | 1.05 | 99 | 6697934 | JWH-018-6-MeO |
| RCS-8 | C25H29NO2 | 7.69 | 375.22051 | 375.21983 | 1.82 | 99 | 3012613 | |
| JWH-200 | C25H24N2O2 | 6.69 | 384.18418 | 384.18378 | 1.04 | 93 | 16634958 | |
| JWH-251 | C22H25NO | 7.43 | 319.19418 | 319.19361 | 1.78 | 75 | 7669899 | |
| JWH-015 | C23H21NO | 7.23 | 327.16331 | 327.16231 | 3.05 | 94 | 10038079 | JWH-073 |
| JWH-250 | C22H25NO2 | 7.33 | 335.18924 | 335.18853 | 2.13 | 88 | 8064283 | JWH-201, JWH-302 |
| JWH-203 | C21H22ClNO | 7.42 | 339.13989 | 339.13899 | 2.64 | 86 | 220661 | |
| JWH-022 | C24H21NO | 7.35 | 339.16313 | 339.16231 | 2.40 | 78 | 563953 | |
| JWH-018 | C24H23NO | 7.46 | 341.18006 | 341.17796 | 6.13 | 64 | 122556 | JWH-016 |
| JWH-016 | C24H23NO | 7.42 | 341.17868 | 341.17796 | 2.09 | 83 | 727863 | JWH-018 |
| JWH-007 | C25H25NO | 7.56 | 355.19409 | 355.19361 | 1.35 | 97 | 2276986 | JWH-019, JWH-122 |
| JWH-018-6-MeO | C25H25NO2 | 7.47 | 371.18949 | 371.18853 | 2.58 | 84 | 179250 | JWH-081 |
| Pravadoline WIN-48098 | C23H26N2O3 | 6.58 | 378.19627 | 378.19434 | 5.10 | 43 | 137908 | |
| JWH-098 | C26H27NO2 | 7.61 | 385.20562 | 385.20418 | 3.75 | 61 | 89646 | |
| AM-1241 | C22H22IN3O3 | 5.45 | 503.07024 | 503.07058 | –0.68 | 83 | 863232 | |
| AM-694 | C20H19FINO | 6.87 | 435.04974 | 435.04954 | 0.46 | 99 | 1099238 | |
| Synthetic cathinones | ||||||||
| Mephedrone | C11H15NO | 3.15 | 177.11515 | 177.11536 | –1.22 | 63 | 116712 | Phenmetrazine, AMMI |
| Methedrone | C11H15NO2 | 2.84 | 193.11062 | 193.11028 | 1.76 | 54 | 296298 | MDMA, 5-methyl-MDA |
| 4'-Methyl-α-pyrrolidino-propiophenone (MPPP) | C14H19NO | 3.42 | 217.14606 | 217.14666 | –2.80 | 93 | 352269 | |
| Butylone | C12H15NO3 | 3.01 | 221.10554 | 221.10519 | 1.57 | 79 | 433487 | Ethylone, metaxalone |
| Ethylone | C12H15NO3 | 2.78 | 221.10605 | 221.10519 | 3.89 | 74 | 268832 | Butylone, metaxalone |
| Methylenedioxy-pyrovalerone (MDPV) | C16H21NO3 | 3.80 | 275.15267 | 275.15214 | 1.92 | 91 | 564883 | |
| Methylone | C11H13NO3 | 2.61 | 207.09019 | 207.08954 | –3.12 | 94 | 1233166 | |
| Pentedrone | C12H17NO | 3.57 | 191.13125 | 191.13101 | –1.27 | 72 | 9596955 | Phendimetrazine, 4-MEC, DMMC |
| αpyrrolidino-propiophenone (α-PPP) | C13H17NO | 2.78 | 203.13121 | 203.13101 | –0.97 | 94 | 7569091 | |
| Naphyrone | C19H23NO | 5.28 | 281.17842 | 281.17796 | 1.63 | 91 | 1244472 | |
| Name . | Formula . | RT (min) . | Mass . | Mass (Tgt) . | Difference (ppm) . | Score . | Area . | Isobaric/isomer . |
|---|---|---|---|---|---|---|---|---|
| A) Stimulants, Benzodiazepines, Opiates and Muscle Relaxants | ||||||||
| Benzodiazepines | ||||||||
| 7-Aminoclonazepam | C15H12ClN3O | 3.63 | 285.06742 | 285.06689 | 1.86 | 99 | 3634238 | |
| 7-Aminoflunitrazepam | C16H14FN3O | 4.03 | 283.11256 | 283.11209 | 1.67 | 95 | 4776594 | |
| α-Hydroxyalprazolam | C17H13ClN4O | 5.79 | 324.07880 | 324.07779 | 3.11 | 97 | 976185 | |
| Alprazolam | C17H13ClN4 | 6.00 | 308.08351 | 308.08287 | 2.07 | 96 | 4959375 | |
| Chlordiazepoxide | C16H14ClN3O | 6.22 | 299.08356 | 299.08254 | 3.42 | 53 | 2049696 | |
| Clonazepam | C15H10ClN3O3 | 5.58 | 315.04196 | 315.04107 | 2.82 | 68 | 465799 | |
| Desalkylflurazepam | C15H10ClFN2O | 6.07 | 288.04695 | 288.04657 | 1.32 | 99 | 1519946 | |
| Diazepam | C16H13ClN2O | 6.47 | 284.07215 | 284.07164 | 1.79 | 98 | 4644744 | |
| Flunitrazepam | C16H12FN3O3 | 5.67 | 313.08731 | 313.08627 | 3.32 | 86 | 1829333 | |
| Lorazepam | C15H10Cl2N2O2 | 5.94 | 320.01432 | 320.01193 | 7.46 | 61 | 320460 | |
| Midazolam | C18H13ClFN3 | 6.16 | 325.07850 | 325.07820 | 0.90 | 97 | 6045516 | |
| Nordiazepam | C15H11ClN2O | 6.33 | 270.05713 | 270.05599 | 4.20 | 93 | 1756261 | |
| Oxazepam | C15H11ClN2O2 | 5.96 | 286.05055 | 286.05091 | –1.24 | 75 | 298293 | |
| Temazepam | C16H13ClN2O2 | 6.12 | 300.06778 | 300.06656 | 4.08 | 92 | 2226199 | |
| Triazolam | C17H12Cl2N4 | 5.95 | 342.04184 | 342.04390 | –6.03 | 90 | 5155100 | |
| Opiates/opioids | ||||||||
| 6-Acetylmorphine | C19H21NO4 | 2.53 | 327.14784 | 327.14706 | 2.39 | 90 | 1594081 | Naloxone |
| Buprenorphine | C29H41NO4 | 6.10 | 467.30399 | 467.30356 | 0.93 | 99 | 3938671 | |
| Morphine | C17H19NO3 | 1.87 | 285.13741 | 285.13649 | 3.20 | 77 | 1706304 | Hydromorphone |
| Hydromorphone | C17H19NO3 | 2.05 | 285.13751 | 285.13649 | 3.58 | 75 | 928698 | Morphine |
| Oxycodone | C18H21NO4 | 2.45 | 315.14930 | 315.14706 | 7.12 | 70 | 2070280 | |
| Oxymorphone | C17H19NO4 | 1.95 | 301.13224 | 301.13141 | 2.76 | 63 | 478331 | |
| Codeine | C18H21NO3 | 2.37 | 299.15311 | 299.15214 | 3.22 | 88 | 3426320 | Hydrocodone |
| Hydrocodone | C18H21NO3 | 2.51 | 299.15266 | 299.15214 | 1.71 | 91 | 2574367 | Codeine |
| Methadone | C21H27NO | 5.57 | 309.21014 | 309.20926 | 2.82 | 97 | 5113103 | |
| 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) | C20H23N | 4.64 | 277.18157 | 277.18305 | –5.35 | 89 | 6308463 | Amitriptyline |
| Fentanyl | C22H28N2O | 4.50 | 336.21862 | 336.22016 | –4.60 | 83 | 4629074 | |
| Tramadol | C16H25NO2 | 3.48 | 263.18810 | 263.18853 | 1.63 | 85 | 23521235 | O-desmethylvenlafaxine |
| Muscle relaxants | ||||||||
| Carisoprodol | C12H24N2O4 | 5.90 | 260.17231 | 260.17361 | –5.00 | 86 | 3693290 | |
| Meprobamate | C9H18N2O4 | 4.43 | 218.12684 | 218.12666 | 0.84 | 83 | 1348511 | |
| Cyclobenzaprine | C20H21N | 5.49 | 275.16828 | 275.16740 | 3.18 | 88 | 25722239 | |
| B) OTC Medications, Antidepressants, Antiepileptics and Hypnotics | ||||||||
| Stimulants | ||||||||
| Amphetamine | C9H13N | 2.76 | 135.10439 | 135.10480 | –3.06 | 98 | 2944608 | |
| Methamphetamine | C10H15N | 2.79 | 149.11999 | 149.12045 | –3.10 | 91 | 4458551 | Phentermine |
| Phentermine | C10H15N | 3.17 | 149.12006 | 149.12045 | –2.63 | 96 | 3246484 | Methamphetamine |
| MDMA | C11H15NO2 | 2.84 | 193.11048 | 193.11028 | 1.04 | 89 | 4508459 | Methedrone, 5-Methyl MDA |
| Methylenedioxy-amphetamine (MDA) | C10H13NO2 | 2.76 | 179.09564 | 179.09463 | 5.63 | 85 | 2926006 | |
| Ephedrine/pseudoephedrine | C10H15NO | 2.51 | 165.11625 | 165.11536 | 5.39 | 85 | 5913477 | p-Methoxyamphetamine |
| Cocaethylene | C18H23NO4 | 3.96 | 317.16290 | 317.16271 | 0.61 | 96 | 1661569 | |
| Cocaine | C17H21NO4 | 3.45 | 303.14774 | 303.14706 | 2.26 | 87 | 725916 | Scopalamine |
| Benzoylecgonine | C16H19NO4 | 3.31 | 289.13166 | 289.13141 | 0.88 | 98 | 8511535 | |
| Lidocaine | C14H22N2O | 3.23 | 234.17065 | 234.17321 | –10.93 | 50 | 95330 | |
| Phencyclidine (PCP) | C17H25N | 4.25 | 243.19764 | 243.19870 | –4.38 | 90 | 4676077 | |
| Methylphenidate | C14H19NO2 | 3.54 | 233.14192 | 233.14158 | 1.44 | 91 | 583504 | |
| Ritalinic acid | C13H17NO2 | 3.34 | 219.12702 | 219.12593 | 4.96 | 85 | 2444074 | |
| OTC/antihistamines | ||||||||
| Acetaminophen | C8H9NO2 | 2.32 | 151.06386 | 151.06333 | 3.51 | 80 | 1938626 | |
| Salicylic acid | C7H6O3 | 2.88 | 138.03159 | 138.03169 | –0.74 | 100 | 164003 | |
| Ibuprofen | C13H18O2 | 6.94 | 206.12960 | 206.13068 | –5.15 | 92 | 7325473 | |
| Naproxen | C14H14O3 | 6.22 | 230.09420 | 230.09429 | –0.62 | 99 | 1202612 | |
| Diphenhydramine | C17H21NO | 4.75 | 255.16152 | 255.16231 | –3.11 | 91 | 24757875 | Atomoxetine |
| Chlorpheniramine | C16H19ClN2 | 4.49 | 274.12361 | 274.12368 | –0.24 | 95 | 29133002 | |
| Promethazine | C17H20N2S | 5.24 | 284.13637 | 284.13472 | 5.81 | 85 | 1773708 | Promazine |
| Dextromethorphan | C18H25NO | 4.70 | 271.19384 | 271.19361 | –0.85 | 89 | 24551563 | |
| Antidepressants | ||||||||
| Mirtazapine | C17H19N3 | 3.87 | 265.16070 | 265.15790 | –10.56 | 84 | 8601329 | |
| Trazodone | C19H22ClN5O | 4.58 | 371.15230 | 371.15129 | –2.72 | 72 | 9515116 | |
| Sertraline | C17H17Cl2N | 5.96 | 305.07354 | 305.07381 | –0.88 | 95 | 19711190 | |
| Venlafaxine | C17H27NO2 | 4.43 | 277.20370 | 277.20418 | 1.73 | 87 | 25447867 | |
| Citalopram | C20H21FN2O | 4.62 | 324.16210 | 324.16379 | 5.21 | 90 | 24889357 | |
| Amitriptyline | C20H23N | 5.67 | 277.18318 | 277.18305 | –0.47 | 79 | 21923939 | EDDP |
| Nortriptyline | C19H21N | 5.74 | 263.16861 | 263.16740 | 4.61 | 91 | 23096205 | |
| Antiepileptics | ||||||||
| Phenytoin | C15H12N2O2 | 5.24 | 252.08940 | 252.08988 | –1.91 | 96 | 12299940 | Oxcarbazepine |
| Topiramate | C12H21NO8S | 4.59 | 339.09840 | 339.09879 | –1.10 | 87 | 34664699 | |
| Hypnotics | ||||||||
| Doxylamine | C17H22N2O | 3.78 | 270.17340 | 270.17321 | –0.70 | 95 | 22908918 | 5-MeO-DALT |
| Zaleplon | C17H15N5O | 5.06 | 305.12896 | 305.12766 | 4.25 | 81 | 1163895 | |
| Zolpidem | C19H21N3O | 4.30 | 307.16669 | 307.16846 | –5.78 | 66 | 4611269 | |
| Zopiclone | C17H17ClN6O3 | 3.46 | 388.10590 | 388.10507 | 2.16 | 92 | 5444754 | |
| C) Synthetic Cannabinoid Spice Compounds and Synthetic Cathinone Bath Salts | ||||||||
| Synthetic cannabinoids | ||||||||
| RCS-4 | C21H23NO2 | 7.29 | 321.17395 | 321.17288 | 3.34 | 83 | 4670598 | |
| JWH-073 | C23H21NO | 7.33 | 327.16322 | 327.16231 | 2.76 | 92 | 6744872 | JWH-015 |
| JWH-019 | C25H25NO | 7.64 | 355.19398 | 355.19361 | 1.03 | 90 | 8203283 | JWH-007, JWH-122 |
| JWH-122 | C25H25NO | 7.64 | 355.19399 | 355.19361 | 1.04 | 98 | 8180911 | JWH-007, JWH-019 |
| AM-2201 | C24H22FNO | 7.10 | 359.16917 | 359.16854 | 1.75 | 97 | 8283323 | |
| JWH-210 | C26H27NO | 7.77 | 369.20959 | 369.20926 | 0.87 | 86 | 467841 | JWH-020 |
| JWH-081 | C25H25NO2 | 7.55 | 371.18892 | 371.18853 | 1.05 | 99 | 6697934 | JWH-018-6-MeO |
| RCS-8 | C25H29NO2 | 7.69 | 375.22051 | 375.21983 | 1.82 | 99 | 3012613 | |
| JWH-200 | C25H24N2O2 | 6.69 | 384.18418 | 384.18378 | 1.04 | 93 | 16634958 | |
| JWH-251 | C22H25NO | 7.43 | 319.19418 | 319.19361 | 1.78 | 75 | 7669899 | |
| JWH-015 | C23H21NO | 7.23 | 327.16331 | 327.16231 | 3.05 | 94 | 10038079 | JWH-073 |
| JWH-250 | C22H25NO2 | 7.33 | 335.18924 | 335.18853 | 2.13 | 88 | 8064283 | JWH-201, JWH-302 |
| JWH-203 | C21H22ClNO | 7.42 | 339.13989 | 339.13899 | 2.64 | 86 | 220661 | |
| JWH-022 | C24H21NO | 7.35 | 339.16313 | 339.16231 | 2.40 | 78 | 563953 | |
| JWH-018 | C24H23NO | 7.46 | 341.18006 | 341.17796 | 6.13 | 64 | 122556 | JWH-016 |
| JWH-016 | C24H23NO | 7.42 | 341.17868 | 341.17796 | 2.09 | 83 | 727863 | JWH-018 |
| JWH-007 | C25H25NO | 7.56 | 355.19409 | 355.19361 | 1.35 | 97 | 2276986 | JWH-019, JWH-122 |
| JWH-018-6-MeO | C25H25NO2 | 7.47 | 371.18949 | 371.18853 | 2.58 | 84 | 179250 | JWH-081 |
| Pravadoline WIN-48098 | C23H26N2O3 | 6.58 | 378.19627 | 378.19434 | 5.10 | 43 | 137908 | |
| JWH-098 | C26H27NO2 | 7.61 | 385.20562 | 385.20418 | 3.75 | 61 | 89646 | |
| AM-1241 | C22H22IN3O3 | 5.45 | 503.07024 | 503.07058 | –0.68 | 83 | 863232 | |
| AM-694 | C20H19FINO | 6.87 | 435.04974 | 435.04954 | 0.46 | 99 | 1099238 | |
| Synthetic cathinones | ||||||||
| Mephedrone | C11H15NO | 3.15 | 177.11515 | 177.11536 | –1.22 | 63 | 116712 | Phenmetrazine, AMMI |
| Methedrone | C11H15NO2 | 2.84 | 193.11062 | 193.11028 | 1.76 | 54 | 296298 | MDMA, 5-methyl-MDA |
| 4'-Methyl-α-pyrrolidino-propiophenone (MPPP) | C14H19NO | 3.42 | 217.14606 | 217.14666 | –2.80 | 93 | 352269 | |
| Butylone | C12H15NO3 | 3.01 | 221.10554 | 221.10519 | 1.57 | 79 | 433487 | Ethylone, metaxalone |
| Ethylone | C12H15NO3 | 2.78 | 221.10605 | 221.10519 | 3.89 | 74 | 268832 | Butylone, metaxalone |
| Methylenedioxy-pyrovalerone (MDPV) | C16H21NO3 | 3.80 | 275.15267 | 275.15214 | 1.92 | 91 | 564883 | |
| Methylone | C11H13NO3 | 2.61 | 207.09019 | 207.08954 | –3.12 | 94 | 1233166 | |
| Pentedrone | C12H17NO | 3.57 | 191.13125 | 191.13101 | –1.27 | 72 | 9596955 | Phendimetrazine, 4-MEC, DMMC |
| αpyrrolidino-propiophenone (α-PPP) | C13H17NO | 2.78 | 203.13121 | 203.13101 | –0.97 | 94 | 7569091 | |
| Naphyrone | C19H23NO | 5.28 | 281.17842 | 281.17796 | 1.63 | 91 | 1244472 | |
The list of drugs found in Table IA is a collection of centrally acting depressants. This panel includes benzodiazepines, opiates and muscle relaxants. In this region, recreational drug abuse often centers on a combination of the latter three, and users choose a representative drug from each class. Because many cases revolve around this type of polypharmacy, the ability to screen and identify each of these drugs on demand can translate to faster confirmations and turnaround times. The benzodiazepine panel contains a mixture of 15 parent drugs and metabolites, which are known for their common presence among driving under the influence (DUI), drug-facilitated sexual assault (DFSA), and drug-related death cases. The opiate panel contains a number of isobaric pairs that can obfuscate the correct identification of morphine versus hydromorphone, or codeine versus hydrocodone. However, these isomers are easily resolvable at retention times of 1.87 versus 2.05 min and 2.37 versus 2.51 min due to their structural differences. The muscle relaxants panel showed good identification scores between 80 and 90%.
The group of drugs in Table IB is a compilation of stimulants, over-the-counter (OTC) medications, antidepressants, antiepileptics, and hypnotics. Some of the amphetamine-based stimulants tend to have mass overlaps and the newly emerging synthetic cathinones. The isobaric methamphetamine and phentermine are separated by approximately 0.4 min at 2.79 versus 3.17 min, but the diastereomers ephedrine and pseudoephedrine were unable to be resolved under these conditions. Likewise, 3,4-methylenedioxy-N-methylamphetamine (MDMA) was indistinguishable from the isobaric bath salt drug methedrone, even after modifying gradient conditions. In the OTC panel, the alkaline drugs gave better responses than the acidic drugs, but the therapeutic ranges for acidic drugs tend to be at least 10-fold higher, which offsets this behavior. The antidepressants tend to be alkaline in nature, so their relative responses and therapeutic dose ranges give robust signals.
In Table IC, there is an extensive panel of commonly known synthetic cannabinoids and cathinone derivatives with commercially available reference standards. This group has quickly become an emerging threat to public safety due to their availability and lack of detection by routine screens. Furthermore, these drugs continue to evolve as manufacturers creatively modify side chains to complicate identification of new drugs and alter drug potency. Twenty-three known synthetic cannabinoid compounds were analyzed to address this problem and many isobaric compounds were found within this class. The isomers JWH-019 and JWH-122 were not distinguishable by this method, because their masses and retention times coincided at 7.64 min. In addition, isobaric JWH-016 and JWH-018 gave retention times of 7.42 and 7.46, respectively, which is a difference of only 2.4 s. In the synthetic cathinone group, 10 possible candidate compounds were analyzed. The mass overlap between butylone and ethylone was already anticipated, but the issue was resolved by their widely spaced retention times at 2.78 and 3.01 min.
Assessment of extraction recovery and matrix effects
The type of extraction technique applied to a wide variety of compounds can have variable effects, usually leading to an overall decrease in the extraction yield when compared to standards. To characterize extraction recovery in this protocol, neat drugs and internal standard were spiked into mobile phase buffer and measured in comparison to the same standards that were spiked into blood and extracted. First, the responses were normalized by dividing drug area counts by the internal standard area counts. Then, the recovery yield was calculated by dividing normalized extracted area counts by the normalized area counts of drugs spiked in the mobile phase buffer. The summary of results by classes of compounds is filed in Table II.
Extraction Recovery and Matrix Effects on Analyzed Compounds
| Compounds . | Average Score . | Average mass error (ppm) . | % Extraction recovery (%) . | Matrix effect (%) . |
|---|---|---|---|---|
| Benzodiazepines and metabolites | 81 | 1.1 | 35–75 | 60–90 |
| Opiates and opioids | 81 | –0.6 | 25–80 | 15–55 |
| Cocaine and metabolites | 83 | –3.4 | 15–70 | 70–95 |
| Amphetamines | 68 | 0.8 | 10–95 | 80–105 |
| Phencyclidine | 63 | –1.6 | 23 | 67 |
| Antidepressants | 97 | 2.3 | 40–95 | 90–120 |
| Carisoprodol/meprobamate | 81 | –1.2 | 60–80 | 70–90 |
| Acetaminophen | 82 | 0.4 | 53 | 84 |
| Hypnotics | 75 | –1 | 65–70 | 115–120 |
| Bath salts (cathinones) | 74 | –0.6 | 25–85 | 80–120 |
| Spice (cannabinoids) | 69 | 4.7 | 15–50 | 30–65 |
| Compounds . | Average Score . | Average mass error (ppm) . | % Extraction recovery (%) . | Matrix effect (%) . |
|---|---|---|---|---|
| Benzodiazepines and metabolites | 81 | 1.1 | 35–75 | 60–90 |
| Opiates and opioids | 81 | –0.6 | 25–80 | 15–55 |
| Cocaine and metabolites | 83 | –3.4 | 15–70 | 70–95 |
| Amphetamines | 68 | 0.8 | 10–95 | 80–105 |
| Phencyclidine | 63 | –1.6 | 23 | 67 |
| Antidepressants | 97 | 2.3 | 40–95 | 90–120 |
| Carisoprodol/meprobamate | 81 | –1.2 | 60–80 | 70–90 |
| Acetaminophen | 82 | 0.4 | 53 | 84 |
| Hypnotics | 75 | –1 | 65–70 | 115–120 |
| Bath salts (cathinones) | 74 | –0.6 | 25–85 | 80–120 |
| Spice (cannabinoids) | 69 | 4.7 | 15–50 | 30–65 |
Extraction Recovery and Matrix Effects on Analyzed Compounds
| Compounds . | Average Score . | Average mass error (ppm) . | % Extraction recovery (%) . | Matrix effect (%) . |
|---|---|---|---|---|
| Benzodiazepines and metabolites | 81 | 1.1 | 35–75 | 60–90 |
| Opiates and opioids | 81 | –0.6 | 25–80 | 15–55 |
| Cocaine and metabolites | 83 | –3.4 | 15–70 | 70–95 |
| Amphetamines | 68 | 0.8 | 10–95 | 80–105 |
| Phencyclidine | 63 | –1.6 | 23 | 67 |
| Antidepressants | 97 | 2.3 | 40–95 | 90–120 |
| Carisoprodol/meprobamate | 81 | –1.2 | 60–80 | 70–90 |
| Acetaminophen | 82 | 0.4 | 53 | 84 |
| Hypnotics | 75 | –1 | 65–70 | 115–120 |
| Bath salts (cathinones) | 74 | –0.6 | 25–85 | 80–120 |
| Spice (cannabinoids) | 69 | 4.7 | 15–50 | 30–65 |
| Compounds . | Average Score . | Average mass error (ppm) . | % Extraction recovery (%) . | Matrix effect (%) . |
|---|---|---|---|---|
| Benzodiazepines and metabolites | 81 | 1.1 | 35–75 | 60–90 |
| Opiates and opioids | 81 | –0.6 | 25–80 | 15–55 |
| Cocaine and metabolites | 83 | –3.4 | 15–70 | 70–95 |
| Amphetamines | 68 | 0.8 | 10–95 | 80–105 |
| Phencyclidine | 63 | –1.6 | 23 | 67 |
| Antidepressants | 97 | 2.3 | 40–95 | 90–120 |
| Carisoprodol/meprobamate | 81 | –1.2 | 60–80 | 70–90 |
| Acetaminophen | 82 | 0.4 | 53 | 84 |
| Hypnotics | 75 | –1 | 65–70 | 115–120 |
| Bath salts (cathinones) | 74 | –0.6 | 25–85 | 80–120 |
| Spice (cannabinoids) | 69 | 4.7 | 15–50 | 30–65 |
Endogenous compounds in biological specimens can introduce interferences or enhancements known as matrix effects. Therefore, decreases or increases in signal are not attributable to extraction loss, but through the influence of pre-existing compounds in the sample that are purified along with the analytes of interest. Using a similar approach mentioned previously, the blood was first extracted without spiking drugs to recover the biological matrix compounds, and then the drugs and internal standard were added. These samples were compared to neat drugs spiked into mobile phase buffer, with area count responses normalized and quotients derived as described previously, and the results are listed in Table II.
There are multiple issues to consider when choosing SPE as the extraction method. One aspect is that it amenable to the automation of high-throughput processing. It also renders a very clean sample for injection onto an ultra-high pressure liquid chromatography system, to achieve sharp separations and avoid column clogging or backpressure. On the other hand, it is difficult to have high yields among compounds with different physicochemical properties. However, these results show modest recovery yields for routine analytes such as benzodiazepines, opiates, cocaine, amphetamines, phencyclidine, acetaminophen and bath salt compounds (Table II). Yields were comparatively better in antidepressants, muscle relaxants and hypnotics. The synthetic cannabinoids known as Spice compounds tended to have poorer recovery, likely due to their strong lipophilic nature.
Matrix effects were also found to influence to detection capacities among the analytical groups. In these calculations, values less than 100% represent a suppression of signal, whereas values greater than 100% reveal signal enhancements. Although benzodiazepines, opiates and cocaine tend to extract moderately well, matrix effects show some signal suppression, with opiates being the most affected (15–55% signal return) out of these groups (Table II). Many of the other classes such as antidepressants, amphetamines and bath salts show mild suppression, which is balanced by signal enhancements of up to 120% signal return. The hypnotic drugs displayed overall positive matrix effects, with enhancements consistently above 100%. Again, the synthetic cannabinoids displayed comparatively poor recovery and significant signal suppression (30–65%), possibly due to the co-extraction of lipids that interfere with ionization. Although extraction yields and negative matrix effects can adversely affect recovery, signals appear to be high enough to deliver passing scores for positive screening detection.
Method application to case specimens
Although the method was developed using model matrix spiked with standard drug samples, the process was validated by screening authentic case samples involving blood, serum and urine. A random selection of drug-positive casework that had already been characterized by conventional means involving immunoassay, GC–MS and LC–MS-MS was chosen for TOF analysis. Aliquots of the case specimens were combined with internal standards, extracted, and applied to the LC–TOF-MS according to the steps and conditions described previously. The validation results are listed with results from conventional screens and confirmations in Table III.
LC–TOF-MS Screening Compared with Screening and Confirmation Results by conventional Methods in Authentic Case Samples
| Case . | Specimen . | Found by LC–TOF-MS . | Confirmed by other means . | Missed by LC–TOF-MS . |
|---|---|---|---|---|
| 1 | Blood | Amphetamine | 0.37 mg/L | |
| 2 | Blood | Oxazepam | 0.73 mg/L | |
| Hydrocodone | 0.21 mg/L | |||
| 3 | Blood | Oxazepam | 0.10 mg/L | |
| Temazepam | 0.97 mg/L | |||
| Benzoylecgonine | 1.6 mg/L | |||
| 4 | Blood | Oxazepam | 1.3 mg/L | |
| Cyclobenzaprine | 0.65 mg/L | |||
| Paroxetine | Present | |||
| 5 | Blood | Lorazepam | 0.17 mg/L | |
| Phenytoin | Present | |||
| 6 | Serum | Codeine | 2.3 mg/L | |
| Morphine | 0.49 mg/L | |||
| Methadone | 0.41 mg/L | |||
| 7 | Serum | Benzoylecgonine/cocaine | Present | |
| 8 | Serum | 7-Aminoclonazepam | Present | |
| 9 | Serum | Metoprolol | Present | |
| 10 | Serum | Tramadol | Present | |
| Desmethyltramadol | Present | |||
| Ketamine | Present | |||
| 11 | Serum | Lorazepam | Present | |
| Carisoprodol/meprobamate | Present | |||
| 12 | Serum | MDMA/MDA | Present | |
| 13 | Serum | Oxycodone | Present | Butalbital |
| 14 | Serum | Acetaminophen | Present | |
| Meperidine/normeperidine | Present | |||
| 15 | Serum | Phenytoin | 13 mg/L | |
| 16 | Serum | Buprenorphine | 0.02 mg/L | |
| Fluoxetine/norfluoxetine | Present | |||
| 17 | Urine | Acetaminophen | Present | |
| Methadone/EDDP | Present | |||
| Codeine/morphine | Present | |||
| 18 | Urine | Trazodone | Present | |
| Temazepam | 0.22 mg/L | |||
| 19 | Urine | MDMA/MDA | Present | |
| 20 | Urine | Acetaminophen | Present | |
| Morphine | 0.10 mg/L | |||
| Meperidine/normeperidine | Present | |||
| 21 | Urine | Buprenorphine | Present | |
| Fluoxetine/Norfluoxetine | Present |
| Case . | Specimen . | Found by LC–TOF-MS . | Confirmed by other means . | Missed by LC–TOF-MS . |
|---|---|---|---|---|
| 1 | Blood | Amphetamine | 0.37 mg/L | |
| 2 | Blood | Oxazepam | 0.73 mg/L | |
| Hydrocodone | 0.21 mg/L | |||
| 3 | Blood | Oxazepam | 0.10 mg/L | |
| Temazepam | 0.97 mg/L | |||
| Benzoylecgonine | 1.6 mg/L | |||
| 4 | Blood | Oxazepam | 1.3 mg/L | |
| Cyclobenzaprine | 0.65 mg/L | |||
| Paroxetine | Present | |||
| 5 | Blood | Lorazepam | 0.17 mg/L | |
| Phenytoin | Present | |||
| 6 | Serum | Codeine | 2.3 mg/L | |
| Morphine | 0.49 mg/L | |||
| Methadone | 0.41 mg/L | |||
| 7 | Serum | Benzoylecgonine/cocaine | Present | |
| 8 | Serum | 7-Aminoclonazepam | Present | |
| 9 | Serum | Metoprolol | Present | |
| 10 | Serum | Tramadol | Present | |
| Desmethyltramadol | Present | |||
| Ketamine | Present | |||
| 11 | Serum | Lorazepam | Present | |
| Carisoprodol/meprobamate | Present | |||
| 12 | Serum | MDMA/MDA | Present | |
| 13 | Serum | Oxycodone | Present | Butalbital |
| 14 | Serum | Acetaminophen | Present | |
| Meperidine/normeperidine | Present | |||
| 15 | Serum | Phenytoin | 13 mg/L | |
| 16 | Serum | Buprenorphine | 0.02 mg/L | |
| Fluoxetine/norfluoxetine | Present | |||
| 17 | Urine | Acetaminophen | Present | |
| Methadone/EDDP | Present | |||
| Codeine/morphine | Present | |||
| 18 | Urine | Trazodone | Present | |
| Temazepam | 0.22 mg/L | |||
| 19 | Urine | MDMA/MDA | Present | |
| 20 | Urine | Acetaminophen | Present | |
| Morphine | 0.10 mg/L | |||
| Meperidine/normeperidine | Present | |||
| 21 | Urine | Buprenorphine | Present | |
| Fluoxetine/Norfluoxetine | Present |
LC–TOF-MS Screening Compared with Screening and Confirmation Results by conventional Methods in Authentic Case Samples
| Case . | Specimen . | Found by LC–TOF-MS . | Confirmed by other means . | Missed by LC–TOF-MS . |
|---|---|---|---|---|
| 1 | Blood | Amphetamine | 0.37 mg/L | |
| 2 | Blood | Oxazepam | 0.73 mg/L | |
| Hydrocodone | 0.21 mg/L | |||
| 3 | Blood | Oxazepam | 0.10 mg/L | |
| Temazepam | 0.97 mg/L | |||
| Benzoylecgonine | 1.6 mg/L | |||
| 4 | Blood | Oxazepam | 1.3 mg/L | |
| Cyclobenzaprine | 0.65 mg/L | |||
| Paroxetine | Present | |||
| 5 | Blood | Lorazepam | 0.17 mg/L | |
| Phenytoin | Present | |||
| 6 | Serum | Codeine | 2.3 mg/L | |
| Morphine | 0.49 mg/L | |||
| Methadone | 0.41 mg/L | |||
| 7 | Serum | Benzoylecgonine/cocaine | Present | |
| 8 | Serum | 7-Aminoclonazepam | Present | |
| 9 | Serum | Metoprolol | Present | |
| 10 | Serum | Tramadol | Present | |
| Desmethyltramadol | Present | |||
| Ketamine | Present | |||
| 11 | Serum | Lorazepam | Present | |
| Carisoprodol/meprobamate | Present | |||
| 12 | Serum | MDMA/MDA | Present | |
| 13 | Serum | Oxycodone | Present | Butalbital |
| 14 | Serum | Acetaminophen | Present | |
| Meperidine/normeperidine | Present | |||
| 15 | Serum | Phenytoin | 13 mg/L | |
| 16 | Serum | Buprenorphine | 0.02 mg/L | |
| Fluoxetine/norfluoxetine | Present | |||
| 17 | Urine | Acetaminophen | Present | |
| Methadone/EDDP | Present | |||
| Codeine/morphine | Present | |||
| 18 | Urine | Trazodone | Present | |
| Temazepam | 0.22 mg/L | |||
| 19 | Urine | MDMA/MDA | Present | |
| 20 | Urine | Acetaminophen | Present | |
| Morphine | 0.10 mg/L | |||
| Meperidine/normeperidine | Present | |||
| 21 | Urine | Buprenorphine | Present | |
| Fluoxetine/Norfluoxetine | Present |
| Case . | Specimen . | Found by LC–TOF-MS . | Confirmed by other means . | Missed by LC–TOF-MS . |
|---|---|---|---|---|
| 1 | Blood | Amphetamine | 0.37 mg/L | |
| 2 | Blood | Oxazepam | 0.73 mg/L | |
| Hydrocodone | 0.21 mg/L | |||
| 3 | Blood | Oxazepam | 0.10 mg/L | |
| Temazepam | 0.97 mg/L | |||
| Benzoylecgonine | 1.6 mg/L | |||
| 4 | Blood | Oxazepam | 1.3 mg/L | |
| Cyclobenzaprine | 0.65 mg/L | |||
| Paroxetine | Present | |||
| 5 | Blood | Lorazepam | 0.17 mg/L | |
| Phenytoin | Present | |||
| 6 | Serum | Codeine | 2.3 mg/L | |
| Morphine | 0.49 mg/L | |||
| Methadone | 0.41 mg/L | |||
| 7 | Serum | Benzoylecgonine/cocaine | Present | |
| 8 | Serum | 7-Aminoclonazepam | Present | |
| 9 | Serum | Metoprolol | Present | |
| 10 | Serum | Tramadol | Present | |
| Desmethyltramadol | Present | |||
| Ketamine | Present | |||
| 11 | Serum | Lorazepam | Present | |
| Carisoprodol/meprobamate | Present | |||
| 12 | Serum | MDMA/MDA | Present | |
| 13 | Serum | Oxycodone | Present | Butalbital |
| 14 | Serum | Acetaminophen | Present | |
| Meperidine/normeperidine | Present | |||
| 15 | Serum | Phenytoin | 13 mg/L | |
| 16 | Serum | Buprenorphine | 0.02 mg/L | |
| Fluoxetine/norfluoxetine | Present | |||
| 17 | Urine | Acetaminophen | Present | |
| Methadone/EDDP | Present | |||
| Codeine/morphine | Present | |||
| 18 | Urine | Trazodone | Present | |
| Temazepam | 0.22 mg/L | |||
| 19 | Urine | MDMA/MDA | Present | |
| 20 | Urine | Acetaminophen | Present | |
| Morphine | 0.10 mg/L | |||
| Meperidine/normeperidine | Present | |||
| 21 | Urine | Buprenorphine | Present | |
| Fluoxetine/Norfluoxetine | Present |
The TOF-derived data reconciles very well with results determined by conventional means. The PCDL database was able to correctly identify the drugs tested in this validation, in addition to other pre-existing drugs in the library, such as paroxetine, metoprolol, ketamine, desmethyltramadol, meperidine/normeperidine and fluoxetine/norfluoxetine. In Case 13, there was an instance in which butalbital was present, but not detected by this method. Barbiturates tend to ionize better in negative mode ionization (22,23), so a shorter and separate negative ion method should be developed and optimized to screen for barbiturates and other acidic compounds.
Discussion
Drug screening by LC–TOF-MS was organized into classes of compounds by pharmacologic action rather than physicochemical relationships. One of the surprising outcomes of analysis was the number of isobaric drug compounds that can confound the initial identification of isomers that cannot or have not been chromatographically resolved. With this knowledge, the liquid chromatography step must be forward-compatible with current and new drugs to provide adequate separation. Therefore, an identity based on chemical formula or accurate mass alone is insufficient, even for a compound that is presumed to be present.
The use of LC–TOF-MS for screening purposes has gained popularity in recent years. Different laboratories have developed screening methods for drugs of abuse, pesticides (18), veterinary drugs (5,14,24), doping agents (20,21,25) and other poisons in different matrices such as blood (26), urine (18,27–31), vitreous humor (32), hair (23,33,34) and food (23,24,27,28,31–33,35). The original fame of accurate mass for confirming theoretical molecular formulas for synthetic compounds has grown to include its use in the identification of unknowns and searching for target compounds in complex matrices (23,29,36). Mass measurements that are accurate to several decimal places (rather than nominal or unit mass measurements of ±0.1 Da), coupled with the creation of a target drug compound list with retention time data allows for rapid, selective and specific screening of target compounds.
In this laboratory, more than 100 pure reference standard drug compounds routinely and newly encountered in forensic toxicology examinations were analyzed, to create an entry in an accurate-mass retention-time (AMRT) Personal Compound Database. The retention time data provide differentiation between isobaric compounds, which lends high confidence to the identification and specificity of the method. Isotope spacing and isotope abundance ratios are additional measurements used to provide additional confidence in the identification of target compounds.
The ability of LC–TOF-MS to acquire accurate mass measurements provides data that will give the analyst confidence in the detection of target compounds. The feature of all-scan, all-the-time TOF data not only allows the detection of known target compounds, but also allows retrospective searching for compounds that may be present but not originally sought or currently identified. This is particularly important in forensic toxicology, in which new designer drugs such as Spice and bath salts are continuously emerging and adapting (37–40) to meet consumer demands and to outflank legal pressures.
Conclusions
In a forensic toxicology environment, it is critical to maintain an expansive and comprehensive screening method that reveals or excludes the presence of drugs as soon as possible. The use of LC–TOF-MS technology helps to address this need by providing fast, broad, inclusive scans that can be retrospectively data-mined as new investigational clues emerge. As this method is expanded, many other drugs will be included that often resist detection by routine means. For example, new additions would include: the antipsychotic drugs such as risperidone, ziprasidone, and haloperidol, which are responsible for neuroleptic malignant syndrome; levetiracetam, lamotrigine, gabapentin, pregabalin, tizanidine and many others that contribute to impairment, combined toxicity or are important in determining compliance with prescribed therapy.
Acknowledgments
This project was supported by NIJ Award #2010-DN-BX-K210, NFSTC #25-6370-08, awarded by the National Institutes of Justice, Office of Justice Programs, and the U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Department of Justice. In addition, the authors are grateful for the assistance provided by Dr. John Hughes and David Presser from Agilent Technologies in method development and technical support.