-
PDF
- Split View
-
Views
-
Cite
Cite
Kohei Nakachi, Junji Furuse, Hiroshi Ishii, Ei-ichiro Suzuki, Masahiro Yoshino, Prognostic Factors in Patients with Gemcitabine-Refractory Pancreatic Cancer, Japanese Journal of Clinical Oncology, Volume 37, Issue 2, February 2007, Pages 114–120, https://doi.org/10.1093/jjco/hyl144
Close - Share Icon Share
Abstract
The purpose of this study was to identify prognostic factors in patients with gemcitabine-refractory pancreatic cancer and to determine criteria for selecting candidates for second-line treatment.
The records of 74 patients who were treated with gemcitabine (GEM) and followed up until disease progression were reviewed retrospectively. Sixteen clinical variables at the time of disease progression after GEM chemotherapy were chosen for analysis in this study. Univariate and multivariate analyses were conducted to identify prognostic factors associated with survival.
At the time of analysis, 71 patients had died because of tumor progression. The overall median survival time was 5.1 months after first-line chemotherapy with GEM was initiated. Median survival time after disease progression was 2.0 months. Three factors, performance status, peritoneal dissemination and C-reactive protein level, were identified as independent prognostic factors in multivariate analysis. Median survival time in the good prognosis group (patients with performance status 0 or 1, no peritoneal dissemination and C-reactive protein <5.0 mg/dl) was 3.4 months.
Performance status, serum level of C-reactive protein and peritoneal dissemination were identified as important prognostic factors in patients with GEM-refractory pancreatic cancer. These factors should be considered in determining the treatment following first-line chemotherapy in patients with advanced pancreatic cancer.
INTRODUCTION
Pancreatic cancer (PC) is the fifth leading cause of cancer deaths in Japan, with approximately 19 000 patients dying from this disease every year (1). Unfortunately, the majority of patients present with the disease already in an unresectable state at the time of diagnosis, due to locally advanced or metastatic spread. In recent years, gemcitabine (GEM), which is associated with more clinical benefits and better survival compared with 5-fluorouracil, has been widely used as a standard first-line chemotherapy agent for unresectable PC (2). Molecular targeted agents have also been developed for pancreatic cancer. It has been demonstrated that erlotinib combined with GEM improve survival (3). Moreover, another epidermal growth factor receptor (EGFR)-inhibitor, namely cetuximab, has been tried in phase II study (4). The efficacy of GEM is still unsatisfactory, and therefore the prognosis for patients remains poor, with a median survival time (MST) of around 6 months. In order to improve survival, it is necessary to develop not only a more effective first-line regimen, but also effective agents for second-line chemotherapy.
Generally PC progresses rapidly, and a patient's general condition often deteriorates too rapidly to perform any additional chemotherapy after failure of first-line treatment with GEM. Some patients may suffer from more serious adverse effects during second-line chemotherapy compared with first-line chemotherapy. Therefore, second-line chemotherapy is difficult to establish and should be initiated with more care than first-line. It is necessary to clarify the natural prognosis for patients with GEM-refractory PC in order to appropriately treat patients with either additional chemotherapy or best supportive care. Furthermore, identification of prognostic factors after GEM failure can help conducting clinical trials for promising second-line chemotherapy agents in patients with advanced PC. Thus, in the present study we investigated survival and prognostic factors in GEM-refractory PC patients and clarified criteria for selecting appropriate candidates for second-line therapy.
PATIENTS AND METHODS
Patients
In Japan, GEM was approved for the treatment of PC by the Ministry of Health, Labor, and Welfare in April 2001. GEM-refractory pancreatic cancer was defined as PC that progresses after chemotherapy with GEM. A total of 210 patients with histologically or cytologically confirmed unresectable PC who had received no other treatment were admitted to our hospital between April 2001 and March 2004. Forty patients were treated with chemoradiotherapy, 33 received palliative treatment and 137 were treated with systemic chemotherapy. Of the 137 patients who received systemic chemotherapy, 100 patients were treated with GEM alone, and 37 were treated with other regimens as part of clinical trials. Twenty-six of the 100 patients treated with GEM alone were excluded from analysis in this study because they were transferred to other hospitals and could not be followed up until disease progression. Data from the remaining 74 patients were analyzed in the current study. These patients were consistent with the criteria of GEM-refractory pancreatic cancer.
Treatment and Assessment of Efficacy
GEM as the first-line chemotherapy agent was administered weekly at a dose of 1000 mg/m2 in a 30-min intravenous infusion for three consecutive weeks, followed by a week of rest. Treatment was continued until disease progression, patient refusal, or unacceptable toxicity.
Tumor response was evaluated by enhanced computed tomography (CT) or magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumors (RECIST) at least every 8 weeks. Disease progression was defined as confirmation of progressive disease (PD) on the RECIST or clinical deterioration of the patient's general condition.
Factors Analyzed
Sixteen clinical variables at the time of disease progression after GEM chemotherapy were chosen in this study. Each variable was divided into two categories based on previous investigations (5–11) as follows: age (<65 or ≥65 years), sex (male or female), performance status (PS; 0, 1 or 2–4), white blood cell count (< 10,000 or ≥ 10 000/μl), hemoglobin level (<8.0 or ≥8.0 g/dl), platelet count (< 100 000 or ≥ 100 000/μl), serum total bilirubin level (<2.0 or ≥2.0 mg/dl), serum albumin level (≤2.8 or > 2.8 g/dl), serum lactate dehydrogenase level (< 400 or ≥400 IU/l), serum C-reactive protein (CRP) level (<5.0 or ≥5.0 mg/dl), serum creatinine level (<1.0 or ≥1.0 mg/dl), size of primary tumor (<50 mm or ≥50 mm), liver metastasis (presence or absence), ascites or peritoneal dissemination (presence or absence), serum carcinoembryonic antigen (CEA) level (< 100 or ≥ 100 ng/ml), and serum carbohydrate antigen 19-9 (CA 19-9) level (< 10 000 or ≥ 10 000 U/ml). PS was evaluated according to the Eastern Cooperative Oncology Group criteria. The size of the primary tumor was measured by enhanced CT. Peritoneal dissemination was defined as recognition of peritoneal nodules in CT scans or accumulation of ascites.
Statistical Methods
Overall survival for first-line GEM treatment was calculated from the day chemotherapy was started to either the day of death or the last day of follow-up. Overall survival after progression was calculated from the day when disease progression was confirmed with imaging examinations to either the day of death or the last day of follow-up. Patients whose disease progression was not evaluated with imaging examinations because of rapid general deterioration were also included in this study and the day GEM chemotherapy was determined to terminate was defined as the day of disease progression. Survival data were analyzed using the Kaplan–Meier method. Differences in survival were evaluated by log-rank tests. The Cox proportional hazards model was used to determine the most significant variables related to survival. Forward and backward stepwise regression procedures based on the partial likelihood ratio were used to determine the major independent predictors of survival. Statistical analyses were performed using the SPSS II 11.0J software package for Windows (SPSS Japan, Tokyo, Japan). All P values presented in this report are of the two-tailed type; P < 0.05 was considered to be statistically significant.
RESULTS
Patient characteristics are shown in Table 1. Seventy (94.6%) of the 74 patients had distant metastasis. In response to the initial treatment with GEM, three patients showed a partial response, 39 showed a stable disease; 23 patients, however, showed a progressive disease. The remaining nine were not evaluable with imaging examinations because of rapid general deterioration due to disease progression. The overall response rate to first-line chemotherapy with GEM alone was thus 4.1% (95% CI: 0.1–11.4%).
Patient characteristics
| Characteristics . | No. of patients (%) . |
|---|---|
| Age [median (range)] | 61.5 (37–90) |
| Gender | |
| Male | 44 (59.5) |
| Female | 30 (40.5) |
| Performance status | |
| 0–1 | 49 (66.2) |
| 2–4 | 25 (33.8) |
| Primary tumor site | |
| head | 23 (31.1) |
| 51 (68.9) | |
| Distant metastasis | |
| absent | 4 (5.4) |
| present | 70 (94.6) |
| liver | 56 |
| peritoneum | 23 |
| lung | 8 |
| distant lymph node | 15 |
| adrenal gland | 2 |
| bone | 2 |
| ovary | 1 |
| Carbohydrate antigen 19-9 (U/ml) | |
| [median (25–75 percentile)] | 1974.5 (305.8–5886.5) |
| Carcinoembryonic antigen (ng/ml) | |
| [median (25–75 percentile)] | 15.7 (7.7–40.3) |
| Characteristics . | No. of patients (%) . |
|---|---|
| Age [median (range)] | 61.5 (37–90) |
| Gender | |
| Male | 44 (59.5) |
| Female | 30 (40.5) |
| Performance status | |
| 0–1 | 49 (66.2) |
| 2–4 | 25 (33.8) |
| Primary tumor site | |
| head | 23 (31.1) |
| 51 (68.9) | |
| Distant metastasis | |
| absent | 4 (5.4) |
| present | 70 (94.6) |
| liver | 56 |
| peritoneum | 23 |
| lung | 8 |
| distant lymph node | 15 |
| adrenal gland | 2 |
| bone | 2 |
| ovary | 1 |
| Carbohydrate antigen 19-9 (U/ml) | |
| [median (25–75 percentile)] | 1974.5 (305.8–5886.5) |
| Carcinoembryonic antigen (ng/ml) | |
| [median (25–75 percentile)] | 15.7 (7.7–40.3) |
Patient characteristics
| Characteristics . | No. of patients (%) . |
|---|---|
| Age [median (range)] | 61.5 (37–90) |
| Gender | |
| Male | 44 (59.5) |
| Female | 30 (40.5) |
| Performance status | |
| 0–1 | 49 (66.2) |
| 2–4 | 25 (33.8) |
| Primary tumor site | |
| head | 23 (31.1) |
| 51 (68.9) | |
| Distant metastasis | |
| absent | 4 (5.4) |
| present | 70 (94.6) |
| liver | 56 |
| peritoneum | 23 |
| lung | 8 |
| distant lymph node | 15 |
| adrenal gland | 2 |
| bone | 2 |
| ovary | 1 |
| Carbohydrate antigen 19-9 (U/ml) | |
| [median (25–75 percentile)] | 1974.5 (305.8–5886.5) |
| Carcinoembryonic antigen (ng/ml) | |
| [median (25–75 percentile)] | 15.7 (7.7–40.3) |
| Characteristics . | No. of patients (%) . |
|---|---|
| Age [median (range)] | 61.5 (37–90) |
| Gender | |
| Male | 44 (59.5) |
| Female | 30 (40.5) |
| Performance status | |
| 0–1 | 49 (66.2) |
| 2–4 | 25 (33.8) |
| Primary tumor site | |
| head | 23 (31.1) |
| 51 (68.9) | |
| Distant metastasis | |
| absent | 4 (5.4) |
| present | 70 (94.6) |
| liver | 56 |
| peritoneum | 23 |
| lung | 8 |
| distant lymph node | 15 |
| adrenal gland | 2 |
| bone | 2 |
| ovary | 1 |
| Carbohydrate antigen 19-9 (U/ml) | |
| [median (25–75 percentile)] | 1974.5 (305.8–5886.5) |
| Carcinoembryonic antigen (ng/ml) | |
| [median (25–75 percentile)] | 15.7 (7.7–40.3) |
Treatment after Failure of GEM Chemotherapy
Generally, we prioritized using agents on clinical trials (e.g. phase I) for treatment after GEM chemotherapy had failed. However, chemotherapy using GEM alone continued if patients were in good general condition, even after disease progression was evident during imaging examinations. Best supportive care alone was provided for patients who refused to continue chemotherapy or in whom general condition deteriorated.
After disease progression, of the 74 patients, 14 (18.9%) continued GEM chemotherapy, two (2.7%) participated in a phase I study of a new anti-cancer agent, and the remaining 58 (78.4%) patients received best supportive care.
Survival
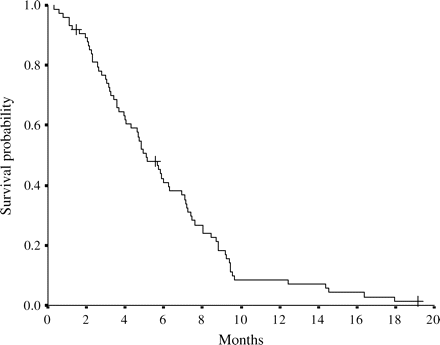
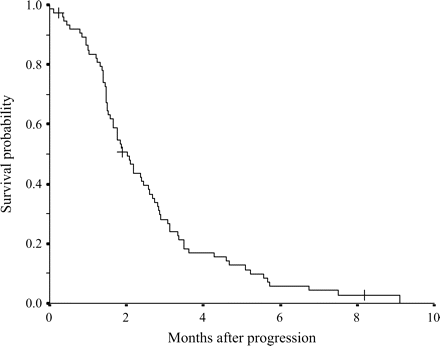
At the time of analysis, 71 patients had died from tumor progression. Overall MST was 5.1 months after first-line chemotherapy with GEM was initiated (Fig. 1). MST after disease progression was 2.0 months (95% CI: 1.7–2.4 months) (Fig. 2).
Overall survival curve for 74 pancreatic cancer patients from the day of the start of gemcitabine chemotherapy. The ‘plus’ sign indicates censored cases. Median survival is 5.1 months.
Overall survival curve for 74 pancreatic cancer patients after progression of the first-line of GEM chemotherapy. The ‘plus’ sign indicates censored cases. Median survival is 2.0 months.
Univariate Analysis
Among the 16 variables, seven variables were identified as being significantly associated with shorter survival time: PS of 2–4, platelet count of <100 000/μl, serum total bilirubin level of ≥2.0 mg/dl, serum albumin level of <2.8 g/dl, serum CRP level of ≥5.0 mg/dl, presence of peritoneal dissemination and serum CA 19-9 level of ≥10 000 U/ml (Table 2).
Univariate analysis
| Variable . | n . | Median survival (months) . | P-value . |
|---|---|---|---|
| Age | |||
| <65 | 47 | 1.9 | 0.9853 |
| ≥65 | 27 | 2.1 | |
| Sex | |||
| Male | 30 | 2.6 | 0.2441 |
| Female | 44 | 1.8 | |
| Performance status | |||
| 0–1 | 49 | 2.6 | 0.0007 |
| 2–4 | 25 | 1.5 | |
| White blood ell | |||
| <10 000/mm3 | 68 | 1.9 | 0.9720 |
| ≥10 000/mm3 | 6 | 2.6 | |
| Hemoglobin | |||
| <8.0 g/dl | 9 | 1.6 | 0.1953 |
| ≥8.0 g/dl | 65 | 2.1 | |
| Platelet | |||
| <100 000/μl | 7 | 1.4 | 0.0498 |
| ≥100 000/μl | 67 | 2.1 | |
| Total bilirubin | |||
| <2.0 mg/dl | 65 | 2.0 | 0.0479 |
| ≥2.0 mg/dl | |||
| Albumin | |||
| <2.8 g/dl | 15 | 1.5 | 0.0023 |
| ≥2.8 g/dl | 59 | 2.2 | |
| Lactate dehydrogenase | |||
| <400 IU/l | 65 | 2.1 | 0.1053 |
| ≥400 IU/l | 9 | 1.7 | |
| C-reactive protein | |||
| <5.0 mg/dl | 56 | 2.4 | <0.0001 |
| ≥5.0 mg/dl | 18 | 1.4 | |
| Serum creatinine | |||
| <1.0 mg/dl | 62 | 2.2 | 0.5390 |
| ≥1.0 mg/dl | 12 | 1.7 | |
| Primary tumor size | |||
| <50 mm | 40 | 2.2 | 0.1402 |
| ≥50 mm | 34 | 1.8 | |
| Liver metastasis | |||
| Absent | 18 | 1.6 | 0.6471 |
| Present | 56 | 2.1 | |
| Carcinoembryonic antigen | |||
| <100 ng/ml | 59 | 2.2 | 0.3337 |
| ≥100 ng/ml | 15 | 1.7 | |
| Carbohydrate antigen 19-9 | |||
| <10 000 U/ml | 62 | 2.1 | 0.0077 |
| ≥10 000 U/ml | 12 | 1.4 |
| Variable . | n . | Median survival (months) . | P-value . |
|---|---|---|---|
| Age | |||
| <65 | 47 | 1.9 | 0.9853 |
| ≥65 | 27 | 2.1 | |
| Sex | |||
| Male | 30 | 2.6 | 0.2441 |
| Female | 44 | 1.8 | |
| Performance status | |||
| 0–1 | 49 | 2.6 | 0.0007 |
| 2–4 | 25 | 1.5 | |
| White blood ell | |||
| <10 000/mm3 | 68 | 1.9 | 0.9720 |
| ≥10 000/mm3 | 6 | 2.6 | |
| Hemoglobin | |||
| <8.0 g/dl | 9 | 1.6 | 0.1953 |
| ≥8.0 g/dl | 65 | 2.1 | |
| Platelet | |||
| <100 000/μl | 7 | 1.4 | 0.0498 |
| ≥100 000/μl | 67 | 2.1 | |
| Total bilirubin | |||
| <2.0 mg/dl | 65 | 2.0 | 0.0479 |
| ≥2.0 mg/dl | |||
| Albumin | |||
| <2.8 g/dl | 15 | 1.5 | 0.0023 |
| ≥2.8 g/dl | 59 | 2.2 | |
| Lactate dehydrogenase | |||
| <400 IU/l | 65 | 2.1 | 0.1053 |
| ≥400 IU/l | 9 | 1.7 | |
| C-reactive protein | |||
| <5.0 mg/dl | 56 | 2.4 | <0.0001 |
| ≥5.0 mg/dl | 18 | 1.4 | |
| Serum creatinine | |||
| <1.0 mg/dl | 62 | 2.2 | 0.5390 |
| ≥1.0 mg/dl | 12 | 1.7 | |
| Primary tumor size | |||
| <50 mm | 40 | 2.2 | 0.1402 |
| ≥50 mm | 34 | 1.8 | |
| Liver metastasis | |||
| Absent | 18 | 1.6 | 0.6471 |
| Present | 56 | 2.1 | |
| Carcinoembryonic antigen | |||
| <100 ng/ml | 59 | 2.2 | 0.3337 |
| ≥100 ng/ml | 15 | 1.7 | |
| Carbohydrate antigen 19-9 | |||
| <10 000 U/ml | 62 | 2.1 | 0.0077 |
| ≥10 000 U/ml | 12 | 1.4 |
Univariate analysis
| Variable . | n . | Median survival (months) . | P-value . |
|---|---|---|---|
| Age | |||
| <65 | 47 | 1.9 | 0.9853 |
| ≥65 | 27 | 2.1 | |
| Sex | |||
| Male | 30 | 2.6 | 0.2441 |
| Female | 44 | 1.8 | |
| Performance status | |||
| 0–1 | 49 | 2.6 | 0.0007 |
| 2–4 | 25 | 1.5 | |
| White blood ell | |||
| <10 000/mm3 | 68 | 1.9 | 0.9720 |
| ≥10 000/mm3 | 6 | 2.6 | |
| Hemoglobin | |||
| <8.0 g/dl | 9 | 1.6 | 0.1953 |
| ≥8.0 g/dl | 65 | 2.1 | |
| Platelet | |||
| <100 000/μl | 7 | 1.4 | 0.0498 |
| ≥100 000/μl | 67 | 2.1 | |
| Total bilirubin | |||
| <2.0 mg/dl | 65 | 2.0 | 0.0479 |
| ≥2.0 mg/dl | |||
| Albumin | |||
| <2.8 g/dl | 15 | 1.5 | 0.0023 |
| ≥2.8 g/dl | 59 | 2.2 | |
| Lactate dehydrogenase | |||
| <400 IU/l | 65 | 2.1 | 0.1053 |
| ≥400 IU/l | 9 | 1.7 | |
| C-reactive protein | |||
| <5.0 mg/dl | 56 | 2.4 | <0.0001 |
| ≥5.0 mg/dl | 18 | 1.4 | |
| Serum creatinine | |||
| <1.0 mg/dl | 62 | 2.2 | 0.5390 |
| ≥1.0 mg/dl | 12 | 1.7 | |
| Primary tumor size | |||
| <50 mm | 40 | 2.2 | 0.1402 |
| ≥50 mm | 34 | 1.8 | |
| Liver metastasis | |||
| Absent | 18 | 1.6 | 0.6471 |
| Present | 56 | 2.1 | |
| Carcinoembryonic antigen | |||
| <100 ng/ml | 59 | 2.2 | 0.3337 |
| ≥100 ng/ml | 15 | 1.7 | |
| Carbohydrate antigen 19-9 | |||
| <10 000 U/ml | 62 | 2.1 | 0.0077 |
| ≥10 000 U/ml | 12 | 1.4 |
| Variable . | n . | Median survival (months) . | P-value . |
|---|---|---|---|
| Age | |||
| <65 | 47 | 1.9 | 0.9853 |
| ≥65 | 27 | 2.1 | |
| Sex | |||
| Male | 30 | 2.6 | 0.2441 |
| Female | 44 | 1.8 | |
| Performance status | |||
| 0–1 | 49 | 2.6 | 0.0007 |
| 2–4 | 25 | 1.5 | |
| White blood ell | |||
| <10 000/mm3 | 68 | 1.9 | 0.9720 |
| ≥10 000/mm3 | 6 | 2.6 | |
| Hemoglobin | |||
| <8.0 g/dl | 9 | 1.6 | 0.1953 |
| ≥8.0 g/dl | 65 | 2.1 | |
| Platelet | |||
| <100 000/μl | 7 | 1.4 | 0.0498 |
| ≥100 000/μl | 67 | 2.1 | |
| Total bilirubin | |||
| <2.0 mg/dl | 65 | 2.0 | 0.0479 |
| ≥2.0 mg/dl | |||
| Albumin | |||
| <2.8 g/dl | 15 | 1.5 | 0.0023 |
| ≥2.8 g/dl | 59 | 2.2 | |
| Lactate dehydrogenase | |||
| <400 IU/l | 65 | 2.1 | 0.1053 |
| ≥400 IU/l | 9 | 1.7 | |
| C-reactive protein | |||
| <5.0 mg/dl | 56 | 2.4 | <0.0001 |
| ≥5.0 mg/dl | 18 | 1.4 | |
| Serum creatinine | |||
| <1.0 mg/dl | 62 | 2.2 | 0.5390 |
| ≥1.0 mg/dl | 12 | 1.7 | |
| Primary tumor size | |||
| <50 mm | 40 | 2.2 | 0.1402 |
| ≥50 mm | 34 | 1.8 | |
| Liver metastasis | |||
| Absent | 18 | 1.6 | 0.6471 |
| Present | 56 | 2.1 | |
| Carcinoembryonic antigen | |||
| <100 ng/ml | 59 | 2.2 | 0.3337 |
| ≥100 ng/ml | 15 | 1.7 | |
| Carbohydrate antigen 19-9 | |||
| <10 000 U/ml | 62 | 2.1 | 0.0077 |
| ≥10 000 U/ml | 12 | 1.4 |
Multivariate Analysis
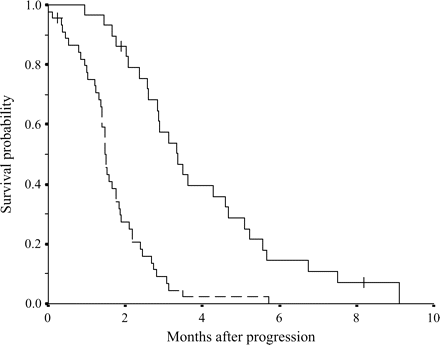
Multivariate regression analysis was conducted for the seven variables found to have prognostic significance in univariate analysis. Three factors, PS, peritoneal dissemination and CRP, were identified as independent prognostic factors (Table 3). In order to apply these findings to clinical practice, the patients were divided into two groups: the good prognosis group (patients with PS 0 or 1, no peritoneal dissemination and CRP <5.0 mg/dl) and the poor prognosis group (positive for at least one of the three prognostic factors). MST in the good prognosis group was 3.4 months, with the 95% CI ranging from 2.6 to 4.1 months, and MST in the poor prognosis group was 1.5 months, with the 95% CI ranging from 1.4 to 1.6 months (Fig. 3). Twenty-nine patients (39.1%) were included in the good prognosis group.
Survival curves for two groups divided: good prognosis group (patients with performance status 0–1, no peritoneal dissemination and C-reactive protein <5.0 mg/dl); and poor prognosis group (positive for at least one among three prognostic factors). Plain line indicates good prognosis group. Dotted line indicates poor prognosis group. There is a significant difference in survival between two groups (P < 0.0001). The ‘plus’ sign indicates censored cases.
Significant prognostic factors identified by multivariate analysis using Cox proportional hazards model
| Variables . | n . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|---|
| C-reactive protein | ||||
| <5.0 mg/dl | 56 | 1 | ||
| ≥5.0 mg/dl | 18 | 3.291 | 1.681–6.444 | 0.001 |
| Performance status | ||||
| 0–1 | 49 | 1 | ||
| 2–4 | 25 | 2.522 | 1.404–4.529 | 0.002 |
| Peritoneal dissemination | ||||
| Absent | 51 | 1 | ||
| Present | 23 | 1.988 | 1.052–3.757 | 0.034 |
| Variables . | n . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|---|
| C-reactive protein | ||||
| <5.0 mg/dl | 56 | 1 | ||
| ≥5.0 mg/dl | 18 | 3.291 | 1.681–6.444 | 0.001 |
| Performance status | ||||
| 0–1 | 49 | 1 | ||
| 2–4 | 25 | 2.522 | 1.404–4.529 | 0.002 |
| Peritoneal dissemination | ||||
| Absent | 51 | 1 | ||
| Present | 23 | 1.988 | 1.052–3.757 | 0.034 |
CI, confidence interval.
Significant prognostic factors identified by multivariate analysis using Cox proportional hazards model
| Variables . | n . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|---|
| C-reactive protein | ||||
| <5.0 mg/dl | 56 | 1 | ||
| ≥5.0 mg/dl | 18 | 3.291 | 1.681–6.444 | 0.001 |
| Performance status | ||||
| 0–1 | 49 | 1 | ||
| 2–4 | 25 | 2.522 | 1.404–4.529 | 0.002 |
| Peritoneal dissemination | ||||
| Absent | 51 | 1 | ||
| Present | 23 | 1.988 | 1.052–3.757 | 0.034 |
| Variables . | n . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|---|
| C-reactive protein | ||||
| <5.0 mg/dl | 56 | 1 | ||
| ≥5.0 mg/dl | 18 | 3.291 | 1.681–6.444 | 0.001 |
| Performance status | ||||
| 0–1 | 49 | 1 | ||
| 2–4 | 25 | 2.522 | 1.404–4.529 | 0.002 |
| Peritoneal dissemination | ||||
| Absent | 51 | 1 | ||
| Present | 23 | 1.988 | 1.052–3.757 | 0.034 |
CI, confidence interval.
DISCUSSION
For patients with advanced PC treated with GEM chemotherapy alone, the prognosis is around 6 months. In the present study, median survival was 5.1 months (95% CI: 4.0–6.2 months), which may be worse than that of previous reports. This may be because patients with better conditions were enrolled in clinical trials, such as chemoradiotherapy of new agents. The patients included in this study were treated with GEM as clinical practice, and these patients might have unfavorable factors for survival. For example, PS of these patients were 39 in score 0, 25 in score 1, 10 in score 2. As a result, overall survival of patients included in this study might be worse than that of some previous clinical trials of GEM chemotherapy for patients with advanced PC.
The efficacy of GEM is still poor and it is important not only to develop more effective first-line therapy but to develop effective second-line chemotherapy. No effective second-line chemotherapy has yet been established; however, clinical trials to develop promising second-line chemotherapy are ongoing. In some patients, systemic condition rapidly deteriorates after GEM failure and not all patients are suitable candidates for second-line chemotherapy. In the present study, we clarified the prognosis and prognostic factors in patients with GEM-refractory PC, and identified appropriate candidates for second-line chemotherapy.
In this study, three factors, PS, peritoneal dissemination and CRP, were identified as independent prognostic factors in patients with GEM-refractory PC. PS has been widely used to evaluate physical conditions of many cancer patients. It has been recognized as an important prognostic factor in many malignancies. Other studies have similarly found that PS has prognostic value in advanced or metastatic PC after first-line chemotherapy (5–7). Because general conditions of patients with PC often rapidly deteriorate after first-line chemotherapy, the indication of second-line chemotherapy should be limited to good performance patients.
Peritoneal dissemination was also recognized as a prognostic factor in this study. PC spreads easily into the peritoneal cavity, resulting in uncontrollable massive ascites and in deterioration of general condition. In an analysis of prognostic factors in patients with metastatic PC from the start of first-line chemotherapy, it was reported that peritoneal dissemination was not a significant factor associated with shorter survival time. The difference in survival between patients with and without peritoneal dissemination was not found to be significant, but MST was 2.2 months and 3.9 months, respectively (5). The prognostic value of peritoneal dissemination may thus possibly be enhanced after GEM chemotherapy failure.
CRP was found to be the most significant prognostic factor in this study. CRP is a biomarker of infection, inflammation and malignancy. CRP is produced by the liver and is induced by proinflammatory cytokines, such as interleukin-6 or tumor necrosis factor-α (12), which are involved in cachexia. These cytokines are associated with hypermetabolism, weight loss and anorexia and, as a result, may reflect shortened survival (13–15). The prognostic value of CRP has been reported for patients with metastatic PC receiving systemic chemotherapy, and the cut-off value was set at 5.0 mg/dl (5,10,11). We used this reported cut-off value for CRP in analyzing prognosis in the present study. As a result, the same conclusion was reached regarding the prognostic value of CRP in patients with GEM-refractory PC. CRP level has been reported to be a prognostic factor in many malignancies, including hepatocellular carcinoma and colorectal cancer (16–23).
In this study, serum lactate dehydrogenase (LDH) is not identified as a prognostic factor. LDH is an important marker of tumor bulk and tumor load for different solid tumors and lymphoma. Although different cut-off levels other than 400 IU/l were analyzed, there were no significant differences in univariate and multivariate analyses.
MST in patients with a CRP level of <5.0 mg/dl was 2.4 months (95% CI: 1.82–2.98), which is significantly better than the 1.4 months for patients with CRP levels of ≥5.0 mg/dl. CRP level is not included as a variable in most clinical studies of first-line chemotherapy, but it is an important parameter for selecting appropriate patients for clinical studies and selecting candidates for second-line chemotherapy.
To clarify conditions for effective second-line chemotherapy, we divided patients into two groups according to the prognostic factors: the good prognosis group (patients with PS 0 or 1, no peritoneal dissemination and CRP <5.0 mg/dl) and the poor prognosis group (positive for at least one of the three prognostic factors). MST in the good prognosis group was 3.4 months (with the 95% CI ranging from 2.6 to 4.1 months), whereas MST in the poor prognosis group was 1.5 months. Twenty-nine patients (39.1%) were included in the good prognosis group, the members of which could expect at least more than 2 months survival. We consider that those patients with favorable factors (i.e. those that comprise the good prognosis group) are good candidates for second-line chemotherapy. Candidates for clinical trials of new second-line chemotherapy agents should be selected from this group to allow accurate evaluation of survival time. In this study, the number of patients was too small to conduct a Cox proportional hazards model. Therefore, a prospective trial should be conducted to validate these results.
Recently, several trials of salvage chemotherapy regimens have been conducted for GEM-refractory PC, with response rates ranging from 0 to 24% and MST ranging from 3.1 to 10.3 months (24–34). Among these regimens, oxaliplatin combination regimens have shown promising results. Cantore et al. reported oxaliplatin/irinotecan regimen in which objective response was 10% and MST was 5.9 months (24). Jacobs et al. conducted a randomized trial using rubitecan as compared with the physician's choice. This large study comprised 409 patients and found an MST of 3.6 months in rubitecan-treated patients, and 3.1 months in control patients (25). In these studies, survival after failure of GEM treatment was much better than that in the present study. One of the reasons for this may be that only patients with good PS were enrolled in the studies above. It remains urgent to develop promising second-line chemotherapy to prolong survival in patients with advanced PC with poor PS.
Most of the patients in the present study did not receive other chemotherapy after GEM failure, because no other agent was approved for the treatment of PC in Japan. In the present study, GEM treatment was continued in 14 of the 74 patients after confirmation of progression if the patient still had good PS, had clinical benefit, or had decreased tumor marker levels, even after disease progression was confirmed by imaging (e.g. by CT). In Japan, some anti-cancer agents, such as irinotecan and S-1, have been reported to have promising anti-cancer effects in clinical studies when administered as monotherapies (35,36). We expect that these agents will become available as second-line chemotherapy in many patients in near future.
In conclusion, serum levels of CRP, PS and peritoneal dissemination were identified as important prognostic factors in patients with GEM-refractory PC. These factors should be considered in determining the treatment following first-line chemotherapy in patients with advanced PC.
Conflict of interest statement
None declared.