-
PDF
- Split View
-
Views
-
Cite
Cite
Mark E. Sherman, Joseph D. Carreon, James V. Lacey, Susan S. Devesa, Impact of Hysterectomy on Endometrial Carcinoma Rates in the United States, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 22, 16 November 2005, Pages 1700–1702, https://doi.org/10.1093/jnci/dji378
Close - Share Icon Share
Abstract
In the United States, endometrial carcinoma incidence rates, uncorrected for hysterectomy prevalence, are higher among white women than black women. We estimated corrected endometrial carcinoma rates by racial/ethnic groups and age (30–74 years) for 1992–2000 using data from the Surveillance, Epidemiology, and End Results program and the Behavioral Risk Factor Surveillance Survey. Hysterectomy prevalence was higher among black women than among Hispanic and white non-Hispanic women. Correcting for hysterectomy prevalence increased age-adjusted endometrial carcinoma rates per 10 5 woman-years from 29.2 to 48.7 (66.8% increase) overall, from 14.6 to 28.5 (95.3% increase) in blacks, from 18.8 to 29.6 (57.6% increase) in Hispanics, and from 33.2 to 54.9 (65.1%) in white non-Hispanics. This correction reduced the rate ratio for white non-Hispanics compared with blacks from 2.27 to 1.93. Among blacks but not Hispanics or white non-Hispanics, the endometrial carcinoma risk factors of obesity and diabetes were more prevalent among hysterectomized than nonhysterectomized women. Failure to correct for hysterectomy prevalence may lead to underestimates of endometrial carcinoma risk, especially among blacks.The high prevalence of hysterectomy among blacks with strong endometrial cancer risk factors may partly account for lower cancer rates in this group.
Incidence rates for uterine corpus cancer are generally determined without eliminating hysterectomized women from the at-risk population, resulting in an underestimate of incidence among women with intact uteri ( 1 – 3 ) . Corpus cancer rates mainly reflect cases of endometrioid adenocarcinoma (referred to herein as “endometrial carcinoma”), which is the predominant histologic tumor type of this cancer. In the United States, reported rates for endometrial carcinoma are substantially higher among white women than among black women ( 4 , 5 ) . This disparity is surprising given that obesity, a strong risk factor for endometrial carcinoma ( 6 ) , is more prevalent among blacks ( 7 ) . However, the observation that hysterectomy prevalence is higher among young blacks as compared with whites ( 8 ) suggests that hysterectomy prevalence by race could contribute to an inflated estimate of the racial disparity in endometrial carcinoma rates. To explore this issue, we reestimated endometrial carcinoma rates among women in the United States with intact uteri, focusing on possible racial/ethnic differences.
Using data for cases diagnosed during 1992–2000 collected by nine registries in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program (Connecticut, metropolitan Atlanta, Iowa, New Mexico, Seattle Puget Sound, Utah, San Francisco–Oakland, San Jose–Monterey, and Los Angeles), we tabulated endometrial carcinoma rates per 10 5 woman-years by racial/ethnic group and age. We identified endometrial carcinomas as tumors with International Classification of Diseases for Oncology ( 9 ) codes 8140 (adenocarcinoma, not otherwise specified, n = 10 275), 8380 (endometrioid adenocarinoma, n = 7285), 8560 (adenosquamous carcinoma, n = 562), and 8570 (adenocarcinoma with squamous metaplasia, n = 635). Our analysis was based on 640 incident cases among blacks, 673 among Hispanics, and 9173 among white non-Hispanics. To reestimate age-specific incidence rates for women with intact uteri, we reduced SEER age-specific at-risk populations by age-specific hysterectomy prevalences (5-year age groups) using 1992–2000 data from the Behavioral Risk Factor Surveillance Survey (BRFSS) for those states that also maintained SEER registries included in this analysis ( 10 , 11 ) . We limited our analysis to women aged 30–74 years (among whom most incident endometrial carcinomas are found). We standardized rates for age in 5-year groups using the 2000 U.S. standard population.
In all age groups, the prevalence of hysterectomy was higher among blacks than among Hispanics and white non-Hispanics. Rates among the latter two groups were similar. Hysterectomy prevalence for blacks and for white non-Hispanics was as follows: ages 30–44 years, 13.7% and 8.5%, respectively; ages 45–59 years, 43.2% and 33.1%, respectively; and ages 60–74 years, 51.6% and 43.8%, respectively. Our results are consistent with previous analyses showing that hysterectomy prevalence is higher among young blacks as compared with whites ( 8 ) . Although the reasons for this difference are unclear, blacks more frequently reside in the South, where hysterectomy procedures have been performed more often ( 8 ) . In addition, factors such as limited education, high parity, and a history of miscarriages—all of which have been associated with increased risk of hysterectomy ( 12 ) —are more common among blacks than among whites.
Endometrial carcinoma rates per 10 5 woman-years for 1992–2000, tabulated without considering hysterectomy prevalence, were 29.2 for all women, 14.6 for blacks, 18.8 for Hispanics, and 33.2 for white non-Hispanics. Endometrial carcinoma rates corrected for hysterectomy prevalence were substantially higher than uncorrected rates, rising to 48.7 overall (a 66.8% increase), to 28.5 among blacks (a 95.3% increase), to 29.6 among Hispanics (a 57.6% increase), and to 54.9 among white non-Hispanics (a 65.1% increase). Therefore, accounting for hysterectomy prevalence reduced the endometrial carcinoma rate ratio for white non-Hispanics compared with blacks from 2.27 to 1.93, although the absolute rate difference increased.
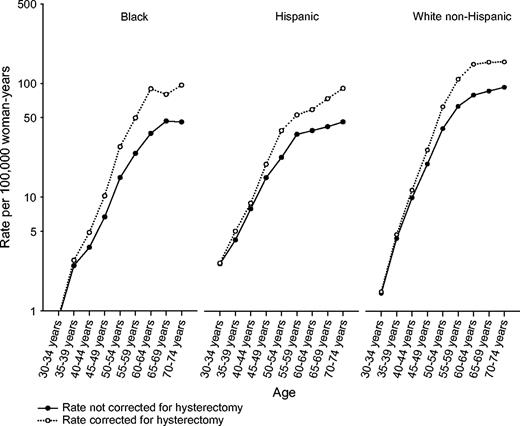
In all three racial/ethnic groups, uncorrected endometrial carcinoma rates increased sharply among women aged 30–59 years and then increased more slowly among older women ( Fig. 1 ). Correcting endometrial cancer rates for hysterectomy prevalence produced a steeper rise with increasing age, especially among blacks and Hispanics.
Semilogarithmic plot of endometrial carcinoma incidence rates per 10 5 woman-years for cases diagnosed during 1992–2000 by age. Solid circles and black lines represent incidence rates, irrespective of hysterectomy status (“uncorrected”); open circles and dotted lines represent rates for women who have retained their uteri (“corrected”). The increase in endometrial carcinoma rates resulting from correction for hysterectomy prevalence was evident at a younger age among blacks than among other racial/ethnic groups. Among Hispanics older than age 59 years, corrected endometrial carcinoma rates increased sharply, a pattern that was not found for the other groups.
Hysterectomy procedures performed for benign indications may selectively reduce the number of women at markedly elevated risk for endometrial carcinoma if women with strong risk factors for endometrial carcinoma are more likely to have their uteri removed than those lacking such factors. In this analysis, two endometrial carcinoma risk factors, obesity and diabetes, were more prevalent among black and Hispanic women than among white non-Hispanic women, regardless of hysterectomy status ( Table 1 ). Moreover, among blacks, the prevalence of obesity and of diabetes was much higher among those who had undergone hysterectomy than among those who had not (by 51% and 69%, respectively). In contrast, among Hispanics and white non-Hispanics, there was less variation in the prevalence of these risk factors by hysterectomy status. Furthermore, Hispanics and white non-Hispanics who underwent hysterectomy included a substantially higher percentage of smokers (in whom endometrial carcinoma risk is reduced) than those who retained their uteri, whereas among blacks smoking varied less by hysterectomy status. The higher relative percentage of blacks with strong endometrial carcinoma risk factors who undergo hysterectomy compared with Hispanics and white non-Hispanics may partly explain the lower endometrial cancer incidence among blacks, even after correcting for hysterectomy.
Prevalence of endometrial cancer risk factors stratified by hysterectomy status (1992–2000) *
| . | Prevalence (%) . | . | . | ||
|---|---|---|---|---|---|
| Risk factor by hysterectomy status † . | Blacks . | Hispanics . | White non-Hispanics . | ||
| Obese ‡ | |||||
| Uterus removed | 44.2 | 22.9 | 15.8 | ||
| Uterus retained | 29.3 | 22.4 | 14.9 | ||
| Diabetic § | |||||
| Uterus removed | 13.2 | 6.2 | 5.0 | ||
| Uterus retained | 7.8 | 6.6 | 3.9 | ||
| Current smoker | |||||
| Uterus removed | 23.9 | 17.4 | 26.1 | ||
| Uterus retained | 20.1 | 12.9 | 18.5 | ||
| . | Prevalence (%) . | . | . | ||
|---|---|---|---|---|---|
| Risk factor by hysterectomy status † . | Blacks . | Hispanics . | White non-Hispanics . | ||
| Obese ‡ | |||||
| Uterus removed | 44.2 | 22.9 | 15.8 | ||
| Uterus retained | 29.3 | 22.4 | 14.9 | ||
| Diabetic § | |||||
| Uterus removed | 13.2 | 6.2 | 5.0 | ||
| Uterus retained | 7.8 | 6.6 | 3.9 | ||
| Current smoker | |||||
| Uterus removed | 23.9 | 17.4 | 26.1 | ||
| Uterus retained | 20.1 | 12.9 | 18.5 | ||
Data for white non-Hispanics and Hispanics were based on results of the Behavioral Risk Factor Surveillance System (BRFSS) for states in which nine of the registries in the Surveillance, Epidemiology, and End Results program (SEER) are located, Connecticut, metropolitan Atlanta, Iowa, New Mexico, Seattle Puget Sound, Utah, San Francisco–Oakland, San Jose–Monterey, and Los Angeles, that is, excluding the registries in Hawaii and metropolitan Detroit. Data for blacks include results from Hawaii and Detroit.
Factors related to endometrial carcinoma risk available in BRFSS.
Obesity was defined as having a body mass index of ≥30 kg/m 2 .
Data for gestational diabetes have not been included.
Prevalence of endometrial cancer risk factors stratified by hysterectomy status (1992–2000) *
| . | Prevalence (%) . | . | . | ||
|---|---|---|---|---|---|
| Risk factor by hysterectomy status † . | Blacks . | Hispanics . | White non-Hispanics . | ||
| Obese ‡ | |||||
| Uterus removed | 44.2 | 22.9 | 15.8 | ||
| Uterus retained | 29.3 | 22.4 | 14.9 | ||
| Diabetic § | |||||
| Uterus removed | 13.2 | 6.2 | 5.0 | ||
| Uterus retained | 7.8 | 6.6 | 3.9 | ||
| Current smoker | |||||
| Uterus removed | 23.9 | 17.4 | 26.1 | ||
| Uterus retained | 20.1 | 12.9 | 18.5 | ||
| . | Prevalence (%) . | . | . | ||
|---|---|---|---|---|---|
| Risk factor by hysterectomy status † . | Blacks . | Hispanics . | White non-Hispanics . | ||
| Obese ‡ | |||||
| Uterus removed | 44.2 | 22.9 | 15.8 | ||
| Uterus retained | 29.3 | 22.4 | 14.9 | ||
| Diabetic § | |||||
| Uterus removed | 13.2 | 6.2 | 5.0 | ||
| Uterus retained | 7.8 | 6.6 | 3.9 | ||
| Current smoker | |||||
| Uterus removed | 23.9 | 17.4 | 26.1 | ||
| Uterus retained | 20.1 | 12.9 | 18.5 | ||
Data for white non-Hispanics and Hispanics were based on results of the Behavioral Risk Factor Surveillance System (BRFSS) for states in which nine of the registries in the Surveillance, Epidemiology, and End Results program (SEER) are located, Connecticut, metropolitan Atlanta, Iowa, New Mexico, Seattle Puget Sound, Utah, San Francisco–Oakland, San Jose–Monterey, and Los Angeles, that is, excluding the registries in Hawaii and metropolitan Detroit. Data for blacks include results from Hawaii and Detroit.
Factors related to endometrial carcinoma risk available in BRFSS.
Obesity was defined as having a body mass index of ≥30 kg/m 2 .
Data for gestational diabetes have not been included.
Our analysis has several limitations. Although it was based on large datasets that are generally representative of women in the United States, our findings may not necessarily apply to populations that are not proportionately represented in SEER or BRFSS. In particular, BRFSS participants are limited to persons with working telephones. Nevertheless, self-reports of hysterectomy are reasonably accurate ( 13 ) , and hysterectomy prevalence determined using BRFSS data has been validated against that found using other data sources ( 14 ) . This analysis was also limited to endometrial cancer risk factors that are available in the BRFSS and did not include expert pathology review. Nevertheless, our analysis demonstrates that failure to account for hysterectomy prevalence can result in grossly underestimated endometrial carcinoma rates in the Unites States, especially for blacks. In addition, patterns of hysterectomy performance among blacks may greatly reduce the prevalence of endometrial carcinoma risk factors (i.e. obesity and diabetes) among blacks who retained their uteri, but only slightly reduce them among Hispanics and white non-Hispanics.
Growing enthusiasm for treating benign uterine diseases without surgery could expand the population of women with intact uteri, thereby increasing the endometrial carcinoma burden in the United States. Although averting hysterectomy is desirable, women with endometrial carcinoma risk factors might experience increased endometrial carcinoma incidence and mortality if hysterectomy prevalence declines. In the future, monitoring of endometrial carcinoma incidence rates using improved methods that correct for hysterectomy prevalence and include information about the characteristics of women who undergo hysterectomy are needed to address these concerns and to provide the basis for prevention efforts.
References
Luoto R, Raitanen J, Pukkala E, Anttila A. Effect of hysterectomy on incidence trends of endometrial and cervical cancer in Finland 1953–2010.
Lyon JL, Gardner JW. The rising frequency of hysterectomy: Its effect on uterine cancer rates.
Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002.
Sherman ME, Devesa S. Analysis of racial differences in incidence, survival and mortality for malignant tumors of the uterine corpus.
Grady D, Ernster VL. Endometrial cancer. In: Schottenfeld D, Fraumeni JF (editors). Cancer epidemiology and prevention. 2nd ed. New York (NY): Oxford University Press,
Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000.
Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance—United States, 1994–1999.
Percy C, Van Holten V, Muir C, editors. International classification of diseases for oncology. 2nd ed. Geneva (Switzerland): World Health Organization;
Merrill RM, Lyon JL, Wiggins C. Comparison of two methods based on cross-sectional data for correcting corpus uterine cancer incidence and probabilities.
National Center for Chronic Disease Prevention and Health Promotion. Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss . [Last accessed: October 14, 2005.]
Brett KM, Marsh JVR, Madans JH. Epidemiology of hysterectomy in the United States demographic and reproductive factors in a nationally representative sample.
Brett KM, Madans JH. Hysterectomy use: The correspondence between self-reports and hospital records.