-
PDF
- Split View
-
Views
-
Cite
Cite
Vahakn B. Shahinian, Yong-Fang Kuo, Jean L. Freeman, James S. Goodwin, Determinants of Androgen Deprivation Therapy Use for Prostate Cancer: Role of the Urologist, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 12, 21 June 2006, Pages 839–845, https://doi.org/10.1093/jnci/djj230
Close - Share Icon Share
Abstract
Background: The use of androgen deprivation therapy for prostate cancer has been increasing, even in settings for which there is weak or no evidence of efficacy. This pattern suggests that factors other than the typical patient and tumor characteristics may be driving its use. We assessed the importance of the physician as a determinant of the use of androgen deprivation therapy in prostate cancer in a population-based, retrospective cohort study using the Surveillance, Epidemiology and End-Results–Medicare linked database. Methods: Participants included 61 717 men with incident prostate cancer diagnosed from January 1, 1992, through December 31, 1999, and 1802 urologists providing care to them within 1 year of cancer diagnosis. Multilevel analyses were used to estimate and partition the variance in use of androgen deprivation therapy within 6 months of diagnosis between patient or tumor characteristics and urologist to examine the relative contribution of each component to androgen deprivation therapy. Results: The percentage of the total variance in the use of androgen deprivation therapy attributable to the urologist was consistently higher than that attributable to tumor or patient characteristics. This pattern was most pronounced for patients diagnosed from January 1, 1997, through December 31, 1999, in which 22.56% of the total variance in use of androgen deprivation therapy was attributable to the urologist, 9.71% to tumor characteristics (stage or grade), and 4.29% to patient characteristics (age, ethnicity, socio-economic status, comorbidity, geographic region, or year of diagnosis). Conclusions: Which urologist a patient sees may be more important in determining whether they will receive androgen deprivation therapy than tumor or patient characteristics.
Androgen deprivation therapy, particularly in the form of gonadotropin-releasing hormone agonists, is a common therapy for prostate cancer that is used in almost half of all patients over the course of their disease ( 1 , 2 ) . This therapy is indicated for the palliation of metastases of prostate cancer and in locally advanced cancer when combined with radiation ( 3 , 4 ) . Although the use of androgen deprivation therapy in other settings remains controversial ( 5 ) , recent studies have shown a dramatic increase in use of androgen deprivation therapy for prostate cancer during the 1990s, even in contexts in which its benefit is unproven or highly improbable ( 2 , 6 ) .
Because androgen deprivation therapy is a costly and potentially toxic therapy ( 7 , 8 ) , it is important to understand the factors responsible for its use. Growth in the use of androgen deprivation therapy, even when there is weak or no evidence of benefit, indicates that factors other than typical patient and tumor characteristics may be driving its use. One possibility is as stated in Wennberg's practice style hypothesis ( 9 , 10 ) —that is, when there is uncertainty about the optimal treatment course, use of medical interventions is determined by characteristics of the physician. In other words, the treatment provided depends more on the physician who is treating the patient than on specific characteristics of the patient's disease. However, previous studies ( 11 – 14 ) that have used multilevel analyses to examine the contribution of the physician to variance in the use of various treatments (i.e., for diabetes or angina) or resources (i.e., hospitalizations or pharmacy expenditures) have found the physician's contribution to be quite small, generally less than 10%.
To assess the importance of the physician as a determinant of the use of androgen deprivation therapy in prostate cancer, we conducted a multilevel analysis in a large population-based retrospective cohort by use of the Surveillance, Epidemiology and End-Results (SEER)-Medicare linked database ( 15 , 16 ) . In particular, we compared the percentage of variance in the use of androgen deprivation therapy that was attributable to patient characteristics with the percentage that was attributable to the physician. Although we acknowledge the difficulties in defining appropriate care, we divided the prostate cancer patients into those for whom androgen deprivation therapy was evidence based (i.e., clinical trials showing survival benefit or improvements in palliation) and those for whom the benefit of androgen deprivation therapy was uncertain (i.e., no clinical trials showing survival benefit, or presence of conflicting data regarding benefits in the literature). We tested the hypothesis that most of the variance in the use of androgen deprivation therapy was attributable to the physician. We also tested the hypothesis that the variance attributable to the physician would be greater for situations in which the benefits of androgen deprivation therapy were uncertain.
P ATIENTS AND M ETHODS
Patient Population
The study protocol was approved by the local institutional review board at the University of Texas Medical Branch at Galveston. Data for this study were obtained from the linked SEER-Medicare database ( 15 , 16 ) . The version of the database used for the analysis included incident prostate cancers through December 31, 1999, and Medicare claims through December 31, 2001.
The study included patients with incident prostate cancer from January 1, 1992, through December 31, 1999, who were at least 66 years old at the time of diagnosis (118 446 subjects). To ensure complete information, patients who were not enrolled in both Medicare Part A and Part B for the 12 months before the diagnosis and the first 6 months after their cancer diagnosis (12 466 cases), were members of a health maintenance organization (24 441 cases), or were diagnosed by autopsy or from a death certificate (1474 cases) were excluded, leaving 80 065 eligible patients.
All physicians providing care to the patients within the first year of diagnosis were identified through use of encrypted Unique Physician Identifier Numbers on Medicare physician claims. For each physician, Medicare claims also contain a two-digit specialty code (e.g., for urology or general surgery), which was reported when the claim was submitted ( 17 ) . From preliminary analyses of Medicare specialty codes of physicians caring for eligible prostate cancer patients, 92.1% of patients were seen by at least one urologist in the year after cancer diagnosis. Moreover, among the 23 642 prostate cancer patients who received androgen deprivation therapy, therapy for 22 304 (94.3%) was administered by a urologist. All physician-level analyses in this study, therefore, used data from urologists only.
We excluded patients who did not see at least one provider identified by Medicare specialty code as an urologist in the year after diagnosis on at least two different days (10 498 patients) or who saw urologists who did not have an assigned Unique Physician Identifier Number in the claims information (827 patients). When patients were seen by two or more urologists, the patient was assigned to the urologist with 75% or more of the urologist visits in the first year after diagnosis. If a patient did not visit one urologist at least 75% of the time, that patient was excluded (7023 patients), which left a total of 61 717 patients for the study. A total of 1802 urologists were identified as providing care within 1 year of cancer diagnosis to these patients.
Because substantial time trends in use of androgen deprivation therapy were identified over the study period, for some analyses, patients were divided into groups by the period of diagnosis, from 1992 through 1996 and from 1997 through 1999. Patients were also divided into those for whom use of androgen deprivation therapy was defined as evidence based (i.e., the evidence-based group) and those for whom its use was defined as uncertain (i.e., the uncertain-benefit group) by use of evidence in the medical literature and on published clinical practice guidelines from the National Comprehensive Cancer Network ( 5 , 18 , 19 ) . Analyses of data from the evidence-based and uncertain-benefit groups were limited to the period from 1997 through 1999, because the literature supporting use of androgen deprivation therapy as adjuvant therapy with radiation therapy was first published in 1997 ( 20 , 21 ) . The evidence-based group included patients receiving radiation therapy who have T3 tumors (regardless of grade) or who have T2 (confined to prostate) tumors with high-grade histology (Gleason score of 8–10). The uncertain-benefit group included all other patients.
Definitions of Variables
Patient demographic and tumor characteristics were derived from the SEER records in the linked database and used to categorize patients by age, ethnicity, SEER region of residence at the time of diagnosis, year of diagnosis, clinical stage (T1 through T4), and grade (well differentiated, Gleason score of 2–4; moderately differentiated, Gleason score of 5–7; poorly or undifferentiated/unknown, Gleason score of 8–10) ( 22 , 23 ) . The American Joint Committee on Cancer staging system was not used because more than 40% of the patients in the SEER database were listed as in the unknown stage within this classification system (reanalysis of the data using the American Joint Committee on Cancer classification for patients with a known stage did not substantially alter the results).
The socioeconomic characteristics of each patient were based on the percentage of adults with fewer than 12 years of education and the percentage of residents living below the poverty level from census tract data. Comorbidity was measured with an adaptation of the Charlson Comorbidity Index ( 24 ) that was developed for use with Medicare physician claims data ( 25 ) .
Surgical androgen deprivation therapy, in the form of orchiectomy, was defined by the presence of the Current Procedural Terminology codes or International Classification of Diseases, 9th revision, procedure codes in the Medicare claims. Administration of a gonadotropin-releasing hormone agonist was identified through specific Medicare claims codes used to designate each dose given of certain specific injectable medications, as described previously ( 2 , 26 ) .
The outcome was receipt of androgen deprivation therapy, which was defined as the receipt of at least one dose of a gonadotropin-releasing hormone agonist or undergoing orchiectomy in the first 6 months after diagnosis of prostate cancer. Consequently, the study was limited to examining the early use of androgen deprivation therapy, without considering whether patients received androgen deprivation therapy later in their disease.
Statistical Analysis
Differences in the percentage of patients receiving androgen deprivation therapy across strata were evaluated with chi-square statistics. Logistic regression models were estimated by use of androgen deprivation therapy as a dependent variable, and patient characteristics (i.e., age, comorbidity, ethnicity, socioeconomic status, SEER region, and year of diagnosis) and tumor characteristics (i.e., stage and grade) were estimated as independent variables. The percentage of variance in use of androgen deprivation therapy that was attributable to each patient and tumor characteristic was calculated with the generalized coefficient of determination (maximum rescaled R2 ) for unadjusted analyses (in which each characteristic was analyzed independently, without adjustment for other variables) and calculated with the partial generalized coefficient of determination for adjusted analyses (in which each characteristic was analyzed with simultaneous adjustment for the other variables) ( 27 ) .
Use of androgen deprivation therapy was further evaluated with multilevel analyses by using hierarchical generalized linear models ( 28 – 30 ) . This method is appropriate when there are various levels of data nested within each other. In this study, patients are nested within urologists. A hierarchical generalized linear model approach allows the estimation and partitioning of variance in use of androgen deprivation therapy between the patient and urologist levels.
As a measure of the importance of the urologist effect on individual use of androgen deprivation therapy, we estimated the percentage of the total variance in the use of androgen deprivation therapy attributable to the urologist, which is defined for this study as the intraclass correlation coefficient (ICC). This value was estimated by use of the threshold model, a previously described method that is appropriate for a binary outcome ( 31 ) . Both “null” models, which do not include any patient or tumor variables, and adjusted models, which include all of these variables, were used. The ICCs from the null models were very similar to those from the adjusted models and are, therefore, not presented. For the adjusted models, results were calculated in two ways. In the first method, a residual ICC was calculated that represents the percentage of variance attributable to the urologist after adjustment for available patient and tumor variables (the denominator in the calculation of this percentage was composed of the variance attributable to the urologist, after adjustment for available patient and tumor variables, and the variance attributable to error plus unexplained patient and tumor variables). In the second method, the variance was further partitioned into contributions from the urologist and from available patient or tumor characteristics to calculate the percentage of total variance from each component (in this case, the denominator was total variance, which also included the variance attributable to available patient and tumor characteristics). In this way, the relative importance of each component could be examined. For the main analyses, urologists who saw at least one patient with prostate cancer over the study period were included.
Alternate techniques (linearization and simulation) have been described for estimating ICCs; these techniques have the advantage that an underlying continuous distribution does not have to be assumed, unlike the threshold model ( 32 ) . However, no method has yet been described for further partitioning of the variance for these techniques. We therefore calculated residual ICCs by these techniques to compare them with those from the threshold model. Because the SEER geographic region could also be considered a random effect, we also used a three-level model that included geographic region as the third level (with patients and urologists as the first and second levels, respectively). The results for urologist-level ICCs from this model were essentially unchanged from the two-level models and were therefore not presented.
As another measure of physician variation in use of androgen deprivation therapy, urologist-specific rates of administering androgen deprivation therapy were calculated with a hierarchical generalized linear model that was adjusted for patient and tumor characteristics and then plotted by rank, from lowest to highest. This model also accounts for the fact that urologists with a smaller panel of patients have less reliable estimates of rates of androgen deprivation use. For each urologist, the term panel of patients refers to the number of prostate cancer patients that were identified as having been cared for by that urologist (the provided care may or may not have included androgen deprivation) within 1 year of cancer diagnosis. Each urologist-specific rate was therefore adjusted toward the mean rate as a factor of the size of the panel of patients. A urologist rate that is based on a large panel of patients will undergo very little adjustment toward the mean rate, whereas a urologist rate that is based on a small panel will have more adjustment. These analyses were limited to urologists who saw at least five patients with prostate cancer during the study period.
Analyses were performed with the software packages SAS version 9.1 (Cary, NC) and Mlwin version 2.02 (London, United Kingdom). All tests of statistical significance were two-sided, and P values of less than .05 were considered statistically significant.
R ESULTS
Patient and Tumor Characteristics
Of the 61 717 patients aged 66 years or older diagnosed with prostate cancer, 19 355 (31.4%) received androgen deprivation therapy within 6 months of diagnosis. Of these 19 355 patients, 15 337 (79.2%) received gonadotropin-releasing hormone agonists, 3688 (19.1%) received orchiectomy, and 330 (1.7%) received both. Table 1 presents the percentage of men who received androgen deprivation therapy by stratum of patient and tumor characteristics. Androgen deprivation therapy was more commonly administered to older patients, to those with more comorbidities, to those with more advanced-stage or higher-grade disease, and to patients diagnosed in recent years. Receipt of this therapy varied substantially by SEER region.
Percentage of men receiving androgen deprivation therapy within 6 months after a diagnosis of prostate cancer across strata of patient characteristics *
| Characteristic . | Strata . | No. . | % of patients receiving androgen deprivation therapy . |
|---|---|---|---|
| All patients | 61 717 | 31.4 | |
| Age, years | 66–69 | 13 854 | 22.7 |
| 70–74 | 20 137 | 26.8 | |
| 75–79 | 15 635 | 34.0 | |
| ≥80 | 12 091 | 45.4 | |
| Ethnicity | White | 50 287 | 30.5 |
| Black | 5273 | 31.3 | |
| White Hispanic | 2438 | 35.3 | |
| Other/unknown | 3719 | 40.6 | |
| SEER region † | San Francisco | 4204 | 33.4 |
| Connecticut | 8002 | 35.4 | |
| Michigan | 11 524 | 26.6 | |
| Hawaii | 1775 | 36.8 | |
| Iowa | 8140 | 32.1 | |
| New Mexico | 3081 | 19.4 | |
| Seattle | 6568 | 28.4 | |
| Utah | 3903 | 27.4 | |
| Georgia | 3580 | 27.2 | |
| San Jose | 2306 | 43.3 | |
| Los Angeles | 8634 | 38.1 | |
| Year of diagnosis | 1992 | 11 338 | 24.1 |
| 1993 | 9583 | 24.0 | |
| 1994 | 7841 | 26.1 | |
| 1995 | 7110 | 29.1 | |
| 1996 | 6553 | 34.3 | |
| 1997 | 6665 | 37.5 | |
| 1998 | 6165 | 41.5 | |
| 1999 | 6462 | 44.9 | |
| Census tract education ‡ | ≤10.5 | 14 966 | 28.7 |
| 10.6–17.5 | 14 920 | 31.3 | |
| 17.6–26.0 | 15 438 | 32.0 | |
| >26 | 15 184 | 33.0 | |
| Census tract poverty § | ≤3.5 | 14 611 | 29.8 |
| 3.6–7.0 | 16 252 | 31.3 | |
| 7.1–12.5 | 14 246 | 31.9 | |
| >12.5 | 15 399 | 32.0 | |
| Comorbidity index ∥ | 0 | 45 694 | 30.3 |
| 1 | 9317 | 32.4 | |
| 2 | 2663 | 37.5 | |
| ≥3 | 4043 | 36.3 | |
| Grade ∥ | Well differentiated | 8164 | 15.4 |
| Moderately differentiated | 35 536 | 27.6 | |
| Poorly differentiated/undifferentiated | 13 467 | 49.3 | |
| Unknown | 4550 | 36.3 | |
| Clinical stage ∥ | T1 | 15 693 | 23.7 |
| T2 | 31 827 | 28.1 | |
| T3 | 3906 | 32.3 | |
| T4 | 4002 | 74.3 | |
| Unknown | 6289 | 39.1 |
| Characteristic . | Strata . | No. . | % of patients receiving androgen deprivation therapy . |
|---|---|---|---|
| All patients | 61 717 | 31.4 | |
| Age, years | 66–69 | 13 854 | 22.7 |
| 70–74 | 20 137 | 26.8 | |
| 75–79 | 15 635 | 34.0 | |
| ≥80 | 12 091 | 45.4 | |
| Ethnicity | White | 50 287 | 30.5 |
| Black | 5273 | 31.3 | |
| White Hispanic | 2438 | 35.3 | |
| Other/unknown | 3719 | 40.6 | |
| SEER region † | San Francisco | 4204 | 33.4 |
| Connecticut | 8002 | 35.4 | |
| Michigan | 11 524 | 26.6 | |
| Hawaii | 1775 | 36.8 | |
| Iowa | 8140 | 32.1 | |
| New Mexico | 3081 | 19.4 | |
| Seattle | 6568 | 28.4 | |
| Utah | 3903 | 27.4 | |
| Georgia | 3580 | 27.2 | |
| San Jose | 2306 | 43.3 | |
| Los Angeles | 8634 | 38.1 | |
| Year of diagnosis | 1992 | 11 338 | 24.1 |
| 1993 | 9583 | 24.0 | |
| 1994 | 7841 | 26.1 | |
| 1995 | 7110 | 29.1 | |
| 1996 | 6553 | 34.3 | |
| 1997 | 6665 | 37.5 | |
| 1998 | 6165 | 41.5 | |
| 1999 | 6462 | 44.9 | |
| Census tract education ‡ | ≤10.5 | 14 966 | 28.7 |
| 10.6–17.5 | 14 920 | 31.3 | |
| 17.6–26.0 | 15 438 | 32.0 | |
| >26 | 15 184 | 33.0 | |
| Census tract poverty § | ≤3.5 | 14 611 | 29.8 |
| 3.6–7.0 | 16 252 | 31.3 | |
| 7.1–12.5 | 14 246 | 31.9 | |
| >12.5 | 15 399 | 32.0 | |
| Comorbidity index ∥ | 0 | 45 694 | 30.3 |
| 1 | 9317 | 32.4 | |
| 2 | 2663 | 37.5 | |
| ≥3 | 4043 | 36.3 | |
| Grade ∥ | Well differentiated | 8164 | 15.4 |
| Moderately differentiated | 35 536 | 27.6 | |
| Poorly differentiated/undifferentiated | 13 467 | 49.3 | |
| Unknown | 4550 | 36.3 | |
| Clinical stage ∥ | T1 | 15 693 | 23.7 |
| T2 | 31 827 | 28.1 | |
| T3 | 3906 | 32.3 | |
| T4 | 4002 | 74.3 | |
| Unknown | 6289 | 39.1 |
All P values were <.001 and were from two-sided chi-square tests for differences in the percentage of patients receiving androgen deprivation therapy across strata for each characteristic.
SEER = Surveillance, Epidemiology and End Results.
Percentage of adults with fewer than 12 years of education.
Percentage of residents living below the poverty level.
Percentage of men receiving androgen deprivation therapy within 6 months after a diagnosis of prostate cancer across strata of patient characteristics *
| Characteristic . | Strata . | No. . | % of patients receiving androgen deprivation therapy . |
|---|---|---|---|
| All patients | 61 717 | 31.4 | |
| Age, years | 66–69 | 13 854 | 22.7 |
| 70–74 | 20 137 | 26.8 | |
| 75–79 | 15 635 | 34.0 | |
| ≥80 | 12 091 | 45.4 | |
| Ethnicity | White | 50 287 | 30.5 |
| Black | 5273 | 31.3 | |
| White Hispanic | 2438 | 35.3 | |
| Other/unknown | 3719 | 40.6 | |
| SEER region † | San Francisco | 4204 | 33.4 |
| Connecticut | 8002 | 35.4 | |
| Michigan | 11 524 | 26.6 | |
| Hawaii | 1775 | 36.8 | |
| Iowa | 8140 | 32.1 | |
| New Mexico | 3081 | 19.4 | |
| Seattle | 6568 | 28.4 | |
| Utah | 3903 | 27.4 | |
| Georgia | 3580 | 27.2 | |
| San Jose | 2306 | 43.3 | |
| Los Angeles | 8634 | 38.1 | |
| Year of diagnosis | 1992 | 11 338 | 24.1 |
| 1993 | 9583 | 24.0 | |
| 1994 | 7841 | 26.1 | |
| 1995 | 7110 | 29.1 | |
| 1996 | 6553 | 34.3 | |
| 1997 | 6665 | 37.5 | |
| 1998 | 6165 | 41.5 | |
| 1999 | 6462 | 44.9 | |
| Census tract education ‡ | ≤10.5 | 14 966 | 28.7 |
| 10.6–17.5 | 14 920 | 31.3 | |
| 17.6–26.0 | 15 438 | 32.0 | |
| >26 | 15 184 | 33.0 | |
| Census tract poverty § | ≤3.5 | 14 611 | 29.8 |
| 3.6–7.0 | 16 252 | 31.3 | |
| 7.1–12.5 | 14 246 | 31.9 | |
| >12.5 | 15 399 | 32.0 | |
| Comorbidity index ∥ | 0 | 45 694 | 30.3 |
| 1 | 9317 | 32.4 | |
| 2 | 2663 | 37.5 | |
| ≥3 | 4043 | 36.3 | |
| Grade ∥ | Well differentiated | 8164 | 15.4 |
| Moderately differentiated | 35 536 | 27.6 | |
| Poorly differentiated/undifferentiated | 13 467 | 49.3 | |
| Unknown | 4550 | 36.3 | |
| Clinical stage ∥ | T1 | 15 693 | 23.7 |
| T2 | 31 827 | 28.1 | |
| T3 | 3906 | 32.3 | |
| T4 | 4002 | 74.3 | |
| Unknown | 6289 | 39.1 |
| Characteristic . | Strata . | No. . | % of patients receiving androgen deprivation therapy . |
|---|---|---|---|
| All patients | 61 717 | 31.4 | |
| Age, years | 66–69 | 13 854 | 22.7 |
| 70–74 | 20 137 | 26.8 | |
| 75–79 | 15 635 | 34.0 | |
| ≥80 | 12 091 | 45.4 | |
| Ethnicity | White | 50 287 | 30.5 |
| Black | 5273 | 31.3 | |
| White Hispanic | 2438 | 35.3 | |
| Other/unknown | 3719 | 40.6 | |
| SEER region † | San Francisco | 4204 | 33.4 |
| Connecticut | 8002 | 35.4 | |
| Michigan | 11 524 | 26.6 | |
| Hawaii | 1775 | 36.8 | |
| Iowa | 8140 | 32.1 | |
| New Mexico | 3081 | 19.4 | |
| Seattle | 6568 | 28.4 | |
| Utah | 3903 | 27.4 | |
| Georgia | 3580 | 27.2 | |
| San Jose | 2306 | 43.3 | |
| Los Angeles | 8634 | 38.1 | |
| Year of diagnosis | 1992 | 11 338 | 24.1 |
| 1993 | 9583 | 24.0 | |
| 1994 | 7841 | 26.1 | |
| 1995 | 7110 | 29.1 | |
| 1996 | 6553 | 34.3 | |
| 1997 | 6665 | 37.5 | |
| 1998 | 6165 | 41.5 | |
| 1999 | 6462 | 44.9 | |
| Census tract education ‡ | ≤10.5 | 14 966 | 28.7 |
| 10.6–17.5 | 14 920 | 31.3 | |
| 17.6–26.0 | 15 438 | 32.0 | |
| >26 | 15 184 | 33.0 | |
| Census tract poverty § | ≤3.5 | 14 611 | 29.8 |
| 3.6–7.0 | 16 252 | 31.3 | |
| 7.1–12.5 | 14 246 | 31.9 | |
| >12.5 | 15 399 | 32.0 | |
| Comorbidity index ∥ | 0 | 45 694 | 30.3 |
| 1 | 9317 | 32.4 | |
| 2 | 2663 | 37.5 | |
| ≥3 | 4043 | 36.3 | |
| Grade ∥ | Well differentiated | 8164 | 15.4 |
| Moderately differentiated | 35 536 | 27.6 | |
| Poorly differentiated/undifferentiated | 13 467 | 49.3 | |
| Unknown | 4550 | 36.3 | |
| Clinical stage ∥ | T1 | 15 693 | 23.7 |
| T2 | 31 827 | 28.1 | |
| T3 | 3906 | 32.3 | |
| T4 | 4002 | 74.3 | |
| Unknown | 6289 | 39.1 |
All P values were <.001 and were from two-sided chi-square tests for differences in the percentage of patients receiving androgen deprivation therapy across strata for each characteristic.
SEER = Surveillance, Epidemiology and End Results.
Percentage of adults with fewer than 12 years of education.
Percentage of residents living below the poverty level.
Percentage of Variance in the Use of Androgen Deprivation Therapy Attributable to Patient and Tumor Characteristics
We investigated the use of androgen deprivation therapy by patient and tumor characteristics with logistic regression models. Table 2 presents the percentage of variance in the use of androgen deprivation therapy that was attributable to each set of patient and tumor characteristics. After adjustment for other variables (i.e., age, ethnicity, year of diagnosis, SEER region, comorbidity, and census tract education and poverty), the combination of tumor stage and grade was associated with the highest percentage of variance, at 11.77%. Ethnicity, the combination of census tract education and poverty, and comorbidity contributed minimally, with each associated with less than 1% of the variance in use of androgen deprivation therapy.
Percentage of variance in use of androgen deprivation therapy attributable to patient and tumor characteristics from logistic regression models ( n = 61 717)
| . | Percentage of variance in use of androgen deprivation therapy * . | . | |
|---|---|---|---|
| Characteristics . | Unadjusted . | Adjusted . | |
| Age | 4.16 | 2.04 | |
| Ethnicity | 0.40 | 0.02 | |
| Census tract education and poverty | 0.20 | 0.10 | |
| SEER region | 1.92 | 1.46 | |
| Year of diagnosis | 3.59 | 4.45 | |
| Comorbidity | 0.27 | 0.04 | |
| Stage and grade | 12.90 | 11.77 | |
| All patient or tumor variables | 21.52 | ||
| . | Percentage of variance in use of androgen deprivation therapy * . | . | |
|---|---|---|---|
| Characteristics . | Unadjusted . | Adjusted . | |
| Age | 4.16 | 2.04 | |
| Ethnicity | 0.40 | 0.02 | |
| Census tract education and poverty | 0.20 | 0.10 | |
| SEER region | 1.92 | 1.46 | |
| Year of diagnosis | 3.59 | 4.45 | |
| Comorbidity | 0.27 | 0.04 | |
| Stage and grade | 12.90 | 11.77 | |
| All patient or tumor variables | 21.52 | ||
The percentage of variance attributable to each characteristic was calculated by the following formula: maximum rescaled R2 × 100%. The R2 values were derived from logistic regression models with use of androgen deprivation therapy as the dependent variable. For the unadjusted analyses, each patient and tumor characteristic was entered separately as an independent variable. For the adjusted analyses, each patient and tumor characteristic was entered as an independent variable, with simultaneous adjustment for the other variables. Patient age was analyzed as a continuous variable. Ethnicity; Surveillance, Epidemiology, and End Results (SEER) region; year of diagnosis; comorbidity; census tract education; and census tract poverty were categorized as in Table 1 . Tumor stage was categorized as T1 through T4 or as unknown. Tumor grade was categorized as well, moderately, poorly or undifferentiated, or unknown.
Percentage of variance in use of androgen deprivation therapy attributable to patient and tumor characteristics from logistic regression models ( n = 61 717)
| . | Percentage of variance in use of androgen deprivation therapy * . | . | |
|---|---|---|---|
| Characteristics . | Unadjusted . | Adjusted . | |
| Age | 4.16 | 2.04 | |
| Ethnicity | 0.40 | 0.02 | |
| Census tract education and poverty | 0.20 | 0.10 | |
| SEER region | 1.92 | 1.46 | |
| Year of diagnosis | 3.59 | 4.45 | |
| Comorbidity | 0.27 | 0.04 | |
| Stage and grade | 12.90 | 11.77 | |
| All patient or tumor variables | 21.52 | ||
| . | Percentage of variance in use of androgen deprivation therapy * . | . | |
|---|---|---|---|
| Characteristics . | Unadjusted . | Adjusted . | |
| Age | 4.16 | 2.04 | |
| Ethnicity | 0.40 | 0.02 | |
| Census tract education and poverty | 0.20 | 0.10 | |
| SEER region | 1.92 | 1.46 | |
| Year of diagnosis | 3.59 | 4.45 | |
| Comorbidity | 0.27 | 0.04 | |
| Stage and grade | 12.90 | 11.77 | |
| All patient or tumor variables | 21.52 | ||
The percentage of variance attributable to each characteristic was calculated by the following formula: maximum rescaled R2 × 100%. The R2 values were derived from logistic regression models with use of androgen deprivation therapy as the dependent variable. For the unadjusted analyses, each patient and tumor characteristic was entered separately as an independent variable. For the adjusted analyses, each patient and tumor characteristic was entered as an independent variable, with simultaneous adjustment for the other variables. Patient age was analyzed as a continuous variable. Ethnicity; Surveillance, Epidemiology, and End Results (SEER) region; year of diagnosis; comorbidity; census tract education; and census tract poverty were categorized as in Table 1 . Tumor stage was categorized as T1 through T4 or as unknown. Tumor grade was categorized as well, moderately, poorly or undifferentiated, or unknown.
Percentage of Variance in Androgen Deprivation Therapy Use Attributable to the Urologist
A total of 1802 urologists were identified as providing care to the patients in this study within the first year of cancer diagnosis. Table 3 presents a multilevel analysis examining the contribution of the urologist to the variance in androgen deprivation therapy use, with models for the overall cohort of patients and also with models stratified by period (1992–1996 versus 1997–1999). For the period from 1997 through 1999, patients were further stratified into evidence-based and uncertain-benefit groups, because that period followed the publication of trials ( 20 , 21 ) that showed a survival benefit for locally advanced cancers treated with the combination of androgen deprivation and radiation. For each model, we have presented the results of two analyses. First, we present the residual ICC, which represents the percentage of variance associated with the urologist after adjustment for available patient and tumor characteristics. Second, we present the further partitioned variance, which represents the percentages of total variance associated with the urologist and with selected patient or tumor variables.
Percentage of variance in use of androgen deprivation therapy attributable to the urologist from multilevel analyses
| . | All patients . | . | . | Evidence-based group (1997–1999) . | Uncertain-benefit group (1997–1999) . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | 1992–1999 . | 1992–1996 . | 1997–1999 . | . | . | ||
| No. of patients | 61 717 | 42 425 | 19 292 | 2329 | 16 963 | ||
| No. of urologists | 1802 | 1577 | 1258 | 789 | 1234 | ||
| Residual ICC * , † , % | 20.63 | 20.38 | 26.55 | 29.09 | 25.48 | ||
| Partitioned variance * , ‡ , % | |||||||
| Stage and grade | 13.06 | 15.40 | 9.71 | 6.63 | 5.34 | ||
| Patient characteristics | 8.99 | 6.58 | 4.29 | 7.27 | 4.99 | ||
| Urologist | 16.00 | 15.60 | 22.56 | 25.38 | 22.68 | ||
| . | All patients . | . | . | Evidence-based group (1997–1999) . | Uncertain-benefit group (1997–1999) . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | 1992–1999 . | 1992–1996 . | 1997–1999 . | . | . | ||
| No. of patients | 61 717 | 42 425 | 19 292 | 2329 | 16 963 | ||
| No. of urologists | 1802 | 1577 | 1258 | 789 | 1234 | ||
| Residual ICC * , † , % | 20.63 | 20.38 | 26.55 | 29.09 | 25.48 | ||
| Partitioned variance * , ‡ , % | |||||||
| Stage and grade | 13.06 | 15.40 | 9.71 | 6.63 | 5.34 | ||
| Patient characteristics | 8.99 | 6.58 | 4.29 | 7.27 | 4.99 | ||
| Urologist | 16.00 | 15.60 | 22.56 | 25.38 | 22.68 | ||
Hierarchical generalized linear model with patient age; comorbidity; ethnicity; Surveillance, Epidemiology, and End Results (SEER) region; tumor stage; grade; year of diagnosis; census tract education; and census tract poverty entered as “level 1” variables and physician identifiers entered as “level 2” variables. ICC = intraclass correlation coefficient.
The percentage of variance attributable to the urologist calculated with a threshold model, after simultaneous adjustment of all available patient and tumor characteristics. The denominator for the calculation of the percentage was composed of the variance attributable to the urologist, after adjustment for available patient and tumor characteristics, and the variance attributable to unexplained patient or tumor variables plus error.
The variance was further partitioned using a threshold model so that the percentages of total variance contributed by the urologist, as well as those contributed by patient and tumor variables, are presented. Results are presented as the percentage of total variance attributable to the indicated characteristic. The denominator is total variance, which is composed of the variance attributable to the urologist after adjustment for available patient and tumor characteristics, the variance attributable to available patient and tumor variables, and the variance attributable to unexplained patient or tumor variables plus error.
Percentage of variance in use of androgen deprivation therapy attributable to the urologist from multilevel analyses
| . | All patients . | . | . | Evidence-based group (1997–1999) . | Uncertain-benefit group (1997–1999) . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | 1992–1999 . | 1992–1996 . | 1997–1999 . | . | . | ||
| No. of patients | 61 717 | 42 425 | 19 292 | 2329 | 16 963 | ||
| No. of urologists | 1802 | 1577 | 1258 | 789 | 1234 | ||
| Residual ICC * , † , % | 20.63 | 20.38 | 26.55 | 29.09 | 25.48 | ||
| Partitioned variance * , ‡ , % | |||||||
| Stage and grade | 13.06 | 15.40 | 9.71 | 6.63 | 5.34 | ||
| Patient characteristics | 8.99 | 6.58 | 4.29 | 7.27 | 4.99 | ||
| Urologist | 16.00 | 15.60 | 22.56 | 25.38 | 22.68 | ||
| . | All patients . | . | . | Evidence-based group (1997–1999) . | Uncertain-benefit group (1997–1999) . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | 1992–1999 . | 1992–1996 . | 1997–1999 . | . | . | ||
| No. of patients | 61 717 | 42 425 | 19 292 | 2329 | 16 963 | ||
| No. of urologists | 1802 | 1577 | 1258 | 789 | 1234 | ||
| Residual ICC * , † , % | 20.63 | 20.38 | 26.55 | 29.09 | 25.48 | ||
| Partitioned variance * , ‡ , % | |||||||
| Stage and grade | 13.06 | 15.40 | 9.71 | 6.63 | 5.34 | ||
| Patient characteristics | 8.99 | 6.58 | 4.29 | 7.27 | 4.99 | ||
| Urologist | 16.00 | 15.60 | 22.56 | 25.38 | 22.68 | ||
Hierarchical generalized linear model with patient age; comorbidity; ethnicity; Surveillance, Epidemiology, and End Results (SEER) region; tumor stage; grade; year of diagnosis; census tract education; and census tract poverty entered as “level 1” variables and physician identifiers entered as “level 2” variables. ICC = intraclass correlation coefficient.
The percentage of variance attributable to the urologist calculated with a threshold model, after simultaneous adjustment of all available patient and tumor characteristics. The denominator for the calculation of the percentage was composed of the variance attributable to the urologist, after adjustment for available patient and tumor characteristics, and the variance attributable to unexplained patient or tumor variables plus error.
The variance was further partitioned using a threshold model so that the percentages of total variance contributed by the urologist, as well as those contributed by patient and tumor variables, are presented. Results are presented as the percentage of total variance attributable to the indicated characteristic. The denominator is total variance, which is composed of the variance attributable to the urologist after adjustment for available patient and tumor characteristics, the variance attributable to available patient and tumor variables, and the variance attributable to unexplained patient or tumor variables plus error.
For the entire cohort of patients during the period from 1992 through 1999, the residual ICC was 20.63%, with an increase to 26.55% when the analysis was limited to patients from 1997 through 1999. The residual ICCs when patients were divided into evidence-based and uncertain-benefit groups were 29.09% and 25.48%, respectively.
When the variance was partitioned further to include the percentage of total variance attributable to patient and tumor variables, the percentage of the total variance attributable to the urologist (16.00%) was higher than that attributable to the combination of tumor stage and grade (13.06%). This pattern was especially pronounced in the period from 1997 through 1999, in which the percentage of the total variance attributable to the urologist was 22.56%, that attributable to the combination of tumor stage and grade was 9.71%, and that attributable to patient characteristics was 4.29%. Similar patterns were found in the evidence-based and uncertain-benefit groups.
In additional analyses, when we limited the analyses to urologists who saw larger numbers of patients with prostate cancer, we found that the ICCs were similar to those obtained with all urologists. For example, the residual ICC was 20.07% when the analysis was limited to urologists who saw at least 50 patients. To determine the potential impact of the data from patients who received only a few doses of gonadotropin-releasing hormone agonist, we repeated the analysis but excluded patients who received one to four doses in the first year. The residual ICC from this analysis was essentially unaffected, at 20.80%. We also calculated residual ICCs by use of different techniques to compare the results with those from the threshold model ( Table 3 ). These results showed similar trends but somewhat lower ICCs than those from the threshold model. For example, the most conservative estimates for the residual ICCs (using a simulation model) were 14.70%, 21.05%, and 18.78% for the entire cohort, evidence-based, and uncertain-benefit group of patients, respectively, compared with 20.63%, 29.09%, and 25.48%, respectively, in the threshold model ( Table 3 ).
Rates of Androgen Deprivation Therapy Use Attributable to the Urologist
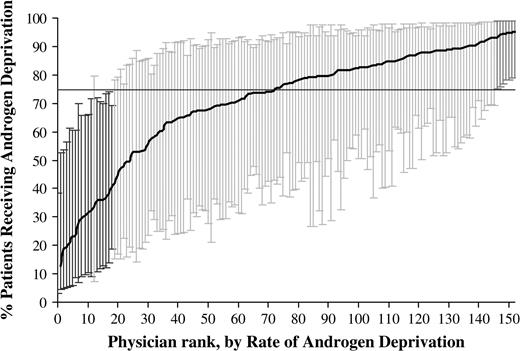
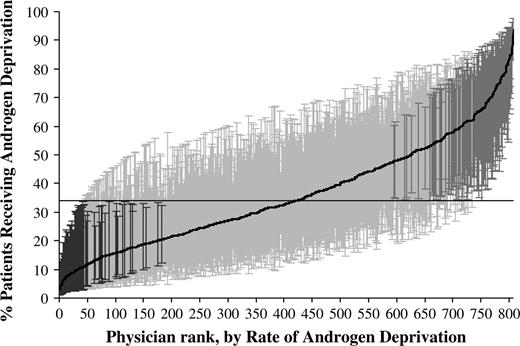
As another measure of physician variation in use of androgen deprivation therapy, urologist-specific rates of administering androgen deprivation therapy were calculated with a hierarchical generalized linear model, adjusted for patient and tumor characteristics, and plotted by rank, from lowest to highest. The analyses were limited to patients diagnosed with prostate cancer from 1997 through 1999, and plots were generated for patients in the evidence-based group ( Fig. 1 ) and patients in the uncertain-benefit group ( Fig. 2 ). For the evidence-based group, 25 (16.3%) of the 153 urologists had a rate of androgen deprivation therapy use that was statistically significantly different (with a cut-off of P <.05) from the mean rate of 71.3%, with 7 (4.6%) of the 153 urologists having a rate above the mean and 18 (11.8%) of them having a rate below the mean. For the uncertain-benefit group of patients, 191 (23.6%) of the 808 urologists had a rate of androgen deprivation therapy use that was statistically significantly (with a cut-off of P <.05) different from the mean rate of 36.0%, with 123 (15.2%) of the 808 urologists having a rate above the mean and 68 (8.4%) having a rate below the mean.
Rates of androgen deprivation therapy use for 153 individual urologists by rank, from lowest to highest, who cared for patients in the evidence-based group diagnosed with prostate cancer from 1997 through 1999. The rates were calculated by use of hierarchical generalized linear modeling, adjusted for patient and tumor characteristics. This model also accounts for differences in reliability of individual rates resulting from variations in the size of the panel of patients for each urologist. Each urologist-specific rate was therefore adjusted toward the mean of the overall rate as a factor of panel size (i.e., a urologist rate that is based on a large panel of patients will result in very little adjustment toward the mean rate, whereas a urologist rate that is based on a small panel will have more adjustment). This analysis was limited to urologists who saw at least five patients. The horizontal line represents the overall mean rate of androgen deprivation. Error bars represent 95% confidence intervals for the rates of individual urologists. Black error bars represent urologists that have rates statistically significantly ( P value range = <.001 to .045) below the mean rate, dark gray bars represent urologists that have rates statistically significantly above the mean rate ( P value range = .009 to .025), and light gray bars represent rates that are not different from the mean rate.
Rates of androgen deprivation therapy use for 808 individual urologists by rank, from lowest to highest, who cared for patients in the uncertain-benefit group diagnosed with prostate cancer from 1997 through 1999. Rates were calculated by use of hierarchical generalized linear modeling, adjusted for patient and tumor characteristics. This model also accounts for differences in the reliability of individual rates resulting from variations in the size of the panel of patients for each urologist. Each urologist-specific rate was therefore adjusted toward the overall rate mean as a factor of panel size (i.e., a urologist rate that is based on a large panel of patients will result in very little adjustment toward the mean rate, whereas a urologist rate that is based on a small panel will have more adjustment). This analysis was limited to urologists who treated at least five patients. The horizontal line represents the overall mean rate of androgen deprivation. Error bars represent 95% confidence intervals for the rates of individual urologists. Black error bars represent urologists that have rates statistically significantly ( P value range = <.001 to .049) below the mean rate, dark gray bars represent urologists that have rates statistically significantly above the mean rate ( P value range = <.001 to .050), and light gray bars represent rates that are not different from the mean rate.
D ISCUSSION
This study is, to our knowledge, the first to use multilevel analyses to examine the contribution of the urologist to the use of androgen deprivation therapy for prostate cancer. Although most of the variance in use of androgen deprivation therapy in this study was unexplained, approximately 21% was associated with the urologist. More of the variance was attributable to the urologist than to available patient and tumor characteristics. Thus, the urologist who sees a patient may be a more important determinant of whether that patient will receive androgen deprivation therapy than the characteristics of the tumor (e.g., stage or grade) or the patient (e.g., age and comorbidity).
With one exception ( 33 ) , previous studies examining the amount of variance in practice behavior attributable to the physician have found the ICCs to be substantially lower than in our study ( 11 – 14 ) . The behaviors examined included resource utilization for care of diabetic patients (ICCs = 1%–4%) ( 11 ) , contribution to managed care pharmacy expenses (ICCs = 0.9%–5.3%) ( 13 ) , patient satisfaction (ICCs = 3.3%–6.5%) ( 12 ) , prescription of beta-blockers to patients with angina (ICC = 7.9%) ( 14 ) , and monitoring of digoxin levels (ICC = 24%) ( 33 ) . Given the generally low ICCs, the authors of these studies concluded that efforts directed at changing physician behavior would likely have a small impact on outcomes and that profiling individual physicians would be unreliable ( 11 , 13 ) .
A number of factors may have explained the higher variance in androgen deprivation use attributable to the urologists in our study. One factor is the uncertainty that surrounds use of androgen deprivation therapy for prostate cancer. For instance, in a national survey of urologists about recommended treatment for patients with localized prostate cancer who were older than 70 years (in which more than one response could be selected) ( 34 ) , 74% chose radiation, 48% chose radical prostatectomy, 31% chose androgen deprivation therapy, and 38% chose observation only. Another factor was the broadening of indications for the use of androgen deprivation therapy during the 1990s ( 2 ) . This factor presumably contributed to the increasing variance attributable to the urologist over time and to the decreasing variance attributable to tumor characteristics. However, we found similar ICCs in the evidence-based and uncertain-benefit groups, suggesting that medical uncertainty was still playing a role in the evidence-based settings or that there were other important factors driving physician decisions to use androgen deprivation therapy. These factors could include financial incentives for the urologist to prescribe gonadotropin-releasing hormone agonists, pressure from patients to prescribe something in the face of a cancer diagnosis, or influences from local opinion leaders ( 35 – 37 ) . Physicians may differ in their response to these influences ( 38 , 39 ) , and these differences may contribute to the variance in use of androgen deprivation therapy.
Use of androgen deprivation therapy in prostate cancer is important to study for several reasons. First, it is a common treatment for a common cancer. There are more than 200 000 new cases of prostate cancer every year, with recent estimates suggesting that more than 80 000 patients will receive androgen deprivation therapy within 6 months of diagnosis ( 2 ) . Second, androgen deprivation therapy in the form of gonadotropin-releasing hormone agonists is very costly, making it the second highest Medicare Part B expenditure, at approximately $1.2 billion in 2003 ( 8 ) . Finally, androgen deprivation therapy is associated with a host of adverse effects, including reduced quality of life, sexual dysfunction, osteoporosis, and fractures ( 7 , 40 ) .
There are several limitations to this study. Because the analyses used information from Medicare claims, the study included only men who were 66 years or older. However, approximately 70% of men with incident prostate cancer are in that age group ( 41 ) , and androgen deprivation therapy is more commonly used for older men ( 2 ) . Another limitation is that we were unable to examine use of androgen deprivation therapy in health maintenance organization settings, in which the patterns of care may differ ( 42 ) . Some of the study exclusions may also limit the generalizability of our results. For instance, we excluded patients who did not see a urologist at all and excluded some patients who received care from several urologists (which may occur in group practices). In addition, we chose to focus on androgen deprivation therapy initiated within 6 months of diagnosis, without accounting for whether therapy was administered later in the treatment of the disease. This choice was based, in part, on the fact that early androgen deprivation therapy is clinically relevant because it is now being used in approximately 40% of patients with incident prostate cancer and is associated with an increased risk of fracture ( 2 , 23 ) . Also, because we only had data on stage and grade of the tumor at the time of diagnosis, examining androgen deprivation therapy beyond the first year may have introduced additional variation due to use for progression of disease, rather than as initial therapy. Most previous studies assessing the amount of variance attributable to the physician examined continuous outcome measures with statistical methods that are not appropriate for a binary outcome, as in our study. However, the optimal statistical approach for binary outcomes has not yet been clarified. We therefore estimated ICCs via several recently described methods ( 32 ) and found that our results were generally consistent across the approaches used. Finally, all potentially relevant variables (e.g., prostate-specific antigen levels) were not available in the study dataset. However, it is unlikely that inclusion of additional tumor variables in the analyses would have substantially altered our results, given that adjustment for other important tumor characteristics, such as stage and grade, had little effect on the ICCs.
There are a number of implications from this study. First, the substantial variations in rates of androgen deprivation use between urologists raise concerns about whether the therapy is being used appropriately ( 10 ) . Second, the ICCs in our study suggest that monitoring of the use of androgen deprivation therapy by individual urologists may be feasible. The reliability of physician profiles increases as the number of patients in the physician's panel increases and as the ICC increases ( 43 ) . Generally, a reliability of 0.8 is considered to be acceptable for making decisions about individual physicians on the basis of their profiles ( 11 , 44 ) . With an ICC of 20%, a urologist would need a panel of 16 patients to achieve a reliability of 0.8 [calculation with the Spearman-Brown prophecy formula ( 43 ) ]. This value compares favorably, for instance, with a physician needing a panel of 100 patients with diabetes to reliably assess that individual's use of hospital resources for diabetes ( 11 ) . The substantial variance in use of androgen deprivation therapy attributable to the urologist, independent of patient factors, suggests that interventions at the level of the urologist (e.g., education or change in reimbursement) may be an effective way to modify the use of this therapy for prostate cancer. Finally, the study findings suggest that primary care physicians should carefully consider the choice of urologist for their patients who may have prostate cancer, because the urologist is an important determinant of how such patients will be managed with regards to androgen deprivation therapy.
This work was supported in part by grants (RO1CA116758, P50CA105631, R24HS011618) from the Public Health Service. The funding bodies had no role in data extraction and analyses, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
References
Meng MV, Grossfeld GD, Sadetsky N, Mehta SS, Lubeck DP, Carroll PR. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer.
Shahinian VB, Kuo Y-F, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for localized prostate cancer.
Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma.
Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff R-O, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial.
Chodak GW, Keane T, Klotz L, The Hormone Therapy Study Group. Critical evaluation of hormonal therapy for carcinoma of the prostate.
Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer.
Holzbeierlein JM, McLaughlin MD, Thrasher JB. Complications of androgen deprivation therapy for prostate cancer.
Part B Physician/Supplier National Data, CY
Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand.
Wennberg J, Gittelsohn A. Variations in medical care among small areas.
Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease.
Sixma HJ, Spreeuwenberg PM, van der Pasch MA. Patient satisfaction with the general practitioner: a two-level analysis.
Cowen ME, Strawderman RL. Quantifying the physician contribution to managed care pharmacy expenses: a random effects approach.
Beaulieu M-D, Blais R, Jacques A, Battista RN, Lebeau R, Brophy J. Are patients suffering from stable angina receiving optimal medical treatment?
Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-medicare data: content, research applications, and generalizability to the United States elderly population.
Baldwin L-M, Adamache E, Klabunde CN, Kenward K, Dahlman C, Warren JL. Linking physician characteristics and medicare claims data: issues in data availability, quality and measurement.
Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis.
Clinical Practice Guidelines in Oncology. Prostate Cancer. The National Comprehensive Cancer Network version 1.
Pilepich MV, Caplan R, Byhardt RW, CA, Gallagher MJ, Mesic JB, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85–31.
Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff R-O, Storme G, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin.
Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer.
Fritz A, Ries L, editors. SEER extent of disease–
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data.
Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use.
Nagelkerke NJD. A note on a general definition of the coefficient of determination.
Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2nd ed. Thousand Oaks (CA): Sage;
Leyland AH, Goldstein H, editors. Multilevel modeling of health statistics. West Sussex (England): John Wiley and Sons, Ltd.;
Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. 5th ed. Cary (NC): SAS Institute;
Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models.
Orav JE, Wright EA, Palmer HR, Hargraves LJ. Issues of variability and bias affecting multisite measurement of quality of care.
Gee WF, Holtgrewe HL, Albertsen PC, Litwin MS, Manyak MJ, O'Leary MP, et al. Practice trends in the diagnosis and management of prostate cancer in the United States.
General Accounting Office Report. Medicare: payments for covered outpatient drugs exceed providers' costs. GAO-01–1118. September 21,
Wasson JH, Fowler FJ, Barry MJ. Androgen deprivation therapy for asymptomatic advanced prostate cancer in the prostate specific antigen era: a national survey of urologist beliefs and practices.
Talcott JA. Androgen deprivation as primary treatment for early prostate cancer: Should we “just do something”?
Komaromy M, Lurie N, Osmond D, Vranizan K, Keane D, Bindman AB. Physician practice style and rates of hospitalization for chronic medical conditions.
Westert GP, Groenewegen PP. Medical practice variations: changing the theoretical approach.
Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of the “Androgen Deprivation Syndrome” in men receiving androgen deprivation for prostate cancer.
Surveillance, Epidemiology, and End Results (SEER). Available at: http://www.seer.cancer.gov . Program Public-Use Data (1973–1999), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2002, based on the November
Riley GF, Potosky AL, Klabunde CN, Warren JL, Ballard-Barbash R. Stage at diagnosis and treatment patterns among older women with breast cancer: an HMO and fee-for-service comparison.
Bravo G, Potvin L. Estimating the reliability of continuous measures with Cronbach's Alpha or the intraclass correlation coefficient: toward the integration of two traditions.