-
PDF
- Split View
-
Views
-
Cite
Cite
Frank Heinzelmann, Verena Jendrossek, Kirsten Lauber, Kerstin Nowak, Therese Eldh, Ruzica Boras, Rene Handrick, Marco Henkel, Christian Martin, Stefan Uhlig, David Köhler, Holger K. Eltzschig, Manfred Wehrmann, Wilfried Budach, Claus Belka, Irradiation-Induced Pneumonitis Mediated by the CD95/CD95-Ligand System, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 17, 6 September 2006, Pages 1248–1251, https://doi.org/10.1093/jnci/djj335
Close - Share Icon Share
Abstract
Pneumonitis is a dose-limiting side effect of radiotherapy. However, the underlying mechanisms of irradiation-induced pneumonitis are unclear. Several observations suggest that the CD95/CD95-ligand (CD95L) system is involved in this process. Therefore, we examined the development of pneumonitis in CD95- and CD95L-deficient and wild-type mice after single irradiation with 0 or 12.5 Gy by measuring breathing frequency, pulmonary resistance, and histopathologic changes. Although wild-type mice developed pathognomonic alterations characteristic of pneumonitis (judged by alveolar wall thickness, interstitial edema, and interstitial and peribronchial inflammation) that paralleled increased breathing frequency ratio on days 5–70 ( P <.03) with a maximum at day 37 (12.5 Gy, mean ratio = 1.05, 95% confidence interval [CI] = 1.01 to 1.08; P = .004 versus 0 Gy, mean ratio = 0.997, 95% CI = 0.976 to 1.02; P = .05) and pulmonary resistance (day 42, 12.5 Gy, mean = 0.51, 95% CI = 0.44 to 0.58 versus 0 Gy, mean = 0.40, 95% CI = 0.32 to 0.47; P = .03) after irradiation, no such changes were detected in CD95- or CD95L-deficient mice. This report demonstrates for the first time, to our knowledge, that the CD95/CD95L system is important for the development of irradiation-induced pneumonitis.
Pneumonitis is a dose-limiting side effect of total-body irradiation and is the main reason for dose restrictions during radiotherapy for any thorax-associated neoplasm. Pneumonitis is clinically associated with symptoms of respiratory failure and results in a mortality rate of up to 10% ( 1 – 3 ) .
Currently, the mechanisms for irradiation-induced pneumonitis are still unclear. Although pneumonitis mostly occurs within the irradiated areas of the lung, it may spread to nonirradiated areas, indicating that humoral factors may be involved ( 4 ) . The current working hypothesis suggests that complex alterations engaging lung epithelial cells (e.g., type 2 pneumocytes) ( 5 ) , endothelial cells ( 6 ) , and a perpetual cascade of cytokine expression patterns ( 7 – 9 ) are important for the induction of pneumonitis ( 10 ) .
CD95 (also known as Fas) and CD95L (also known as FasL or CD178) are expressed in various cells and tissues, including the lung (e.g., bronchiolar and alveolar cells) ( 11 ) . CD95 and CD95L are involved in the induction of apoptosis ( 12 , 13 ) , proinflammatory cytokine responses (e.g., tumor necrosis factor-α, interleukin-8) ( 14 , 15 ) , and the attraction of neutrophils ( 16 , 17 ) . In this regard, it has been shown that acute lung injury induced by bacterial infection ( 18 ) , bleomycin treatment ( 19 ) , or intrapulmonary deposition of IgG immune complexes ( 20 ) may result in increased CD95 and CD95L expression and the induction of apoptosis and inflammatory responses, including secretion of defensins and/or cytokines. Moreover, the expression of CD95 and CD95L is increased after irradiation ( 21 , 22 ) .
The present study was designed to define the contribution of CD95 and CD95L in the pathogenesis of irradiation-induced pneumonitis. Therefore, we assessed the development of pneumonitis after single irradiation (0 Gy [sham] or 12.5 Gy) of the right hemithorax in C57BL6/J mice with intact CD95 and CD95L (wild-type), CD95-deficient lpr mice, and CD95L-deficient gld mice with respect to physiologic (breathing frequency, pulmonary resistance, and pulmonary compliance) and histopathologic (cumulative inflammation score) changes.
Four- to six-week-old female C57BL6/J wild-type (n = 67), CD95 receptor-deficient (lpr) (n = 53), or CD95L-deficient (gld) (n = 61) mice (Charles River laboratories, Sulzfeld, Germany) were housed (up to five mice per cage) in a standard barrier facility at room temperatures of 20–22 °C with a 12-hour light/dark cycle. Food and drinking water were provided ad libitum. Mice were subsequently enrolled to the study protocol with a body weight of approximately 20 g after adaptation to a total-body plethysmograph for 14 days. All mouse protocols were approved by the University of Tuebingen animal protection board in conjunction with the regional council Tuebingen (Regierungspraesidium Tuebingen). Animal care was provided in accordance with the guidelines for care and use of laboratory animals (newest edition 25.05.1998 BGBI. I S. 1105; animal experiment R 3/01; R 1/04).
Mice were anesthetized with 2% isoflurane and placed in holders, and their bodies, excluding the right hemithorax, were shielded with 60 mm of lead. Mice were then irradiated with a single dose of 0 Gy (sham) or 12.5 Gy using a linear accelerator (dose rate = 4.7 Gy/min; n ≥ 5 mice per dose group).
After irradiation, breathing frequency was measured using a total-body plethysmograph with a chamber volume of 960 cm 3 . Mice were placed individually in the chamber, and pressure changes in the chamber were monitored, converted into an electric signal, which was filtered to remove interfering signals not caused by respiration, and amplified. The amplified signal was calibrated from 1 to 8 Hz (i.e., breaths per second) using an oscillator. Breathing frequencies at rest were measured for 2 minutes and 40 seconds at the same time of day to minimize circadian changes. Breathing frequency was measured twice weekly for up to 30 weeks, and the breathing frequency ratio (breathing frequency at day x/breathing frequency at day 0) was plotted as a function of time (Supplementary Data 1, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol98/issue17 ).
We analyzed pulmonary resistance and pulmonary compliance, additional parameters of lung physiology that may be indicative of lung injury, in wild-type, gld, and lpr mice at day 42, on which the maximum increase of breathing frequency ratio in irradiated C57BL6/J mice occurred. To do this, mice were deeply anesthetized with pentobarbital and their lungs prepared and perfused at the indicated time points after irradiation, as described ( 23 , 24 ) (Supplementary Data 2, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol98/issue17 ).
In addition, we histologically examined the lungs of wild-type, lpr, and gld mice for pathognomonic alterations of pneumonitis using standard methods ( 5 , 19 , 25 ) . After cervical dislocation, lungs were fixed in formalin and embedded in paraffin. Two 5-μm-thick sections were cut from each lobe and stained with hematoxillin and eosin. In accordance with previously reported findings ( 5 , 19 , 25 ) , lungs were analyzed at days 1, 21, 42, and 84 for the onset of the pneumonitic reaction and at day 210 to detect potential late effects. Histopathologic changes, i.e., alveolar wall thickness, interstitial edema, and interstitial and peribronchial inflammation, were judged by two independent investigators (T. Eldh, K. Nowak) in a blinded manner. For each of these morphologic alterations, a numerical score was determined. Scoring was assessed according to previously published scoring criteria ( 5 , 19 , 25 ) as follows: 0 = alterations in less than 10% of the fields viewed, 1 = in 10%–30%, 2 = in 30%–50%, 3 = in 50%–70%, and 4 = in more than 70%. A cumulative inflammation score was then determined for each group of mice (Supplementary Data 3, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol98/issue17 ). Due to the development of lymphadenopathy in lpr and gld mice, these analyses were restricted to days 1–84 ( 26 , 27 ) .
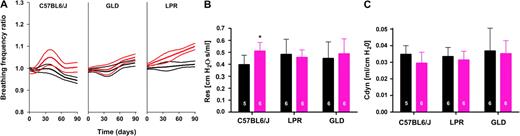
Determination of lung physiologic parameters revealed that in sham-irradiated wild-type mice, breathing frequency ratio decreased over time, whereas that of sham-irradiated lpr and gld mice increased, most probably due to lymphadenopathy, e.g., in the thorax, cervical lymph nodes, and lungs, as previously described ( 26 , 27 ) . In contrast to sham-irradiated mice, wild-type mice treated with 12.5 Gy had a statistically significant increase in breathing frequency ratio on days 5–70 ( P <.03) with a maximum at day 37 (12.5 Gy, mean ratio = 1.05, 95% CI = 1.01 to 1.08; P = .004 versus 0 Gy, mean ratio = 0.997, 95% CI = 0.976 to 1.02; P = .05; Fig. 1, A ). This peak in breathing frequency occurred during the acute phase of lung injury ( 28 ) , namely pneumonitis.
Ionizing radiation and alterations of lung physiologic parameters in C57BL6/J wild-type, CD95 receptor-deficient (LPR), and CD95L-deficient (GLD) mice. Mice were individually irradiated at the right hemithorax with 0 Gy (sham irradiated) or 12.5 Gy (irradiated). A ) The breathing frequency ratio (breathing frequency at day x/breathing frequency at day 0) of sham-irradiated and irradiated mice was measured twice weekly from day 0 to 210 and fitted by a nonlinear regression model that allowed for a sinus function between days 5 and 70 after irradiation. Regression lines with 95% confidence intervals are shown from day 0 to 90 (details in Supplementary Data 1, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol98/issue17 ). C57BL6/J mice: sham irradiated ( black lines ), decreased breathing frequency ratio over time ( P <.001), no pneumonitic peak; irradiated ( red lines ), no change in breathing frequency over time, but a pneumonitic peak was observed ( P <.03). GLD mice: sham irradiated ( black lines ), increased breathing frequency ratio over time ( P <.001), no pneumonitic peak observed; irradiated ( red lines ), breathing frequency ratio increased over time ( P <.001), no pneumonitic peak. LPR mice: sham irradiated ( black lines ), increased breathing frequency ratio over time ( P <.001), no pneumonitic peak; irradiated ( red lines ), breathing frequency ratio increased over time ( P <.001), no pneumonitic peak. B ) Pulmonary resistance (Res) was measured in sham-irradiated mice ( black bars ) and irradiated mice ( red bars ) 42 days after irradiation. * P = .03, irradiated C57BL6/J mice compared with sham-irradiated control mice. Pulmonary resistance was not altered in LPR ( P = .349) and GLD ( P = .349) mice. C ) Pulmonary compliance (Cdyn) was measured in sham-irradiated mice ( black bars ) and irradiated mice ( red bars ) 42 days after irradiation. Pulmonary compliance was not altered in any mouse strain. P = .14 (C57BL6/J), P = .37 (LPR), P = .40 (GLD). Each series represents six independent experiments. Experimental details are provided in Supplementary Data 2 (available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol98/issue17 ). Pulmonary resistance and pulmonary compliance are expressed in absolute values. Means and upper 95% confidence intervals are shown. The number of independent experiments performed is indicated on each bar. P values (one-sided t test) were corrected for multiple comparisons according to the false-discovery rate procedure using the “R” statistical package.
Furthermore, this peak was not detectable in lpr or gld mice (day 37, lpr mice, 12.5 Gy, mean = 1.05, 95% CI = 1.03 to 1.07; P = .99 versus 0 Gy, mean = 1.00, 95% CI = 0.98 to 1.02; P = .92; gld mice, 12.5 Gy, mean = 0.995, 95% CI = 0.97 to 1.02; P = .09 versus 0 Gy, mean = 0.99, 95% CI = 0.97 to 1.01; P = .151; Fig. 1, A ). Although breathing frequency was recorded until day 210, only values through day 90 could be considered for further analysis. From day 90 onward, breathing frequencies of gld and lpr mice increased independent of irradiation, most probably due to lymphadenopathy, e.g., in the thorax, the mediastinum, and the bowel, which impairs normal breathing ( 26 , 27 ) .
Pulmonary resistance and pulmonary compliance were also compared in wild-type, gld, and lpr mice at day 42 after irradiation ( Fig. 1, B and C ). Irradiated wild-type mice had statistically significantly higher airway resistance than sham-irradiated mice at day 42 (day 42, 12.5 Gy, mean = 0.51, 95% CI = 0.44 to 0.58 versus 0 Gy, mean = 0.40, 95% CI = 0.32 to 0.47; P = .03; Fig. 1, B ). Thus, in irradiated wild-type mice, at the time point of maximal increase in breathing frequency, a second physiologic parameter indicative of inflammatory lung injury was increased. In contrast, no differences were observed in irradiated and sham-irradiated gld ( P = .349) and lpr ( P = .349) mice ( Fig. 1, B ).
To further confirm the physiological significance of CD95 and CD95L in the development of irradiation-induced pneumonitis, we histologically examined the lungs of wild-type, lpr, and gld mice for characteristic pathognomonic alterations of pneumonitis. In sham-irradated wild-type mice, no morphologic changes in lung tissue were detected throughout the observation period (days 1–84). In contrast, following 12.5-Gy exposure, a noteworthy augmentation in alveolar wall thickness, occurrence of interstitial edema, and interstitial and peribronchial inflammation in irradiated right lungs of wild-type mice was detected ( Fig. 2, A ) resulting in increased inflammation scores on days 21, 42, and 84, compared with sham-irradiated mice ( Fig. 2, B ). Moreover, a less pronounced yet clearly detectable inflammatory reaction was observed in the lead-shielded left lungs supporting the interpretation that humoral factors, like inflammatory cytokines or chemokines, might be involved in this form of secondary lung injury ( Fig. 2, A and B ).
Ionizing radiation and histopathologic alterations of pneumonitis in wild-type and CD95/CD95L-deficient mice. A ) Images of histological sections. At days 1 and 21, histological sections were taken from lungs of sham-irradiated and irradiated C57BL6/J, lpr (CD95 deficient), and gld (CD95L deficient) mice; stained with hematoxillin and eosin; and analyzed at ×10 and ×40 ( inset ) magnification. B ) Semiquantitative histological analysis of pulmonary inflammation. In contrast to CD95/CD95L-deficient mice, C57BL6/J mice show a strongly elevated pulmonary inflammation sum score after irradiation. Histological sections were prepared at days 1, 21, 42, and 84 as described in Fig. 2, A and semiquantitatively analyzed to obtain the pulmonary inflammation score (numerical score consisting of alveolar wall thickness, interstitial edema, and interstitial and peribronchial inflammation). C57BL6/J—right and left lung: day 1, n.s.; days 21, 42, and 84, P <.001. gld —right and left lung: day 1, P <.001 (sum inflammation score statistically significantly lower); days 21, 42, and 84, n.s. lpr —right and left lung: days 1, 21, 42, and 84, n.s. C ) Differences in pulmonary inflammation score of irradiated and sham-irradiated littermates. On the basis of the data from Fig. 2, B the Δ inflammation score values ( Δ inflammation score = inflammation score [irradated mice] − inflammation score [sham-irradiated mice]) were calculated and plotted against time. For histopathological analysis at least 5 C57BL6/J, gld , and lpr mice per dose and time point were examined as indicated in more detail in Supplementary Data 3.
In strong contrast to wild-type mice, the histopathologic analyses revealed no signs of a pulmonary inflammatory response in gld mice. Irradiated right lungs of gld mice remained undistinguishable from nonirradiated or shielded left lungs at all time points ( Fig. 2, A ).
A slight but statistically nonsignificant inflammatory response was observed in the right lungs of lpr mice at days 21 and 42 upon 12.5-Gy exposure when compared with sham-irradiated lpr mice ( Fig. 2, A and B ). However, the difference in the respective inflammation score was clearly less pronounced in lpr mice (approximately 2 points above nonirradiated littermates) compared with wild-type mice (approximately 5 points above nonirradiated littermates; P <.001) ( Fig. 2, C ). Similarly, no statistically significant histologic differences were observed in the shielded left lungs of mice treated with 0 and 12.5 Gy ( Fig. 2, A and B ).
In conclusion, our data demonstrate a central role of CD95 and CD95L in the development of irradiation-induced pneumonitis. In response to hemithoracic irradiation, a statistically significant increase in breathing frequency ratio and pulmonary resistance accompanied by histologic signs of pulmonary inflammation was only detected in wild-type mice. This pneumonitic reaction was considerably diminished in lpr mice and completely absent in gld mice. These data are consistent with recent findings of reduced lung injury induced in gld and lpr mice compared with wild-type mice by intrapulmonary deposition of IgG immune complexes ( 20 ) .
However, it has to be taken into account that the time curve and severity of a given pneumonitic response in mice is strain-dependent. Thus, a potential limitation of our study is the fact that all conclusions are based on the use of C57BL6/J mice.
Nevertheless, our findings identify CD95 and CD95L as potential therapeutic targets in irradiation-induced pneumonitis. Future studies will define whether pharmacologic inhibition of CD95 and CD95L might be suitable for the prevention or treatment of the inflammatory response to oncologic irradiation therapy.
Supported by grants of the fortune program of the University of Tuebingen (F. Heinzelmann and W. Budach), the Doctoral Program of the Deutsche Forschungsgemeinschaft (DFG) “Cellular mechanisms of immune-associated processes,” GK 794, the DFG grant EL274/2-2 (H. K. Eltzschig), as well as the “Federal Ministry of Education and Research” (Foe. 01KS9602) and the Interdisciplinary Center of Clinical Research Tuebingen (IZKF) (to V. Jendrossek and C. Belka) and a grant from the Mildred Scheel Stiftung (C. Belka and V. Jendrossek). The sponsors had no role in the study design, data collection, analysis, interpretation of the data, or the writing of the manuscript.
We are highly grateful to D. Karp (Department of Pulmonary Pharmacology, Research Center Borstel) for the help with the determinations of lung physiologic parameters.
Funding to pay the Open Access publication charges for this article was provided by the Department of Radiation Oncology, University Tübingen, Germany.
References
Movsas B, Raffin TA, Epstein AH, Link CJ Jr. Pulmonary radiation injury.
Roach M 3rd, Gandara DR, Yuo HS, Swift PS, Kroll S, Shrieve DC, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors.
Girinsky T, Benhamou E, Bourhis JH, Dhermain F, Guillot-Valls D, Ganansia V, et al. Prospective randomized compariso of single-dose versus hyperfractionated total-body irradiation in patients with hematologic malignancies.
Roberts CM, Foulcher E, Zaunders JJ, Bryant DH, Freund J, Cairns D, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction.
Penney DP, Siemann DW, Rubin P, Maltby K. Morphological correlates of fractionated radiation of the mouse lung: early and late effects.
Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation.
Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis.
Rube CE, Uthe D, Wilfert F, Ludwig D, Yang K, Konig J, et al. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation.
Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, et al. Compartmental responses after thoracic irradiation of mice: strain differences.
Trott KR, Herrmann T, Kasper M. Target cells in radiation pneumopathy.
Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, et al. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium.
Hagimoto N, Kuwano K, Kawasaki M, Yoshimi M, Kaneko Y, Kunitake R, et al. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation.
Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, et al. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages.
Seino K, Iwabuchi K, Kayagaki N, Miyata R, Nagaoka I, Matsuzawa A, et al. Chemotactic activity of soluble Fas ligand against phagocytes.
Ottonello L, Tortolina G, Amelotti M, Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes.
Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, Lang F, et al. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa.
Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis.
Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, Gao H, et al. Relationship of acute lung inflammatory injury to Fas/FasL system.
Belka C, Marini P, Budach W, Schulze-Osthoff K, Lang F, Gulbins E, et al. Radiation-induced apoptosis in human lymphocytes and lymphoma cells critically relies on the up-regulation of CD95/Fas/APO-1 ligand.
Nishioka A, Ogawa Y, Kubonishi I, Kataoka S, Hamada N, Terashima M, et al. An augmentation of Fas (CD95/APO-1) antigen induced by radiation: flow cytometry analysis of lymphoma and leukemia cell lines.
Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs.
Held HD, Uhlig S. Mechanisms of endotoxin-induced airway and pulmonary vascular hyperreactivity in mice.
Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays.
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis.
Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand.


![Ionizing radiation and histopathologic alterations of pneumonitis in wild-type and CD95/CD95L-deficient mice. A ) Images of histological sections. At days 1 and 21, histological sections were taken from lungs of sham-irradiated and irradiated C57BL6/J, lpr (CD95 deficient), and gld (CD95L deficient) mice; stained with hematoxillin and eosin; and analyzed at ×10 and ×40 ( inset ) magnification. B ) Semiquantitative histological analysis of pulmonary inflammation. In contrast to CD95/CD95L-deficient mice, C57BL6/J mice show a strongly elevated pulmonary inflammation sum score after irradiation. Histological sections were prepared at days 1, 21, 42, and 84 as described in Fig. 2, A and semiquantitatively analyzed to obtain the pulmonary inflammation score (numerical score consisting of alveolar wall thickness, interstitial edema, and interstitial and peribronchial inflammation). C57BL6/J—right and left lung: day 1, n.s.; days 21, 42, and 84, P <.001. gld —right and left lung: day 1, P <.001 (sum inflammation score statistically significantly lower); days 21, 42, and 84, n.s. lpr —right and left lung: days 1, 21, 42, and 84, n.s. C ) Differences in pulmonary inflammation score of irradiated and sham-irradiated littermates. On the basis of the data from Fig. 2, B the Δ inflammation score values ( Δ inflammation score = inflammation score [irradated mice] − inflammation score [sham-irradiated mice]) were calculated and plotted against time. For histopathological analysis at least 5 C57BL6/J, gld , and lpr mice per dose and time point were examined as indicated in more detail in Supplementary Data 3.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jnci/98/17/10.1093_jnci_djj335/2/m_jncidjj335f02_4c.jpeg?Expires=1716533413&Signature=xkVOu9Y9JTv8MgcPgOuM-r33a0ASKnkH9sodK6IQ1-FXFNhgVejgJyhm6fV-qfU16Z-EQsqgB~Sch2GXF2xeqIBXlMlRBcZsaoyQ-9HqQN9~Sxa4TezbPQ3fwsyGCuGS3ecNkTwej0Y-MUckWckvKD836TgKzDV5Zs9PNbIbhfTQZE5OTKZCySryp6c3e3jXlBaDC~M9QeNLIho2SsBr~X97-bN8SG4QsAZmoVCInxF6VGWCi1IqiBLwth1tBnyIuutxajoQ-N3lrda2GWFir7Qdtclx95SRXr14jGZ7p02EouGZf1HSEztWmmo-0HCMO2KDjRxUtClXzRuCdk25-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
